Abstract
Detection of medial olivocochlear-induced (MOC) changes to transient-evoked otoacoustic emissions (TEOAE) requires high signal-to-noise ratios (SNR). TEOAEs associated with synchronized spontaneous (SS) OAEs exhibit higher SNRs than TEOAEs in the absence of SSOAEs, potentially making the former well suited for MOC assays. Although SSOAEs may complicate interpretation of MOC-induced changes to TEOAE latency, recent work suggests SSOAEs are not a problem in non-latency-dependent MOC assays. The current work examined the potential benefit of SSOAEs in TEOAE-based assays of the MOC efferents. It was hypothesized that the higher SNR afforded by SSOAEs would permit detection of smaller changes to the TEOAE upon activation of the MOC reflex. TEOAEs were measured in 24 female subjects in the presence and absence of contralateral broadband noise. Frequency bands with and without SSOAEs were identified for each subject. The prevalence of TEOAEs and statistically significant MOC effects were highest in frequency bands that also contained SSOAEs. The median TEOAE SNR in frequency bands with SSOAEs was approximately 8 dB higher than the SNR in frequency bands lacking SSOAEs. After normalizing by TEOAE amplitude, MOC-induced changes to the TEOAE were similar between frequency bands with and without SSOAEs. Smaller MOC effects were detectable across a subset of the frequency bands with SSOAEs, presumably due to a higher TEOAE SNR. These findings demonstrate that SSOAEs are advantageous in assays of the MOC reflex.
Keywords: auditory efferent system, cochlear processing, female, normal hearing
INTRODUCTION
Otoacoustic emissions (OAEs) provide a means to noninvasively assay the influence of the medial-olivocochlear reflex (MOCR) on cochlear processing. Activation of the MOCR reduces OAE amplitude (e.g., Moulin et al. 1993; Collet 1993; Berlin et al. 1994), decreases the latencies of transient-evoked (TE) OAEs (Giraud et al. 1996; Francis and Guinan 2010; Mishra and Dinger 2016) and shifts the frequencies of distortion product (DP; Henin et al. 2011) and spontaneous (S) OAEs (Mott et al. 1989; Zhao and Dhar 2010). Both the reduction in OAE amplitudes and phase-related changes (whether in terms latency or frequency shifts) are consistent with input from the MOC bundle to the cochlear outer hair cells reducing cochlear amplification and tuning (Murugasu and Russell 1996; see Cooper and Guinan 2006 for review).
Although OAEs provide a convenient means to evaluate the MOCR, MOCR-induced changes to OAEs are small. Reported magnitude and latency changes are approximately 25 % and 5 %, respectively (e.g., Francis and Guinan 2010; Mishra and Dinger 2016). Statistical techniques such as bootstrapping are necessary to determine the significance of changes, especially when the goal is to detect the response in individual subjects (Backus and Guinan 2007; Goodman et al. 2013; Marshall et al. 2014; Mertes and Goodman 2016). Additionally, the measurement paradigm must be carefully constructed to control for potentially confounding factors such as activation of the middle-ear muscle reflex (MEMR).
Assuming that the experiment design is robust to changes in middle-ear impedance, detection of MOC-induced changes to the OAE requires a signal-to-noise ratio (SNR) of at least 6 dB (e.g., Mishra and Lutman 2013). Moreover, the SNR required to detect a response increases as the size of the response decreases (Goodman et al. 2013). Increasing the number of averages and/or the evoking-stimulus level can increase OAE SNR. In research protocols investigating MOC effects in normal-hearing adults, increasing the number of averages is often feasible despite the associated increase in test time. However, in clinical applications, increasing the test time is not always practical—especially among difficult-to-test populations. In such cases, increasing the OAE-evoking stimulus level is not a desirable means to increase OAE SNR, as higher stimulus levels may activate the MEMR (Guinan et al. 2003).
TEOAEs in frequency bands with SOAEs are potentially well suited for evaluating the auditory efferent system’s influence on cochlear processing and/or the integrity of the efferent pathways. One class of SOAEs particularly relevant to TEOAEs is synchronized spontaneous (SS) OAEs. SSOAEs present as slow-decaying components of the TEOAE, which, in some cases, persist for hundreds of milliseconds post-presentation of the click (Sisto et al. 2001; Jedrzejczak et al. 2008; Keefe 2012). Like SOAEs, SSOAEs may arise through repeated intra-cochlear reflections, stemming from an impedance mismatch between the oval window and characteristic frequency place (Shera 2004). Near frequencies of SSOAEs, the TEOAE spectrum exhibits magnitude peaks and high SNRs (Kulawiec and Orlando 1995). SSOAEs are prevalent in normal-hearing ears from both males and females; although, prevalence is higher in females (Sisto et al. 2001; Jedrzejczak et al. 2008), like SOAEs (Talmadge et al. 1993; Penner and Zhang 1997). The high SNR of TEOAEs associated with SSOAEs may facilitate detection of small MOCR effects.
SOAEs (including SSOAEs) are traditionally viewed as a potential confound in OAE-based assays of the MOCR. In stimulus-frequency (SF) OAE paradigms, it is common for investigators to avoid the use of stimulus frequencies within 30–100 Hz of an SOAE due to potential interactions between the SOAE and SFOAE (as well as the stimulus; Burns et al. 1984; Backus and Guinan 2007; Lilaonitkul and Guinan 2009; Francis and Guinan 2010). Several studies cite the potential for SSOAEs to complicate interpretation of MOC-induced changes to TEOAEs (Marshall et al. 2014; Mertes and Goodman 2016; Mishra and Dinger 2016). It is not clear as to why SSOAEs may be problematic in TEOAE-based assays of the MOCR. However, one may speculate based on how SSOAEs affect TEOAE latency. When the amount of time between adjacent stimulus presentations is shorter than the decay time of the SSOAE, the SSOAE contaminates the early-time portion of the adjacent recording and may shift the TEOAE latency estimate to shorter values (Jedrzejczak et al. 2008; Keefe 2012). Upon activation of the MOCR, SSOAE amplitude is reduced (Meric and Collet 1994), as is the amount of SSOAE energy contaminating the early time portion of the adjacent recording. The reduction in SSOAE energy within the early-time portion of the recording upon MOCR activation may result in slightly longer latencies, compared to the condition where the MOCR is not active. Longer latencies during MOCR activation would suggest a sharpening of tuning. At least one study excluded frequency bands with SSOAEs in their analysis of the efferent influence on TEOAE latency (Mishra and Dinger 2016).
Findings from Marshall et al. (2014) and Mertes and Goodman (2016) suggest that SSOAEs are not problematic in TEOAE-based assays of the MOCR, at least for applications that do not rely on latency measures. Marshall et al. (2014) reported no significant difference in the strength of the MOCR between frequency bands with and without SSOAEs. Mertes and Goodman (2016) observed that the presence of SSOAEs neither prevented detection of statistically significant MOCR effects nor increased the variability of MOC effects within or across measurement sessions. Whereas the work by Marshall et al. (2014) and Mertes and Goodman (2016) address concerns regarding SSOAEs in TEOAE-based MOC assays, the current work aims to identify an advantage associated with SSOAEs. It was hypothesized that smaller MOC effects are detectable when SSOAEs are present, due to higher TEOAE SNRs. The ability to detect small MOC-induced changes to the TEOAE is expected to be useful when investigating the influence of attentional, maturational and disease processes on efferent-modulation of cochlear sensitivity.
METHODS
Subjects
Twenty-five adult females (18–39 years) participated in the study. Only females were recruited as the prevalence of SOAEs is higher in females. All subjects had air-conduction, pure-tone behavioral thresholds at or below 20 dB HL at the octave frequencies between 0.25 and 8 kHz, type-A 226-Hz tympanograms (compensated static admittance between 0.5 and 2.1 mmho, tympanometric peak pressure within ± 100 daPa) and present ipsilateral (both right and left) MEMR in response to white-noise at a fixed level of 80 dB HL. The type-A tympanograms and present MEMRs served to rule-out middle-ear pathology. Data collection was completed over the course of a single 2-h visit. To equate visual attention across subjects, all subjects watched closed-captioned movies during data collection. Subjects were compensated for their participation. The Institutional Review Board at the University of Tennessee-Knoxville approved all testing.
Signal Presentation and Data Acquisition
MATLAB (The Mathworks, Inc.) was used to generate stimuli at a sample rate of 48 kHz. An RME Babyface 24-bit sound card converted the analog signals to their digital equivalent. A Rane HC6S Headphone Console amplified the stimuli. Transient stimuli to evoke the OAE were routed through one channel of a pair of Etymotic ER2 headphones connected to an ER10B+ probe-microphone system. An Etymotic ER10D impedance tip coupled the ER10B+ probe to the subject’s ear. Broadband noise was routed through the second channel of the ER2 headphones, which was coupled to the contralateral ear. The ER10B+ probe microphone system recorded and amplified (20 dB gain) the ipsilateral ear-canal sound pressure before directing it to the sound card. Recordings were stored for offline analysis. The MATLAB-based software ARLas (provided by Dr. Shawn S. Goodman at the University of Iowa) controlled stimulus presentation and data acquisition. OAE measurements were performed in a sound-attenuating booth with the subject seated in a recliner.
A band-limited click shaped to have a flat magnitude spectrum from 0.25–12 kHz evoked the OAEs. The following steps were used to create the stimulus: (1) The fast Fourier transform (FFT) converted the impulse response of an IEC711 ear simulator to the frequency domain. (2) The reciprocal of the impulse-response’s normalized magnitude was calculated. (3) Magnitudes above 12 kHz and below 0.25 kHz were specified as 0. (4) A phase vector was created with 0 phase at all frequencies. (5) The magnitude and phase data were converted to the time-domain via the inverse fast Fourier transform (IFFT). (6) A 3-ms Blackman window truncated the time-domain response to yield the evoking stimulus. The level of the stimulus was 58 dB peak SPL (pSPL; as generated in the IEC711 coupler); the presentation rate was 6.3 stimuli/s (inter-stimulus interval of 159 ms). Stimuli were presented using a linear paradigm and directed to the subject’s right ear concurrent with either silence or continuous broadband-noise directed to the left ear [50 dB SPL (root mean square; RMS) in an IEC711 simulator]. The spectrum of the noise matched that of the band-limited click. Quiet and noise conditions alternated every 15 s. A total of 4224 stimuli were presented across the two conditions (2112 for the quiet condition, 2112 for the noise condition).
Analysis
Detection of the MEMR
The contralateral noise may inadvertently activate the MEMR. If activated, the MEMR, as opposed to the MOCR, may underlie changes in the OAE between Quiet and Noise conditions. To determine if the MEMR was activated during the Noise condition, in situ stimulus levels measured for the Quiet condition were compared to those measured for the Noise condition. Stimulus levels are predicted to be different between conditions when the MEMR is activated, due to a change in the impedance of the middle ear and, as a byproduct, the amount of sound power reflected at the plane of the tympanic membrane. After low-pass filtering (1.5-kHz cutoff frequency; 256 order), ear-canal sound-pressure recordings from the Quiet and Noise conditions were combined into a single data set. Resampling with replacement was then performed to create two subsets of the combined data, each of which was composed of approximately 2000 waveforms. Within each data subset, the mean waveform was calculated, from which the stimulus level was determined as the RMS level across the 3-ms stimulus duration. The difference in stimulus levels between the resampled subsets was then calculated. This process was repeated 10,000 times to estimate the 99 % confidence intervals for the difference in stimulus levels between resampled data subsets. The null hypothesis was that stimulus levels between the Quiet and Noise conditions were equal (Goodman et al. 2013). The mean measured difference between stimulus levels was compared to the bootstrapped confidence intervals. Activation of the MEMR was suspected if the mean measured difference fell outside of the confidence interval.
Detection of MOC-Induced Changes to the OAE
Ear-canal sound-pressure recordings were mapped to a time vector with time = 0 ms occurring at the peak amplitude of the evoking stimulus. Stimulus onset occurred at − 1.5 ms and offset occurred at + 1.5 ms. The portion of the ear-canal recordings extending from − 1.5 to + 3.5 ms was discarded to eliminate stimulus energy. The rising phase of a Hann function onset windowed the subsequent 1 ms of the retained recordings. Measurements made in an IEC711 ear simulator confirmed that the windowing procedure effectively removed stimulus energy from the recordings. Specifically, the SNR of the IEC711 recordings between 3.5 and 20-ms post-stimulus (re: stimulus peak amplitude) was calculated for 1/3-octave frequency bands with center frequencies ranging from 0.75–4.76 kHz. As discussed below, an SNR of at least 6 dB within 20-ms post-stimulus was required to classify a TEOAE as present. The SNR measured in the IEC711 ear simulator consistently fell below 6 dB across all frequency bands. Thus, any residual stimulus energy was insufficient to be mistakenly identified as TEOAE energy.
Recordings were high-pass filtered (low-frequency cutoff = 500 Hz; order = 512). An artifact rejection algorithm (based on both the RMS level and crest-factor of the ear-canal recording) identified and removed recordings contaminated by high-level noise. For each condition (Noise and Quiet), retained recordings were divided into odd-numbered and even-numbered recordings. The signal (which may contain emission energy) was estimated by synchronously averaging and then summing the odd- and even-numbered waveforms. The noise was estimated by synchronously averaging and then subtracting the odd- and even-numbered waveforms.
For each subject, the time-domain signal measured in Quiet was analyzed for the presence/absence of TEOAE and SSOAE energy in nine frequency bands (1/3-octave wide; center frequencies from 0.75–4.76 kHz). First, the signal and noise recordings were constrained to a time window spanning 3.5–20 ms relative to the peak amplitude of the evoking stimulus. Emission energy within this time window was attributed to the TEOAE. A 1024-point FFT converted the time-domain signal and noise to their frequency-domain equivalents (waveforms were zero-padded by 232 samples). Within each frequency band, the RMS pressure levels of the signal and noise were calculated and compared. A TEOAE was classified as present if the SNR within the frequency band was at least + 6 dB. A similar analysis was used to identify the presence/absence of SSOAE energy within each frequency band; however, the time window spanned 20–40-ms re: peak amplitude of the stimulus. This window was expected to remove the fast-decaying TEOAE energy, leaving only the slow-decaying SSOAE energy. An SSOAE was defined as present if the SNR in the frequency band was at least + 6 dB.
Two metrics were used to quantify the effect of the MOCR on the TEOAE. For the first metric, the complex difference between frequency domain representations of the TEOAE measured in Quiet (P Q) and Noise (P N) was calculated,
| 1 |
where f indicates the FFT frequency bin. The RMS value of |∆P| was then calculated across each of the previously defined 1/3-octave frequency bands,
| 2 |
where f L and f H are the lower- and upper-frequency limits, respectively, of the frequency band with center-frequency f C, and F was the number of frequency bins spanning the upper- and lower-frequency limits. P MOCT describes the total MOCR-induced change to the emission as it captures changes in both magnitude and phase (the “T” in the subscript “MOCT” indicates “total”).
The second metric was sensitive only to magnitude differences between the emissions measured in Quiet and Noise. The magnitude difference between P Q and P N, Δ|P|, was first calculated by
| 3 |
The RMS level of ∆|P| was then calculated across the different frequency bands,
| 4 |
P MOCM describes the magnitude change to the emission stemming from the MOCR (the “M” in the subscript “MOCM” indicates “magnitude”). P MOCM is insensitive to phase changes.
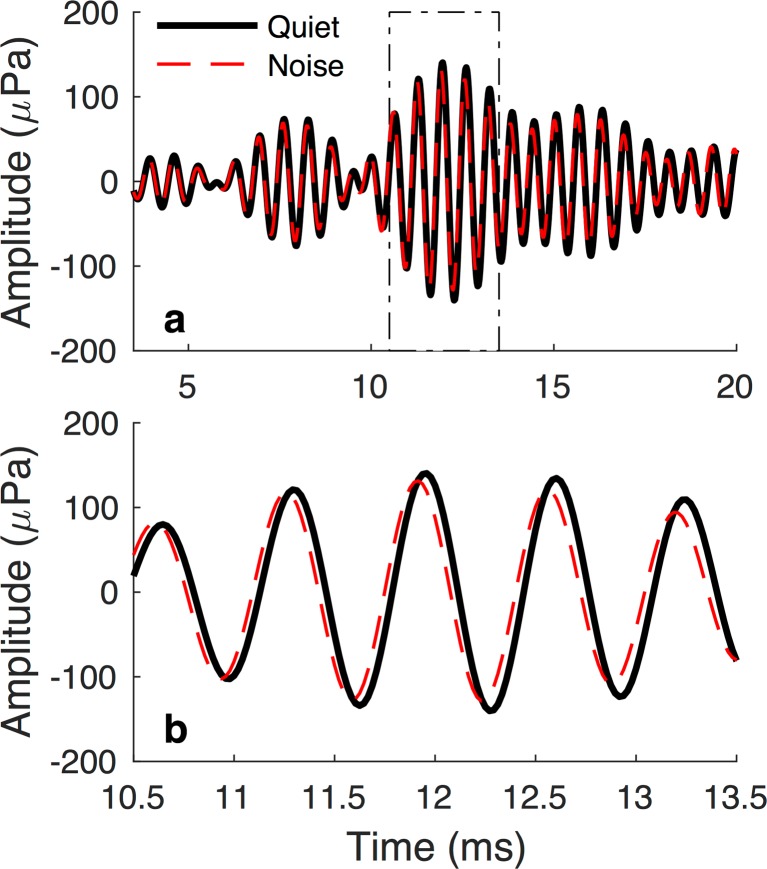
P MOCT and P MOCM are equal when activation of the MOCR induces a change only in TEOAE magnitude. However, when only the phase of the TEOAE or both the phase and magnitude of the TEOAE are changed, P MOCT exceeds P MOCM (Henin et al. 2011; Mertes and Goodman 2016; Mishra and Dinger 2016). Figure 1 illustrates an MOC-induced change in TEOAE phase. The top panel shows the TEOAE waveform within the initial 20-ms post-stimulus; the bottom panel restricts the time window to the peak-amplitude region of the OAE to facilitate visualization of phase changes.
Fig. 1.
Example of an MOC-induced phase change to the TEOAE waveform. a Emission waveforms measured in Quiet and Noise conditions (thick, solid black line and thin, broken red line, respectively) across the duration of the TEOAE analysis window. b Waveforms constrained to a time window spanning 10.5–13.5 ms post-stimulus. Activation of the MOCR resulted in a slight amplitude decrease and phase shift
For each subject, bootstrapping was used to determine the 99 % confidence intervals (0.5–99.5 %) of P MOCT and P MOCM. Like the MEMR analysis, waveforms from the Quiet and Noise conditions were combined into a single data set. Resampling with replacement was performed to create two data subsets, from which P MOCT and P MOCM were then calculated. This process was repeated 10,000 times to estimate the 99 % confidence intervals. The null hypothesis was that the TEOAEs in the Quiet and Noise conditions were measured under the same cochlear state, i.e., the MOCR was not activated. The measured P MOCT and P MOCM were statistically significant when they exceeded the upper 99.5 % of the bootstrapped samples. The upper 99.5 % of the bootstrapped samples is hereafter referred to as δ 99.5%.
RESULTS
Across subjects, the mean signed and unsigned differences in stimulus levels between Quiet and Noise conditions were 0.013 dB (range − 0.58 to 0.54 dB) and 0.17 dB (range 0.003 to 0.58 dB). The average 99 % CI describing the expected difference in Quiet and Noise stimulus levels under the null hypothesis that the contralateral noise did not active the MEMR was [− 0.51, 0.51] dB. Activation of the MEMR by the contralateral noise was indicated for a single subject. The mean difference in stimulus levels between Quiet and Noise conditions for the subject was 0.19 dB (99 % bootstrapped CI: [− 0.13, 0.13]). This subject’s data were not further analyzed; thus, the results detailed below rely on data from 24 subjects.
Influence of SSOAEs on TEOAE Magnitude and SNR
Every subject had a least one frequency band where an SSOAE was present. Table 1 provides the number of subjects in each frequency band that had present TEOAEs and absent SSOAEs (1st and 2nd columns). Table 2 provides the number of subjects in each frequency band that had present TEOAEs and SSOAEs. Note that subjects were classified based on SSOAE status on a frequency band by frequency band case. Across the different frequency bands, TEOAEs accompanied by SSOAEs (117 instances) were more common than TEOAEs in the absence of SSOAEs (59 instances). The 1.19- and 1.5-kHz frequency bands exhibited the highest number of subjects with both TEOAEs and SSOAEs, and the lowest number of subjects with TEOAEs alone.
Table 1.
The prevalence of TEOAEs and statistically significant MOCR metrics when SSOAEs were absent. Column 2 (“present TEOAE”) shows the number of subjects (N) for each frequency band that had present TEOAEs (an SNR ≥ 6 dB). Column 3 (“present TEOAE and significant P MOCT) and column 4 (“present TEOAE and significant P MOCM) show the number and percentage of subjects with TEOAEs (“n” and “% of N”, respectively) that also had statistically significant MOCR effects
| Absent SSOAEs | |||||
|---|---|---|---|---|---|
| Center Frequency (kHz) | Present TEOAE | Present TEOAE and significant P MOCT | Present TEOAE and significant P MOCM | ||
| N | n | % of N | n | % of N | |
| 0.75 | 6 | 1 | 16.7 % | 0 | 0 % |
| 0.94 | 6 | 0 | 0 % | 0 | 0 % |
| 1.19 | 3 | 0 | 0 % | 0 | 0 % |
| 1.5 | 3 | 0 | 0 % | 0 | 0 % |
| 1.89 | 7 | 3 | 42.9 % | 1 | 14.3 % |
| 2.38 | 9 | 3 | 33.3 % | 4 | 44.4 % |
| 3 | 9 | 4 | 44.4 % | 3 | 33.3 % |
| 3.78 | 9 | 3 | 33.3 % | 0 | 0 % |
| 4.76 | 7 | 3 | 42.9 % | 2 | 28.6 % |
| Total | 59 | 17 | 28.9 % | 10 | 16.9 % |
Table 2.
The prevalence of TEOAES and statistically significant MOCR metrics when SSOAEs were present. Column 2 (“present TEOAE”) shows the number of subjects (N) for each frequency band that had present TEOAEs (an SNR ≥ 6 dB). Column 3 (“present TEOAE and significant P MOCT) and column 4 (“present TEOAE and significant P MOCM) show the number and percentage of subjects with TEOAEs (“n” and “% of N”, respectively) that also had statistically significant MOCR effects
| Present SSOAEs | |||||
|---|---|---|---|---|---|
| Center frequency (kHz) | Present TEOAE | Present TEOAE and significant P MOCT | Present TEOAE and significant P MOCM | ||
| N | n | % of N | n | % of N | |
| 0.75 | 7 | 2 | 28.5 % | 3 | 42.9 % |
| 0.94 | 12 | 9 | 75 % | 5 | 41.7 % |
| 1.19 | 18 | 13 | 72 % | 11 | 61.1 % |
| 1.5 | 20 | 15 | 75 % | 11 | 55 % |
| 1.89 | 16 | 14 | 87.5 % | 9 | 56.3 % |
| 2.38 | 13 | 13 | 100 % | 8 | 61.5 % |
| 3 | 13 | 12 | 92.3 % | 8 | 61.5 % |
| 3.78 | 13 | 12 | 92.3 % | 8 | 61.5 % |
| 4.76 | 5 | 5 | 100 % | 4 | 80 % |
| Total | 117 | 95 | 81.2 % | 67 | 57.2 % |
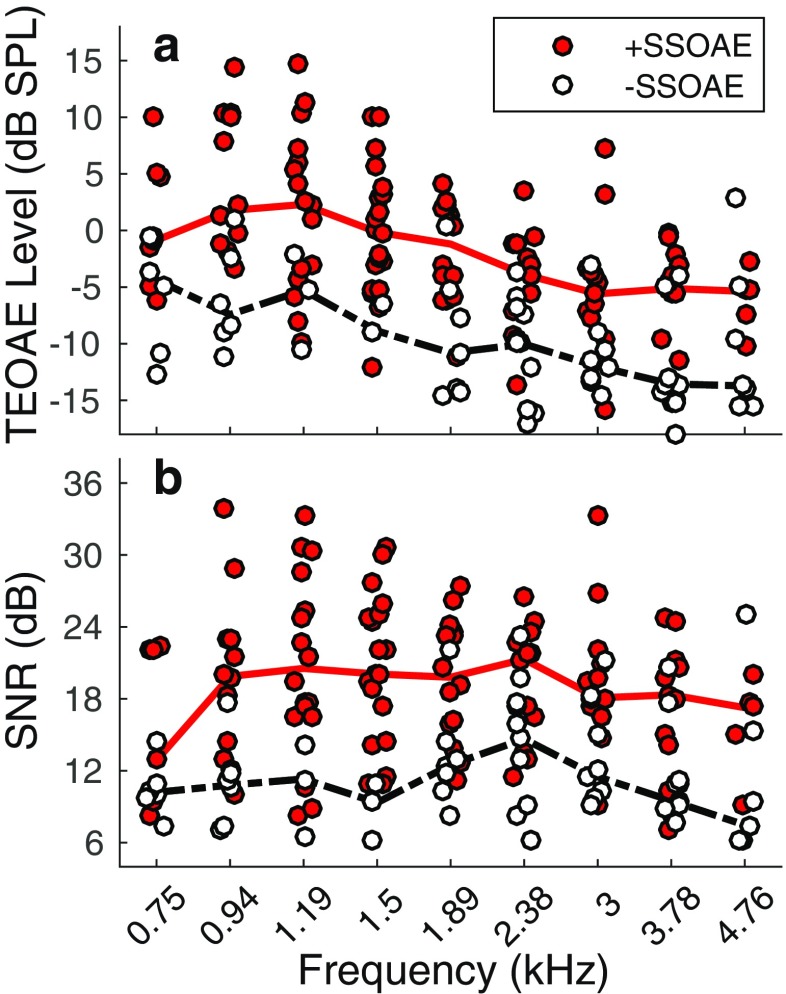
Figure 2a shows TEOAE magnitudes in instances where SSOAEs were present and absent, for each frequency band. The overlaid lines indicate median magnitudes. TEOAE magnitudes were often higher when SSOAEs were present. Across frequency, the median TEOAE magnitude when accompanied by an SSOAE was − 2.4 dB SPL (95 % CI: [− 13.5, 11.2]), compared to − 10.6 dB SPL (95 % CI: [− 17.9, 1.2]) for TEOAEs in the absence of SSOAEs. Figure 2b shows SNRs for TEOAEs in the presence and absence of SSOAEs. Across frequency, the median SNR of TEOAEs accompanied by SSOAEs was 19.3 dB (95 % CI: [9.2, 30.2]), compared to 10.8 dB (95 % CI: [6.2, 23.3]) for TEOAEs in the absence of SSOAEs. TEOAE magnitude and SNR were correlated—higher magnitudes predicted higher SNRs (pairwise linear correlation coefficients of 0.79 and 0.64 for TEOAEs with and without SSOAEs, respectively).
Fig. 2.
TEOAE magnitudes (a) and SNRs (b) in instances where the TEOAE was measured in the presence and absence of an SSOAE (+SSOAE and −SSOAE, respectively). +SSOAE data are indicated by filled symbols, −SSOAE data are indicated by open symbols. The solid lines in each plot represent the median (across frequency and subject) data for TEOAEs with SSOAEs, whereas dashed lines represent the median data for TEOAEs without SSOAEs
MOC Effects in Frequency Bands With and Without SSOAEs
In frequency bands lacking SSOAEs, statistically significant MOC-induced changes to the TEOAE were less common than in frequency bands with SSOAEs (columns 3 and 4 in Tables 1 and 2). P MOCT was statistically significant in 28.9 % of all occurrences where a TEOAE was measured in the absence of an SSOAE, compared to 81.2 % of occurrences where a TEOAE was accompanied by an SSOAE. The prevalence of statistically significant MOC effects decreased when phase information was discarded from the MOC metric: statistically significant values of P MOCM were detected in 16.9 % of cases where an SSOAE was not detected, and in 57.2 % of cases with present SSOAEs. For both MOC metrics, statistically significant MOC effects were most prevalent in frequency bands above 1.5 kHz, regardless of SSOAE status.
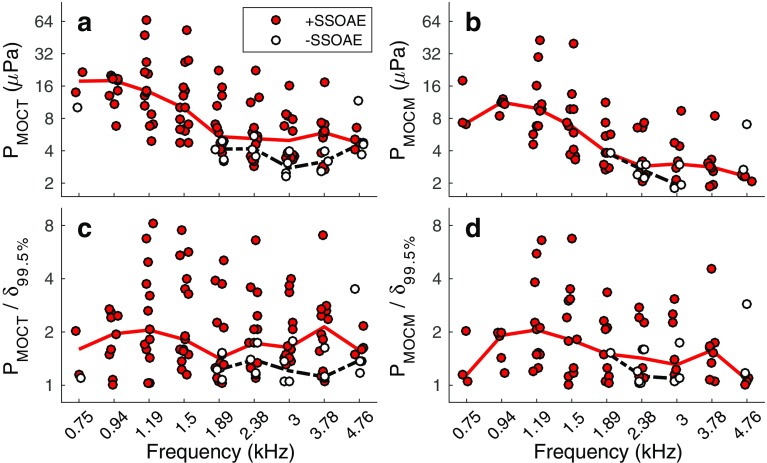
Figure 3a, b shows statistically significant values of P MOCT and P MOCM, respectively, for TEOAEs with and without SSOAEs. P MOCT and P MOCM tended to be larger for TEOAEs accompanied by SSOAEs. The MOC metrics decreased with increasing frequency for TEOAEs accompanied by SSOAEs, as indicated by the median values (solid lines in panels a and b). Across frequency, the median value of P MOCT was 6.91 μPa (95 % CI: [2.83, 48.69]) for TEOAEs accompanied by SSOAEs, compared to 3.95 μPa (95 % CI: [2.29, 11.76]) for TEOAEs lacking SSOAEs. The median value of P MOCM was 5.47 μPa (95 % CI: [1.89, 38.04]) for TEOAEs with SSOAEs and 2.8 μPa (95 % CI: [1.77, 6.94]) for TEOAEs without SSOAEs.
Fig. 3.
MOC metrics (a, b) and SNRs (c, d) in instances where the TEOAE was measured in the presence and absence of an SSOAE. The solid lines in each plot represent the median data for TEOAEs with SSOAEs, whereas dashed lines represent the median data for TEOAEs without SSOAEs.
Figure 3c, d shows P MOCT and P MOCM divided by the 99.5 % upper-limit of the bootstrapped samples, δ 99.5%. Recall that the MOC metrics had to exceed δ 99.5% to be considered statistically significant. As such, the resulting metric may be interpreted as a sort of SNR, quantifying the robustness of P MOCT and P MOCM. For both MOC metrics, higher values were often measured for TEOAEs accompanied by SSOAEs. Across frequency, the median P MOCT/δ 99.5% was 1.73 (95 % CI: [1.03, 7.1]) for TEOAEs with SSOAEs and 1.22 (95 % CI: [1.04, 3.52]) for TEOAEs without SSOAEs. When converted to dB SNR values (20log10 P MOCT/δ 99.5%), the median SNR was 4.8 and 1.8 dB for TEOAEs with and without SSOAEs, respectively. The median P MOCM/δ 99.5% was 1.52 (95 % CI: [1.01, 6.43] when SSOAEs were present and 1.18 (95 % CI: [1.02, 2.85] when SSOAEs were absent. In units of dB SNR (20log10 P MOCM/δ 99.5%), the median values were 3.6 dB and 1.5 dB for TEOAEs with and without SSOAEs, respectively.
Relationship Between MOC Metrics and TEOAE Magnitude
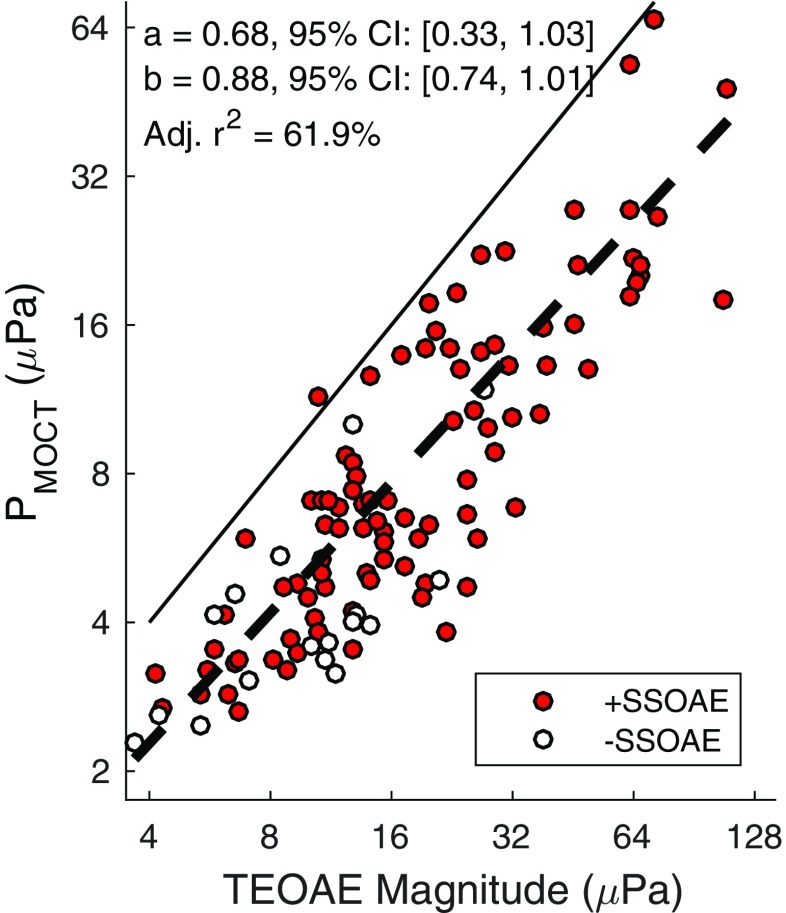
Figure 4 illustrates the dependency of P MOCT on TEOAE magnitude. P MOCT increased with TEOAE magnitude both in cases of present and absent SSOAEs. Overlaid on the individual data points is a 1st-order power-fit of the form y = ax b, where y is P MOCT in μPa, x is TEOAE magnitude (μPa), and [a, b] are model coefficients. Data across frequency bands and SSOAE status were combined into a single data set for the fit. Coefficient values and 95 % confidence intervals are provided in the graph. The model accounted for approximately 62 % of the variance in P MOCT. Although not shown, P MOCM was similarly dependent on TEOAE magnitude.
Fig. 4.
PMOCT as a function of TEOAE magnitude in cases where SSOAEs were present and absent. The solid line represents a 1st-order power-fit to the combined +SSOAE and -SSOAE data. Coefficient values and the goodness of fit are provided in the plot
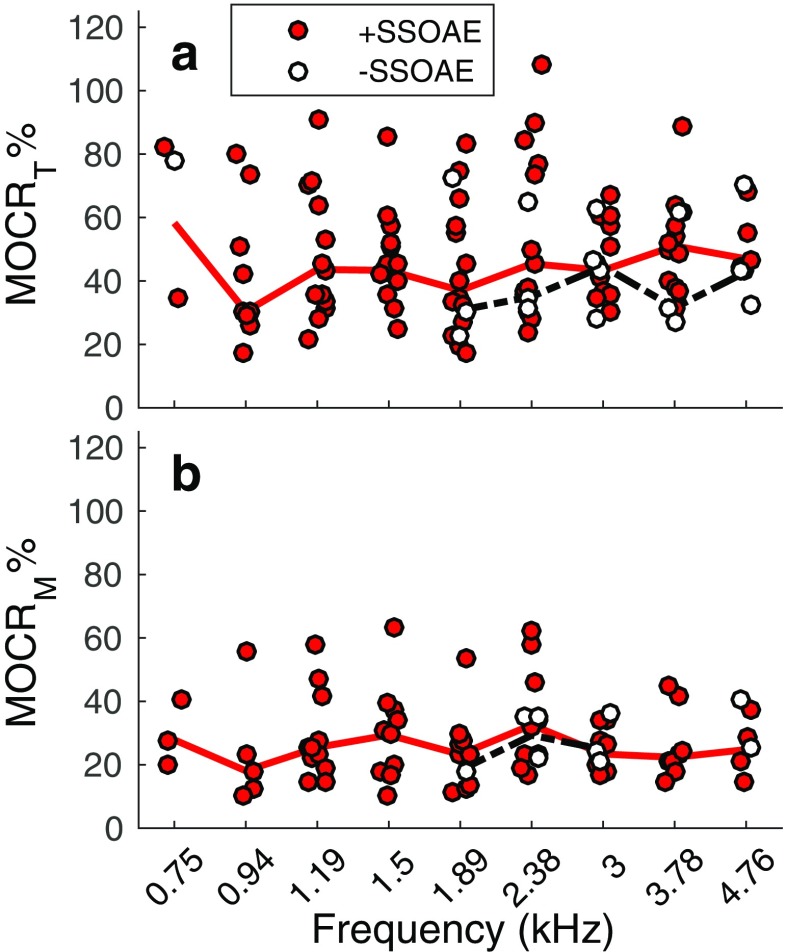
To remove the dependence of P MOCT and P MOCM on TEOAE magnitude, these values were normalized by the TEOAE magnitude measured in Quiet (Backus and Guinan 2007; Marshall et al. 2014; Mishra and Dinger 2016). Normalized values were then multiplied by 100 (MOCR%) to yield an estimate of the MOCR strength (Marshall et al. 2014). Higher values of MOCR% are presumably consistent with a stronger MOCR. Figure 5 shows MOCR% derived from P MOCT (MOCRT%; panel a) and P MOCM (MOCRM%; panel b). For TEOAEs lacking SSOAEs, MOCRT% and MOCRM% fell within the range of those for TEOAEs accompanied by SSOAEs. Across frequency, the median MOCRT% was 44.2 % (95 % CI: [19 %, 90.1 %]) in cases of present SSOAEs and 42.8 % (95 % CI: [23.2 %, 77.7 %]) in cases of absent SSOAEs. The median MOCRM% was 23.7 % (95 % CI: [10.9 %, 61.4 %]) when SSOAEs were present and 25.1 % (95 % CI: [18.3 %, 40.5 %]) when SSOAEs were absent.
Fig. 5.
MOCR% estimated from PMOCT (MOCRT%, a) and PMOCM (MOCRM%, b) within different frequency bands for instances where SSOAEs were present and absent. The solid lines in each plot indicate the frequency-specific median values of MOCR% for the +SSOAE data, whereas the broken lines indicate the median values of MOCR% for −SSOAE data
Dependence of Minimum Detectable MOCR% on TEOAE SNR
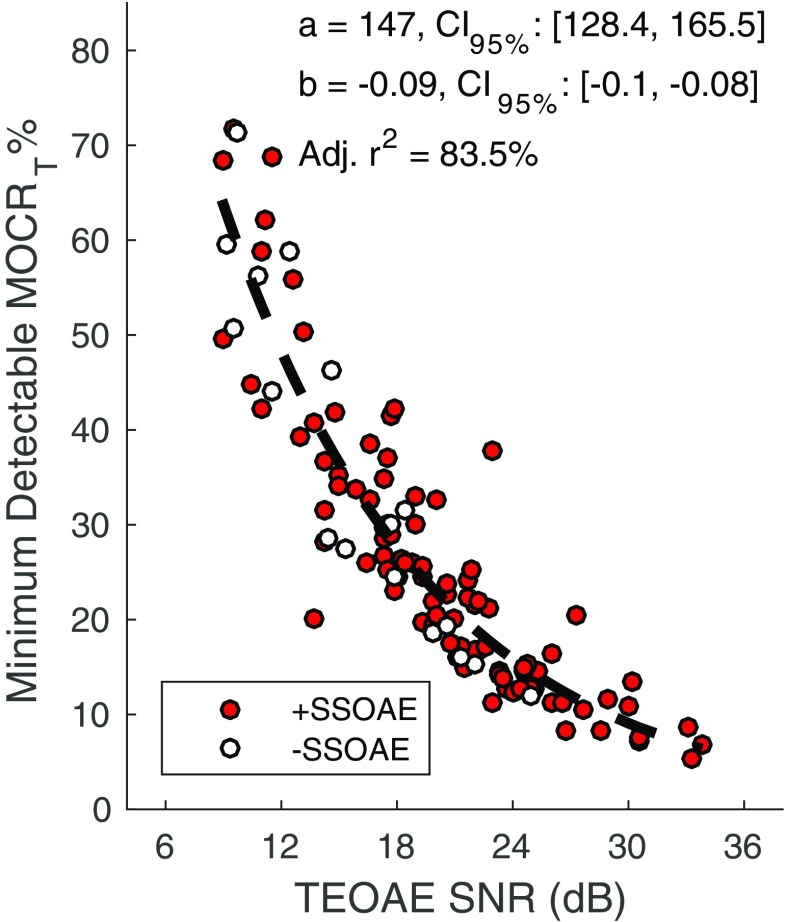
Figure 6 shows the minimum detectable MOCRT% as a function of TEOAE SNR for frequency bands with and without SSOAEs. The minimum detectable MOCRT% was calculated as δ 99.5% divided by TEOAE magnitude (in μPa). Regardless of SSOAE status, higher SNRs permit detection of smaller MOCRT%. Because of the higher SNRs for a subset of the data with present SSOAEs, smaller MOC-induced changes to the TEOAE can, theoretically, be detected. For instance, the minimum detectable MOCRT% in frequency bands lacking SSOAEs is approximately 12 % (at a SNR of 25 dB), compared to 6 % in frequency bands with SSOAEs (at a SNR of 34 dB). Overlaid on the plot is a 1st-order exponential fit of the form y = ae bx, where y is the minimum detectable MOCRT%, x is TEOAE SNR (dB), and [a,b] are model coefficients. SSOAE status was ignored and data were combined into a single data set for the fit. Coefficient values and their 95 % CIs are provided in the plot. The model accounted for approximately 84 % of the variance. Although not shown, the minimum-detectable MOCRM% was similarly dependent on TEOAE SNR.
Fig. 6.
Minimum detectable MOCRT% as a function of TEOAE SNR. The minimum detectable MOCRT% was calculated as δ99.5% normalized by TEOAE magnitude. As TEOAE SNR increases, smaller values of MOCRT% are detectable. The broken dashed line represents a 1st-order exponential fit to the combined +SSOAE and -SSOAE data. Coefficient values and the goodness of fit are provided in the plot
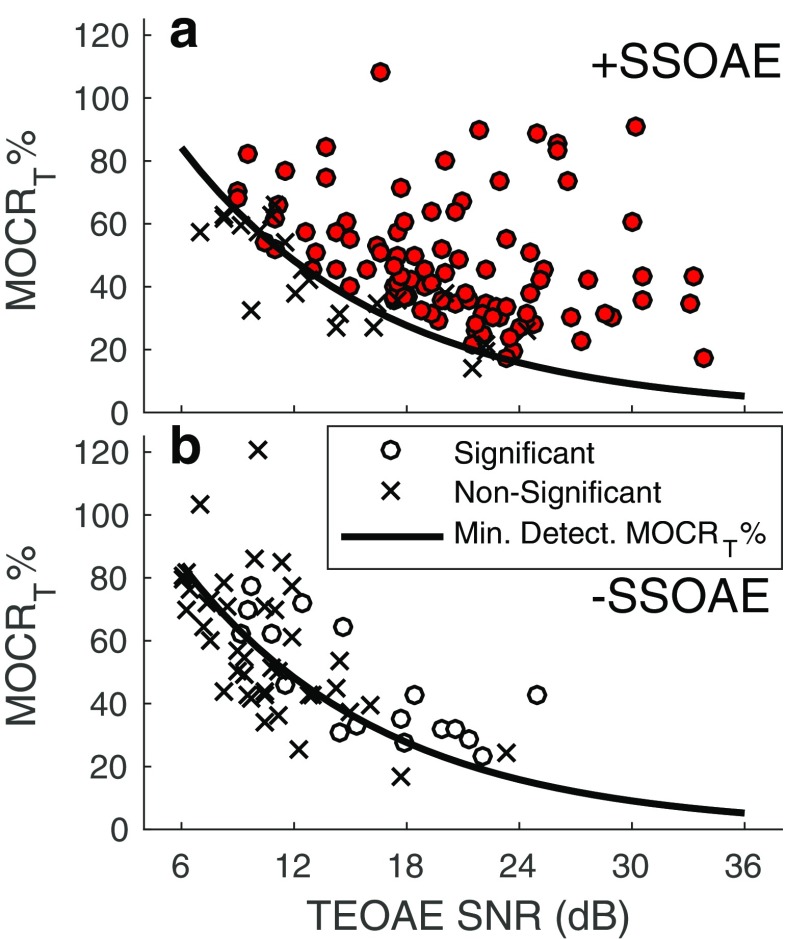
Given the dependence of the minimum-detectable MOCR% on TEOAE SNR, analysis was performed to determine if the TEOAEs lacking statistically significant MOCR effects had lower SNRs, compared to TEOAEs that did show statistically significant MOCR effects. If this were the case, a larger MOCRT% would have been required for detection among the former group. Figure 7 shows MOCRT% for statistically significant and non-significant values of P MOCT, as a function of TEOAE SNR. Regardless of SSOAE status, statistically significant MOCR effects were infrequent for SNRs below 12 dB. Moreover, at SNRs below 12 dB in cases of absent SSOAEs, MOCRT% tended to be lower than the predicted minimum-detectable effect. Across the 42 occurrences where P MOCT was not statistically significant for TEOAEs lacking SSOAEs, the median SNR was 9.9 dB (95 % CI: [6.1, 20.7]). In contrast, for the 17 occurrences where P MOCT was statistically significant, the median SNR was 15.2 dB (95 % CI: [9.2, 25]). SNRs were also higher for statistically significant values of P MOCT when the TEOAE was accompanied by SSOAEs: The median SNR for non-significant values of P MOCT was 12.7 dB (95 % CI: [7.1, 24.2]), compared to a median SNR of 19.3 dB (95 % CI: [9.3, 30.2]) when P MOCT was statistically significant. A similar trend was observed for P MOCM.
Fig. 7.
Statistically significant and non-significant values of MOCRT% as a function of TEOAE SNR when SSOAEs were present (a) and absent (b). The circle markers indicate a statistically significant MOC-induced change to the TEOAE, whereas cross markers indicate a non-significant change. Overlaid on the plots is the 1st-order exponential fit describing the minimum-detectable MOCRT% as a function of TEOAE SNR (from Fig. 6)
DISCUSSION
The Prevalence of SSOAEs, TEOAEs and Statistically Significant MOCR Effects
Findings from the current study demonstrate that SSOAEs are prevalent in young-adult, normal-hearing females. All subjects had at least 1 SSOAE. The subject population was, however, homogenous in terms of racial composition (most, is not all, subjects were white). Jedrzejczak et al. (2008) similarly report 100 % prevalence of SSOAEs among normal-hearing females, albeit using a higher stimulus level (80 dB pSPL compared to 58 dB pSPL). The prevalence of SSOAEs in males appears to be less than in females. For instance, Sisto et al. (2001) reported 70 % of male subjects had at least one SSOAE.
TEOAEs in the absence of SSOAEs were uncommon, at least between 0.75–5 kHz. Across the 24 subjects and 9 frequency bands, there were 176 instances of present TEOAEs. The TEOAE presented without an SSOAE only 34 % of the time. The higher prevalence of TEOAEs accompanied by SSOAEs may be attributed, in part, to the SNR advantage afforded by SSOAEs. Consistent with previous work (Kulawiec and Orlando 1995), TEOAE magnitudes and, by extension, SNRs were higher when an SSOAE was present. The SSOAE-SNR advantage is potentially desirable in TEOAE-based assays of the MOCR given the importance of using low stimulus levels. Specifically, transient stimuli are effective elicitors of the MOCR when presented at moderate-to-high stimulus levels and fast repetition rates (Guinan et al. 2003; Boothalingam and Purcell 2015). Activation of the MOCR by the OAE-evoking stimulus is not ideal since the difference in the TEOAE between Quiet and Noise conditions is reduced. For this reason, low-level stimuli are routinely used; however, a consequence is that TEOAEs may not be detectable in many ears due to reduced magnitudes and poorer SNRs. Indeed, the current findings demonstrate that when a TEOAE is detectable, it is more-often-than-not accompanied by an SSOAE.
Detectable (i.e., statistically significant) MOC-induced changes to TEOAEs unaccompanied by SSOAEs were also uncommon. Statistically significant MOC effects (based on the combined phase and magnitude change) were detected in only 29 % of cases where a SSOAE was absent, compared to 81 % of cases where a SSOAE was present. The lower prevalence of statistically significant MOCR effects in frequency bands lacking SSOAEs does not mean that the TEOAEs were less sensitive to efferent activation. Rather, the lower SNRs of the TEOAEs are hypothesized to preclude detection of statistically significant MOCR effects. The median SNR across TEOAEs that lacked SSOAEs and did not exhibit a statistically significant MOCR effect was nearly 10 dB. An MOCRT% of approximately 60 % is needed for detection when the SNR is 10 dB (see Fig. 6). A statistically significant MOCRT% equal to or greater than 60 % was observed only six times when TEOAEs were unaccompanied by SSOAEs.
SSOAEs are commonly cited as potential confounds in TEOAE-based assays of the efferent system (Marshall et al. 2014; Mertes and Goodman 2016; Mishra and Dinger 2016). At least one study excluded TEOAEs from frequency bands where SSOAEs were present (Mishra and Dinger 2016). In the current study, exclusion of TEOAEs accompanied by SSOAEs would have resulted in discarding nearly 70 % of the data. Given the high prevalence of TEOAEs accompanied by SSOAEs, it is important to determine whether the presence of SSOAEs necessarily requires exclusion of the associated TEOAEs. One approach to answering this question is to compare MOCR effects between frequency bands with and without SSOAEs (e.g., Marshall et al. 2014).
The pressure differences (P MOCT and P MOCM, in μPa) between TEOAEs measured in Quiet and Noise were higher for TEOAEs accompanied by SSOAEs. However, larger values of P MOCT and P MOCM are not necessarily an indicator of a stronger MOCR, as these metrics are highly correlated with emission magnitude (Backus and Guinan 2007). Of course, the dependence of P MOCT and P MOCM on TEOAE magnitude does not necessarily invalidate their usefulness. Normative values of P MOCT and/or P MOCM for a given TEOAE magnitude could perhaps be established to determine whether the MOCR effect is abnormal. Alternatively, the dependence of these metrics on TEOAE magnitude may be removed by expressing the MOC-induced change relative to the magnitude of the TEOAE, i.e., MOCR% (Backus and Guinan 2007; Marshall et al. 2014; Mishra and Dinger 2016). The measured MOCR% in frequency bands lacking SSOAEs fell within the range of MOCR% in frequency bands with SSOAEs (see Fig. 5). This observation is consistent with that reported by Marshall et al. (2014) and suggests that the presence of SSOAEs is not a confounding factor when measuring MOC-induced changes to TEOAEs.
The influence of SSOAEs on estimates of TEOAE latency during MOCR activation was not addressed in the current study. The MOCR-induced change to TEOAE latency is an area of interest as it provides an assay of top-down modulation of cochlear tuning (Francis and Guinan 2010; Shera et al. 2010; Mishra and Dinger 2016). As mentioned in the Introduction, SSOAEs may potentially confound interpretation of TEOAE latency if the inter-stimulus interval is sufficiently short and the SSOAE contaminates the early time portion of the adjacent recording. It is worth noting that the current work utilized a longer inter-stimulus interval (159 ms), compared to that traditionally used in TEOAE-based MOCR assays (20–50 ms). Thus, contamination of the early time window by SSOAE energy was likely reduced.
Minimum Detectable MOC Effects With and Without SSOAEs
Based on the relationship between TEOAE SNR and minimum-detectable MOCR effect from Goodman et al. (2013), it was hypothesized that the higher TEOAE SNRs associated with SSOAEs would permit detection of smaller MOCR effects. Indeed, as shown in Fig. 6, it is theoretically possible to detect smaller MOC effects for TEOAEs associated with SSOAEs, compared to those without SSOAEs. The highest TEOAE SNR across frequency bands lacking SSOAEs was approximately 25 dB, compared to 34 dB across frequency bands with SSOAEs. The minimum detectable MOCRT% predicted for a SNR of 25 dB is approximately 15 %, compared to 7 % for a SNR of 34 dB (per the 1st-order exponential fit in Fig. 6).
The ability to detect small MOCR effects may be advantageous when investigating the influence of selective attention on cochlear processing. Multiple studies have found that both auditory and visual selective attention alter peripheral auditory responses, including the cochlear microphonic and compound action potential (Delano et al. 2007; León et al. 2012), TEOAEs (Puel et al. 1988; de Boer and Thornton 2007; Garinis et al. 2011; Smith and Cone 2015), distortion-product (DP) OAEs (Smith et al. 2012; Srinivasan et al. 2012; Wittekindt et al. 2014), and SFOAEs (Giard et al. 1994). To evaluate top-down influences on the MOCR, the aforementioned OAE studies compared the OAE between conditions requiring varying degrees of attentional demands, either within a sensory modality (e.g., Garinis et al. 2011; Smith and Cone 2015) or across sensory modalities (e.g., de Boer and Thornton 2007; Smith et al. 2012; Wittekindt et al. 2014). A common finding is that when a difference between conditions occurs, the size of the MOC effect is less than that when assayed using passive Quiet and Noise conditions (akin to those used in the current study). For instance, Garinis et al. (2011) report a difference in TEOAE amplitudes between passive- and active-listening conditions of ~ 0.2 dB. In contrast, the difference associated with the MOCR (using a passive Quiet vs. Noise paradigm) was closer to 3 dB. Statistical analyses across these studies were also restricted to group data, as opposed to individual data. Although small, yet significant changes to the OAE associated with attention may be detected at the group level, detecting changes at the individual level may pose greater challenges unless measurement conditions (i.e., OAE SNR) are optimized. It is worth noting, however, that at least one study has reported no effect of selective attention (visual) on TEOAEs near frequencies of SSOAEs (Meric and Collet 1994).
OAE-based assays of the auditory efferent system that are sensitive to small MOC effects may also be important when the goal is to identify changes in efferent function over time, whether due to maturational, aging or disease processes. An important consideration in determining the utility of OAE-based metrics of the MOC response is the normal variability of the response both within and across measurement sessions. Mertes and Goodman (2016) note that the variability of the MOCR effect measured using TEOAEs in frequency bands with SSOAEs is not significantly different from that for TEOAEs in frequency bands lacking SSOAEs.
Origin of the SSOAE-SNR Advantage
The current work defined the TEOAE as emission energy occurring within 20-ms post-stimulus; the SSOAE was defined as energy occurring later than 20-ms post-stimulus. Although these are common definitions (e.g., Keefe 2012; Mishra and Dinger 2016), they are somewhat arbitrary. Perhaps a more mechanistic definition of the TEOAE is emission energy that originates from the initial reflection of stimulus energy within the cochlea. This energy is predicted to present to the canal with latency that depends on the round-trip travel time between the canal and characteristic-frequency place (Shera et al. 2002, 2010; Shera and Guinan 2003). The SSOAE may then be associated with emission energy that persists beyond the latency of the TEOAE, presumably arising through repeated intra-cochlear reflections between the oval window and characteristic-frequency place (Shera 2004). Shera et al. (2002) and Shera and Guinan (2003) model the relationship between SFOAE latency (τ, ms; in response to 40-dB SPL tones) and cochlear-frequency place (f, kHz) as
| 5 |
The parameters α and β are 0.37 and 11, respectively. When applied to low-level TEOAEs, which are thought to be generated through the same mechanism as low-level SFOAEs (Shera and Guinan 1999; Kalluri and Shera 2007), Eq. 5 predicts latency to decrease from approximately 14 ms at 0.75 kHz to 4 ms by 5 kHz. As such, an analysis window that extends to 20-ms post-stimulus will capture not only emission energy stemming from the initial cochlear reflection but also energy from repeated reflections, especially at higher frequencies.
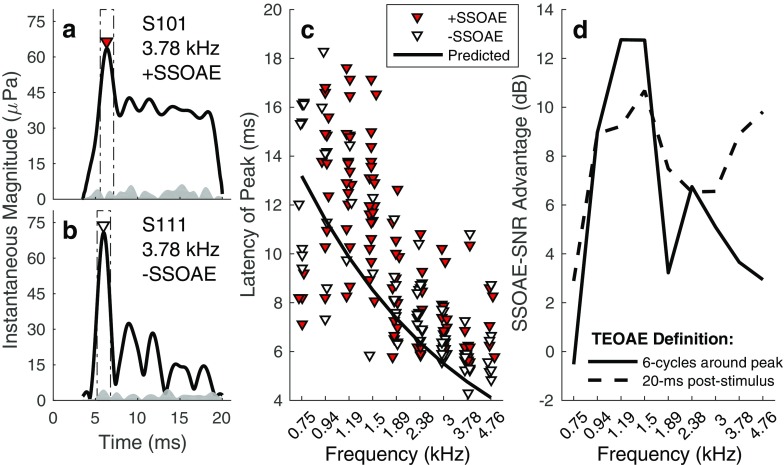
Figure 8a illustrates the presence of SSOAE energy within the initial 20-ms post-stimulus. In this graph, the 3.78-kHz band OAE waveform envelope (measured in quiet) is shown for a subject with an SSOAE. [The waveform was calculated via an inverse FFT (IFFT) of the 3.78-kHz 1/3 octave frequency band.] An amplitude peak occurred at approximately 6 ms, presumably associated with the initial reflection of stimulus energy occurring in the cochlea. Following the peak, emission amplitude decreased rapidly before achieving a steady-state level. The steady-state portion of the emission is associated with repeated internal cochlear reflections. Panel B shows the 3.78-kHz band OAE waveform envelope for a subject without an SSOAE. As for the subject with a SSOAE, an amplitude peak occurred around 6 ms. However, instead of persisting at a steady level across the remainder of the window, emission energy decayed across the duration of the time window, eventually falling into the noise floor.
Fig. 8.
a, b Examples of TEOAE time-waveform envelopes in frequency bands with and without SSOAEs, respectively. Each panel shows the 3.78-kHz band emission waveform envelope measured in Quiet (solid line) and an estimate of the noise (shaded region). In both cases, the emission exhibits an amplitude peak around 6 ms (indicated by the inverted triangle marker). Following the amplitude peak, emission energy persists at a steady level throughout the duration of the analysis window for the subject with a SSOAE. In contrast, for the subject lacking a SSOAE, emission energy decayed into the noise floor within the 20-ms window. c Latencies of the emission’s peak amplitude. Overlaid on the plot is the predicted relationship between SFOAE latency and frequency from Shera and Guinan (2003); see Eq. 5. d SNR advantage associated with present SSOAEs for different definitions of the TEOAE. The solid line indicates data for when the TEOAE is defined over a duration of 6 cycles re: latency of the peak amplitude. The broken line indicates data for when the TEOAE is defined based on the energy within an analysis window extending to 20-ms post-stimulus
The presence of SSOAE energy within the initial 20-ms post-stimulus explains, in part, the higher SNRs measured in frequency bands with SSOAEs. To demonstrate, the TEOAE was first redefined from the energy occurring within 20-ms post-stimulus to the energy within a time window spanning 6 cycles around the emission’s peak amplitude (an IFFT transformed frequency-band-specific responses to the time domain). The rectangles overlaid in Fig. 8a, b illustrate the new TEOAE analysis window. Constraining the time window to a narrow range around the emission peak eliminates contributions from slow-decaying/non-decaying OAE energy. Figure 8c shows the latencies of the emission amplitude peaks in cases of present and absent SSOAEs. Overlaid on the plot are the predicted latencies for low-level SFOAEs (Eq. 5). Latencies were similar regardless of SSOAE status and decreased with increasing frequency. The frequency dependence mirrors that predicted from SFOAEs, although the latencies are slightly longer. The SNR difference between the redefined TEOAE in frequency bands with and without SSOAEs was then compared to that calculated using the original 20-ms TEOAE analysis window.
In Fig. 8d, the differences in the median SNRs between frequency bands with and without SSOAEs are compared across the two TEOAE definitions. Except for the 0.75-kHz band, an SSOAE-SNR advantage (i.e., higher SNR for present SSOAEs) was observed at all frequencies, regardless of TEOAE definition. However, for frequencies above 1.5 kHz, the SSOAE-SNR advantage was larger when the TEOAE was defined as energy within 20-ms post-stimulus. This observation is consistent with the examples shown in Fig. 8a, b. In the case of present SSOAEs (Fig. 8a), inclusion of the waveform through 20-ms post-stimulus is advantageous since high-level emission energy is present. In contrast, when SSOAEs are absent, inclusion of the waveform beyond the peak amplitude of the emission effectively reduces the relative contribution of the high-SNR peak-amplitude region to the overall SNR. Interestingly, a larger SSOAE-SNR advantage was observed for the TEOAE defined relative to the peak amplitude at frequencies below 1.89 kHz (with the exception of the 0.75-kHz band). Below 1.89 kHz, latencies (Fig. 8c) were often later than 10 ms. Assuming the inter-stimulus interval of 159 ms was sufficient to permit significant decay of SSOAEs, the initial 10 ms of the original TEOAE analysis window was likely dominated by noise energy. The inclusion of this energy in the 20-ms TEOAE window would have resulted in lower SNRs and a smaller SSOAE-SNR advantage.
The observations from Fig. 8 raise the question: What is the ideal analysis window in TEOAE-based assays of the MOCR? In cases where a SSOAE is not present, it makes sense to use an analysis window narrowly defined around the TEOAE, as a broader time window would simply increase the relative amount of noise. However, when a SSOAE is present, it may be advantageous to extend the analysis window to include the SSOAE. SSOAEs (as well as SOAEs) are affected by activation of the MOCR and may themselves be useful tools to evaluate the integrity/function of the system (Mott et al. 1989; Meric and Collet 1994; Zhao and Dhar 2010; Dewey et al. 2014).
Acknowledgments
Thanks to Britney Ometz, Rebecca Walston, and Rachael Mackey for their assistance in data collection; Dr. Shawn S. Goodman for helpful comments on a previous version of this manuscript; and three anonymous reviewers for their critique of this paper.
Compliance with Ethical Standards
Subjects were compensated for their participation. The Institutional Review Board at the University of Tennessee, Knoxville, approved all testing.
Conflict of Interest
The author declares that he has no conflict of interest.
References
- Backus BC, Guinan JJ., Jr Measurement of the distribution of medial olivocochlear acoustic reflex strengths across normal-hearing individuals via otoacoustic emissions. J Assoc Res Otolaryngol. 2007;8(4):484–496. doi: 10.1007/s10162-007-0100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin CI, Hood LJ, Hurley A, Wen H. Contralateral suppression of otoacoustic emissions: an index of the function of the medial olivocochlear system. Otolaryngol—Head Neck Surg. 1994;110(1):3–21. doi: 10.1177/019459989411000102. [DOI] [PubMed] [Google Scholar]
- Boothalingam S, Purcell DW. Influence of the stimulus presentation rate on medial olivocochlear system assays. J Acoust Soc Am. 2015;137(2):724–732. doi: 10.1121/1.4906250. [DOI] [PubMed] [Google Scholar]
- Burns EM, Strickland EA, Tubis A, Jones K. (1984) Interactions among spontaneous otoacoustic emissions. I. Distortion products and linked emissions. Hear Res 16(3):271-278. [DOI] [PubMed]
- Collet L. Use of otoacoustic emissions to explore the medial olivocochlear system in humans. Br J Audiol. 1993;27(2):155–159. doi: 10.3109/03005369309077907. [DOI] [PubMed] [Google Scholar]
- Cooper NP, Guinan JJ., Jr Efferent-mediated control of basilar membrane motion. J Physiol. 2006;576(1):49–54. doi: 10.1113/jphysiol.2006.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer J, Thornton ARD. Effect of subject task on contralateral suppression of click evoked otoacoustic emissions. Hear Res. 2007;233(1):117–123. doi: 10.1016/j.heares.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Delano PH, Elgueda D, Hamame CM, Robles L. Selective attention to visual stimuli reduces cochlear sensitivity in chinchillas. J Neurosci. 2007;27(15):4146–4153. doi: 10.1523/JNEUROSCI.3702-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey JB, Lee J, Dhar S. Effects of contralateral acoustic stimulation on spontaneous otoacoustic emissions and hearing threshold fine structure. J Assoc Res Otolaryngol. 2014;15(6):897–914. doi: 10.1007/s10162-014-0485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis NA, Guinan JJ., Jr Acoustic stimulation of human medial olivocochlear efferents reduces stimulus-frequency and click-evoked otoacoustic emission delays: Implications for cochlear filter bandwidths. Hear Res. 2010;267(1):36–45. doi: 10.1016/j.heares.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garinis AC, Glattke T, Cone BK. The MOC reflex during active listening to speech. J Speech Lang Hear Res. 2011;54(5):1464–1476. doi: 10.1044/1092-4388(2011/10-0223). [DOI] [PubMed] [Google Scholar]
- Giard MH, Bouchet P, Pernier J. Modulation of human cochlear activity during auditory selective attention. Brain Res. 1994;633:353–356. doi: 10.1016/0006-8993(94)91561-X. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Perrin E, Chéry-Croze S, Chays A, Collet L. Contralateral acoustic stimulation induces a phase advance in evoked otoacoustic emissions in humans. Hear Res. 1996;94(1):54–62. doi: 10.1016/0378-5955(96)00002-0. [DOI] [PubMed] [Google Scholar]
- Goodman SS, Mertes IB, Lewis JD, Weissbeck DK. Medial olivocochlear-induced transient-evoked otoacoustic emission amplitude shifts in individual subjects. J Assoc Res Otolaryngol. 2013;14(6):829–842. doi: 10.1007/s10162-013-0409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Backus BC, Lilaonitkul W, Aharonson V. Medial olivocochlear efferent reflex in humans: otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs. J Assoc Res Otolaryngol. 2003;4(4):521–540. doi: 10.1007/s10162-002-3037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henin S, Thompson S, Abdelrazeq S, Long GR. Changes in amplitude and phase of distortion-product otoacoustic emission fine-structure and separated components during efferent activation. J Acoust Soc Am. 2011;129(4):2068–2079. doi: 10.1121/1.3543945. [DOI] [PubMed] [Google Scholar]
- Jedrzejczak WW, Blinowska KJ, Kochanek K, Skarzynski H. Synchronized spontaneous otoacoustic emissions analyzed in a time-frequency domain. J Acoust Soc Am. 2008;124(6):3720–3729. doi: 10.1121/1.2999556. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Shera CA. Near equivalence of human click-evoked and stimulus-frequency otoacoustic emissions. J Acoust Soc Am. 2007;121(4):2097–2110. doi: 10.1121/1.2435981. [DOI] [PubMed] [Google Scholar]
- Keefe DH. Moments of click-evoked otoacoustic emissions in human ears: group delay and spread, instantaneous frequency and bandwidth. J Acoust Soc Am. 2012;132(5):3319–3350. doi: 10.1121/1.4757734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulawiec JT, Orlando MS. The contribution of spontaneous otoacoustic emissions to the click evoked otoacoustic emissions. Ear Hear. 1995;16(5):515–520. doi: 10.1097/00003446-199510000-00008. [DOI] [PubMed] [Google Scholar]
- León A, Elgueda D, Silva MA, Hamamé CM, Delano PH. Auditory cortex basal activity modulates cochlear responses in chinchillas. PLoS One. 2012;7(4):e36203. doi: 10.1371/journal.pone.0036203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ., Jr Human medial olivocochlear reflex: effects as functions of contralateral, ipsilateral, and bilateral elicitor bandwidths. J Assoc Res Otolaryngol. 2009;10(3):459–470. doi: 10.1007/s10162-009-0163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Miller JAL, Guinan JJ, Jr, Shera CA, Reed CM, Perez ZD, Delhorne LA, Boege P. Otoacoustic-emission-based medial-olivocochlear reflex assays for humans. J Acoust Soc Am. 2014;136(5):2697–2713. doi: 10.1121/1.4896745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meric C, Collet L. Differential effects of visual attention on spontaneous and evoked otoacoustic emissions. Int J Psychophysiol. 1994;17(3):281–289. doi: 10.1016/0167-8760(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Mertes IB, Goodman SS. Within-and across-subject variability of repeated measurements of medial olivocochlear-induced changes in transient-evoked otoacoustic emissions. Ear Hear. 2016;37(2):e72–e84. doi: 10.1097/AUD.0000000000000244. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Dinger Z. Influence of medial olivocochlear efferents on the sharpness of cochlear tuning estimates in children. J Acoust Soc Am. 2016;140(2):1060–1071. doi: 10.1121/1.4960550. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Lutman ME. Repeatability of click-evoked otoacoustic emission-based medial olivocochlear efferent assay. Ear Hear. 2013;34(6):789–798. doi: 10.1097/AUD.0b013e3182944c04. [DOI] [PubMed] [Google Scholar]
- Mott JB, Norton SJ, Neely ST, Warr WB. Changes in spontaneous otoacoustic emissions produced by acoustic stimulation of the contralateral ear. Hear Res. 1989;38(3):229–242. doi: 10.1016/0378-5955(89)90068-3. [DOI] [PubMed] [Google Scholar]
- Moulin A, Collet L, Duclaux R. Contralateral auditory stimulation alters acoustic distortion products in humans. Hear Res. 1993;65(1):193–210. doi: 10.1016/0378-5955(93)90213-K. [DOI] [PubMed] [Google Scholar]
- Murugasu E, Russell IJ. The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea. J Neurosci. 1996;16(1):325–332. doi: 10.1523/JNEUROSCI.16-01-00325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner MJ, Zhang T. Prevalence of spontaneous otoacoustic emissions in adults revisited. Hear Res. 1997;103(1):28–34. doi: 10.1016/S0378-5955(96)00162-1. [DOI] [PubMed] [Google Scholar]
- Puel JL, Bonfils P, Pujol R. Selective attention modifies the active micromechanical properties of the cochlea. Brain Res. 1988;447(2):380–383. doi: 10.1016/0006-8993(88)91144-4. [DOI] [PubMed] [Google Scholar]
- Shera CA. Mammalian spontaneous otoacoustic emissions are amplitude-stabilized cochlear standing waves. J Acoust Soc Am. 2004;114(1):244–262. doi: 10.1121/1.1575750. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ., Jr Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for mammalian OAEs. J Acoust Soc Am. 1999;105(2):782–798. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ., Jr Stimulus-frequency-emission group delay: a test of coherent reflection filtering and a window on cochlear tuning. J Acoust Soc Am. 2003;113(5):2762–2772. doi: 10.1121/1.1557211. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ, Jr, Oxenham AJ. Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements. Proc Natl Acad Sci. 2002;99(5):3318–3323. doi: 10.1073/pnas.032675099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ, Jr, Oxenham AJ. Otoacoustic estimation of cochlear tuning: validation in the chinchilla. J Assoc Res Otolaryngol. 2010;11(3):343–365. doi: 10.1007/s10162-010-0217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisto R, Moleti A, Lucertini M. Spontaneous otoacoustic emissions and relaxation dynamics of long decay time OAEs in audiometrically normal and impaired subjects. J Acoust Soc Am. 2001;109(2):638–647. doi: 10.1121/1.1336502. [DOI] [PubMed] [Google Scholar]
- Smith SB, Cone B. The medial olivocochlear reflex in children during active listening. Int J Audiol. 2015;54(8):518–523. doi: 10.3109/14992027.2015.1008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DW, Aouad RK, Keil A. Cognitive task demands modulate the sensitivity of the human cochlea. Front Psychol. 2012;3:30. doi: 10.3389/fpsyg.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Keil A, Stratis K, Carr KW, Smith DW. Effects of cross-modal selective attention on the sensory periphery: cochlear sensitivity is altered by selective attention. Neuroscience. 2012;223:325–332. doi: 10.1016/j.neuroscience.2012.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge CL, Long GR, Murphy WJ, Tubis A. New off-line method for detecting spontaneous otoacoustic emissions in human subjects. Hear Res. 1993;71(1–2):170–182. doi: 10.1016/0378-5955(93)90032-V. [DOI] [PubMed] [Google Scholar]
- Wittekindt A, Kaiser J, Abel C. Attentional modulation of the inner ear: a combined otoacoustic emission and EEG study. J Neurosci. 2014;34(30):9995–10002. doi: 10.1523/JNEUROSCI.4861-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Dhar S. The effect of contralateral acoustic stimulation on spontaneous otoacoustic emissions. J Assoc Res Otolaryngol. 2010;11(1):53–67. doi: 10.1007/s10162-009-0189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]