Abstract
Neural crest cells (NCC) can migrate into different parts of the body and express their strong inductive potential. In addition, they are multipotent and are able to differentiate into various cell types with diverse functions. In the primitive gut, NCC induce differentiation of muscular structures and interstitial cells of Cajal (ICC), and they themselves differentiate into the elements of the enteric nervous system (ENS), neurons and glial cells. ICC develop by way of mesenchymal cell differentiation in the outer parts of the primitive gut wall around the myenteric plexus (MP) ganglia, with the exception of colon, where they appear simultaneously also at the submucosal border of the circular muscular layer around the submucosal plexus (SMP) ganglia. However, in a complex process of reciprocal induction of NCC and local mesenchyma, c‐kit positive precursors are the first to differentiate, representing probably the common precursors of ICC and smooth muscle cells (SMC). C‐kit positive precursors could represent a key impact factor regarding the final differentiation of NCC into neurons and glial cells with neurons subsequently excreting stem cell factor (SCF) and other signalling molecules. Under the impact of SCF, a portion of c‐kit positive precursors lying immediately around the ganglia differentiate into ICC, while the rest differentiate into SMC.
Keywords: interstitial cells of Cajal, neural crest cells, enteric nervous system, human, digestive tract
| • Introduction |
| • Development of the ENS |
| • Interstitial cells of Cajal |
| • Differentiation of c‐kit positive cells in the human digestive tract |
| • Differentiation of ICC from c‐kit positive cells |
| • Conclusion |
| • Acknowledgements |
| • Conflict of interests |
Introduction
NCC migrate into different parts of the body and express their strong inductive potential (inducing differentiation of numerous structures) and multipotency (they themselves differentiate into various cell types with diverse functions). One of the sites of NCC migration is the digestive tube, where the cells from two different neural crest segments, vagal and sacral, can be found 1, 2, 3, 4. In the digestive tube, NCC induce differentiation of muscular structures and ICC along a rostrocaudal gradient, and they themselves differentiate into neurons and glial cells of the ENS 5, 6, 7, 8. Smooth muscle cells, ICC and enteric neurons are required for the onset of peristalsis, a prerequisite for normal bowel function. Some authors have suggested another possible migration wave of neural tube cells—the ventrally emigrating neural tube (VENT) cells into the foregut. Foregut is a part of primitive gut giving rise to the oesophagus, stomach and first part of duodenum. It is thought that VENT cells are able to differentiate into nerve, glial cells and intramuscular subtypes of ICC in these portions of the digestive tube 9, 10. Our hypothesis is that NCC induced mesenchymal cell differentiation into ICC, but in the manner that mesenchymal cells differentiated first into c‐kit positive precursors common for both ICC and SMC. C‐kit positive precursors could have an impact on the guidance of NCC migration, as well as on their final differentiation into neurons and glial cells and formation of myenteric ganglia. Neurons are one of the sources of SCF 11, 12 and other signalling molecules, determining differentiation of c‐kit positive precursors. Recent data have shown that besides the aforementioned Kit signalling pathway, there are additional signalling pathways which play a role in the differentiation and proliferation of ICC (neuronally derived nitric oxide, serotonin signalling through the 5‐HT2B receptor, interleukin 9, insulin and IGF‐1 signalling through stem cell factor) 13, 14, 15, 16. Under the influence of SCF, a portion of c‐kit positive precursors lying immediately around the ganglia differentiate into ICC, while the rest differentiate into SMC.
Development of the ENS
Most of the ENS develops from the vagal segment that arise at the level of somites 1–7, while the sacral (that lies caudally to somite 28) contributes to the ENS along the postumbilical gut 2, 17, 18, 19, 20. Vagal NCC leave NC at week 4 and populate the region of pharyngeal arches. They enter the posterior wall of the anterior gut, surrounding it and continue their rostrocaudal migration route 20, 21. NCC migrate into the external portion of the gut wall 6, 7, immediately beneath the serosa, in the form of an uninterrupted chain of cells that continues in the caudal direction 22, 23, 24. On their way, NCC intensely divide, interacting with the surrounding cells and differentiate into neurons and glial cells of the MP 7, 25, 26, 27. It has been proposed that NCC transit through several phenotypes before reaching the mature neuron form 28, with frontal cells retaining the NCC phenotype. These frontal cells coexpress Sox 10, RET, p75 and Phox2b, a NCC markers, but they do not express neuronal markers, confirming the assumption that the migrating frontal cells have not commenced their differentiation into neurons 23, 28, 29, 30, 31. Behind the undifferentiated NCC, cells are at different stages of differentiation, with neurons appearing before glial cells 28, 32. Several studies have shown that when NCC differentiate into neurons, they lose their migratory potential 33, 34.
The local mesoderm excretes a number of factors which primarily secure the survival of NCC, then prolong their proliferation and support their migration along the digestive tube; above all, these are GDNF (glial cell line‐derived neurotrophic factor), Endothelin‐3 (ET‐3), BMP2/4 and others 35, 36, 37, 38. Mesenchymal cells thus postpone differentiation of NCC into ganglia and enable their propagation along the primitive gut.
NCC contain the RET receptor 31, 39, 40, 41, mediating local mesodermal influences via GDNF production 26, 42, 43, providing thus the proliferation of frontal NCC. In particular, GDNF is not only a mitogenic, but also a chemotactic factor, determining rostrocaudal continuation of the NCC chain 44, 45, 46, 47. The wave of maximum GDNF expression shifts rostrocaudally and pulls along the tip of the NCC chain made of newly proliferated cells with the highest migratory potentials 33, 34, 48. Because GDNF attracts NCC, it is possible that the GDNF gradient is important in leading the advance of NCC down the gut 48.
A body of experimental data shows that neurons do not migrate, but that the majority of immature neurons are capable of migrating, although slower and at shorter distances compared to NCC 49. Neuroblasts are situated within the chain formed by migrating NCC 30, 32, 50, and they probably first slow down the migration and at a later stage fall behind and group together to form ganglia 51, 52. After a MP is formed, 2 or 3 weeks later, the ganglia of the SMP are formed in the human digestive tract. They develop during the secondary migration wave from the cells that migrate centripetally from the MP region 53, 54. The development of ENS in the oesophagus, stomach and small bowel follows this pattern, while the development in the large bowel is different. On their route through the digestive tube, migrating NCC stay in the caecum for a while and then continue their migration through the large bowel, following a somewhat different route 55. The reason for this arrest of NCC in the region of caecum is not known 56. It should be stressed that caecum has been the place of maximum GDNF and ET‐3 expression 43, 57, 58. While they migrate to the caecum in the form of an uninterrupted chain of cells localized in the outer portion of the wall, immediately beneath the serosa 23, 24, in the caecum and later in the colon, they continue their migration as individual, isolated cells that occassionally group together 55. A significant difference regarding the ENS development in the large bowel is also the fact that the cells of the sacral neural crest segment also contributes to the enteric neurons and glial cells of both the myenteric and the submucosal plexuses; sacral NCC migrate in the caudorostral direction and meet the vagal NCC 2, 22, 59, 60, 61.
Interstitial cells of Cajal
ICC are specialized network‐forming cells distributed within and around the smooth muscle wall of the digestive tract, capable of generating and propagating the electric slow waves 62, 63, 64. In addition to their pacemaker role, ICC are implicated in enteric neurotransmission and acting as stretch receptors in the gastrointestinal tract 65, 66, 67, 68. It has been shown that there are several ICC subtypes depending on their anatomical locations, morphologic and functional criteria as follows: ICC lying between the circular and longitudinal muscle layer and around the MP ganglia (termed the ICC‐MP subtype), ICC located in muscle bundles, between muscle cells (the ICC‐IM subtype), ICC situated along the submucosal margin of the circular muscle layer (the ICC‐SM subtype), ICC lying within the connective tissue septa which surround bundles of the muscle (the ICC‐SEP subtype) and ICC located in the small intestine at the level of the deep muscular plexus (the ICC‐DMP subtype). Throughout the digestive tube, the ICC lying around the MP ganglia (termed the ICC‐MP subtype) play the pacemaker role 62, 63, 69; only in the colon, in addition to ICC‐MP, the ICC‐SMP play the pacemaker role as well 70, 71, 72. Other ICC subtypes are functionally intercalated between the ENS and SMC 66, 73 or they function as mechanoreceptors 74, 75. Although it has been thought in the past that ICC represent a kind of neuron, it has been later reliably established that they are mesenchymal by origin 12, 30, 76, 77. ICC express the gene product of c‐kit, a proto‐oncogene that encodes the receptor tyrosine kinase. Stimulation of the Kit receptor (the natural ligand of which is SCF) is essential for their differentiation and survival 62, 78, 79. Most ICC subtypes, ICC‐MP and ICC‐SM included can be identified by labelling with c‐Kit antibody 80, 81. This fact has markedly facilitated ICC identification and made possible the study of their appearance in the wall of the digestive tube. In addition, VENT cells as well can have a role in the development of particular ICC subtypes (ICC‐IM) in the oesophagus, stomach and the first part of duodenum, portions of the digestive tube that arise from the foregut 9, 10.
Differentiation of c‐kit positive cells in the human digestive tract
During the development of human digestive tract, c‐kit positive cells, morphologically different from mature ICC, appear at the end of the embryonal period. C‐kit positive cells appear firstly in the oesophagus and stomach, then in the small bowel and finally in the large bowel 55, 82, 83, 84, 85. C‐kit positive cells emerge along the digestive tube following the rostrocaudal gradient, in the same way, NCC colonizing the digestive tube. What is important is that c‐kit positive cells appear just at the level of the NCC migration route, immediately around the chain formed by NCC during their migration along the digestive tube.
Consecutive longitudinal sections of the human embryonal oesophagus (Fig. 1A, B) showed that c‐kit positive cells were present exactly at the spot where the differentiation of the MP ganglia inception started and distally from that spot, that is, in the direction of NCC migration along the digestive tube. The inception of MP ganglia (Fig. 1A) and c‐kit positive cells (Fig. 1B) started to differentiate from the same proximal spots located in the posterior wall of the oesophagus. These c‐kit positive cells are much more abundant and morphologically different from the described mature ICC 82, 83, 84. C‐kit positive cells are very similar to blast cells, with a small pleomorphic body, containing a large nucleus and numerous but short cellular processes. They form a wide belt of cells in the outer portion of the wall of the oesophagus, stomach and first part of the duodenum and surround the NCC in the chain that represent future MP ganglia 55, 82, 83, 84. The environment is thus changed for all NCC in the chain of migration except for the frontal group of cells (newly proliferated NCC cells), which are the only ones remaining in direct contact with mesenchymal cells. Exactly at the front of the chain, there is the spot of maximum GDNF expression 43, 86 and that maximum shifts in the rostrocaudal direction following the prolonged migration wave. C‐kit positive cells probably reduce GDNF production and as they surround the NCC chain on the sides, they act as a kind of ‘funnel’ that orients chain continuation in the caudal direction. The cells in the chain slow down their migration and start to differentiate into neurons and glial cells and group together into inception of MP ganglia.
Figure 1.
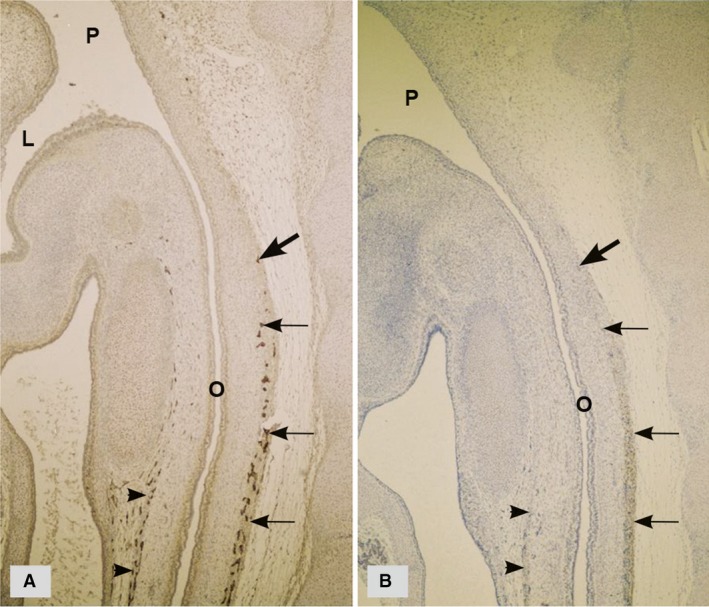
Sagittal sections of a human embryo at 8 weeks of development (two very close sections). (A) PGP9,5—immunohistochemistry; the inceptions of MP ganglia are numerous in the posterior wall (arrows) and slightly less abundant in the anterior of the primitive oesophagus (arrow heads). (B) c‐kit immunohistochemistry; c‐kit positive cells in the posterior (arrows) and anterior wall of the primitive oesophagus (arrow heads) form a wide belt of cells around the inceptions of MP ganglia. Large arrows indicate the spot from which MP ganglia (A) and c‐kit positive cells (B) started to differentiate, as both differentiate along the digestive tube following the rostrocaudal gradient. O, oesophagus; P, pharynx; L, larynx. (Radenkovic, unpublished data).
The appearance of c‐kit positive cells in the oesophagus, stomach and first part of duodenum follows this pattern 82, 83, 84. In the rest of the small bowel, all the way to the caecum, c‐kit positive cells appear in a similar way, although in the form of a very narrow chain of cells lying in the outer portion of the wall, immediately beneath the serosa, around the migrating NCC, that is inception of MP ganglia 84, 85, 87, 88. The difference in the appearance of c‐kit positive cells in the oesophagus, stomach and the first part of duodenum compared to the rest of the small bowel could perhaps be explained by the presence of VENT cells which populate only the foregut 9, 10. VENT cells could potentially contribute to that larger number of c‐kit positive cells observed in the organs developing from the foregut. A reduced number of c‐kit positive cells along the small intestine may be a result of the diminished proliferative capacity of NCC 47. However, this assumption requires confirmation by future studies.
The observed difference in the appearance of c‐kit positive cells in the large bowel is significant. In particular, c‐kit positive cells appear in the form of two parallel belts of cells, the first being situated around the inception of MP ganglia, and the second at the submucosal border of the circular muscle layer, around the inception og SMP ganglia 85, 89 (Fig. 2).
Figure 2.
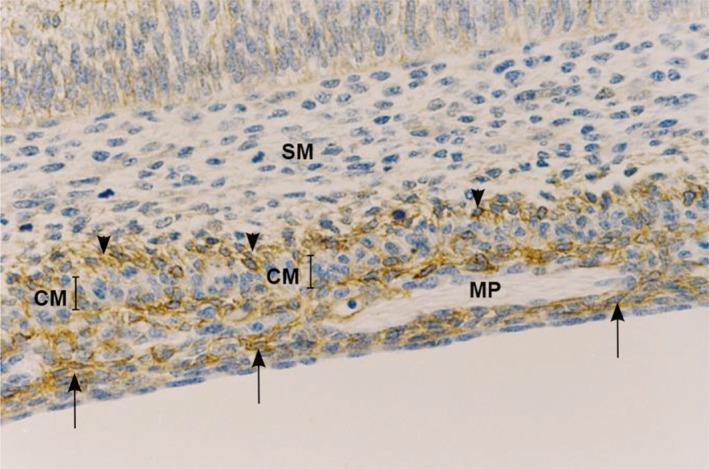
Longitudinal section of the proximal human colon at 9–10 weeks. C‐kit immunohistochemistry; c‐kit positive cells are present in the form of two parallel bands of cells: beneath the serosa, around the inception of MP ganglia (arrows) and at the submucosal border of the circular muscular layer, around the inception of SMP ganglia (arrow heads). MP, myenteric plexus; SM, submucosa; CM, circular muscle.
The reason for their simultaneous two‐level differentiation can be explained by the differences in the manner of migration of NCC in the colon compared to the rest of the digestive tube 55, as described in the previous chapter. Vagal NCC are scattered widely throughout the mesenchyme of the wall of the colon, and they migrate in a wide wave that covers almost the entire width of the wall from serosa to submucosa, although the presumptive submucosal region was sparsely populated 86, 89. Such a distribution is possible as muscle layers are still undifferentiated in the colon. We should be aware of the fact that in the colon, the cells from the sacral neural crest segment are involved in the development of ENS 59, 60, which may have an impact on the pattern of differentiation of c‐kit positive cells. A recent study has shown an additional pathway for NCC migration to the colon. Due to close proximity of the midgut and hindgut, some NCC pass directly from the midgut to the hindgut as isolated cells via the mesentery 90. We may say that the process of differentiation of c‐kit positive cells and their feedback impact on the slowing down of migration, differentiation and grouping of NCC into inception of ganglia takes place simultaneously at two levels in the colon, in the MP and in SMP regions.
Differentiation of ICC from c‐kit positive cells
In the weeks of development to follow, the number of c‐kit positive cells is markedly reduced, and the rest of them assume the morphological characteristics of mature ICC, as described in a later foetal period and after birth 82, 83, 84, 85. In the period when the number of c‐kit positive cells is being reduced, SMC of the longitudinal layer appear as well, so it seems possible that a part of c‐kit positive cells differentiate into SMC 84, 91, 92. This hypothesis is corroborated by the papers about ICC transdifferentiation, that is, the fact that in the absence of KIT receptor stimulation ICC can differentiate into SMC as well as the morphological and functional similarities between ICC and SMC 93, 94.
The probability exists as well that a some of these c‐kit positive cells differentiate into ICC and specifically into those lying immediately adjacent to the MP ganglia so that ICC‐MP cells are the first to appear in the oesophagus, stomach and small bowel [55, 82, 83, 84, 85, 88, 95]. They lie at the MP ganglia borders (Fig. 3), surrounding it entirely with their bodies and processes, but they do not present within the ganglia. Experimental data showed that enteric neurons express SCF 11, 12, 96. Expression of SCF by neurons might induce differentiation of c‐kit positive cells situated immediately around them into ICC‐MP. Taking into consideration the hypothesis by Huizinga and White, the above‐described c‐kit positive cells represent ICC precursors, which are capable of differentiating into ICC (depending on the Kit receptor stimulation), or in absence of such stimulation, into SMC 97. Recent data suggest that in the adult gut, there are precursor/stem cells the differentiation of which can produce not only ICC but also SMC and perhaps other cell types involved in the maintenance of ICC network 98. Chen et all. have proposed the hypothesis that some c‐kit positive cells maintain the characteristics of ICC progenitor cells after birth as well, thus being able to differentiate into ICC if there is a need 99. Recent data have shown that bone marrow‐derived Kit+ cells are able to repopulate the small intestine in response to intestinal injury 100. After MP ganglia differentiation, the neurons migrate centripetally through the circular layer to the submucosal border where they differentiate into SMP so that SMP appears 2–3 weeks after MP. ICC‐SMP appear 2–3 weeks after diferentiation of ICC‐MP 53, 55, 82, 83. This occurs throughout the digestive tube except in the colon, where ICC differentiation follows a different pattern. ICC‐MP and ICC‐SMP appear simultaneously in the colon, and the reason for this is that c‐kit positive cells simultaneously differentiate at the MP and SMP levels. As previously stated in the chapter about ICC, in all portions of the digestive tube, the ICC with pacemaker role 73, 101, 102 are the first to differentiate in all portions of the human digestive tube.
Figure 3.
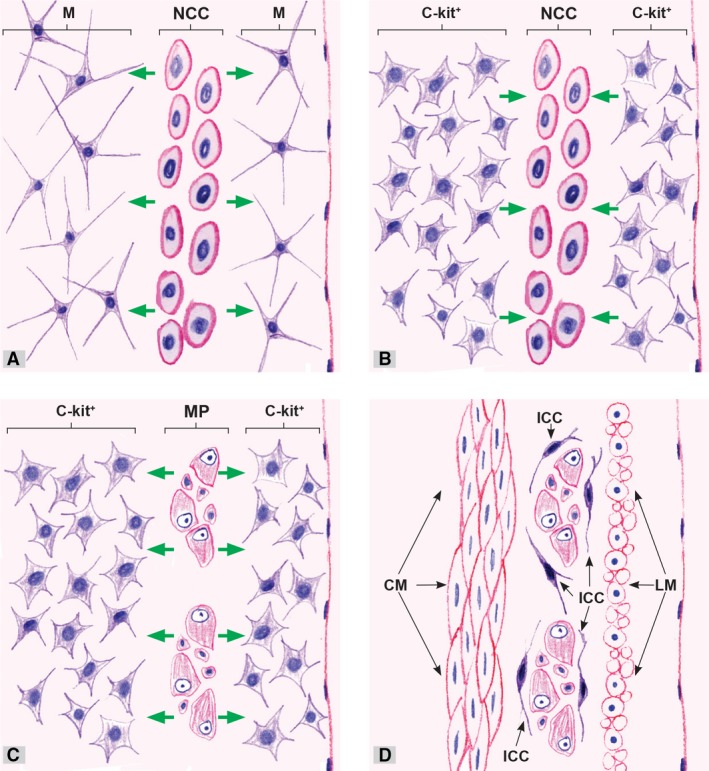
Possible pathways of interaction and reciprocal induction of NCC and mesenchymal cells in the wall of the human primitive gut. (A) NCC populate the outer portion of the wall of the primitive gut and under the action of growth factors (GDNF, ET‐3, BMP2/4) excreted by mesenchymal cells they rapidly proliferate. At the same time, NCC induce differentiation of the adjacent mesenchymal cells into c‐kit positive cells. (B) c‐kit positive cells probably reduce GDNF production and induce differentiation of NCC into neurons (cells with a neuronal phenotype) and glial cells, which slow down their migration and group to form the inception of MP ganglia. (C) Neurons as one of the sources of STF induce differentiation of c‐kit positive cells situated immediately around them into ICC‐MP. (D) The rest of c‐kit positive cells differentiate into SMC, mostly of the longitudinal layer. M, mesenchymal cells; NCC, neural crest cells (NCC); c‐kit+, c‐kit positive cells; MP, myenteric plexus; ICC, interstitial cells of Cajal; CM, circular muscle; LM, longitudinal muscle.
During the NCC migration along the digestive tube, they induce differentiation of mesenchymal cell into c‐kit positive precursors. These precursors perhaps induce NCC differentiation into neurons and glial cells. The impact of c‐kit positive precursors on NCC cannot be definitely confirmed at the moment, but experimental findings have indicated the possibility. Zhao et all. demonstrated in vitro that ICC can promote the differentiation of neuroepithelial stem cells into neurons 103. These observations suggest that c‐kit positive precursors can affect the differentiation of NCC into mature neurons during the development of the human ENS.
Conclusion
In conclusion, NCC have a decisive impact on the differentiation of ICC in the human digestive tract. Nevertheless, in a complex process of reciprocal induction of NCC and local mesenchyma, c‐kit positive precursors are the first to differentiate, representing probably the common precursors of ICC and SMC. These precursors could have an impact on NCC differentiation into neurons and glial cells. Finally, neurons induce differentiation of c‐kit positive precursors lying immediately around them into mature ICC, while the remaining precursors, in an absence of KIT stimulation, differentiate into SMC.
Conflict of interest
The authors declare that they have no conflict of interests.
Acknowledgements
This work was supported by the Internal project of Medical faculty of Nis number 22.
References
- 1. Yntema CL, Hammond WS. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J Comp Neurol. 1954; 101: 515–41. [DOI] [PubMed] [Google Scholar]
- 2. Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973; 30: 31–48. [PubMed] [Google Scholar]
- 3. Pomeranz HD, Gershon MD. Colonization of the avian hindgut by cells derived from the sacral neural crest. Dev Biol. 1990; 137: 378–94. [DOI] [PubMed] [Google Scholar]
- 4. Serbedzija GN, Burgan S, Fraser SE, et al Vital dye labelling demonstrates a sacral neural crest contribution to the enteric nervous system of chick and mouse embryos. Development. 1991; 111: 857–66. [DOI] [PubMed] [Google Scholar]
- 5. Sãnchez MP, Silos‐Santiago I, Frisén J, et al Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996; 382: 70–3. [DOI] [PubMed] [Google Scholar]
- 6. Kapur RP. Colonization of the murine hindgut by sacral crest‐derived neural precursors: experimental support for an evolutionarily conserved model. Dev Biol. 2000; 227: 146–55. [DOI] [PubMed] [Google Scholar]
- 7. Young HM, Newgreen D. Enteric neural crest‐derived cells: origin, migration and differentiation. Anat Rec. 2001; 262: 1–15. [DOI] [PubMed] [Google Scholar]
- 8. Fu M, Chi Hang Lui V, Har Sham M, et al HOXB5 expression is spatially and temporarily regulated in human embryonic gut during neural crest cell colonization and differentiation of enteric neuroblasts. Dev Dyn. 2003; 228: 1–10. [DOI] [PubMed] [Google Scholar]
- 9. Dickinson DP, Machnicki M, Ali MM, et al Ventrally emigrating neural tube (VENT) cells: a second neural tube‐derived cell population. J Anat. 2004; 205: 79–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sohal GS, Ali MM, Farooqui FA. A second source of precursor cells for the developing enteric nervous system and interstitial cells of Cajal. Int J Dev Neurosci. 2002; 20: 619–26. [DOI] [PubMed] [Google Scholar]
- 11. Torihashi S, Yoshida H, Nishikawa S, et al Enteric neurons express Steel factor‐lacZ transgene in the murine gastrointestinal tract. Brain Res. 1996; 738: 323–8. [DOI] [PubMed] [Google Scholar]
- 12. Wu JJ, Rothman TP, Gershon MD. Development of the interstitial cell of Cajal: origin, kit dependence and neuronal and nonneuronal sources of kit ligand. J Neurosci Res. 2000; 59: 384–401. [DOI] [PubMed] [Google Scholar]
- 13. Ye J, Zhu Y, Khan WI, et al IL‐9 enhances growth of ICC, maintains network structure and strengthens rhythmicity of contraction in culture. J Cell Mol Med. 2006; 10: 687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horvath VJ, Vittal H, Lorincz A, et al Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006; 130: 759–70. [DOI] [PubMed] [Google Scholar]
- 15. Choi KM, Gibbons SJ, Nguyen TV, et al Heme oxygenase‐1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008; 135: 2055–64. 2064 e1–2064 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wouters MM, Gibbons SJ, Roeder JL, et al Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5‐HT2B receptors. Gastroenterology. 2007; 133: 897–906. [DOI] [PubMed] [Google Scholar]
- 17. Hearn C, Newgreen D. Lumbo‐sacral neural crest contributes to the avian enteric nervous system independently of vagal neural crest. Dev Dyn. 2000; 218: 525–30. [DOI] [PubMed] [Google Scholar]
- 18. Burns AJ, Le Douarin NM. Enteric nervous system development: analysis of the selective developmental potentialities of vagal and sacral neural crest cells using quail–chick chimeras. Anat Rec. 2001; 262: 16–28. [DOI] [PubMed] [Google Scholar]
- 19. Burns AJ. The migration of neural crest‐derived enteric nervous system precursor cells to and within the gastrointestinal tract. Int J Dev Biol. 2005; 49: 143–50. [DOI] [PubMed] [Google Scholar]
- 20. Burns AJ, Le Douarin NM. The sacral neural crest contributes neurons and glia to the post‐umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development. 1998; 125: 4335–47. [DOI] [PubMed] [Google Scholar]
- 21. Kapur R, Yost C, Palmiter R. A transgenic model for studying development of the enteric nervous system in normal and aganglionic mice. Development. 1992; 116: 167–75. [DOI] [PubMed] [Google Scholar]
- 22. Pomeranz HD, Rothman TP, Gershon MD. Colonization of the post‐umbilical bowel by cells derived from the sacral neural crest: direct tracing of cell migration using an intercalating probe and a replication‐deficient retrovirus. Development. 1991; 111: 647–55. [DOI] [PubMed] [Google Scholar]
- 23. Young HM, Hearn CJ, Ciampoli D, et al A single rostrocaudal colonization of the rodent intestine by enteric neuron precursors is revealed by the expression of Phox2b, Ret, and p75 and by explants grown under the kidney capsule or in organ culture. Dev Biol. 1998; 202: 67–84. [DOI] [PubMed] [Google Scholar]
- 24. Conner PJ, Focke PJ, Noden DM, et al Appearance of neurons and glia with respect to the wavefront during colonization of the avian gut by neural crest cells. Dev Dyn. 2003; 226: 91–8. [DOI] [PubMed] [Google Scholar]
- 25. Heuckeroth RO, Lampe PA, Johnson EM, et al Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro . Dev Biol. 1998; 200: 116–29. [DOI] [PubMed] [Google Scholar]
- 26. Young HM, Hearn CJ, Farlie CJ, et al GDNF is a chemoattractant for enteric neural cells. Dev Biol. 2001; 229: 503–16. [DOI] [PubMed] [Google Scholar]
- 27. Gershon MD. Developmental determinants of the independence and complexity of the enteric nervous system. Trends Neurosci. 2010; 33: 446–56. [DOI] [PubMed] [Google Scholar]
- 28. Young HM, Bergner AJ, Muller T. Acquisition of neuronal and glial markers by neural crest‐derived cells in the mouse intestine. J Comp Neurol. 2003; 456: 1–11. [DOI] [PubMed] [Google Scholar]
- 29. Southard‐Smith EM, Kos L, Pavan WJ. Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat Genet. 1998; 18: 60–4. [DOI] [PubMed] [Google Scholar]
- 30. Young HM, Ciampoli D, Hsuan J, et al Expression of Ret‐, p75(NTR)‐, Phox2a, Phox2b‐, and tyrosine hydroxylase‐immunoreactivity by undifferentiated neural crest‐derived cells and different classes of enteric neurons in the embryonic mouse gut. Dev Dyn. 1999; 216: 137–52. [DOI] [PubMed] [Google Scholar]
- 31. Anderson RB, Stewart AL, Young HM. Phenotypes of neural‐crest‐derived cells in vagal and sacral pathways. Cell Tissue Res. 2006; 323: 11–25. [DOI] [PubMed] [Google Scholar]
- 32. Hao MM, Young HM. Development of enteric neuron diversity. J Cell Mol Med. 2009; 13: 1193–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hearn CJ, Murphy M, Newgreen D. GDNF and ET‐3 differentially modulate the numbers of avian enteric neural crest cells and enteric neurons in vitro . Dev Biol. 1998; 197: 93–105. [DOI] [PubMed] [Google Scholar]
- 34. Wu JJ, Chen J‐X, Rothman TP, et al Inhibition of in vitro enteric neuronal development by endothelin‐3: mediation by endothelin B receptors. Development. 1999; 126: 1161–73. [DOI] [PubMed] [Google Scholar]
- 35. Baynash AG, Hosoda K, Giaid A, et al Interaction of endothelin‐3 with endothelin‐B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994; 79: 1277–85. [DOI] [PubMed] [Google Scholar]
- 36. Hosoda K, Hammer RE, Richardson JA, et al Targeted and natural (piebald‐lethal) mutations of endothelin‐B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994; 79: 1267–76. [DOI] [PubMed] [Google Scholar]
- 37. Puffenberger EG, Hosoda K, Washington SS, et al A missense mutation of the endothelin‐B receptor gene in multigenic Hirschsprung's disease. Cell. 1994; 79: 1257–66. [DOI] [PubMed] [Google Scholar]
- 38. Goldstein AM, Brewer KC, Doyle AM, et al BMP signaling is necessary for neural crest cell migration and ganglion formation in the enteric nervous system. Mech Dev. 2005; 122: 821–33. [DOI] [PubMed] [Google Scholar]
- 39. Pachnis V, Mankoo B, Costantini F. Expression of the c‐ret proto‐oncogene during mouse embryogenesis. Development. 1993; 119: 1005–17. [DOI] [PubMed] [Google Scholar]
- 40. Durbec PL, Larsson‐Blomberg LB, Schuchardt A, et al Common origin and developmental dependence on c‐ret of subsets of enteric and sympathetic neuroblasts. Development. 1996; 122: 349–58. [DOI] [PubMed] [Google Scholar]
- 41. Attie‐Bitach T, Abitbol M, Gerard M, et al Expression of the RET proto‐oncogene in human embryos. Am J Med Genet. 1998; 80: 481–6. [DOI] [PubMed] [Google Scholar]
- 42. Hellmich HL, Kos L, Cho ES, et al Embryonic expression of glial cell‐line derived neurotrophic factor (GDNF) suggests multiple developmental roles in neural differentiation and epithelial‐mesenchymal interactions. Mech Dev. 1996; 54: 95–105. [DOI] [PubMed] [Google Scholar]
- 43. Natarajan D, Marcos‐Gutierrez C, Pachnis V, et al Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous progenitor cells during mammalian embryogenesis. Development. 2002; 129: 5151–60. [DOI] [PubMed] [Google Scholar]
- 44. Moore MW, Klein RD, Fariñas I, et al Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996; 382: 76–9. [DOI] [PubMed] [Google Scholar]
- 45. Pichel JG, Shen L, Sheng HZ, et al Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996; 382: 73–6. [DOI] [PubMed] [Google Scholar]
- 46. Taraviras S, Marcos‐Gutierrez CV, Durbec P, et al Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development. 1999; 126: 2785–97. [DOI] [PubMed] [Google Scholar]
- 47. Gianino S, Grider JR, Cresswell J, et al GDNF availability determines enteric neuron number by controllingprecursor proliferation. Development. 2003; 130: 2187–98. [DOI] [PubMed] [Google Scholar]
- 48. Chalazonitis A, Rothman TP, Chen J, et al Age‐dependent differences in the effects of GDNF and NT‐3 on the development of neurons and glia from neural crest‐derived precursors immunoselected from the fetal rat gut: Expression of GFRalpha‐1 in vitro and in vivo . Dev Biol. 1998; 204: 385–406. [DOI] [PubMed] [Google Scholar]
- 49. Hao MM, Anderson RB, Kobayashi K, et al The migratory behaveior of immature enteric neurons. Dev Neurobiol. 2009; 69: 22–35. [DOI] [PubMed] [Google Scholar]
- 50. Baetge G, Gershon MD. Transient catecholaminergic (TC) cells in the vagus nerves and bowel of fetalmice: relationship to the development of enteric neurons. Dev Biol. 1989; 132: 189–211. [DOI] [PubMed] [Google Scholar]
- 51. Faure C, Chalazonitis A, Rheaume C, et al Gangliogenesis in the enteric nervous system: roles of the polysialylation of the neural cell adhesion molecule and its regulation by bone morphogenetic protein ‐4. Dev Dyn. 2007; 236: 44–59. [DOI] [PubMed] [Google Scholar]
- 52. Fu M, Vohra BP, Wind D, et al BMP signaling regulates murine enteric nervous system precursor migration, neurite fasciculation and patterning via altered Ncam1 polysialic acid addition. Dev Biol. 2006; 299: 137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jiang Y, Liu MT, Gershon MD. Netrins and DCC in the guidance of migrating neural crest‐derived cells in the developing bowel and pancreas. Dev Biol. 2003; 258: 364–84. [DOI] [PubMed] [Google Scholar]
- 54. Fu M, Tam PK, Sham MH, et al Embryonic development of the ganglion plexuses and the concentric layer structure of human gut: a topographical study. Anat Embryol. 2004; 208: 33–41. [DOI] [PubMed] [Google Scholar]
- 55. Wallace AS, Burns AJ. Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res. 2005; 319: 367–82. [DOI] [PubMed] [Google Scholar]
- 56. Druckenbrod NR, Epstein ML. The pattern of neural crest advance in the cecum and colon. Dev Biol. 2005; 287: 125–33. [DOI] [PubMed] [Google Scholar]
- 57. Leibl MA, Ota T, Woodward MN. Expression of endothelin 3 by mesenchymal cells of embryonic mouse caecum. Gut. 1999; 44: 246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Barlow A, de Graaff E, Pachnis V. Enteric nervous system progenitors are coordinately controlled by the G protein‐coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron. 2003; 40: 905–16. [DOI] [PubMed] [Google Scholar]
- 59. Burns AJ, Champeval D, Le Douarin NM. Sacral neural crest cells colonise aganglionic hindgut in vivo but fail to compensate for lack of enteric ganglia. Dev Biol. 2000; 219: 30–43. [DOI] [PubMed] [Google Scholar]
- 60. Burns AJ, Delalande JM, Douarin NM. In ovo transplantation of enteric nervous system precursors from vagal to sacral neural crest results in extensive hindgut colonisation. Development. 2002; 129: 2785–96. [DOI] [PubMed] [Google Scholar]
- 61. Wang X, Chan AKK, Sham MH, et al Analysis of the sacral neural crest cell contribution to the hindgut enteric nervous system in the mouse embryo. Gastroenterology. 2011; 141: 992–1002. [DOI] [PubMed] [Google Scholar]
- 62. Ward SM, Burns AJ, Torihashi S, et al Mutation of the proto‐oncogene c‐kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol (Lond). 1994; 480: 91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Huizinga JD, Thuneberg L, Kluppel M, et al W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995; 373: 347–9. [DOI] [PubMed] [Google Scholar]
- 64. Sanders KM, Koh SD, Ordog T, et al Ionic conductances involved in generation and propagation of electrical slow waves in phasic gastrointestinal muscles. Neurogastro Mot. 2004; 16: 100–5. [DOI] [PubMed] [Google Scholar]
- 65. Burns AJ, Lomax AEJ, Torihashi S, et al Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Nat Acad Sci USA. 1996; 93: 12008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ward SM, Beckett EAH, Wang X‐Y, et al Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000; 20: 1393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Beckett EAH, Horiguchi K, Khoyi M, et al Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sl (d) mice. J Physiol. 2002; 543: 871–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Suzuki H, Ward SM, Bayguinov YR, et al Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J Physiol. 2003; 546: 751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Torihashi S, Ward SM, Nishikawa S‐I, et al c‐kit‐dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tiss Res. 1995; 280: 97–111. [DOI] [PubMed] [Google Scholar]
- 70. Smith TK, Reed JB, Sanders KM. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am J Physiol. 1987; 252: C215–24. [DOI] [PubMed] [Google Scholar]
- 71. Barajas‐Lopez C, Huizinga JD. Different mechanisms of contraction generation in circular muscle of canine colon. Am J Physiol. 1989; 256: G570–80. [DOI] [PubMed] [Google Scholar]
- 72. Rae MG, Fleming N, McGregor DB. Control of motility patterns in the human colonic circular muscle layer by pacemaker activity. J Physiol (Lond). 1998; 510: 309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ward SM, Morris G, Reese L, et al Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998; 115: 314–29. [DOI] [PubMed] [Google Scholar]
- 74. Fox EA, Phillips RJ, Byerly MS, et al Selective loss of vagal intramuscular mechanoreceptors in mice mutant for steel factor, the c‐Kit receptor ligand. Anat Embryol (Berl). 2002; 205: 325–42. [DOI] [PubMed] [Google Scholar]
- 75. Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci USA. 2005; 102: 14913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lecoin L, Gabella G, LeDouarin N. Origin of the c‐kit‐positive interstitial cells in the avian bowel. Development. 1996; 122: 725–33. [DOI] [PubMed] [Google Scholar]
- 77. Young HM, Ciampoli D, Southwell BR, et al Origin of interstitial cells of Cajal in the mouse intestine. Dev Biol. 1996; 180: 97–107. [DOI] [PubMed] [Google Scholar]
- 78. Zsebo KM, Williams DA, Geissler EN, et al Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c‐kit tyrosine kinase receptor. Cell. 1990; 63: 213–24. [DOI] [PubMed] [Google Scholar]
- 79. Ward SM, Burns AJ, Torihashi S, et al Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995; 269: C1577–85. [DOI] [PubMed] [Google Scholar]
- 80. Maeda H, Yamagata A, Nishikawa S, et al Requirement of c‐kit for development of intestinal pacemaker system. Development. 1992; 116: 369–75. [DOI] [PubMed] [Google Scholar]
- 81. Komuro T, Tokui K, Zhou DS. Identification of the interstitial cells of Cajal. Histol Histopathol. 1996; 11: 769–86. [PubMed] [Google Scholar]
- 82. Radenkovic G, Savic V, Mitic D, et al Development of c‐kit immunopositive interstitial cells of Cajal in the human stomach. J Cell Mol Med. 2010; 14: 1125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Radenkovic G, Ilic I, Zivanovic D, et al C‐kit‐immunopositive interstitial cells of Cajal in human embryonal and fetal oesophagus. Cell Tissue Res. 2010; 340: 427–36. [DOI] [PubMed] [Google Scholar]
- 84. Radenkovic G. Two patterns of development of interstitial cells of Cajal in the human duodenum. J Cell Mol Med. 2012; 16: 185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Abramovic M, Radenkovic G, Velickov A. Appearance of interstitial cells of Cajal in the human midgut. Cell Tissue Res. 2014; 356: 9–14. [DOI] [PubMed] [Google Scholar]
- 86. Wang H, Hughes I, Planer W, et al The timing and location of glial cell line‐derived neurotrophic factor expression determine enteric nervous system structure and function. J Neurosci. 2010; 30: 1523–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Faussone‐Pellegrini MS, Vannucchi MG, Alaggio R, et al Morphology of the interstitial cells of Cajal of the human ileum from foetal to neonatal life. J Cell Mol Med. 2007; 11: 482–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wester T, Eriksson L, Olsson Y, et al Interstitial cells of Cajal in the human fetal small bowel as shown by c‐kit immunohistochemistry. Gut. 1999; 44: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Radenkovic G, Abramovic M. Differantiation of interstitial cells of Cajal in the human distal colon. Cells Tissues Organs. 2012; 196: 463–9. [DOI] [PubMed] [Google Scholar]
- 90. Nishiyama C, Uesaka T, Manabe T, et al Trans‐mesenteric neural crest cells are the principal source of the colonic enteric nervous system. Nat Neurosci. 2012; 15: 1211–8. [DOI] [PubMed] [Google Scholar]
- 91. Torihashi S, Ward SM, Sanders KM. Development of c‐Kit‐positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology. 1997; 112: 144–55. [DOI] [PubMed] [Google Scholar]
- 92. Kluppel M, Huizinga JD, Malysz J, et al Developmental origin and Kit‐dependent development of the interstitial cells of cajal in the mammalian small intestine. Dev Dyn. 1998; 211: 60–71. [DOI] [PubMed] [Google Scholar]
- 93. Mei F, Han J, Huang Y, et al Plasticity of interstitial cells of cajal: a study in the small intestine of adult Guinea pigs. Anat Rec(Hoboken). 2009; 292: 985–93. [DOI] [PubMed] [Google Scholar]
- 94. Torihashi S, Nishi K, Tokutomi Y, et al Blockade of kit signaling induces transdifferentiation of interstitial cells of cajal to a smooth muscle phenotype. Gastroenterology. 1999; 117: 140–8. [DOI] [PubMed] [Google Scholar]
- 95. Kenny SE, Connell G, Woodward MN, et al Ontogeny of interstitial cells of Cajal in the human intestine. J Pediatr Surg. 1999; 34: 1241–7. [DOI] [PubMed] [Google Scholar]
- 96. Young HM, Torihashi S, Ciampoli D, et al Identification of neurons that express stem cell factor in the mouse small intestine. Gastroenterology. 1998; 115: 898–908. [DOI] [PubMed] [Google Scholar]
- 97. Huizinga JD, White EJ. Progenitor cells of interstitial cells of Cajal: on the road to tissue repair. Gastroenterology. 2008; 134: 1252–4. [DOI] [PubMed] [Google Scholar]
- 98. Lorincz A, Redelman D, Horvath VJ, et al Progenitors of interstitial cells of cajal in the postnatal murine stomach. Gastroenterology. 2008; 134: 1083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chen ZH, Zhang YC, Jiang WF, et al Characterization of Interstitial Cajal Progenitors Cells and Their Changes in Hirschsprung's Disease. PLoS One. 2014; 9: e86100 https://doi.org/10.1371/journal.pone.0086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Liu D, Wang F, Zou Z, et al Bone marrow derivation of interstitial cells of cajal in small intestine following intestinal injury. J Biomed Biotechnol. 2010; 2010: 164986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pluja L, Alberti E, Fernandez E, et al Evidence supporting presence of two pacemakers in rat colon. Am J Physiol Gastrointest Liver Physiol. 2001; 281: G255–66. [DOI] [PubMed] [Google Scholar]
- 102. Faussone‐Pellegrini MS, Vannucchi MG, Ledder O, et al Plasticity of interstitial cells of Cajal: a study of mouse colon. Cell Tissue Res. 2006; 325: 211–7. [DOI] [PubMed] [Google Scholar]
- 103. Zhao B, Liu W, Wu R. Co‐culture of neuroepithelial stem cells with interstitial cells of Cajal results in neuron differentiation. Int J Clin Exp Med. 2015; 8: 10437–43. [PMC free article] [PubMed] [Google Scholar]


