Abstract
Objective
To compare the efficacy and safety of mifepristone followed by misoprostol with misoprostol alone in the management of early pregnancy failure (EPF).
Study Design
A randomized double-blind placebo-controlled clinical trial.
Methods
Ninety-two women with EPF ≤12 weeks were recruited and randomly allocated to receive either mifepristone 200 mg (n = 46) or placebo (n = 46). Forty-eight hours later, patients in both the groups were given 800 µg misoprostol per-vaginum. If no expulsion occurred within 4 h, repeat doses of 400 µg misoprostol were given orally at 3-hourly interval to a maximum of 2 doses in women ≤9 weeks by scan and 4 doses in women >9 weeks by scan.
Results
Pre-treatment of misoprostol with mifepristone significantly increased the complete abortion rate (86.7 vs. 57.8%, p = 0.009) and, hence, reduced the need for surgical evacuation (13.3 vs. 42.2%, p = 0.002), induction to expulsion interval (4.74 ± 2.24 vs. 8.03 ± 2.77 h, p = 0.000), mean number of additional doses of misoprostol required (0.68 vs. 1.91, p = 0.000), and side effects.
Conclusion
Use of mifepristone prior to misoprostol in EPF significantly improves the efficacy and reduces the side effects of misoprostol alone.
Keywords: Early pregnancy failure, Mifepristone, Misoprostol
Introduction
Early pregnancy failure (EPF), ≤12 weeks, is one of the most common complications of pregnancy, accounting for almost 50% of conceptions and 12–15% of all clinically diagnosed pregnancies [1]. Surgical evacuation has been the standard treatment for years. However, considering the long-term sequelae of surgical management in subsequent pregnancies, the role of medical management becomes utmost important [2].
Non-viable pregnancies contain viable trophoblast tissue, which produces hormones that make these pregnancies more susceptible to antihormone therapy and uterotonics. Based on this theory, various drugs (mifepristone, misoprostol, gemeprost, dinoprostone, and methotrexate) with different dosage schedule and routes have been used in different studies for the management of EPF [3].
Mifepristone is a progesterone receptor antagonist that inhibits transcription, causes decidual necrosis, detaches products of gestation, and promotes myometrial excitability. It is widely used in elective medical termination of pregnancy along with misoprostol. However, its use in medical treatment of EPF is not well established as studies have shown inconsistent results regarding the efficacy of mifepristone pre-treatment which needs to be resolved.
Although ACOG and NICE guidelines [4, 5] do not recommend mifepristone prior to misoprostol for medical treatment of EPF, various studies using this combination have shown higher success rate [3, 6, 7]. Therefore, more trials are needed to compare the efficacy of mifepristone followed by misoprostol with misoprostol alone. Moreover, no double-blind placebo-controlled trial has been conducted, and the studies so far are limited to only retrospective studies or randomized prospective trials.
The present study is the first double-blind placebo-controlled trial conducted to compare the efficacy and safety of mifepristone followed by misoprostol with misoprostol alone for treatment of EPF.
Materials and Methods
This double-blind randomized placebo-controlled trial was performed in the Department of Obstetrics and Gynaecology at University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, from October 2011 to April 2013. Prior ethical clearance was obtained from the institutional ethical committee and trial was registered in Clinical Trial Registry of India (CTRI 2013/03/003492).
Ninety-two women with EPF ≤12 weeks of gestation were recruited. EPF was defined as: Intrauterine gestation with gestational sac > 20 mm with no evidence of an embryo/yolk sac OR Intrauterine gestation with CRL > 6 mm without cardiac activity OR Intrauterine gestation with gestational sac < 20 mm/CRL < 6 mm with no growth in 7 days OR other radiological signs of abnormal pregnancy—irregular sac/debris within the gestational sac. Exclusion criteria included incomplete abortion, inevitable abortion, hemodynamic instability, Hb < 8 g%, bleeding disorder, obvious infection, and known allergy to mifepristone/misoprostol. Sample size was calculated assuming a baseline success rate of 65% in control group and expecting an increase in success rate to 85% in the intervention group at a significant level of 0.05 and a power of 0.80.
Similar looking 92 coded packets containing either 200 mg mifepristone or similar looking placebo (calcium 500 mg tablet) were made. Calcium was chosen as placebo as it is similar looking to mifepristone and has no effects on the ongoing pregnancy. The sealed packets were numbered from 1 to 92 by simple randomization using computer generated random tables on a master list which was available with the third party. The third party used to dispense the coded sealed packet to the treating obstetrician. Hence, both the treating obstetrician and the patient were blinded regarding the nature of the drug.
After the written informed consent, all recruited women were subjected to detailed history and examination including systemic and per-vaginal examination. Baseline routine investigations including ABO and rhesus group, hemoglobin levels and coagulation profile were obtained. The subjects were given sealed numbered envelope containing either the active drug/placebo on outpatient basis.
Forty-eight hours after administration of mifepristone/placebo, patients were admitted in the labor room. Per-vaginal examination was done following which 800 µg of misoprostol was given per-vaginally. If no expulsion occurred within 4 h, a repeat dose of misoprostol 400 µg was given orally at 3-hourly interval to a maximum of 2 doses in women ≤9 weeks by scan and to a maximum of 4 doses in women >9 weeks by scan.
The patients were discharged 6 h after the last dose of misoprostol and were asked to come on day 14 for clinical interview regarding their bleeding pattern and transvaginal sonography (TVS). If the patient did not bleed within 48 h of the last dose of misoprostol, she was advised to report for a TVS the same day. They were also instructed to report in case of heavy bleeding and were prescribed antibiotics and paracetamol tablets for pain.
Surgical evacuation was done if no bleeding occurred within 48 h of completion of protocol with scan suggestive of intact gestational sac OR the patient had excessive bleeding anytime OR at 2-week follow-up visit, if TVS was suggestive of intact gestational sac/endometrial thickness ≥15 mm. The products of conception were sent for histopathological examination.
Primary outcome of the study was complete abortion rate, defined as a well-defined endometrial line with a maximum thickness of <15 mm on TVS on day 14 combined with absence of vaginal bleeding [3]. Secondary outcomes included the need for surgical evacuation, induction to expulsion interval, mean number of additional doses of misoprostol required, side effects, and number of women who would choose medical management in future. Success was defined as complete expulsion of products with no need for surgical evacuation.
Decoding of sealed envelopes was done at the time of data analysis, and subjects were divided into two groups: Group I: Patient taking active drug, i.e., mifepristone (study group) and Group II: Patient taking placebo (control group).
Statistical analysis was performed using Pearson’s Chi-square test and Fisher’s exact test to compare qualitative data with the help of SPSS Version 20.0. Unpaired t test was used to compare quantitative data. Multivariate logistic regression was used to correlate primary outcomes with baseline parameters. p value smaller than 0.05 was considered significant.
Results
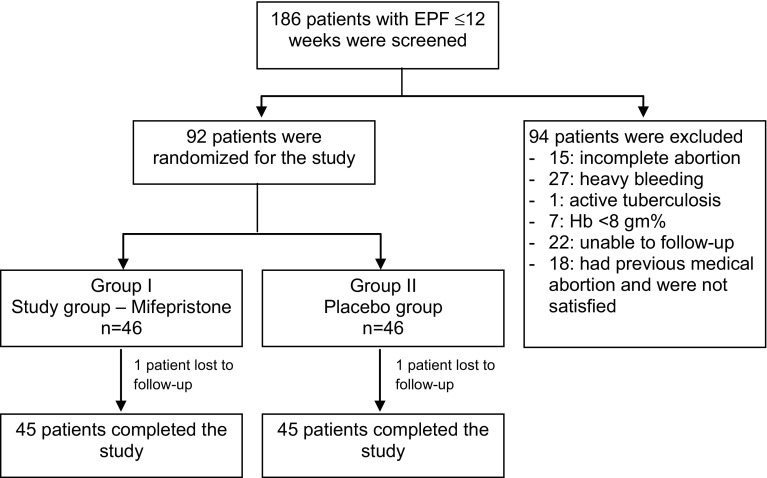
A total of 92 women with EPF (≤12 weeks) were recruited for the present double-blind placebo-controlled study. Group I, i.e., the study group (n = 46) received mifepristone prior to misoprostol, and Group II, i.e., the control group (n = 46) received placebo prior to misoprostol (Fig. 1). Both the groups were matching with respect to sociodemographic profile, obstetrical parameters, clinical symptoms, and laboratory findings (Table 1).
Fig. 1.
Flow diagram to depict consort statement of the study
Table 1.
Comparison of pre-therapy evaluation parameters in both the groups
| Characteristics | Group I (Mifepristone) n = 45 | Group II (Placebo) n = 45 | p value |
|---|---|---|---|
| Age (years, mean ± SD) | 24.69 ± 3.67 | 25.74 ± 4.42 | 0.534 |
| BMI (kg/m2, mean ± SD) | 21.98 ± 1.49 | 22.10 ± 1.53 | 0.684 |
| Parity (median) | 1.0 | 1.0 | 1.000 |
| POG (LMP) (days, mean ± SD) | 72.44 ± 10.58 | 71.36 ± 9.72 | 0.674 |
| POG (USG) (days, mean ± SD) | 48.78 ± 7.78 | 51.62 ± 8.55 | 0.710 |
| Type of EPF | |||
| Missed miscarriage | 30 (66.7) | 41 (91.1) | 0.003 |
| Blighted ovum | 15 (33.3) | 4 (08.8) | |
| Hb (g%) | 11.75 ± 1.36 | 11.75 ± 1.37 | 0.877 |
| PT (s) | 13.73 ± 1.34 | 13.76 ± 1.25 | 0.794 |
| PTTK (s) | 28.78 ± 2.61 | 28.32 ± 2.07 | 0.316 |
Significant p < 0.05 value is in bold
One patient in each group was lost to follow-up during study period, and the data were analyzed by intent to treat analysis by replacing missing value with mean.
The success rate, determined as no need for surgical evacuation, was 88.7% in Group I as compared to 57.8% in Group II. This effect of mifepristone was statistically significant (p = 0.009).
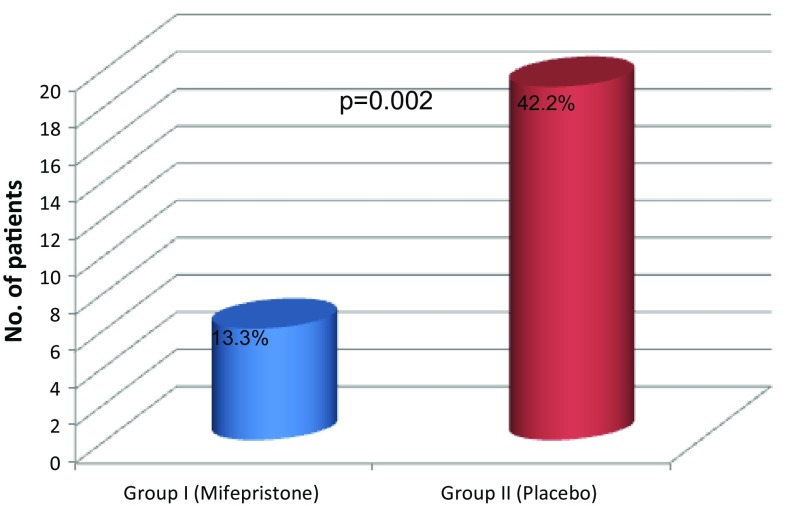
The need for surgical evacuation was significantly less in Group I compared to Group II (13.34 vs. 42.2%, p = 0.002) (Fig. 2). In Group I, 6 of 45 patients underwent surgical evacuation; 2 (4.4%) of them because of suspected retained products of gestation, while 3 patients (6.6%) had persistent gestational sac on TVS on day 14. In Group II, 19 of 45 patients underwent surgical evacuation; 10 (22.2%) of them due to incomplete expulsion and 8 patients (17.8%) had persistent gestational sac on TVS on day 14. Emergency curettage was performed in one patient (2.2%) in each group because of heavy bleeding (Table 2). On histopathology, products of conception were confirmed in all the specimens.
Fig. 2.
Comparison of need for surgical evacuation in both the groups
Table 2.
Comparison of outcome on day 14 in both the groups
| Characteristics | Group I (Mifepristone) n = 45 (%) | Group II (Placebo) n = 45 (%) | p value |
|---|---|---|---|
| Expulsion of POCs within 48 h of mifepristone | 3 (06.7) | 1 (02.2) | 0.675 |
| Expulsion of POCs within 4 h of first dose of 800 µg misoprostol | 29 (65.9) | 5 (11.4) | 0.000 |
| Additional doses of oral misoprostol 400 mg required | |||
| First dose | 10 (22.7) | 15 (34.1) | 0.000 |
| Second dose | 5 (11.4) | 20 (45.5) | |
| Third dose | 0 | 2 (04.5) | |
| Fourth dose | 0 | 2 (04.5) | |
| Outcome on day 14 | |||
| Complete abortion | 39 (86.7) | 26 (57.8) | 0.009 |
| Incomplete abortion | 3 (06.7) | 11 (24.4) | 0.016 |
| Persistent gestational sac | 3 (06.6) | 8 (17.8) | 0.126 |
Significant p < 0.05 values are in bold
Occurrence of vaginal bleeding within 48 h of administration of mifepristone (Group I) was significantly more as compared to after placebo (Group II) (88.9 vs. 35.6%, p = 0.000). Three out of 45 patients (6.7%) in Group I expelled the products within 48 h (Table 2).
Although bleeding per-vaginum within 4 h of initial dose of 800 µg misoprostol was comparable in both the groups, 66% of patients in the Group I expelled the products of conception as compared to only 11.4% of patients in Group II. This difference was highly significant (p = 0.000) (Table 2).
Patients who did not expel the products of conception with initial dose of misoprostol (34.1 vs. 88.6%) were given additional doses of oral misoprostol 400 µg at 3-hourly interval. The difference in the need for additional doses of misoprostol was highly significant (p = 0.000) (Table 2). For two patients, one in each group who did not bleed even after receiving additional doses of misoprostol, a TVS was done 48 h after the completion of protocol following which a surgical evacuation was done due to intact gestational sac.
The mean induction to expulsion interval (4.74 ± 2.24 vs. 8.03 ± 2.77 h, p = 0.000) was significantly less in the Group I as compared to Group II. Induction to expulsion interval was defined as expulsion of the products of conception following initial dose of misoprostol.
The number of days of bleeding (6.20 vs. 6.22 days, p = 0.384) and mean hemoglobin reduction (0.6 vs. 0.62 g%, p = 0.848) was comparable in both the groups. None of the patients in either group required blood transfusion. The number of patients developing nausea and vomiting in Group II was significantly high compared to Group I (42.2 vs. 17.8%, p = 0.009). The overall procedure was more acceptable to patients in Group I (84.4 vs. 60%, p = 0.012).
On analyzing the factors that might influence the primary outcome, i.e., the complete abortion rate, it was found that sociodemographic profile, parity, previous abortions, period of gestation, clinical symptoms, and size of gestational sac had no significant effect on the complete abortion rate (Table 3).
Table 3.
Correlation of primary outcome (complete abortion rate) with pre-therapy evaluation parameters in both the groups
| Parameter | Group I (Mifepristone) n = 45 | Group II (Placebo) n = 45 | ||||
|---|---|---|---|---|---|---|
| No. of patients without complete expulsion (n = 6) | No. of patients with complete expulsion (n = 39) | p value | No. of patients without complete expulsion (n = 19) | No. of patients with complete expulsion (n = 26) | p value | |
| Age (years) | 25.67 ± 3.62 | 24.29 ± 3.41 | 0.366 | 26.63 ± 3.69 | 25.11 ± 4.92 | 0.261 |
| BMI (kg/m2) | 21.27 ± 0.87 | 22.10 ± 1.56 | 0.212 | 22.21 ± 1.63 | 22.03 ± 1.52 | 0.706 |
| Mean parity | ||||||
| Nulliparous | 3 | 25 | 0.293 | 6 | 13 | 0.052 |
| Multiparous | 3 | 14 | 13 | 13 | ||
| Previous abortions (mean) | ||||||
| 0 | 3 | 26 | 0.576 | 14 | 20 | 0.453 |
| ≥1 | 3 | 13 | 5 | 6 | ||
| POG (days) (mean ± SD) | 74.67 ± 8.14 | 72.39 ± 10.94 | 0.630 | 69.26 ± 11.02 | 72.44 ± 8.62 | 0.278 |
| POG (scan) (mean ± SD) | 53.33 ± 10.80 | 48.13 ± 7.23 | 0.134 | 53.21 ± 8.51 | 50.15 ± 8.46 | 0.234 |
| Bleeding P/V | 4 | 24 | 1.000 | 12 | 21 | 0.497 |
| Pain abdomen | 2 | 9 | 1.000 | 8 | 5 | 0.081 |
| Size of gestational sac | ||||||
| GS < 18 mm | 1 | 7 | 1 | 1 | 0.562 | |
| GS > 18 mm | 0 | 7 | 1 | 1 | ||
Discussion
Complete abortion rate as revealed by TVS on day 14 was significantly more in Group I as compared to Group II (86.7 vs. 57.8%, p = 0.009). Patient’s age, parity, previous abortions, period of gestation, clinical symptoms, and size of gestational sac did not affect the primary outcome which was assessed by multivariate regression analysis (Table 3).
In our study, only 13.34% (6/45) of the patients in Group I required surgical evacuation as compared to 42.2% (9/45) in Group II. This effect of mifepristone is statistically significant (p = 0.002). Better results in the mifepristone group can be explained by the fact that pre-treatment with mifepristone reduces the activity of prostaglandin dehydrogenase leading to marked decrease in the metabolism of locally produced prostaglandin, thereby enhancing the effect of misoprostol and also induces cervical ripening.
In Group I, 6.7% of patients expelled the products of conception with mifepristone alone. This could be attributed to its antiprogesterone effect. Expulsion in the placebo group could be attributed to natural process.
The expulsion rate within 4 h of 800 µg misoprostol was significantly higher in Group I (65.9 vs. 11.4%). In our study, mifepristone significantly reduced the man number of additional doses of misoprostol required to achieve expulsion (0.68 vs. 1.91, p = 0.000). The mean induction to expulsion interval was also less in Group I as compared to Group II (4.74 ± 2.24 vs. 8.03 ± 2.77 h).
The number of days of bleeding and mean hemoglobin reduction was similar in both the groups despite the fact that there was less failure and less dose of misoprostol required in Group I. Side effect of nausea was significantly high in Group II which could be due to higher doses of misoprostol given to patients in Group II. The overall procedure was more acceptable to patients in Group I (p = 0.012).
Studies using a combination of mifepristone and misoprostol in the treatment of EPF have reported complete abortion rate ranging from 52 to 93.3% [3, 6–9]. The complete abortion rate was lower in studies by Stockheim et al. [3] (65.5%), Colleselli et al. [8] (61%) and van den Berg et al. [9] (66.8%) which could be attributed to differences in patient selection criteria, dosing protocols, route of administration of drugs, follow-up regime, and definition of success. Colleselli et al. [8] used low doses of misoprostol (400 µg) after mifepristone pre-treatment. Stockheim et al. [3] used 800 µg of misoprostol, but repeat doses of misoprostol were not used. The complete abortion rate was higher in study by Scheiber et al. [6] (93%). This may be due to differences in the definition of complete abortion, i.e., absence of gestational sac on TVS. Had this definition been the criteria for complete abortion in our study, the complete abortion rate would have been 91.1%.
The expulsion rate with initial dose of misoprostol after mifepristone pre-treatment ranges from 52 to 74% in various studies [3, 6, 7]. However, Schreiber et al. [6] and Kollitz et al. [7] have reported higher expulsion rates varying from 80 to 90%. Kollitz et al. [7] used 600 mg mifepristone, while Schreiber et al. used 200 mg mifepristone, but most of their patients were symptomatic at the time of recruitment and the sample size was small (30 patients).
The strength of the present study is that it is a double-blind randomized placebo-controlled trial. Statistically also, it is a sound study as the sample size taken is adequate.
Conclusion
Pre-treatment of misoprostol with mifepristone for treatment of EPF significantly increases the complete abortion rate. There was a significant decrease in the surgical evacuation rate, induction to expulsion interval, mean total dose of misoprostol required and side effects and hence, increased acceptability among the patients. The present study strongly supports the sequential combination of mifepristone and misoprostol for treatment of EPF.
Acknowledgement
We acknowledge the help extended by the Department of Biostatistics for the analysis.
Dr. Priya Sinha
passed her MBBS from Lady Hardinge Medical College, New Delhi, and MS (Obs & Gynae) from UCMS & GTB Hospital, Delhi. She is currently working as Senior Resident in Department of Obstetrics & Gynaecology at UCMS & GTB Hospital, Delhi. Her field of interest is reproductive medicine.
Compliance with Ethical Standards
Conflict of interest
None.
Footnotes
Priya Sinha passed her MBBS from Lady Hardinge Medical College and is currently working as Senior Resident in Department of Obstetrics & Gynaecology at UCMS & GTB Hospital, Delhi, India. Amita Suneja Director Professor & Head, Department of Obstetrics & Gynaecology at UCMS & GTB Hospital, Delhi, India. Kiran Guleria Director Professor, Department of Obstetrics & Gynaecology at UCMS & GTB Hospital, Delhi, India. Richa Aggarwal Assistant Professor, Department of Obstetrics & Gynaecology at UCMS & GTB Hospital, Delhi, India. Neelam B Vaid Director Professor, Department of Obstetrics & Gynaecology at UCMS & GTB Hospital, Delhi, India.
References
- 1.Speroff L, Glass RH, Kase NG. Clinical gynecologic endocrinology and infertility. 8. Philadelphia: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 2.Gariepy AM, Chen BA. Management of early pregnancy failure. In: Berghella V, editor. Obstetric evidence based guidelines. 2. London: Informa Healthcare; 2012. pp. 261–265. [Google Scholar]
- 3.Stockheim D, Machtinger R, Wiser A, et al. A randomized prospective study of misoprostol or mifepristone followed by misoprostol when needed for the treatment of women with early pregnancy failure. Fertil Steril. 2006;86:956–960. doi: 10.1016/j.fertnstert.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 4.Committee on Practice Bulletins—Gynecology The American College of Obstetricians and Gynecologists practice bulletin no. 150. Early pregnancy loss. Obstet Gynecol. 2015;125(5):1258–1267. doi: 10.1097/01.AOG.0000465191.27155.25. [DOI] [PubMed] [Google Scholar]
- 5.NICE: Ectopic pregnancy and miscarriage: diagnosis and initial management in early pregnancy of ectopic pregnancy and miscarriage. (Clinical Guidelines 154). 2012. http://www.nice.org.uk/CG154diagnosis. [PubMed]
- 6.Schreiber CA, Creinin MD, Reever MF, et al. Mifepristopne and misoprostol for the treatment of early pregnancy failure: a pilot clinical trial. Contraception. 2006;74(6):458–462. doi: 10.1016/j.contraception.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Kollitz KM, Meyn LA, Lohr PA, et al. Mifepristone and misoprostol for early pregnancy failure: a cohort analysis. Am J Obstet Gynecol. 2011;204(5):386-e1–386-e6. doi: 10.1016/j.ajog.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Colleselli V, Schreiber CA, D’Costa E, et al. Medical management of early pregnancy failure (EPF): a retrospective analysis of a combined protocol of mifepristone and misoprostol used in clinical practice. Arch Gynecol Obstet. 2014;289(6):1341–1345. doi: 10.1007/s00404-013-3105-4. [DOI] [PubMed] [Google Scholar]
- 9.van den Berg J, van den Bent JM, Snijders MP, et al. Sequential use of mifepristone and misoprostol in treatment of early pregnancy failure appears more effective than misoprostol alone: a retrospective study. Eur J Obstet Gynecol Reprod Biol. 2014;183:16–19. doi: 10.1016/j.ejogrb.2014.10.010. [DOI] [PubMed] [Google Scholar]