Abstract
Leptin is now considered an important signalling molecule of the reproductive system, as it regulates the production of gonadotrophins, the blastocyst formation and implantation, the normal placentation, as well as the foeto‐placental communication. Leptin is a peptide hormone secreted mainly by adipose tissue, and the placenta is the second leptin‐producing tissue in humans. Placental leptin is an important cytokine which regulates placental functions in an autocrine or paracrine manner. Leptin seems to play a crucial role during the first stages of pregnancy as it modulates critical processes such as proliferation, protein synthesis, invasion and apoptosis in placental cells. Furthermore, deregulation of leptin levels has been correlated with the pathogenesis of various disorders associated with reproduction and gestation, including polycystic ovary syndrome, recurrent miscarriage, gestational diabetes mellitus, pre‐eclampsia and intrauterine growth restriction. Due to the relevant incidence of the mentioned diseases and the importance of leptin, we decided to review the latest information available about leptin action in normal and pathological pregnancies to support the idea of leptin as an important factor and/or predictor of diverse disorders associated with reproduction and pregnancy.
Keywords: leptin, reproduction, placenta, polycystic ovary syndrome, recurrent miscarriage, pre‐eclampsia, gestational diabetes, growth restriction
|
• Introduction • Leptin mediates the crosstalk between adipose tissue and reproduction • Role of leptin in placenta development • Leptin as an immunomodulator during pregnancy • Leptin and pathologies associated with pregnancy ‐ Polycystic ovary syndrome ‐ Recurrent miscarriage |
‐ Gestational diabetes mellitus ‐ Pre‐eclampsia ‐ Intrauterine growth restriction • Conclusions • Acknowledgements • Conflicts of interests |
Introduction
Adipose tissue acts as an endocrine organ, secreting different molecules or adipokines 1. Leptin is produced and secreted predominantly from adipose tissue into the circulation. Circulating leptin levels reflect adipose tissue size and also change with nutritional state 2. Furthermore, leptin is considered as a pleiotropic hormone that regulates not only bodyweight but many other functions, including vascular function, bone and cartilage growth, immune system and systemic inflammatory response as well as the normal physiology of the reproductive system 3, 4.
A link between bodyweight, adipokines and success of pregnancy has been proposed, although it is not fully understood 5, 6, 7. The observations that human and rodents with congenital leptin deficiencies are sterile and that anorexia and obesity modify the onset of puberty in opposite ways, led to the idea that leptin is an important player in reproduction 8. In this way, leptin was the first adipokine claimed to be the ‘missing link’ between fat and reproduction 9.
Leptin mediates its effects by binding to leptin receptors (LepRs) expressed in the brain and peripheral tissues 2. Different variants of LepR have been described, but the long isoform of LepR (LepRb) is primarily responsible for leptin signalling. LepRb is strongly expressed in specific nuclei of the hypothalamus, a region of the brain that is involved in the control of appetite, and there it regulates energy homoeostasis and neuroendocrine function, among other functions 10. In addition, leptin has direct effects on many cell types on the periphery. LepRb is expressed in lung, kidney, adipocytes, endothelial cells, blood cells, stomach, muscle, liver, pancreatic islets, osteoblast, endometrium, placenta and umbilical cord 2, 11.
Leptin or LepR deficiencies not only cause severe obesity but also abnormalities in haematopoiesis, immunity, angiogenesis, bone formation, blood pressure and reproduction. Mutations in the leptin gene, in human and/or mouse models, result in infertility or significant reproductive dysfunction 8, 12. Leptin is required for the release of gonadotrophin‐releasing hormone (GnRH) from the pituitary, and as a consequence, female ob/ob mice (deficient in leptin) have reduced oestrogen levels and exhibit low uterine weight 13, 14. Male ob/ob mice also show reduced GnRH levels and diminished production of luteinizing hormone (LH) and follicle‐stimulating hormone (FSH) as well as testosterone, an essential hormone for the maintenance of male fertility and testicular function 15.
Therefore, leptin can act as metabolic switch connecting the nutritional status of the body to high energy consuming processes. The energy requirements of pregnancy are those desired for correct maternal gain to ensure the growth of the foetus, placenta and associated maternal tissues 12. Another key observation that built on the link between leptin and reproduction is the secretion of leptin from human placenta, further establishing an association between leptin and pregnancy 8, 16. Placental formation during human gestation is crucial for embryonic progress and successful pregnancy outcome, allowing metabolic exchange and production of steroids, hormones, growth factors and cytokines that are critical for the maintenance of pregnancy 17, 18. Trophoblast cells play an essential role in the development of placenta. These cells differentiate into two distinct types: extravillous and villous trophoblast. In the extravillous pathway, cytotrophoblasts (CT) proliferate, differentiate into an invasive phenotype and penetrate into the maternal decidua and myometrium. Meanwhile, in the villous pathway, mononuclear CT fuse to form a specialized multinuclear syncytium called syncytiotrophoblast (ST) 19.
In normal pregnancy, trophoblast invasion is a critical step in remodelling the maternal spiral arteries to adequately perfuse the developing placenta and foetus 20. Failure of invasion processes may lead to miscarriage or pregnancy disorders such as pre‐eclampsia (PE) or intrauterine growth restriction (IUGR) 21, 22. In this sense, deregulation of leptin levels has been implicated in the pathogenesis of various disorders of reproduction and gestation, such as polycystic ovary syndrome (PCOS), recurrent miscarriage, gestational diabetes mellitus (GDM), PE and IUGR 23.
Leptin mediates the crosstalk between adipose tissue and reproduction
Reproductive function depends on the energy reserves stored in adipose tissue and the reproductive system. The large energy needs for pregnancy was the original rationale to explain the disruption of reproductive function by low fat reserves 24. This led to the hypothesis of an endocrine signal that conveys information to the brain about the size of fat stores 25. Thus, leptin was the first adipokine claimed to be the ‘missing link’ between fat and reproduction 9. Leptin modulates satiety and energy homoeostasis 26, 27, but is also produced by placenta. Thus, it was suggested that the effects of placental leptin on the mother may contribute to endocrine‐mediated alterations in energy balance, such as the mobilization of maternal fat, which occurs during the second half of pregnancy 28, 29. In addition, leptin has been found to influence several reproductive functions, including embryo development and implantation 30. Moreover, animal models have demonstrated that leptin‐deficient mice are infertile, and fertility can be restored by exogenous leptin 31. This adipokine may therefore play a critical role in regulating both energy homoeostasis and the reproductive system 32.
Leptin increases the secretion of gonadotrophin hormones, by acting centrally at the hypothalamus 33. In addition, because leptin has been shown to be influenced by steroid hormones and can stimulate LH release, leptin may act as a permissive factor in the development of puberty 34.
Leptin can also regulate ovary functions 35, 36, 37, 38. Thus, leptin resistance and/or hyperleptinaemia in obesity lead to altered follicle function, whereas conditions in which nutritional status is suboptimal, leptin deficiency results in hypothalamic–pituitary gonadal axis dysfunction 39, 40.
In addition, a significant role of leptin in embryo implantation was proposed. Leptin and leptin receptor are specifically expressed at the blastocyst stage 41, and it was also reported that leptin is present in conditioned media from human blastocysts, promoting embryo development, suggesting a function in the blastocyst–endometrial dialog 42.
Role of leptin in placenta development
The implantation process involves complex and synchronized molecular and cellular interplay between the uterus and the implanting embryo, and these events are regulated by paracrine and autocrine factors 18. In 1997, leptin was described as a new placental hormone in humans 29. Circulating leptin levels are significantly increased during pregnancy and decreased after birth, revealing an important role of leptin during gestation 43, 44. Placental production of leptin is one of major source of higher levels in maternal circulating leptin other than maternal gain of fat mass 45. Leptin is now considered an important regulator during the first stages of pregnancy which has physiological effects on the placenta, including angiogenesis, growth and immunomodulation 28, 46, 47, 48, 49, 50, 51. Figure 1 highlights the main actions of leptin in the maternal–foetal interface.
Figure 1.

Leptin action during the first stages of pregnancy. Leptin seems to play a crucial role during the first stages of pregnancy as it modulates critical processes such as implantation and placentation, as well as foetus development. The figure summarizes the most important actions of leptin during early gestation (green), highlighting its role in the maternal–foetal interface. It is also shown the different type of cell which expresses leptin receptor (LEPR) and produce leptin (violet). CT: cytotrophoblast, ST: syncytiotrophoblasts.
The control of cell proliferation is critical for a correct placental development, and it is finely regulated 52. During placentation, CT and ST keep a subset of cells in direct contact to the villous basement membranes. In the extravillous compartment, cell proliferation favours the invasion of the uterine stroma. Similarly, in the villous compartment cells undergo syncytial fusion directed by specific transcription factors 53. It was shown that leptin induces proliferative activity in many human cell types 54, 55, 56, via mitogen‐activated protein kinase (MAPK) activation 57. We have demonstrated that leptin promotes proliferation of trophoblast cells by this MAPK pathway 46, 51 and stimulates protein synthesis by the activation of translation machinery in trophoblastic JEG‐3 cells, a human placenta choriocarcinoma cell line 47, 58.
In this line, multiple signal transduction pathways are activated in response to leptin both in trophoblastic JEG‐3 cell culture and in human term placenta 46. Leptin receptor requires activation of receptor‐associated kinases of the Janus family (JAK) 59. After ligand binding, JAKs autophosphorylate and tyrosine phosphorylate various signal transducers and activators of transcription (STATs). In this context, leptin is able to stimulate Janus kinase (JAK)–STAT pathway by mainly promoting JAK‐2, the most important JAK isoform to mediate physiological effects of leptin 60, and STAT‐3 tyrosine phosphorylation in the human placenta choriocarcinoma JEG‐3 cell line, as well as in trophoblast cells from human term placenta. STAT‐3 activity has been correlated with trophoblast invasiveness 61. In this context, it is interesting to mention the role of Sam68, an RNA‐binding protein originally identified as the substrate of Src kinase during mitosis and a member of the signal transduction and activation of RNA metabolism (STAR) family 62, 63. Leptin stimulates Tyr‐phosphorylation of Sam68 in the trophoblast, mediating the dissociation from RNA, suggesting that leptin signalling could modulate RNA metabolism 64, 65. Moreover, phosphorylated Sam68 interacts with STAT‐3 in response to leptin in trophoblastic JEG‐3 cells 66, suggesting that Sam68 seems to play an important role mediating biological function of leptin 66.
In human trophoblastic cells, it has also been demonstrated that leptin induces the phosphorylation of the extracellular signal‐regulated MEK and the extracellular signal‐regulated ERK 1/2 46. Moreover, it is well‐established that the ERK pathway is essential for reproduction in general, and for the control of trophoblast penetration and invasion 67, as well as placental development 68.
Besides, leptin activation of phosphatidylinositol 3‐kinase (PI3K) pathway has been described in many systems, including placenta 47, 58, leading to phosphorylation of Protein kinase B (PKB), also known as Akt and inactivation of glycogen synthase kinase 3 (GSK‐3), as well as the activation of the translation machinery.
In placental villi, cell turnover is tightly regulated, via apoptotic cascade 69. In normal pregnancy, apoptosis is an essential feature of placental development and it is well‐established that trophoblast apoptosis increases with placental growth and advancing gestation 70. Leptin prevents early and late events of apoptosis via MAPK pathway 46, 51. The role of leptin was also studied under different stress conditions such as serum deprivation and hyperthermia. Under serum deprivation, leptin increased the anti‐apoptotic B‐cell lymphoma 2 (BCL‐2) protein expression, while down‐regulated the pro‐apoptotic BAX and BH3 interacting domain death agonist (BID) proteins expression as well as caspase‐3 active form and cleaved poly [ADP‐ribose] polymerase 1 (PARP‐1) fragment in Swan‐71 cells, a first‐trimester trophoblast cells isolated from a 7‐week normal placenta 71 and placental explants. In addition, it was demonstrated that p53 and its phosphorylation of serine 46 (Ser‐46), phosphorylation involved in the selectivity of apoptotic target genes, are down‐regulated by leptin suggesting that leptin plays a pivotal role for apoptotic signalling by inhibiting p53 48. Recent studies have demonstrated that MAPK and PI3K pathways are necessaries for this anti‐apoptotic leptin action and it was also demonstrated that murine double‐minute type 2 also known as E3 ubiquitin–protein ligase (MDM‐2) expression is regulated by leptin 49. In placental explants cultured at high temperatures (40°C and 42°C), the extent of Ser‐46 phosphorylation of p53 and the expressions of p53‐regulated apoptosis‐inducing protein 1 (p53AIP1), a potential mediator of apoptosis depending on p53, p21 and Caspase‐3 are increased and these effects are significantly attenuated by leptin, indicating that leptin is a pro‐survival placental cytokine 50. Figure 1 highlights the main actions of leptin in the maternal–foetal interface.
Leptin as an immunomodulator during pregnancy
One of the most important placental functions is to prevent embryo rejection by the maternal immune system to enable its correct development 72. To ensure normal pregnancy, trophoblast differentiation requires potent immunomodulatory mechanisms to prevent rejection of ST and invasive trophoblast by alloreactive lymphocytes and natural killer (NK) cells present in maternal blood and decidua 73. Inflammatory mediators such as interleukin‐1 β (IL‐1β), interleukin‐6 (IL‐6), tumour necrosis factor α (TNFα) and prostaglandins are produced and secreted by the human placenta and these cytokines play an important role in a number of normal and abnormal inflammatory processes, including the initiation and progression of human labour 74, 75, 76. There are several homologies between the expression and regulation of cytokines and inflammation‐related genes in the placenta and in the white adipose tissue. In this regard, leptin effects include the promotion of inflammation and the modulation of innate and adaptive immunity 64, 77, 78. Thus, placental leptin acts as an immune modulator, regulating the generation of matrix metalloproteinases (MMPs), arachidonic acid products, nitric oxide production and T‐cell cytokines 76. Interestingly, leptin expression is also regulated by interleukin‐1 α (IL‐1α), IL‐1β, IL‐6 and interferon‐ϒ (IFN‐ϒ) 44, 79, 80.
It was reported that leptin stimulates IL‐6 secretion in human trophoblast cells 81, 82. In addition, TNFα release from human placenta is also stimulated by leptin, and it was demonstrated that nuclear transcription factor NF‐kappa B (NF‐ҡB) and peroxisome proliferator‐activated receptor γ (PPAR‐γ) are important mediators of this effect 83. Recently, we have found that leptin induces human leucocyte antigen G (HLA‐G) expression in placenta. HLA‐G has potent immunosuppressive effects promoting apoptosis of activated CD8+ T lymphocytes, the generation of tolerogenic antigen‐presenting cells and the prevention of NK cell‐mediated cytotoxicity. These data place leptin as a placental cytokine which confers to trophoblast cells a tolerogenic phenotype to prevent immunological damage during the first steps of pregnancy 84.
Pro‐inflammatory leptin actions may also have significant implications in the pathogenesis of various disorders associated with pregnancy, such as GDM and PE, which are characterized by increased leptin expression. In this sense, placental leptin may contribute to the incremented circulating levels of pro‐inflammatory mediators that are evident in these pregnancy diseases, whereas successful pregnancy is associated with down‐regulation of intrauterine pro‐inflammatory cytokines 23, 85, 86.
Leptin and pathologies associated with pregnancy
Polycystic ovary syndrome
PCOS, the most common endocrine disorder in females and major cause of anovulatory infertility, affects approximately 15% of women during reproductive ages 32. It is characterized by hyperandrogenism, chronic oligoanovulation and polycystic ovarian morphology 87. Peripheral insulin resistance appears to play a crucial role in the pathogenesis of PCOS 88. However, the aetiology of PCOS is not fully understood yet. The deregulated secretion of adipokines, including leptin, plays a role in the pathogenesis of PCOS 89. Besides, PCOS could be associated with increased prevalence of gestational disorders such as miscarriage, GDM and PE 90.
Different studies have shown augmented leptin levels in women with PCOS 91, 92, 93. A recent work confirmed that leptin serum concentrations are increased in obese women with PCOS, while adiponectin levels are decreased 94. Furthermore, the authors suggested the higher leptin levels may be related to the hyperinsulinaemic characteristic of obesity and PCOS 94. In this sense, as women with PCOS also commonly present overweight and obesity 95, the symptoms mentioned could be a consequence of the hyperleptinaemia due to the gain of fat mass. A recent preliminary investigation proposed leptin as strong biomarker of hyperandrogenic PCOS women, suggesting metabolic and inflammatory biomarkers may be increased in PCOS. Interestingly, offspring from PCOS patients have increased inflammatory markers such as matrix metalloproteinase‐9 (MMP‐9) and S100 calcium‐binding protein A8 or calgranulin A (S100A8), suggesting that these children may exhibit increased chronic low‐grade inflammation 96. In fact, it has been reported that increased leptin concentrations may be correlated with insulin resistance, metabolic disorder, infertility and even cardiovascular disease risk in PCOS, which may contribute to the aetiology and development of PCOS 97. Elevated leptin levels could be one of the mechanisms underlying insulin‐mediated ovarian androgen production, as high leptin levels are associated with elevated testosterone levels 98.
Recurrent miscarriage
Recurrent miscarriage is defined as the loss of three or more consecutives pregnancies before the 20th week of gestation with or without previous live births. Genetic, endocrine, anatomical, immunological, thrombophilic and environmental factors have been implicated in recurrent miscarriage. However, no cause can be found in up to 50% of cases. In patients who have early recurrent miscarriages, some proteins such as human chorionic gonadotrophin (hCG), glycodelin and galectin‐1 are down‐regulated in the ST. Moreover, animal and human studies indicate that alterations in leptin signalling may increase the risk for pregnancy loss 99.
Serum leptin concentration was found elevated in women with recurrent miscarriage in comparison to control group 100. However, in women who subsequently miscarried, it was found that at weeks 5–6 and 7–8 plasma leptin concentrations are also lower than women who subsequently had a term birth 101. In addition, low serum leptin concentrations were observed in women suffering spontaneous miscarriage during the first trimester 102. However, Tommaselli et al. did not find significant differences in maternal serum leptin levels, probably due to the heterogeneity of miscarriage in terms of pathogenesis 103.
Single nucleotide polymorphisms (SNP) of LEPR within domains necessary for receptor activation or the cytoplasmic domains may be associated with impaired signalling capacity. In this line, the A223G polymorphism of LEPR is associated with increased risk of pregnancy disorders like PE 104. On the other hand, Chin et al. 105 did not find a correlation between this polymorphism and recurrent miscarriage. However, other studies have reported that these genetic variants are associated with pregnancy recurrent loss (Table 1) 100, 106.
Table 1.
LEP and LEPR single nucleotide polymorphisms (SNPs) in pathologies associated with pregnancy
| Pathologies associated with pregnancy | SNP | Description |
|---|---|---|
| Polycystic ovary syndrome | – | No LEP or LEPR SNPs have been described |
| Recurrent miscarriage | LEP‐2548G/A | GA genotype and G allele are associated with risk of RM |
| Gestational diabetes mellitus | LEP‐2548G/A | A allele is associated with risk of gestational diabetes mellitus (GDM) |
| Pre‐eclampsia | LEP‐2548G/A | A allele is associated with PE |
| LEPR A223G | G allele is associated with increased risk of severe PE | |
| LEPRG1019A | GA genotype and G allele are associated with severe PE | |
| LEPR A668G | A allele is associated with severe PE | |
| Intrauterine growth restriction | – | No LEP or LEPR SNPs have been described |
Gestational diabetes mellitus
GDM is the most common pregnancy metabolic disorder and is defined as the type of glucose intolerance that develops in the second trimester and third trimester of the pregnancy, resulting in hyperglycaemia of variable severity 107. Aberrant development and functioning of the placenta, including placental overgrowth, have been implicated as important factors that contribute to GDM‐associated complications 108, 109. GDM is associated with a high perinatal morbidity and mortality as well as insulin resistance, hyperinsulinaemia and hyperleptinaemia, and these GDM‐associated conditions disturb placental nutrient transport and foetal nutrient supply 110, 111. It has been found that leptin and leptin receptor expressions are increased in placenta from GDM 23, 85 and, in fact, leptin was proposed as a first‐trimester biochemical predictor of GDM 112, 113. In addition, it was suggested that hyperinsulinaemia may regulate placental leptin production acting as a circulating signal to control foetal homoeostasis 114, 115. Furthermore, it is though that maternal glucose regulates cord blood leptin levels and this could explain why newborns exposed to GDM have an increased risk of obesity 116. Comparison of the placental gene expression profile between normal and diabetic pregnancies indicates that increased leptin synthesis in GDM is correlated with higher production of pro‐inflammatory cytokines such as IL‐6 and TNFα, causing a chronic inflammatory environment that enhances leptin production 117.
Our group has reported that insulin induces leptin expression in trophoblastic cells by increasing leptin promoter activity 118. It is known that leptin and insulin share several signalling pathways, such as JAK2/STAT‐3, MAPK and PI3K. Moreover, we could demonstrate that in GDM placenta is increased the basal phosphorylation of STAT‐3, MAPK 1/3 and PKB, with resistance to a further stimulation with leptin or insulin in vitro, suggesting synergistic interaction and a crosstalk between insulin and leptin signalling in human placenta 23.
On the other hand, GDM is associated with increased incidence of polyhydramnios, due to an increase in amniotic fluid volume, suggesting that aquaporins (AQP), such as AQP9 expression could be altered in GDM 119, 120. Besides, when maternal circulating glucose levels are controlled they have normal amniotic fluid volume. AQP9 is also a transporter for glycerol and may also provide this substrate to the foetus. In this context, we have found that AQP9 messenger RNA (mRNA) and protein expressions are elevated in placentas from women with GDM. These data could suggest that during GDM the overexpression of AQP9, which correlates with higher leptin plasma levels, increments glycerol transport to the foetus and may help to cover the increase in energy needs that occur during this gestational metabolic disorder 121.
Pre‐eclampsia
PE is a potentially life‐threatening hypertensive disorder affecting ~2–7% of all pregnancies. Approximately 1% of cases are severe, causing stillbirth or the need for extreme preterm delivery. It is characterized by hypertension, systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg after 20 weeks of gestation, and proteinuria. Some of the established risk factors are age younger than 20 years or older than 40 years, primiparity, excess bodyweight of the mother, multifoetal pregnancy and familiar and individual history of PE 122. Increasing evidence supports that the pathogenesis of PE involves improper placental development, due to dysfunctional proliferation, migration and invasion of CT into the uterus. This leads to inappropriate spiral artery remodelling, decreased placental blood flow and placental hypoxia 123.
Leptin expression is increased in pre‐eclamptic placentas, and many studies suggest a positive correlation between elevated serum levels and PE 124, 125, 126. Moreover, it has been shown that leptin concentration is higher in term PE but not in preterm PE 126. Thus, leptin has been proposed as a link between body mass index and PE, but the role of obesity or leptin in the pathogenesis of PE is not obvious 126. Leptin up‐regulation could be attributed to placental stress, mainly by the hypoxia present in pre‐eclamptic placenta. Furthermore, serum leptin levels seem elevated in PE even before the clinical onset of the disease, suggesting a possible prognostic value 127. In addition, leptin inhibits increased apoptosis of placental cells during PE. Also, as leptin is a potent angiogenic factor, enhanced placental leptin could increase blood supply to the placenta by neovascularization. Furthermore, leptin is involved in the regulation of placental nutrient transporters, suggesting that hyperleptinaemia in PE is a compensatory response to boost nutrient delivery to the underperfused placenta 117.
However, the role of leptin in PE should be evaluated cautiously as it has recently been found no association of leptin levels with PE 128. SNPs in the LEPR gene have also been investigated in relation to severe PE. In this sense, it was reported that variants of LEPR such as A223G polymorphism may individually modify the risk of severe PE (Table 1) 104.
Intrauterine growth restriction
The failure of arterial remodelling results in malperfusion of the placenta 129. The incapacity of the placenta to deliver an adequate supply of nutrients to the foetus is termed placental insufficiency and results in IUGR, affecting up to 5–10% of pregnancies in developed countries. IUGR is characterized by a birthweight of <2.5 kg and is associated with a high incidence of perinatal morbidity and mortality and increased risk of cardiovascular and metabolic diseases in adulthood 130. IUGR represents a period of true foetal malnutrition followed by a period of weight recovery after birth, which leads to changes in adipose tissue with important long‐term consequences 131. Specifically, IUGR is frequently associated with inflammation and infarcts within the villi, implying abnormal villous development 132. At the same time, several growth factors and signalling molecules have been implicated in IUGR, including vascular endothelial growth factor and leptin 133.
Diverse studies demonstrated lower circulating leptin levels in IUGR neonates at birth, due to reduced fat mass and/or lower placental production, suggesting leptin as a growth factor that intervenes during foetal intrauterine development 134, 135. Compared with normal birthweight controls, leptin levels become higher in IUGR children and adults, suggesting an adaptive leptin resistance beneficial for catch‐up growth or an adipocyte dysfunction associated with IUGR 136. However, other studies have reported that maternal serum leptin concentrations were significantly higher in pregnancies complicated by foetal growth restriction and growth‐restricted foetuses show umbilical cord leptin concentrations lower than those in normal foetuses, suggesting that it could be due to a compensatory mechanism in which small placentas produce more leptin 137. Simultaneously, it was suggested that placental insufficiency is associated with an increase in placental leptin production 138. Furthermore, some studies demonstrated that leptin levels are lower in IUGR, but differences were not significant 139, 140, and it was also reported that cord blood leptin levels did not differ significantly in IUGR compared to normal pregnancies 141. These data suggest that the association between leptin and IUGR is controversial. Finally, it was reported that the mother of foetuses with growth restriction has a body composition pattern characterized by slightly increased fraction of fat mass and increased serum leptin levels 142.
Conclusions
In conclusion, it could be affirmed that leptin plays an integral role in the normal physiology of the reproductive system. Leptin controls reproduction depending on the energy state of the body, and sufficient leptin levels are a prerequisite for the maintenance of reproductive capacity. The present review was focused in placental leptin effects during gestation, when leptin levels are increased due to leptin production by trophoblastic cells. Thus, leptin has a wide range of biological functions on trophoblast cells and a role in successful establishment of pregnancy. In this sense, leptin promotes proliferation, protein synthesis and survival of placental cells. These actions are very important as cell proliferation and apoptotic cascades are critical for the correct placental development and function. Moreover, leptin is involved in the promotion of trophoblast invasion which represent a key event during early pregnancy. Besides, it is suggested an important role of leptin in the regulation of immune mechanisms at the maternal interface.
On the other hand, observational studies have demonstrated that states of leptin overabundance, deficiency or resistance can be associated with abnormal reproductive function. Clinical studies demonstrate an impact of obesity on the risk of infertility, and it is also established that obesity may lead to deregulation in leptin function that results in maternal disease 143. In this context, leptin deregulation has been implicated in the pathogenesis for at least some disorders associated with reproduction and pregnancy, such as PCOS, recurrent miscarriage, GDM, PE and IUGR. It is well accepted that increased leptin levels are detected in women with PCOS and that may be correlated with insulin resistance, metabolic disorders and infertility. Recurrent miscarriages are associated with altered leptin levels, but the relationship is open for discussion. On the other hand, SNPs of leptin and LEPR genes are risk factors for miscarriage. Leptin and leptin receptor expressions are increased in placentas from GDM, which may be relevant to control foetal homoeostasis. PE is also characterized by enhanced leptin concentrations, even before the clinical onset of the disease, suggesting a possible prognostic significance. Finally, the association between IUGR and leptin levels is controversial. Figure 2 summarizes the link between leptin and the mentioned diseases, including suggested causes and consequences of these pathologies.
Figure 2.
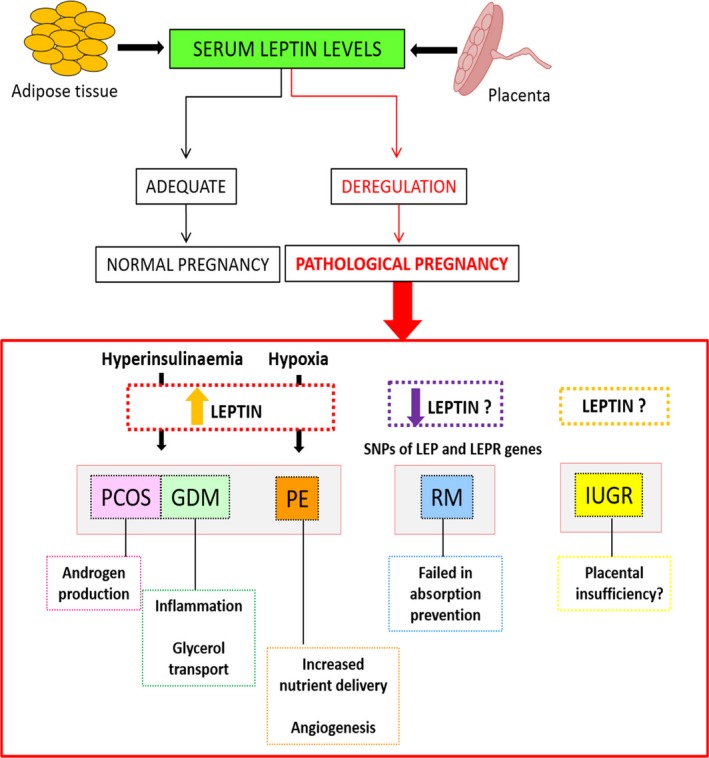
Leptin association pregnancy disorders. Deregulation of leptin levels has been correlated with the pathogenesis of various disorders associated with reproduction and gestation, including polycystic ovary syndrome (PCOS), recurrent miscarriage (RM), gestational diabetes mellitus (GDM), pre‐eclampsia (PE) and intrauterine growth restriction (IUGR). The figure summarizes the link between leptin and the mentioned diseases, including suggested causes and consequences of these pathologies.
Different therapeutic strategies based on leptin administration have been described. Patients with leptin mutations show a marked restoration of fertility as well as weight loss and improvements in immune function after leptin therapy. Furthermore, leptin replacement therapy improves the reproductive abnormalities associated with hypothalamic amenorrhoea (such as failure to menstruate, infertility and premature osteoporosis) 144. On the other hand, compounds that could reverse leptin resistance and act as leptin sensitizers could be beneficial to treat pathologies associated with hyperleptinaemia 145. A number of evidence suggested that leptin might have potential as a treatment for diverse pathologies including the malfunctioning of the reproductive system.
Further investigation is needed to fully elucidate the association of leptin with pathological pregnancy and to establish leptin as a biomarker for pathologies associated with pregnancy.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
Funding source: This work was supported by a Grant from the Instituto de Salud Carlos III (ISCIII PI09/00119, PS12/01172, and PI15/01535) funded in part by FEDER funds.
References
- 1. El Husseny MW, Mamdouh M, Shaban S, et al Adipokines: potential therapeutic targets for vascular dysfunction in type II diabetes mellitus and obesity. J Diabetes Res. 2017; 1–11. https://doi.org/10.1155/2017/8095926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Münzberg H, Morrison CD. Structure, production and signaling of leptin. Metabolism. 2015; 64: 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pérez‐Pérez A, Sánchez‐Jiménez F, Maymó J, et al Role of leptin in female reproduction. Clin Chem Lab Med. 2015; 53: 15–28. [DOI] [PubMed] [Google Scholar]
- 4. Behnes M, Brueckmann M, Lang S, et al Alterations of leptin in the course of inflammation and severe sepsis. BMC Infect Dis. 2012; 12: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lekva T, Roland MCP, Michelsen AE, et al Large reduction in adiponectin during pregnancy is associated with large for gestational age newborns. J Clin Endocrinol Metab. 2017; 102: 2552–9. [DOI] [PubMed] [Google Scholar]
- 6. Bao W, Baecker A, Song Y, et al Adipokine levels during the first or early second trimester of pregnancy and subsequent risk of gestational diabetes mellitus: a systematic review. Metabolism. 2015; 64: 756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Howell KR, Powell TL. Effects of maternal obesity on placental function and fetal development. Reproduction. 2017; 153: R97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chehab FF. 20 YEARS OF LEPTIN: Leptin and reproduction: past milestones, present undertakings, and future endeavors. J Endocrinol. 2014; 223: T37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998; 395: 763–70. [DOI] [PubMed] [Google Scholar]
- 10. Park H‐K, Ahima RS. Leptin signaling. F1000prime Rep. 2014; 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hegyi K, Fülöp K, Kovács K, et al Leptin‐induced signal transduction pathways. Cell Biol Int. 2004; 28: 159–69. [DOI] [PubMed] [Google Scholar]
- 12. Wauman J, Zabeau L, Tavernier J. The Leptin Receptor complex: heavier than expected? Front Endocrinol (Lausanne). 2017; 8: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turner RT, Philbrick KA, Kuah AF, et al Role of estrogen receptor signaling in skeletal response to leptin in female ob/ob mice. J Endocrinol. 2017; 233: 357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herrid M, Palanisamy SK, Ciller UA, et al An updated view of leptin on implantation and pregnancy: a review. Physiol Res. 2014; 63: 543–57. [DOI] [PubMed] [Google Scholar]
- 15. Martins FF, Aguila MB, Mandarim‐de‐Lacerda CA. Impaired steroidogenesis in the testis of leptin‐deficient mice (ob/ob‐/‐). Acta Histochem. 2017; 119: 508–515. [DOI] [PubMed] [Google Scholar]
- 16. Reitman ML, Bi S, Marcus‐Samuels B, et al Leptin and its role in pregnancy and fetal development – an overview. Biochem Soc Trans. 2001; 29(Pt 2): 68–72. [DOI] [PubMed] [Google Scholar]
- 17. Pollheimer J, Knofler M. Signalling pathways regulating the invasive differentiation of human trophoblasts: a review. Placenta. 2005; 26(Suppl. A): S21–30. [DOI] [PubMed] [Google Scholar]
- 18. Staun‐Ram E, Shalev E. Human trophoblast function during the implantation process. Reprod Biol Endocrinol. 2005; 3: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carson DD, Bagchi I, Dey SK, et al Embryo implantation. Dev Biol. 2000; 223: 217–37. [DOI] [PubMed] [Google Scholar]
- 20. E Davies J, Pollheimer J, Yong HE, et al Epithelial‐mesenchymal transition during extravillous trophoblast differentiation. Cell Adh Migr. 2016; 10: 310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weiss G, Sundl M, Glasner A, et al The trophoblast plug during early pregnancy: a deeper insight. Histochem Cell Biol. 2016; 146: 749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sheikh AM, Small HY, Currie G, et al Systematic review of micro‐RNA expression in pre‐eclampsia identifies a number of common pathways associated with the disease. PLoS ONE. 2016; 11: e0160808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pérez‐Pérez A, Guadix P, Maymó J, et al Insulin and leptin signaling in placenta from gestational diabetic subjects. Horm Metab Res. 2016; 48: 62–9. [DOI] [PubMed] [Google Scholar]
- 24. King JC. Physiology of pregnancy and nutrient metabolism. Am J Clin Nutr. 2000; 71: 1218s–25s. [DOI] [PubMed] [Google Scholar]
- 25. Elmquist JK. Anatomic basis of leptin action in the hypothalamus. Front Horm Res. 2000; 26: 21–41. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Y, Proenca R, Maffei M, et al Positional cloning of the mouse obese gene and its human homologue. Nature. 1994; 372: 425–32. [DOI] [PubMed] [Google Scholar]
- 27. Houseknecht KL, Portocarrero CP. Leptin and its receptors: regulators of whole‐body energy homeostasis. Domest Anim Endocrinol. 1998; 15: 457–75. [DOI] [PubMed] [Google Scholar]
- 28. Hoggard N, Haggarty P, Thomas L, et al Leptin expression in placental and fetal tissues: does leptin have a functional role? Biochem Soc Trans. 2001; 29(Pt 2): 57–63. [DOI] [PubMed] [Google Scholar]
- 29. Masuzaki H, Ogawa Y, Sagawa N, et al Nonadipose tissue production of leptin: leptin as a novel placenta‐derived hormone in humans. Nat Med. 1997; 3: 1029–33. [DOI] [PubMed] [Google Scholar]
- 30. Acconcia F, Kumar R. Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett. 2006; 238: 1–14. [DOI] [PubMed] [Google Scholar]
- 31. González RR, Simón C, Caballero‐Campo P, et al Leptin and reproduction. Hum Reprod Update. 2000; 6: 290–300. [DOI] [PubMed] [Google Scholar]
- 32. Sartori C, Lazzeroni P, Merli S, et al From placenta to polycystic ovarian syndrome: the role of adipokines. Mediators Inflamm. 2016; 14. https://doi.org/10.1155/2016/4981916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Louis GW, et al Molecular mapping of the neural pathways linking leptin to the neuroendocrine reproductive axis. Endocrinology. 2011; 152: 2302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jin L, Zhang S, Burguera BG, et al Leptin and leptin receptor expression in rat and mouse pituitary cells 1. Endocrinology. 2000; 141: 333–9. [DOI] [PubMed] [Google Scholar]
- 35. Karlsson C, Lindell K, Svensson E, et al Expression of functional leptin receptors in the human ovary 1. J Clin Endocrinol Metab. 1997; 82: 4144–8. [DOI] [PubMed] [Google Scholar]
- 36. Archanco M, Muruzábal FJ, Llopiz D, et al Leptin expression in the rat ovary depends on estrous cycle. J Histochem Cytochem. 2003; 51: 1269–77. [DOI] [PubMed] [Google Scholar]
- 37. Cioffi JA, Van Blerkom J, Antczak M, et al The expression of leptin and its receptors in pre‐ovulatory human follicles. Mol Hum Reprod. 1997; 3: 467–72. [DOI] [PubMed] [Google Scholar]
- 38. Ricci AG, Di Yorio MP, Faletti AG. Inhibitory effect of leptin on the rat ovary during the ovulatory process. Reproduction. 2006; 132: 771–80. [DOI] [PubMed] [Google Scholar]
- 39. Farooqi IS, Jebb SA, Langmack G, et al Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999; 341: 879–84. [DOI] [PubMed] [Google Scholar]
- 40. Sir‐Petermann T, Recabarren SE, Lobos A, et al Secretory pattern of leptin and LH during lactational amenorrhoea in breastfeeding normal and polycystic ovarian syndrome women. Hum Reprod. 2001; 16: 244–9. [DOI] [PubMed] [Google Scholar]
- 41. Cervero A, Horcajadas JA, Domínguez F, et al Leptin system in embryo development and implantation: a protein in search of a function. Reprod Biomed Online. 2005; 10: 217–23. [DOI] [PubMed] [Google Scholar]
- 42. Kawamura K, Sato N, Fukuda J, et al Leptin promotes the development of mouse preimplantation embryos in vitro . Endocrinology. 2002; 143: 1922–31. [DOI] [PubMed] [Google Scholar]
- 43. Bajoria R, Sooranna SR, Ward BS, et al Prospective function of placental leptin at maternal‐fetal interface. Placenta. 2002; 23: 103–15. [DOI] [PubMed] [Google Scholar]
- 44. Henson MC, Castracane VD. Leptin in pregnancy: an update. Biol Reprod. 2006; 74: 218–29. [DOI] [PubMed] [Google Scholar]
- 45. Lin K‐C. Increase of maternal plasma leptin concentrations during pregnancy: comparison with nonpregnant women. Kaohsiung J Med Sci. 1999; 15: 640–5. [PubMed] [Google Scholar]
- 46. Pérez‐Pérez A, Maymó J, Dueñas JL, et al Leptin prevents apoptosis of trophoblastic cells by activation of MAPK pathway. Arch Biochem Biophys. 2008; 477: 390–5. [DOI] [PubMed] [Google Scholar]
- 47. Pérez‐Pérez A, Maymó J, Gambino Y, et al Leptin stimulates protein synthesis‐activating translation machinery in human trophoblastic cells. Biol Reprod. 2009; 81: 826–32. [DOI] [PubMed] [Google Scholar]
- 48. Toro AR, Maymó JL, Ibarbalz FM, et al Leptin is an anti‐apoptotic effector in placental cells involving p53 downregulation. PLoS ONE. 2014; 9: e99187 doi.org/10.1371/journal.pone.0099187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Toro AR, Pérez‐Pérez A, Corrales Gutiérrez I, et al Mechanisms involved in p53 downregulation by leptin in trophoblastic cells. Placenta. 2015; 36: 1266–75. [DOI] [PubMed] [Google Scholar]
- 50. Pérez‐Pérez A, Toro AR, Vilarino‐Garcia T, et al Leptin reduces apoptosis triggered by high temperature in human placental villous explants: the role of the p53 pathway. Placenta. 2016; 42: 106–13. [DOI] [PubMed] [Google Scholar]
- 51. Magariños MP, Sánchez‐Margalet V, Kotler M, et al Leptin promotes cell proliferation and survival of trophoblastic cells. Biol Reprod. 2007; 76: 203–10. [DOI] [PubMed] [Google Scholar]
- 52. Genbacev O, Miller R. Post‐implantation differentiation and proliferation of cytotrophoblast cells: in vitro models—a review. Placenta. 2000; 21: S45–9. [DOI] [PubMed] [Google Scholar]
- 53. Huppertz B, Kadyrov M, Kingdom JC. Apoptosis and its role in the trophoblast. Am J Obstet Gynecol. 2006; 195: 29–39. [DOI] [PubMed] [Google Scholar]
- 54. Martín‐Romero C, Santos‐Alvarez J, Goberna R, et al Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000; 199: 15–24. [DOI] [PubMed] [Google Scholar]
- 55. Santos‐Alvarez J, Goberna R, Sanchez‐Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999; 194: 6–11. [DOI] [PubMed] [Google Scholar]
- 56. Steppan CM, Crawford DT, Chidsey‐Frink KL, et al Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000; 92: 73–8. [DOI] [PubMed] [Google Scholar]
- 57. Najib S, Sanchez‐Margalet V. Human leptin promotes survival of human circulating blood monocytes prone to apoptosis by activation of p42/44 MAPK pathway. Cell Immunol. 2002; 220: 143–9. [DOI] [PubMed] [Google Scholar]
- 58. Pérez‐Pérez A, Gambino Y, Maymó J, et al MAPK and PI3K activities are required for leptin stimulation of protein synthesis in human trophoblastic cells. Biochem Biophys Res Commun. 2010; 396: 956–60. [DOI] [PubMed] [Google Scholar]
- 59. Ghilardi N, Skoda RC. The leptin receptor activates janus kinase 2 and signals for proliferation in a factor‐dependent cell line. Mol Endocrinol. 1997; 11: 393–9. [DOI] [PubMed] [Google Scholar]
- 60. Kloek C, Haq AK, Dunn SL, et al Regulation of Jak kinases by intracellular leptin receptor sequences. J Biol Chem. 2002; 277: 41547–55. [DOI] [PubMed] [Google Scholar]
- 61. Corvinus FM, Fitzgerald JS, Friedrich K, et al Evidence for a correlation between trophoblast invasiveness and STAT3 activity. Am J Reprod Immunol. 2003; 50: 316–21. [DOI] [PubMed] [Google Scholar]
- 62. Fumagalli S, Totty NF, Hsuan JJ, et al A target for Src in mitosis. Nature. 1994; 368: 871–4. [DOI] [PubMed] [Google Scholar]
- 63. Taylor SJ, Shalloway D. An RNA‐binding protein associated with Src through its SH2 and SH3 domains in mitosis. Nature. 1994; 368: 867. [DOI] [PubMed] [Google Scholar]
- 64. Sánchez‐Margalet V, Martín‐Romero C, Santos‐Alvarez J, et al Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clin Exp Immunol. 2003; 133: 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sánchez‐Jiménez F, Pérez‐Pérez A, González‐Yanes C, et al Leptin receptor activation increases Sam68 tyrosine phosphorylation and expression in human trophoblastic cells. Mol Cell Endocrinol. 2011; 332: 221–7. [DOI] [PubMed] [Google Scholar]
- 66. Sánchez‐Jiménez F, Pérez‐Pérez A, González‐Yanes C, et al Sam68 mediates leptin‐stimulated growth by modulating leptin receptor signaling in human trophoblastic JEG‐3 cells. Hum Reprod. 2011; 26: 2306–15. [DOI] [PubMed] [Google Scholar]
- 67. Fitzgerald JS, et al Signal transduction in trophoblast invasion In Immunology of gametes and embryo implantation (Vol. 88). Karger Publishers; 2005: pp. 181–99. [DOI] [PubMed] [Google Scholar]
- 68. Hatano N, Mori Y, Oh‐hora M, et al Essential role for ERK2 mitogen‐activated protein kinase in placental development. Genes Cells. 2003; 8: 847–56. [DOI] [PubMed] [Google Scholar]
- 69. Heazell AE, Lacey HA, Jones CJ, et al Effects of oxygen on cell turnover and expression of regulators of apoptosis in human placental trophoblast. Placenta. 2008; 29: 175–86. [DOI] [PubMed] [Google Scholar]
- 70. Sharp AN, Heazell AE, Crocker IP, et al Placental apoptosis in health and disease. Am J Reprod Immunol. 2010; 64: 159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Straszewski‐Chavez SL, Abrahams VM, Alvero AB, et al The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta. 2009; 30: 939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maltepe E, Bakardjiev AI, Fisher SJ. The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Investig. 2010; 120: 1016–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hutter H, Hammer A, Dohr G, et al HLA expression at the maternal‐fetal interface. J Immunol Res. 1998; 6: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lappas M, Rice G. Phospholipase A 2 isozymes in pregnancy and parturition. Prostaglandins Leukot Essent Fatty Acids. 2004; 70: 87–100. [DOI] [PubMed] [Google Scholar]
- 75. Rice GE. Cytokines and the initiation of parturition In The endocrinology of parturition (Vol. 27). Karger Publishers; 2001: pp. 113–46. [DOI] [PubMed] [Google Scholar]
- 76. Lappas M, Yee K, Permezel M, et al Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus‐complicated pregnancies. J Endocrinol. 2005; 186: 457–65. [DOI] [PubMed] [Google Scholar]
- 77. Lam Q, Lu L. Role of leptin in immunity. Cell Mol Immunol. 2007; 4: 1–13. [PubMed] [Google Scholar]
- 78. Fernández‐Riejos P, Najib S, Santos‐Alvarez J, et al Role of leptin in the activation of immune cells. Mediator Inflamm. 2010; 8. https://doi.org/10.1155/2010/568343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fontana VA, Sanchez M, Cebral E, et al Interleukin‐1β regulates metalloproteinase activity and leptin secretion in a cytotrophoblast model. Biocell. 2010; 34: 37–43. [PubMed] [Google Scholar]
- 80. Fontana VA, Sanchez M, Cebral E, et al Interferon‐γ Inhibits metalloproteinase activity and cytotrophoblast cell migration. Am J Reprod Immunol. 2010; 64: 20–6. [DOI] [PubMed] [Google Scholar]
- 81. Cameo P, Bischof P, Calvo JC. Effect of leptin on progesterone, human chorionic gonadotropin, and interleukin‐6 secretion by human term trophoblast cells in culture 1. Biol Reprod. 2003; 68: 472–7. [DOI] [PubMed] [Google Scholar]
- 82. Soh E, Mitchell M, Keelan J. Does leptin exhibit cytokine‐like properties in tissues of pregnancy? Am J Reprod Immunol. 2000; 43: 292–8. [DOI] [PubMed] [Google Scholar]
- 83. Lappas M, Permezel M, Georgiou HM, et al Nuclear factor kappa b regulation of proinflammatory cytokines in human gestational tissues in vitro 1. Biol Reprod. 2002; 67: 668–73. [DOI] [PubMed] [Google Scholar]
- 84. Barrientos G, Toro A1, Moschansky P, et al Leptin promotes HLA‐G expression on placental trophoblasts via the MEK/Erk and PI3K signaling pathways. Placenta. 2015; 36: 419–26. [DOI] [PubMed] [Google Scholar]
- 85. Pérez‐Pérez A, Maymó JL, Gambino YP, et al Activated translation signaling in placenta from pregnant women with gestational diabetes mellitus: possible role of leptin. Horm Metab Res. 2013; 45: 436–42. [DOI] [PubMed] [Google Scholar]
- 86. Qiu C, Williams MA, Vadachkoria S, et al Increased maternal plasma leptin in early pregnancy and risk of gestational diabetes mellitus. Obstet Gynecol. 2004; 103: 519–25. [DOI] [PubMed] [Google Scholar]
- 87. Li L, Lee KJ, Choi BC, et al Relationship between leptin receptor and polycystic ovary syndrome. Gene. 2013; 527: 71–4. [DOI] [PubMed] [Google Scholar]
- 88. Sepilian VP, Crochet JR, Nagamani M. Serum soluble leptin receptor levels and free leptin index in women with polycystic ovary syndrome: relationship to insulin resistance and androgens. Fertil Steril. 2006; 85: 1441–7. [DOI] [PubMed] [Google Scholar]
- 89. Behboudi‐Gandevani S, Ramezani Tehrani F, Bidhendi Yarandi R, et al The association between polycystic ovary syndrome, obesity, and the serum concentration of adipokines. J Endocrinol Invest. 2017; 40: 859–66. [DOI] [PubMed] [Google Scholar]
- 90. De Leo V, Musacchio MC, Cappelli V, et al Genetic, hormonal and metabolic aspects of PCOS: an update. Reprod Biol Endocrinol. 2016; 14: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Micić D, Macut D, Popović V, et al Leptin levels and insulin sensitivity in obese and non‐obese patients with polycystic ovary syndrome. Gynecol Endocrinol. 1997; 11: 315–20. [DOI] [PubMed] [Google Scholar]
- 92. Rizk NM, Sharif E. Leptin as well as free leptin receptor is associated with polycystic ovary syndrome in young women. Int J Endocrinol. 2015; 10 http://dx.doi.org/10.1155/2015/927805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Brzechffa PR, Jakimiuk AJ, Agarwal SK, et al Serum immunoreactive leptin concentrations in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1996; 81: 4166–9. [DOI] [PubMed] [Google Scholar]
- 94. Behboudi‐Gandevani S, Ramezani Tehrani F, Bidhendi Yarandi R, et al The association between polycystic ovary syndrome, obesity, and the serum concentration of adipokines. J Endocrinol Invest. 2017; 40: 859–66. [DOI] [PubMed] [Google Scholar]
- 95. Gambineri A, Patton L, Altieri P, et al Polycystic ovary syndrome is a risk factor for type 2 diabetes. Diabetes. 2012; 61: 2369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Daan NM, Koster MP, de Wilde MA, et al Biomarker profiles in women with PCOS and PCOS offspring. A pilot study. PLoS ONE. 2016; 11: e0165033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zheng S‐H, Du D‐F, Li X‐L. Leptin levels in women with polycystic ovary syndrome: a systematic review and a meta‐analysis. Reprod Sci. 2017; 24: 656–70. [DOI] [PubMed] [Google Scholar]
- 98. Chakrabarti J. Serum leptin level in women with polycystic ovary syndrome: correlation with adiposity, insulin, and circulating testosterone. Ann Med Health Sci Res. 2013; 3: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Garrido‐Gimenez C, Alijotas‐Reig J. Recurrent miscarriage: causes, evaluation and management. Postgrad Med J. 2015; 91: 151–62. [DOI] [PubMed] [Google Scholar]
- 100. Zidan HE, Rezk NA, Alnemr AA, et al Interleukin‐17 and leptin genes polymorphisms and their levels in relation to recurrent pregnancy loss in Egyptian females. Immunogenetics. 2015; 67: 665–73. [DOI] [PubMed] [Google Scholar]
- 101. Laird SM, Quinton ND, Anstie B, et al Leptin and leptin‐binding activity in women with recurrent miscarriage: correlation with pregnancy outcome. Hum Reprod. 2001; 16: 2008–13. [DOI] [PubMed] [Google Scholar]
- 102. Kratzsch J, Höckel M, Kiess W. Leptin and pregnancy outcome. Curr Opin Obstet Gynecol. 2000; 12: 501–5. [DOI] [PubMed] [Google Scholar]
- 103. Tommaselli GA, Di Spiezio Sardo A, Di Carlo C, et al Do serum leptin levels have a role in the prediction of pregnancy outcome in case of threatened miscarriage? Clin Endocrinol. 2006; 65: 772–5. [DOI] [PubMed] [Google Scholar]
- 104. Rigó J, Szendei G, Rosta K, et al Leptin receptor gene polymorphisms in severely pre‐eclamptic women. Gynecol Endocrinol. 2006; 22: 521–5. [DOI] [PubMed] [Google Scholar]
- 105. Chin JR, Heuser CC, Eller AG, et al Leptin and leptin receptor polymorphisms and recurrent pregnancy loss. J Perinatol. 2013; 33: 589–92. [DOI] [PubMed] [Google Scholar]
- 106. Müller A, Wagner J, Hodžić A, et al Genetic variation in leptin and leptin receptor genes is a risk factor for idiopathic recurrent spontaneous abortion. Croat Med J. 2016; 57: 566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chiefari E, Arcidiacono B, Foti D, et al Gestational diabetes mellitus: an updated overview. J Endocrinol Invest. 2017; 1–11. [DOI] [PubMed] [Google Scholar]
- 108. Ericsson A, Säljö K, Sjöstrand E, et al Brief hyperglycaemia in the early pregnant rat increases fetal weight at term by stimulating placental growth and affecting placental nutrient transport. J Physiol. 2007; 581: 1323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Taricco E, Radaelli T, Nobile de Santis MS, et al Foetal and placental weights in relation to maternal characteristics in gestational diabetes. Placenta. 2003; 24: 343–7. [DOI] [PubMed] [Google Scholar]
- 110. Desoye G, Hauguel‐de S. Mouzon, The human placenta in gestational diabetes mellitus. Diabetes Care. 2007; 30(Suppl. 2): S120–6. [DOI] [PubMed] [Google Scholar]
- 111. Araújo JR, Keating E, Martel F. Impact of gestational diabetes mellitus in the maternal‐to‐fetal transport of nutrients. Curr DiabRep. 2015; 15: 1–10. [DOI] [PubMed] [Google Scholar]
- 112. Powe CE. Early pregnancy biochemical predictors of gestational diabetes mellitus. Curr DiabRep. 2017; 17: 12. [DOI] [PubMed] [Google Scholar]
- 113. Iciek R, Wender‐Ozegowska E, Zawiejska A, et al Placental leptin and its receptor genes expression in pregnancies complicated by type 1 diabetes. J Physiol Pharmacol. 2013; 64: 579–85. [PubMed] [Google Scholar]
- 114. Lepercq J, Cauzac M, Lahlou N, et al Overexpression of placental leptin in diabetic pregnancy: a critical role for insulin. Diabetes. 1998; 47: 847–50. [DOI] [PubMed] [Google Scholar]
- 115. Uzelac PS, Li X, Lin J, et al Dysregulation of leptin and testosterone production and their receptor expression in the human placenta with gestational diabetes mellitus. Placenta. 2010; 31: 581–8. [DOI] [PubMed] [Google Scholar]
- 116. Côté S, Gagné‐Ouellet V, Guay SP, et al PPARGC1α gene DNA methylation variations in human placenta mediate the link between maternal hyperglycemia and leptin levels in newborns. Clin Epigenetics. 2016; 8: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Miehle K, Stepan H, Fasshauer M. Leptin, adiponectin and other adipokines in gestational diabetes mellitus and pre‐eclampsia. Clin Endocrinol. 2012; 76: 2–11. [DOI] [PubMed] [Google Scholar]
- 118. Pérez‐Pérez A, Maymó J, Gambino Y, et al Insulin enhances leptin expression in human trophoblastic cells. Biol Reprod. 2013; 89: 20. [DOI] [PubMed] [Google Scholar]
- 119. Castro Parodi M, Farina M, Dietrich V, et al Evidence for insulin‐mediated control of AQP9 expression in human placenta. Placenta. 2011; 32: 1050–6. [DOI] [PubMed] [Google Scholar]
- 120. Bednar AD, Beardall MK, Brace RA, et al Differential expression and regional distribution of aquaporins in amnion of normal and gestational diabetic pregnancies. Physiol Rep. 2015; 3: e12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Vilariño‐García T, et al Increased expression of aquaporin 9 in trophoblast from gestational diabetic patients. Horm Metab Res. 2016; 48: 535–9. [DOI] [PubMed] [Google Scholar]
- 122. Kingdom JC, Drewlo S. Is heparin a placental anticoagulant in high‐risk pregnancies? Blood. 2011; 118: 4780–8. [DOI] [PubMed] [Google Scholar]
- 123. Spradley FT, Palei AC, Granger JP. Increased risk for the development of preeclampsia in obese pregnancies: weighing in on the mechanisms. Am J Physiol Regul Integr Comp Physiol. 2015; 309: R1326–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Song Y, Gao J1, Qu Y, et al Serum levels of leptin, adiponectin and resistin in relation to clinical characteristics in normal pregnancy and preeclampsia. Clin Chim Acta. 2016; 458: 133–7. [DOI] [PubMed] [Google Scholar]
- 125. Kalinderis M, Papanikolaou A, Kalinderi K, et al Serum levels of leptin and IP‐10 in preeclampsia compared to controls. Arch Gynecol Obstet. 2015; 292: 343–7. [DOI] [PubMed] [Google Scholar]
- 126. Taylor BD, Ness RB, Olsen J, et al Serum leptin measured in early pregnancy is higher in women with preeclampsia compared with normotensive pregnant women novelty and significance. Hypertension. 2015; 65: 594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chrelias G, Makris GM, Papanota AM, et al Serum inhibin and leptin: risk factors for pre‐eclampsia? Clin Chim Acta. 2016; 463: 84–7. [DOI] [PubMed] [Google Scholar]
- 128. Doster Y, Cetinkaya Demir B, Atalay MA, et al The possible role of serum leptin in preeclampsia. Clin Exp Obstet Gynecol. 2015; 43: 98–102. [PubMed] [Google Scholar]
- 129. Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003; 69: 1–7. [DOI] [PubMed] [Google Scholar]
- 130. Zhang S, Regnault TR, Barker PL, et al Placental adaptations in growth restriction. Nutrients. 2015; 7: 360–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Dessì A, Pravettoni C, Cesare Marincola F, et al The biomarkers of fetal growth in intrauterine growth retardation and large for gestational age cases: from adipocytokines to a metabolomic all‐in‐one tool. Expert Rev Proteomics. 2015; 12: 309–16. [DOI] [PubMed] [Google Scholar]
- 132. Briffa JF, McAinch AJ, Romano T, et al Leptin in pregnancy and development: a contributor to adulthood disease? Am J Physiol Endocrinol Metab. 2015; 308: E335–50. [DOI] [PubMed] [Google Scholar]
- 133. Scott‐Finley M, Woo JG, Habli M, et al Standardization of amniotic fluid leptin levels and utility in maternal overweight and fetal undergrowth. J Perinatol. 2015; 35: 547–52. [DOI] [PubMed] [Google Scholar]
- 134. Catov JM, Patrick TE, Powers RW, et al Maternal leptin across pregnancy in women with small‐for‐gestational‐age infants. Am J Obstet Gynecol. 2007; 196: 558. e1‐558. e8. [DOI] [PubMed] [Google Scholar]
- 135. Laivuori H, Gallaher MJ, Collura L, et al Relationships between maternal plasma leptin, placental leptin mRNA and protein in normal pregnancy, pre‐eclampsia and intrauterine growth restriction without pre‐eclampsia. Mol Hum Reprod. 2006; 12: 551–6. [DOI] [PubMed] [Google Scholar]
- 136. Briana DD, Malamitsi‐Puchner A. Intrauterine growth restriction and adult disease: the role of adipocytokines. Eur J Endocrinol. 2009; 160: 337–47. [DOI] [PubMed] [Google Scholar]
- 137. Pighetti M, et al Maternal serum and umbilical cord blood leptin concentrations with fetal growth restriction. Obstet Gynecol. 2003; 102: 535–43. [DOI] [PubMed] [Google Scholar]
- 138. Lepercq J, Guerre‐Millo M, André J, et al Leptin: a potential marker of placental insufficiency. Gynecol Obstet Invest. 2003; 55: 151–5. [DOI] [PubMed] [Google Scholar]
- 139. Milenković S, Jankovic B, Mirković L, et al Lipids and adipokines in cord blood and at 72 h in discordant dichorionic twins. Fetal Pediatr Pathol. 2017; 36: 106–122. [DOI] [PubMed] [Google Scholar]
- 140. Davidson S, Hod M, Merlob P, et al Leptin, insulin, insulin‐like growth factors and their binding proteins in cord serum: insight into fetal growth and discordancy. Clin Endocrinol. 2006; 65: 586–92. [DOI] [PubMed] [Google Scholar]
- 141. Aydin HI, Eser A, Kaygusuz I, et al Adipokine, adropin and endothelin‐1 levels in intrauterine growth restricted neonates and their mothers. J Perinat Med. 2016; 44: 669–76. [DOI] [PubMed] [Google Scholar]
- 142. Ferrero S, Mazarico E, Valls C, et al Relationship between foetal growth restriction and maternal nutrition status measured by dual‐energy X‐ray absorptiometry, leptin, and insulin‐like growth factor. Gynecol Obstet Invest. 2015; 80: 54–9. [DOI] [PubMed] [Google Scholar]
- 143. Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity's impact. Fertil Steril. 2017; 107: 840–7. [DOI] [PubMed] [Google Scholar]
- 144. Friedman J. The long road to leptin. J Clin Investig. 2016; 126: 4727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lee J, Liu J, Feng X, et al Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nat Med. 2016; 22: 1023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]


