Abstract
Instead of the scepticism on animal numerical understanding that characterized the first half of the twentieth century, in recent decades, a large and increasing body of the literature has shown that adult animals can master a variety of non-symbolic (in the absence of symbols such as mathematical words) numerical tasks. Nonetheless, evidence proving early numerical abilities in non-human animals was sparse. In this paper, I report the ongoing work to investigate numerical cognition in the day-old domestic chick (Gallus gallus). Unlike previous studies on adult animals, chicks can be tested very early in life, which gives us the opportunity to discover the origins of numerical comprehension. Here, I discuss studies revealing that day-old domestic chicks can: (i) discriminate between different numbers of objects; (ii) solve rudimentary arithmetic operations; and (iii) use ordinal information, identifying a target element (e.g. the fourth) in a series of identical elements, on the basis of its serial-numerical position. Some of these abilities are number-specific, while others underlie the interplay between number and continuous extents (continuous-quantity cues that covary with number, such as area and perimeter). These data are discussed in terms of ontogenetic development of mathematical comprehension.
This article is part of a discussion meeting issue ‘The origins of numerical abilities’.
Keywords: numerical cognition, numerical abilities, number sense, arithmetic, ordinal competences, domestic chick
1. Rationales for numerical cognition in non-human animals
Mathematics is usually referred to as a variety of abstract and complex calculations that are uniquely human. These abstract capabilities are rooted in a variety of symbols and comprise numerical words, numerals and operations that can be performed on numbers; hence, these can be defined as symbolic numerical abilities. With some certainty, we can say such symbolic-based mathematical comprehension is mastered solely by humans who have received intense mathematical education [1–3]. Yet, educated adult humans are able to master a subset of non-symbolic numerical tasks (i.e. extrapolating numerical magnitudes from arrays of elements) [4,5] when, under specific experimental conditions, language is prevented [6]. Non-symbolic numerical abilities are therefore preserved in adult humans and can be compared to other beings such as pre-verbal infants and non-human animals, which do not use symbols [6,7].
Non-symbolic numerical cognition is considered to be based on two separate systems: (i) one that represents small numbers, called the object file system (OFS) and (ii) one that represents large numerical magnitudes, called the analogue magnitude system (AMS). OFS is an object-based attention mechanism that represents each perceived object as a discrete file in the working memory. This system is capable of individuating a new object when it is introduced in a real scene, dedicating to it a new file in the working memory. Such a system may have initially evolved to represent objects, but it implicitly represents object number, according to the number limit (which is usually 4 or less) of object-files that can be simultaneously attended to and held in the working memory [8]. The discrimination of larger magnitudes (i.e. 4 or more) seems to be supported by the AMS, which is ratio-dependent, according to Weber's law; as the ratio between the numerousness to be discriminated becomes smaller, response times decrease and accuracy increases [1].
These similar effects reported in different species, humans included, suggest there is a shared, ancient, non-symbolic mechanism for number comprehension [7]. Although its relative importance is currently debated [9], better proficiency in non-symbolic numerical tasks seems to be related to higher symbolic mathematic achievement. It has been suggested that (i) non-verbal number sense in six-month-old infants is predictive of mathematical ability in early childhood [10], (ii) training the non-symbolic number sense improves proficiency in mathematics [11], and (iii) AMS and OFS both contribute to cardinal number learning in 3–4-year-old children [12,13]. Non-symbolic numerical cognition seems therefore to facilitate numerical symbol acquisition and mathematical calculations. Uniquely, human mathematical abilities appear fundamentally linked to an ontogenetically precocious and evolutionarily ancient ‘number sense’ [2,14–16], which emerges in the first days of human life [17]. From this perspective, precocious non-symbolic number sense can be considered as a developmental building block for symbolic mathematic acquisition [18]. Comprehension of aspects favouring non-symbolic numerical development may therefore open a door for educational interventions to improve children's number sense, even before learning to count.
2. Historical outline of non-symbolic numerical cognition
Scientific interest in animal numerical competence has been documented for almost 100 years [19]. However, the past three decades saw a significant increase in comparative numerical cognition studies [20]. A lack of inquiries into animal numerical cognition in the first half of the twentieth century is a consequence of an ‘unfortunate’ start to this topic's examination. An initial attempt to establish numerical abilities in animals is the famous story of Clever Hans. In the first decade of the twentieth century, a horse named Hans had been trained by his owner, Wilhelm von Osten, to perform a variety of arithmetic calculations, which ranged from summations, subtraction, long divisions and square roots. When von Osten presented Hans with an arithmetical operation, he responded by tapping his hoof on the ground the correct number of times. Other than a few sceptics, the majority of scientists were rather convinced by Hans' counting abilities. Some years later, it turned out that such extraordinary abilities were due to an artefact: the horse was able to solve numerical problems only when the experimenter knew the answer and when Hans could see his handler while responding [21]. Hans had no mathematical intelligence—but social intelligence: he was surprisingly skilled in detecting and interpreting subtle behavioural signals made by the experimenter, as he reached the correct count with his hoof tapping.
This observation caused a revolution due to Hans’ fame as an intelligent animal, and the belief in animal numerical capabilities plummeted. Scientific opinion reverted to Aristotelian thought, according to which logos, including numerical knowledge, was the purview of only human minds [22]. From this perspective, all cognition was believed to be firmly related to language and therefore to symbols. As a consequence, all creatures unable to use language and symbols (mainly pre-verbal infants and non-human animals) were considered incapable of reaching any mathematical comprehension. Such scepticism about animal numerical competence lasted for a long time.
After a few decades, the German zoologist Otto Koehler [23,24] was the first to reconsider numerical abilities in various avian species. He proved that pigeons (Columba livia), jackdaws (Corvus monedula) and budgerigars (Melopsittacus undulates) were able to solve numerical tasks, such as the identification of a given number in a matching-to-sample task, action enumeration or the identification of a target number. In all these experiments, Koehler made every possible effort to avoid any cue that Hans had possibly used. In Koehler's works, the experimenter hid behind a screen out of a bird's sight to avoid direct contact between animals and experimenter. Mechanical devices were used to give rewards to prevent the experimenter unconsciously biasing an animal's choice, and birds were filmed for the experiment's duration, providing an objective record of their behaviour. However, despite his efforts to eliminate extraneous cues, Koehler's works had been criticized for their lack of control for non-numerical cues such as size, shape, colour, brightness, texture and odour during experimental procedures [25]. It is noteworthy that numerical abilities studied by Koehler were simpler than tasks presented to Hans, both for their required numerical computation and for the numbers used. Moreover, such tasks implied a more ecologically relevant numerical comprehension with respect to the abstract and symbolic mathematical abilities that were initially attributed to Clever Hans. Nevertheless, scepticism towards such simple numerical cognition persisted. It was claimed that although animals can be trained to solve numerical tasks, they will do this only as a strategy of last resort, when (i) extensive training had been provided and (ii) all other non-numerical cues like overall area, volume, perimeter, surface area, odour and similar are useless for resolving tasks [26].
For the first point, (i), various types of proto-numerical competences have been shown in non-human species in the absence of specific numerical training. Lions hearing recordings of intruder lions roaring from hidden loudspeakers could assess their own troop's size and could compare it to the number of rivals. Depending on the number of rivals roaring from the loudspeakers and their own troops' sizes, lions reacted differently. They approached the sound only if they had at least a two-to-one numerical advantage over their rivals [27]. Conversely, fish, when presented with two groups of conspecifics differing in numerousness, spontaneously join the larger shoal [28]. Also in foraging decisions [29], animals faced with numerically different food options spontaneously prefer those providing the greatest energetic gain [30–34].
In summary, these findings support the idea that species as diverse as salamanders [30], rats [32] and coyotes [33] are able to discriminate between two numerousness. However, an adequate control for possible use of quantity information has not been performed in these kinds of studies; therefore, animals could rely both on numerical and on other continuous-quantity cues.
Overall, these works support the idea that animals can spontaneously, in the absence of intensive numerical training, use proto-numerical information to maximize their fitness in different contexts. Spontaneous proto-numerical capability could therefore be considered as a biologically based knowledge system that might have originated from adaptation to the external world under specific evolutionary pressures. However, alternative explanations sustaining that discrimination is founded on numerical cues (e.g. overall area, volume, perimeter, surface area) should be considered and eliminated, and here we come to the second point, (ii), as raised by Davis & Pérusse [26]. In fact, changes in number correlate with changes in other continuous-quantity cues, such as contour length, filled area, surface area or volume. All these continuous-quantity variables, precisely because they covary with numbers, are also called ‘continuous variables’ or ‘continuous extents’. Unless continuous-quantity variables are controlled, it is impossible to conclude that discrimination is based on numerical cues.
Even if an increasing number of studies in the comparative literature are focused on proto-numerical abilities, some aspects have nevertheless been poorly considered; namely, (i) whether animals can discriminate solely on the basis of numerical cues whenever a rigorous control for continuous-quantity variables is performed; and (ii) whether animals approach number versus continuous extent or an interplay between them [7]. Both aspects are two faces of the same coin.
On the one side, to talk about numerical competences, it is necessary that all continuous-quantity cues have been controlled for, but on the flipside, the inherent nature of a non-symbolic number being intrinsically related to real objects has to be considered. To deeply comprehend this last aspect, what a ‘non-symbolic number is’ must be taken into account [4,5]. Therefore, given that objects are characterized by space, mass, volume, surface area and similar, non-symbolic numbers should be regarded as a part of a more general system for representing quantity, whether discrete or continuous [35]. A future challenge in numerical cognition could be to disentangle the relative role and weight of various cues like number and continuous extents, and how they interact to influence numerical estimation. From this perspective, it is fascinating to understand how early animals can start to use numerical information.
Most research has been conducted on adult subjects, prohibiting an understanding of how much experience is needed to grasp a numerical ability and how soon animals can start mastering numerical information. Whether precocious numerical knowledge is available soon after birth is difficult to prove for animals with an altricial pattern of development. Therein rests the advantage of animal models like the domestic chick (Gallus gallus), which is extremely precocial regarding motor development pattern, yielding both (i) sophisticated behavioural analyses at an early age (soon after hatching) and (ii) very precise control (even in ovo) over sensory experience. Hence, this animal model allows us to pull nature and nurture apart.
Evidence from this precocious bird has revealed the variety of numerical concepts that very young animals can master soon after birth. This viewpoint offers a battle-ground for tackling the empirical investigation of nature–nurture issues on the origins of numerical knowledge. By comparing data obtained under different paradigms and stimulus types, I will try to point out both the importance of numerical over continuous-quantity cues, as well as their interplay—as recently suggested by the ‘sense of magnitude theory’ [36]—in non-symbolic numerical comprehension.
3. Proto-numerical and numerical discrimination
Relative numerousness discrimination [26] requires an ability to make size difference judgements between two sets, such as ‘more than … ,’ ‘less than … ’ [37]. Numerical discrimination has been considered a first and elementary level of numerical comprehension, at the basis of natural and spontaneous behaviours.
In one of the very first studies on number discrimination learning in day-old domestic chicks [38], their capability to amodally complete partially occluded objects [39] was exploited as a strategy to control for continuous-quantity cues. In fact, previous studies had shown that chicks perceptually completed the missing parts of objects, recognizing partly occluded objects as the corresponding whole objects [39]. This perceptive process had been previously described in humans and it is known as ‘amodal completion’ [40].
Day-old chicks were trained, for food reinforcement, to discriminate small sets of identical elements—1 versus 2, 2 versus 3—by pecking at a stimulus depicting a target numerousness, while ignoring the other one [38]. For example, a group of birds was trained to peck at the one-element stimulus and to ignore the two-element stimulus; and vice versa for the other group. During training, both in the 1 versus 2 and the 2 versus 3 comparison, a single pair of stimuli was used. As all dots were identical, chicks could distinguish the stimuli on the basis of numerical as well as continuous-quantity cues, such as overall area and perimeter.
Chicks then underwent different (unrewarded) tests in which different cues were controlled. In one of these tests, chicks were required to respond to ten new pairs of stimuli depicting dots identical to the one experienced during training, but differently displaced on the cardboard stimulus. Even when the spatial disposition of dots was randomly changed, chicks in both numerical comparisons continued to peck at the target number of dots. An interesting case arose when animals were presented with two stimuli, each depicting both elements and a black bar, which functioned as an occluder. The visible parts of the elements were calculated in each pair of stimuli in such a way that the two stimuli were characterized by an identical area or perimeter. For example, in the 1 versus 2 comparison, the bar did not overlap the dot in the one-element stimulus, while it overlapped exactly half of each dot in the two-element stimulus. Birds also succeeded in these tasks (1 versus 2 and 2 versus 3), continuing to peck at the number acquired during training, though the two stimuli depicted the same amount of area. However, using the same paradigm, chicks failed when they were presented with a larger number of objects such as 3 versus 4 and 4 versus 6, though the object sizes were identical, allowing them to rely both on numerical and continuous-quantity cues [38]. These findings showed that day-old birds can learn to discriminate, between small groups of elements, up to three, only on the basis of the number cue. What should be noted here is that training was conducted using only a single pair of stimuli, in which number covaried with several continuous-quantity cues on which chicks could rely in order to gain their reward. However, at test, when the items' spatial disposition and the overall area or perimeter of the two stimuli were identical, chicks continued to select the previously reinforced numerical value. This indicates that very young birds encode number rather than, or as well as, continuous-quantity cues, suggesting that number is natural and relevant information, encoded by non-human animals even when other continuous-quantity cues were available.
However, even if the chicks’ training lasted about an hour, there remained a need to understand whether animals could rely on numerical cues spontaneously (i.e. in the absence of any specific numerical training). To try to avoid this limitation, a new procedure to test numerical discrimination in very young animals has been designed.
In a new series of experiments, we exploited the chicks' memories for their rearing object. We reared different groups of chicks with various numbers of objects that through exposure were treated by the chicks as social companions [41]. A group of chicks was reared with a single object (figure 1a) and another group with three identical objects (figure 1b). On their third day of life, birds underwent a 6 min free choice test, during which they were allowed to approach one of the two numerical groups: one familiar and one unfamiliar. A group of animals underwent an absolute discrimination 1 versus 3 test (figure 1c). A second group underwent a relative discrimination test. In the latter, both groups were composed of four objects in total: one comprised three familiar objects and one new object; the other comprised one familiar and three new objects (figure 1d). In both tests, chicks reared with a single object approached the new group, while chicks reared with three objects approached the familiar number of objects. In other words, irrespective of their rearing condition, chicks approached the larger group, but not the larger group in an absolute sense; instead, the larger number of familiar objects. This means that they firstly discriminate between familiar and unfamiliar objects, and secondly on the basis of numerousness. Nevertheless, in both tests, chicks could discriminate on the basis of number as well as on the basis of other continuous-quantity cues such as the overall surface area or volume. To control for the possible use of continuous-quantity cues, different-sized objects were employed to create sets for the comparisons 1 versus 4, 1 versus 6 or 1 versus 3. In particular, in some sets, the volume, and in others, the surface area of the single object was identical to the total volume or surface area of objects in the plural set. As a result, each numerical comparison was characterized by a large single object and a plurality of smaller objects. In all numerical comparisons, chicks did not approach the more numerous object set, but the single bigger object. In these cases, birds were affected by continuous physical cues. Maybe it occurred because the bigger artificial social companion was more attractive, thus it is difficult to disentangle whether birds also have encoded numerical information.
Figure 1.

Spontaneous proto-numerical discrimination. (a) A chick reared with one object; (b) a chick reared with three objects. The two groups of objects used in the: (c) absolute discrimination test; and (d) relative discrimination test. (Online version in colour.)
Therefore, with the same aim to understand whether chicks could distinguish solely on numerical cues, heterogeneous objects were used. Two different groups of chicks were reared with objects differing from one another in colour, shape and size. One group was reared with two objects (figure 2a) and another group with three objects (figure 2b). At test, we used completely novel objects, different from the rearing ones in colour, shape and size (figure 2c). Further, the overall surface area and volume of the two testing groups were identical. Both groups of animals approached the familiar number; plus, chicks reared with two objects approached the familiar number and not the larger one. This suggests that whenever no other cues are available, chicks can rely on number.
Figure 2.
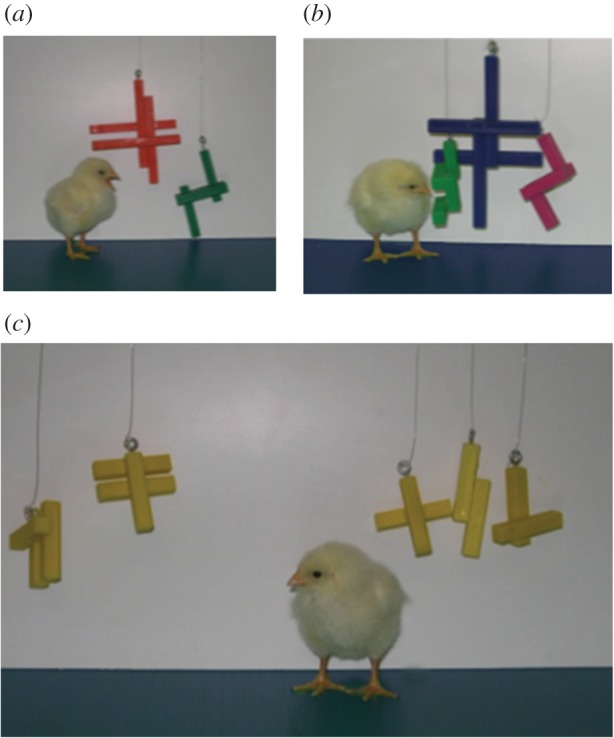
Spontaneous numerical discrimination. (a) A chick reared with the two-element stimulus; (b) a chick reared with the two-element stimulus. (c) The two groups of objects used during testing. (Online version in colour.)
Therefore, using the same paradigm, chicks (i) approached the more numerous set whenever the objects were identical in size, (ii) approached the single bigger object whenever this was significantly larger than the others, (iii) approached the familiar number, when the rearing objects were heterogeneous, and test objects were totally different from the rearing ones and the two groups of objects were identical for both surface area and volume. Stimuli characteristics experienced during rearing and testing seem therefore to elicit a quantitative versus number processing. Data on chicks are consistent with data on month-old infants. Six-month-old infants also discriminate between an array depicting different numbers of elements when continuous extents were controlled [42–43]. Conversely, other studies have reported that six- to eight-month-old infants are sensitive to the continuous properties (e.g. overall perimeter and area) of visual stimuli, but not to number [44–46].
Overall, on the basis of these first results, only discrimination of up to three items seems possible for day-old domestic chicks on a numerical basis. It had been initially hypothesized that chicks could rely only on a small number representation system and not on a large representation system.
A much debated issue in the numerical cognition literature is the domain of interest of the two non-symbolic numerical systems. What is unquestionable is that the OFS can process small numerical values [8] and the AMS larger ones [1]. However, contrasting evidence has been reported either indicating that the AMS can process small numerical magnitudes [7,47,48] or that these are processed only via the OFS [49]. From the latter viewpoint, the relevant factor that could trigger processing via OFS or via AMS could be the to-be-evaluated numerical values. Numbers smaller than four would trigger an OFS processing and numbers larger than four, an AMS processing. Nevertheless, Hyde & Spelke [50] suggested that attention could take a crucial role in a selective functioning of the OFS or the AMS. A simultaneous presentation of an entire set of elements would drive attention to the whole collection, thus activating AMS processing. Conversely, a sequential (one after another) presentation of elements would focus attention on each object, thus activating OFS processing.
In subsequent experiments, a chick's capacity to compare small and large numbers by AMS was studied [48]. With three being the largest number that the OFS can handle in day-old chicks [38], birds were given a choice among exclusively small numbers of less than four and exclusively large numbers of greater than four. Moreover, numbers that lie on different sides of the set size boundary were also compared to ascertain whether there was continuity in their processing of the numerical continuum.
During rearing, chicks were exposed for approximately 2 days to a situation in which food could be found in proximity to a group of stimuli representing a certain numerical amount. For example, birds could learn that food was behind a panel, depicting a positive numerousness (either two or three squares, for different groups of animals) and not behind a second panel, depicting a neutral numerousness (either three or two squares, complementary to the positive numerousness). The terms ‘positive’ and ‘neutral’ therefore are not related to the numerical magnitude per se, but to the association of a certain numerousness with the reward. At test, which occurred on the third day of life, chicks were presented with new stimuli depicting either the numerical amount associated or not associated with food. The former depicted the same number of elements as did the positive stimulus but differing for the elements' spatial disposition from the rearing ones; the latter depicted the same number of elements as in the neutral rearing stimulus but different again for their spatial disposition. Testing consisted of a free choice, therefore chicks could freely approach (i) the positive, (ii) the neutral stimulus or (iii) neither of them. Within 6 min, the time spent by each bird near either stimulus was recorded. In conditions in which both continuous-quantity and numerical cues were available (with the element size identical in rearing and in testing stimuli), the chicks approached the number associated with food in numerical comparisons that involved (i) two small numerousness (2 versus 3), (ii) a small and a large numerousness (2 versus 8) and (iii) two large numbers (6 versus 9, 8 versus 12, 8 versus 14), also when continuous variables (overall area and perimeter) were controlled [48]. It is to be noticed here that by equating the overall perimeter of square elements, an inverse correlation between number of elements and overall area occurs [5]. This means that chicks could not base their responses either on overall area or perimeter. Moreover, 3-day-old chicks also succeeded in a task that implies the discrimination between larger numbers, when overall area, overall perimeter, area and density were contemporarily controlled [51]. This indicates that while feeding, chicks spontaneously encoded numerical information. They in fact were not trained by operant conditioning, nor were they required to respond by pecking on the stimuli for food reinforcement.
Data gathered in these experiments, and especially the success in comparing a small (two) and a larger numbers (eight), support and expand to the dominion of very young non-humans animals, the hypothesis that AMS can distinguish quantities along the numerical continuum. Evidence in this direction was previously reported in adult humans performing a non-symbolic numerical task [6], seven-month-old infants [52] and other species [7,33,47]. These results also indicate that chicks can discriminate two numerosities, characterized by a 2/3 ratio. Further studies are needed to better understand the upper limit of discrimination by AMS in this species.
Difference in the upper limit in numerical discriminations reported with different paradigms should prompt reflection on functioning and specificity of the two non-symbolic numerical systems. Differences between these and previous findings in the same animal species can be explained by the dissimilar behaviours animals were required to emit for responding, such as pecking versus walking. As pecking can be considered a grasping action, birds would probably focus their attention on one or a few elements depicted on the stimuli, rather than on the overall collection. Watching chicks responding to these kind of tasks, it appeared they did not peck equally at any part of the stimuli, instead pecks were principally directed towards one of the elements. This behaviour could cause an activation of the system dedicated to object representation (i.e. the OFS), producing a lack of discrimination for sets comprising numbers larger than four.
Taken together, these findings are consistent with Hyde & Spelke's [50] theory that numerical systems seem not to be specialized for processing small or large numbers; instead, an attentional selection would activate an analysis of the stimuli by the AMS or the OFS. When attention is focused on single elements of a set, the representation is supported by the OFS, whereas when attention is focused on the overall set, the representation is performed by the AMS.
4. Arithmetic abilities
A more complex non-symbolic numerical capacity involves the manipulation of numerical representations by adding or subtracting one or more elements to produce a new representation [37]. If a certain scepticism has long accompanied a belief that non-symbolic creatures could master numerical discrimination, such a scepticism was even more remarkable concerning arithmetic, which does not simply require number representation, but also the ability to operate on such representations. Arithmetic, more than other numerical abilities, has been considered likely to be a uniquely human capacity, only performed by acculturated human beings who had received specific arithmetic instruction. Nonetheless, an increasing number of studies have proven that adult non-human animals and month-infants are capable of some form of arithmetic calculation [2,53].
The majority of research on non-symbolic arithmetic, with the only exception being human infants, has been investigated in adult non-human animals. Up to now, the only evidence showing that arithmetic calculation can be correctly solved in the very first day of life has been found in domestic chicks.
To test arithmetic calculation in chicks, their preference for a larger group of identical rearing objects has been exploited [54]. Chicks were reared soon after hatching with five identical objects: rearing objects. On their third day of life, they underwent testing. During testing, chicks were initially confined in a starting box behind a transparent partition, from where they could see two identical opaque panels positioned within the arena. They saw the objects sequentially (one after the other) disappear: two objects disappeared behind a panel and three objects behind the other panel. Immediately after, the chicks were released by lifting a transparent partition, and they were free to move wherever they wanted inside the arena. Chicks spontaneously circumnavigated the panel hiding the larger group. The same preference for the larger group was found both when the objects had an identical size and when the dimension of the objects in the three-element group was reduced, using a two-dimensional object, so that the overall area or the overall perimeter of the two sets were equal. It is noteworthy that when the overall perimeter of two arrays of two-dimensional squares is equated, a negative correlation between the numbers and the overall area occurs [5]. This means that chicks could not rely on overall area or on overall perimeter. These findings show that day-old birds are able to create two representations of two groups of objects by adding an element to the other one, then they can compare the two representations in order to find the larger group [55]. Chicks at the same age are also able to solve sequential addition and subtraction to finally find the larger group. In this case, chicks were presented with two sequences of events, such as (4 − 1) versus (1 + 1), (5 − 2) versus (0 + 2), (4 − 2) versus (1 + 2) and (5 − 3) versus (0 + 3), all giving ‘two versus three’ as final results. During the first sequence of events (shown in bold font), the task required them to understand that a ‘large’ number of objects (four or five) was hidden behind a panel and that a ‘small’ number of objects (zero, one or two) was hidden behind the other panel. During the second event (in italics), one, two or three object/s was/were moved from behind the panel hiding the larger group, to behind the other one. At the end of the second sequence of events, the larger group was in some comparisons, (4 − 1) versus (1 + 1) and (5 − 2) versus (0 + 2), behind the panel that initially hid the larger group (figure 3a); and in others (4 − 2) versus (1 + 2) and (5 − 3) versus (0 + 3), behind the panel that initially hid the smaller group (figure 3b). This also implies that, at the end of the second sequence of events, the larger group was hidden solely in half comparisons behind the panel where the chicks saw the last object disappear. Overall, this means that to find the larger group, chicks could not simply consider the result of the first sequence of events, nor circumnavigate the panel behind which the last object disappeared. To be solved, the task required an update of the two representations over event occurrence [55]. It should be noted here that in these numerical comparisons, for their task resolution, the chicks needed to discriminate between small and large numbers, at least in the first sequence of events. Plus, it is unlikely their discrimination was based on the OFS, because this would imply an OFS's storage capacity is larger than four, but the upper limit of the OFS in this species at this age has been identified as the number three [38]. These findings seem therefore to support the idea, discussed in the previous paragraph, according to which the AMS could also process small numbers, or that the two systems could, in some way, communicate [6,7,47,52]. Nevertheless, on this topic, the scientific literature reported contrasting evidence. Contrarily, some data suggest that small numbers can be treated solely via OFS [56]. For example, nine-month-old infants (who can solve summations between large numbers, i.e. 5 + 5 [57]), when presented with two sets of crackers subsequently hidden in one of two opaque containers, chose the larger set in 1 versus 1 + 1, 1 versus 1 + 1 + 1 and 1 + 1 versus 1 + 1 + 1 but not in 1 versus 1 + 1 + 1 + 1 numerical comparison. Taken together, these data seem to validate the hypothesis that there are two separate, independent systems accounting for the non-verbal representation of numbers: one for small values (less than 4) seemingly unable to track larger ones, and one for large magnitudes (greater than 4) seemingly unable to process smaller ones.
Figure 3.
The arithmetic task. A schematic representation of the objects' presentation during the first and the second series of events for the: (a) (4 − 1) versus (1 + 1), (b) (4 − 2) versus (1 + 2). (Online version in colour.)
When the critical numerical comparison 1 versus 1 + 1 + 1 + 1 was tested, using the paradigm previously described, chicks succeeded both when social attractors (rearing objects) and also when food attractors (mealworms) were used [58]. Further, chicks succeeded in 1 + 1 versus 1 + 1 + 1 + 1 discrimination also when perimeter and area were controlled. The latter comparison possibly contradicts the explanation of the chicks’ performance by a strategy of singular versus a plural discrimination, indicating that there is continuity in processing small and large numbers [58].
However, when the same arithmetic paradigm [55,58] was used to test whether chicks of the same age could summate large numbers (5 versus 10 and 6 versus 9 condition in which the objects were presented one at a time), chicks succeeded solely when all objects had the same dimensions, but not when quantity information were controlled, by changing the elements' size in the two sets [59]. This possibly indicates that whenever a task's difficulty increases, computation is possible solely when animals can rely on a redundancy of information, continuous-quantity and numerical. This hypothesis, which has also been formulated for findings obtained with other paradigms [6,7,31,33,52] (see also previous and subsequent paragraphs), should cause reflection on the interplay of numerical and continuous-quantity cues to achieve non-symbolic numerical competences.
5. Ordinal abilities
An additional central aspect of number cognition refers to the ability to identify a target element, in a series of identical elements, on the exclusive basis of its ordinal position. Rats are capable of learning to enter a target tunnel solely on the basis of its ordinal position in an array of six [60] or 18 [61]. Their performance was maintained also when the tunnel size and distance between tunnels were changed to prevent animals finding the target tunnel by relying on spatial cues. Honeybees (Apis mellifera) can find a food source after they have passed a precise number (up to four) of landmarks [62–64].
Plus, day-old animals can use ordinal information to find a food reward. Different groups of 5-day-old chicks were trained, for food reinforcement, to peck at the third, fourth or sixth element in a series of ten identical, equidistant and sagittally (with respect to the chicks' starting point) aligned elements. All groups of animals mastered the task, and at test they pecked the target element alone at significantly above chance. This first evidence suggested that chicks could encode serial-ordinal information. However, because during training and during testing the elements’ position remained identical, chicks could rely both on numerical and on spatial cues, such as the distance of the target from the starting point to a series' beginning or end. To disentangle which information the chicks were using, a new group of chicks was trained to peck at the fourth element in a series of ten identical elements, maintained in fixed position. At test, the number of elements in the series was reduced to five and the inter-element distance was enlarged to create a conflict between the ordinal and the spatial information (e.g. the distance separating the starting point and the target). In the new series, the second element was in the position previously occupied by the fourth, and the fourth was farther away (i.e. in the position previously occupied by the tenth element). Chicks pecked above chance solely the fourth element, while all the others were pecked at chance. This means that although the chicks during training can learn to identify a food source both on the basis of spatial and ordinal cues, whenever these are in conflict, they rely on numerical cues. This suggests that number is relevant information that very young animals process even though other cues are available. Moreover, birds also continued to respond in a differently oriented series. Day-old chicks and adult Clark's nutcrackers (Nucifraga columbiana) were initially trained to peck at a target element (either at the fourth or at the sixth for different groups of birds) in a series of 16 identical elements, maintained in fixed positions and sagittally arranged with respect to the birds' starting point. At test, the series was maintained identical, but it was rotated by 90°. Therefore, it was fronto-parallel oriented, from left to right. Both species correctly identified the target, although showing a left bias. They directed their pecks at the fourth element from the left end of the series, almost neglecting the fourth on the right side [65]. This left bias could be related to either numerical or spatial cues. In the fronto-parallel series, the target was located at a distance, with respect to each end of the series, that was identical to the distance separating the target and the closest end of the series that they had experienced during training. To disentangle the role of these cues in determining the left bias, in a subsequent experiment, chicks were trained to peck at the fourth element in a sagittally oriented series in which the elements were maintained in fixed position. In this way, the target could be identified both by numerical and spatial cues. At fronto-parallel test, the distance between elements was modified to create a conflict between the two cues. For half of the birds, the inter-element distance was reduced (but kept identical throughout the series) in such a way that the fifth element was in the position occupied at training by the fourth one. For the other half of the chicks, the inter-element distance was increased, so that the third element was in the position previously occupied by the fourth one. In both tests, chicks neglected the elements located at the correct distance from either end of the series. They instead directed their pecks at the numerically correct elements, but in this case, they chose identically the left and the right target element [66]. Also, when the possible use of spatial information was prevented by changing the inter-element distances from trial to trial, both during training and during fronto-parallel test, chicks identified as correct both the left and the right target elements. Moreover, the lack of bias could not be ascribed to a novelty effect related to a general change that occurred between the training and the testing series. In fact, when chicks experienced a modification in the elements’ colour between training and testing, they showed a left bias, pecking above chance solely the target of the left side of the series. Overall, these data show that in ordinal tasks, chicks (i) can use exclusively numerical cues, (ii) preferentially rely on numerical over spatial cues, and (iii) manifest a left bias only when they can code, during training and recover during test, both spatial and numerical information.
Given that chicks are able to use proto-‘cardinal’ numerical cues [38,48,54] and because they can comprehend ordinality [65,66], a future challenge would be to ascertain whether they can integrate these two aspects of numerical knowledge.
6. Conclusion
The history of the study of non-symbolic numerical skills (i.e. those calculations that can be solved in the absence of numerical symbols such as words and numerals) is characterized by a quite arduous beginning, which caused a great skepticism on animal numerical capability. After the unfortunate beginning of the Clever Hans story [21], a certain scepticism on numerical competences of non-human animals and pre-verbal infants pervaded numerical research. For a long time it was believed that non-symbolic numerical comprehension could occur solely after intensive numerical training and if (and only if) any other continuous extent could guide animal behaviour [26]. In recent decades, it has been demonstrated that purely numerical skills (when the possible use of continuous extents was prevented) are widespread within the animal kingdom [17,67–69]. Nevertheless, studies proving early numerical abilities in non-human animals were sparse. To investigate the ontogenetic origins of numerical knowledge, research outlined here has exploited domestic chicks (G. gallus) as an animal model. Here, I discussed studies revealing that day-old domestic chicks can (i) discriminate between different numbers of artificial social companions (i.e. objects they were exposed to soon after hatching); (ii) solve rudimentary arithmetic calculations, such as 1 + 1 + 1 versus 1 + 1; and (iii) use ordinal information, identifying a target element (e.g. the fourth) in a series of identical elements, on the basis of its numerical position in the series. Animals with very reduced experience naturally extrapolate and use numerical cues, suggesting that numerical processing constitutes a crucial ability for animal survival. Numerical competences are therefore a cognitive tool that animals could precociously use in their life. This does not detract any relevance from the powerful effects of experience and, for humans, scholastic education exerted on initial knowledge to achieve a more complex numerical comprehension. Nonetheless, non-symbolic number sense [2] is considered to be a necessary core knowledge, allowing interactions with the environment and onto which knowledge grows through experience [4]. Still chicks, and non-symbolic creatures in general, master different types of numerical comprehension whenever the possible use of continuous-quantity cues is prevented. However, the availability of either numerical information only or a redundancy of numerical and continuous-quantity cues causes differences in the animal behaviour. Chicks, in approaching artificial social companions, choose the larger group whenever continuous-quantity and numerical cues are accessible, but the numerically familiar group whenever solely numerical information is available [54]. Redundancy in numerical and continuous-quantity information also allows birds to solve summations in the domain of larger number, while this is unattainable on numerical cues only when continuous-quantitative information is controlled [59]. When both continuous magnitudes and numerical cues are accessible and consistent, non-symbolic creatures can solve increasingly complex numerical tasks [31,70], suggesting that the possibility of maintaining and integrating multiple sources of information can be exploited as a strategy to increase mathematical abilities. It is important to notice that any inherent sensitivity to numerousness and continuous-quantities may emerge later in development or be more experience dependent in its expression in altricial rather than precocial species. However, a deeper comprehension of non-symbolic mathematical abilities is not only of interest to deepen our knowledge. It could also open new and challenging educational issues because non-symbolic numerical abilities are considered as the foundations of more complex numerical reasoning [10,71], and training the non-symbolic number sense improves proficiency in mathematics [11].
In ordinal tasks, chicks show a left bias whenever they can rely on ordinal and spatial information, but do not show a sided-bias when numerical information only is provided [66]. Numerical information does not interact solely with the overall continuous extension of the objects. Evidence in this direction emerged even more directly in other kinds of contexts. Newborn chicks, once they have learnt to find a food reward in proximity of a target number, were biased towards the left side of space when presented with numerical values that were smaller than the target, but biased towards the right side of space when presented with numerical values that were larger than the target [51]. These findings suggest that chicks associated number onto space. Moreover, it could support the idea that numbers are inter-related with a variety of continuous extents. This is probably related to the inherent nature of non-symbolic numbers, which are extrapolated in the environment from perceived events that occur in time and space, and it is exactly in these spatial and temporal coordinates that animals naturally act in relation to numbers.
Non-symbolic numbers refer to the information that can be extrapolated from physical events. Weber's law describes discriminability between two numbers in the same way that it describes discriminability between values on a perceptual continua such as line length, brightness or weight [72]. Even though the bulk of research to date has focused on the exclusion of all possible continuous extents in non-symbolic numerical tasks (this is possibly a reaction to the last resort hypothesis [26]; see previous paragraph), one of the future challenges in this field could be to understand the relative roles and weights of different information (number and continuous extents) and how they interact to influence estimation and behaviour.
Ethics
The experiments reviewed here complied with all applicable Italian and European laws concerning the use of animals in research.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
This work was supported by the German Academic Exchange Service or DAAD (German: Deutscher Akademischer Austauschdienst) funding programme: Research Stays for University Academics and Scientists, 2017—N. 91644645 to R.R.
References
- 1.Gallistel CR, Gelman R. 1992. Preverbal and verbal counting and computation. Cognition 44, 43–74. ( 10.1016/0010-0277(92)90050-r) [DOI] [PubMed] [Google Scholar]
- 2.Dehaene S. 2011. The number sense: how the mind creates mathematics. New York, NY: Oxford University Press. [Google Scholar]
- 3.Carey S. 2004. Bootstrapping and the origin of concept. Daedalus 133, 59–68. ( 10.1162/001152604772746701) [DOI] [Google Scholar]
- 4.Feigenson L, Dehaene S, Spelke E. 2004. Core systems of number. Trends. Cogn. Sci. 8, 307–314. ( 10.1016/j.tics.2004.05.002) [DOI] [PubMed] [Google Scholar]
- 5.Rugani R, Castiello U, Priftis K, Spoto A, Sartori L. 2017. What is a number? The interplay between number and continuous magnitudes. Behav. Brain Sci. 40, e187 ( 10.1017/S0140525X16002259) [DOI] [PubMed] [Google Scholar]
- 6.Cordes S, Gelman R, Gallistel CR, Whalen J. 2001. Variability signatures distinguish verbal from nonverbal counting for both small and large numbers. Psychon. Bull. Rev. 8, 698–707. ( 10.3758/bf03196206) [DOI] [PubMed] [Google Scholar]
- 7.Cantlon JF, Brannon EM. 2007. How much does number matter to a monkey? J. Exp. Psychol. Anim. Behav. Process 33, 32–41. ( 10.1037/0097-7403.33.1.32) [DOI] [PubMed] [Google Scholar]
- 8.Trick LM, Pylyshyn ZW. 1994. Why are small and large numbers enumerated differently? A limited-capacity preattentive stage in vision. Psychol. Rev. 101, 80–102. ( 10.1037/0033-295X.101.1.80) [DOI] [PubMed] [Google Scholar]
- 9.De Smedt B, Noel MP, Gilmore C, Ansari D. 2013. The relationship between symbolic and non-symbolic numerical magnitude processing and the typical and atypical development of mathematics: a review of evidence from brain and behavior. Trends Neurosci. Educ. 2, 48–55. ( 10.1016/j.tine.2013.06.001) [DOI] [Google Scholar]
- 10.Starr A, Libertus ME, Brannon EM. 2013. Number sense in infancy predicts mathematical abilities in childhood. Proc. Natl Acad. Sci. USA 110, 18 116–18 120. ( 10.1073/pnas.1302751110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J, Brannon EM. 2013. Training the approximate number system improves math proficiency. Psychol. Sci. 24, 2013–2019. ( 10.1177/0956797613482944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mou Y, vanMarle K. 2014. Two core systems of numerical representation in infants. Dev. Rev. 34, 1–25. ( 10.1016/j.dr.2013.11.001) [DOI] [Google Scholar]
- 13.vanMarle K, Chu FW, Mou Y, Seok JH, Rouder J, David C, Geary DC. 2016. Attaching meaning to the number words: contributions of the object tracking and approximate number systems. Dev. Sci. ( 10.1111/desc.12495) [DOI] [PubMed] [Google Scholar]
- 14.Butterworth B. 2005. The development of arithmetical abilities. J. Child Psychol. Psychiatry 46, 3–18. ( 10.1111/j.1469-7610.2004.00374.x) [DOI] [PubMed] [Google Scholar]
- 15.Butterworth B. 2010. Foundational numerical capacities and the origins of dyscalculia. Trends. Cogn. Sci. 14, 534–541. ( 10.1016/j.tics.2010.09.007) [DOI] [PubMed] [Google Scholar]
- 16.Mussolin C, Nys J, Content A, Leybaert J. 2014. Symbolic number abilities predict later approximate number system acuity in preschool children. PLoS ONE 9, e91839 ( 10.1371/journal.pone.0091839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izard V, Sann C, Spelke ES, Streri A. 2009. Newborn infants perceive abstract numbers. Proc. Natl Acad. Sci. USA 106, 10 382–10 385. ( 10.1073/pnas.0812142106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spelke ES. 2000. Core knowledge. Am. Psychol. 55, 1233–1243. ( 10.1037/0003-066X.55.11.1233) [DOI] [PubMed] [Google Scholar]
- 19.Rilling M. 1993. Invisible counting animals: a history of contributions from comparative psychology, ethology and learning theory. In The development of numerical competence: animal and human models (eds Boysen ST, Capaldi EJ), pp. 3–37. Hillsdale, NJ: Erlbaum. [Google Scholar]
- 20.Haun DB, Jordan FM, Vallortigara G, Clayton NS. 2010. Origins of spatial, temporal and numerical cognition: insights from comparative psychology. Trends. Cogn. Sci. 12, 552–560. ( 10.1016/j.tics.2010.09.006) [DOI] [PubMed] [Google Scholar]
- 21.Pfungst O. 1907. Das Pferd von Herrn Osten. Leipzig, reprinted 1977 as: Der kluge Hans. Ein Beitrag zur nicht-verbalen Kommunikation. Frankfurt am Main, Germany: Fachbuchhandlung für Psychologie. [Google Scholar]
- 22.Houndé O, Tzourio-Mazoyer N. 2003. Neural foundations of logical and mathematical cognition. Nat. Rev. Neurosci. 4, 507–514. ( 10.1038/nrn1117) [DOI] [PubMed] [Google Scholar]
- 23.Koehler O. 1941. Vom Erlernen unbenannter Anzahlen bei Vögeln [On the learning of unnamed numerosities by birds]. Naturwissenschaften 29, 201–218. ( 10.1007/BF01481755) [DOI] [Google Scholar]
- 24.Koehler O. 1943. ‘Zähl’—Versuche an einem Kolkraben und Vergleichsversuche an Menschen. Z. f. Tierpsychol. 5, 575–712. ( 10.1111/j.1439-0310.1943.tb00665.x) [DOI] [Google Scholar]
- 25.Wesley F. 1961. The number concept: a phylogenetic review. Psychol. Bull. 58, 420–428. ( 10.1037/h0045746) [DOI] [PubMed] [Google Scholar]
- 26.Davis H, Pérusse R. 1988. Numerical competence in animals: definitional issues, current evidence, and new research agenda. Behav. Brain Sci. 11, 561–615. ( 10.1017/S0140525X00053437) [DOI] [Google Scholar]
- 27.McComb K, Packer C, Pusey A. 1994. Roaring and numerical assessment in contests between groups of female lions, (Panthera leo). Anim. Behav. 47, 379–387. ( 10.1006/anbe.1994.1052) [DOI] [Google Scholar]
- 28.Agrillo C, Bisazza A. 2017. Understanding the origin of number sense: a review of fish studies. Phil. Trans. R. Soc. B 373, 20160511 ( 10.1098/rstb.2016.0511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krebs JR. 1974. Colonial nesting and social feeding as strategies for exploiting food resources in the great blue heron (Ardea herodias). Behaviour 51, 99–130. ( 10.1163/156853974X00165) [DOI] [Google Scholar]
- 30.Uller C, Jaeger R, Guidry G. 2003. Salamanders (Plethodon cinereus) go for more: rudiments of number in an amphibian. Anim. Cogn. 6, 105–112. ( 10.1007/s10071-003-0167-x) [DOI] [PubMed] [Google Scholar]
- 31.Stancher G, Rugani R, Regolin L, Vallortigara G. 2015. Numerical discrimination by frogs (Bombina orientalis). Anim. Cogn. 18, 219–229. ( 10.1007/s10071-014-0791-7) [DOI] [PubMed] [Google Scholar]
- 32.Cox L, Montrose VT. 2016. Quantity discrimination in domestic rats, Rattus norvegicus. Animals 6, 46 ( 10.3390/ani6080046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker JM, Shivik J, Jordan KE. 2011. Tracking of food quantity by coyotes (Canis latrans). Behav. Process. 88, 72–75. ( 10.1016/j.beproc.2011.08.006) [DOI] [PubMed] [Google Scholar]
- 34.Call J. 2000. Estimating and operating on discrete quantities in orangutans (Pongo pygmaeus). J. Comp. Psychol. 114, 136–147. ( 10.1037/0735-7036.114.2.136) [DOI] [PubMed] [Google Scholar]
- 35.Gallistel CR. 2011. Prelinguistic thought. Lang. Learn. Dev. 7, 253–262. ( 10.1080/15475441.2011.578548) [DOI] [Google Scholar]
- 36.Leibovich T, Katzin N, Harel M, Henik A. 2016. From ‘sense of number’ to ‘sense of magnitude’. Behav. Brain Sci. 40, e164 ( 10.1017/S0140525X16000960) [DOI] [PubMed] [Google Scholar]
- 37.Vallortigara G, Chiandetti C, Sovrano VA, Rugani R, Regolin L. 2010. Animal cognition. Wiley. Interdiscip. Rev. Cogn. Sci. 1, 882–893. ( 10.1002/wcs.75) [DOI] [PubMed] [Google Scholar]
- 38.Rugani R, Regolin L, Vallortigara G. 2008. Discrimination of small numerosities in young chicks. J. Exp. Psychol. Anim. Behav. Process 34, 388–399. ( 10.1037/0097-7403.34.3.388) [DOI] [PubMed] [Google Scholar]
- 39.Regolin L, Vallortigara G. 1995. Perception of partly occluded objects by young chicks. Percept. Psychophys. 57, 971–976. ( 10.3758/BF03205456) [DOI] [PubMed] [Google Scholar]
- 40.Grossberg S, Mingolla E. 1985. Neural dynamics of form perception: boundary completion, illusory figures, and neon colour spreading. Psychol. Rev. 92, 173–211. ( 10.1037/0033-295X.92.2.173) [DOI] [PubMed] [Google Scholar]
- 41.Regolin L, Rugani R, Pagni P, Vallortigara G. 2005. Delayed search for social and nonsocial goals by young domestic chicks, Gallus gallus domesticus. Anim. Behav. 70, 855–864. ( 10.1016/j.anbehav.2005.01.014) [DOI] [Google Scholar]
- 42.Xu F, Spelke ES, Gottard S. 2005. Number sense in human infants. Dev. Sci. 8, 88–101. ( 10.1111/j.1467-7687.2005.00395.x) [DOI] [PubMed] [Google Scholar]
- 43.Libertus ME, Brannon EM. 2010. Stable individual differences in number discrimination in infancy. Dev. Sci. 13, 900–906. ( 10.1111/j.1467-7687.2009.00948.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clearfield MW, Mix KS. 1999. Number versus contour length in infants' discrimination of small visual sets. Psychol. Sci. 10, 408–411. ( 10.1111/1467-9280.00177) [DOI] [Google Scholar]
- 45.Clearfield MW, Mix KS. 2001. Amount versus number: infants' use of area and contour length to discriminate small sets. J. Cogn. Dev. 2, 243–260. ( 10.1207/S15327647JCD0203_1) [DOI] [Google Scholar]
- 46.Feigenson L, Carey S, Spelke E. 2002. Infants' discrimination of number versus continuous extent. Cogn. Psychol. 44, 33–66. ( 10.1006/cogp.2001.0760) [DOI] [PubMed] [Google Scholar]
- 47.Pepperberg MI. 2012. Further evidence for addition and numerical competence by a Grey parrot (Psittacus erithacus). Anim. Cogn. 15, 711–717. ( 10.1007/s10071-012-0470-5) [DOI] [PubMed] [Google Scholar]
- 48.Rugani R, Vallortigara G, Regolin L. 2014. From small to large. Numerical discrimination by young domestic chicks. J. Comp. Psychol. 128, 163–171. ( 10.1037/a0034513) [DOI] [PubMed] [Google Scholar]
- 49.Feigenson L, Carey S. 2005. On the limits of infants' quantification of small object arrays. Cognition 97, 295–313. ( 10.1016/j.cognition.2004.09.010) [DOI] [PubMed] [Google Scholar]
- 50.Hyde DC, Spelke ES. 2011. Neural signatures of number processing in human infants: evidence for two core systems underlying numerical cognition. Dev. Sci. 14, 360–371. ( 10.1111/j.1467-7687.2010.00987.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rugani R, Vallortigara G, Priftis K, Regolin L. 2015. Number-space mapping in the newborn chick resembles humans’ mental number line. Science 347, 534–536. ( 10.1126/science.aaa1379) [DOI] [PubMed] [Google Scholar]
- 52.Cordes S, Brannon EM. 2009. Crossing the divide: infants discriminate small from large numerosities. Dev. Psychol. 45, 1583–1594. ( 10.1037/a0015666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brannon EM, Roitman JD. 2003. Nonverbal representations of time and number in animals and human infants. In Functional and neural mechanism of interval timing (eds Brannon EM, Terrace HS), pp. 143–182. Boca Raton, FL: CRC Press. [Google Scholar]
- 54.Rugani R, Regolin L, Vallortigara G. 2010. Imprinted numbers: newborn chicks' sensitivity to number vs. continuous extent of objects they have been reared with. Dev. Sci. 13, 790–797. ( 10.1111/j.1467-7687.2009.00936.x) [DOI] [PubMed] [Google Scholar]
- 55.Rugani R, Fontanari L, Simoni E, Regolin L, Vallortigara G. 2009. Arithmetic in newborn chicks. Proc. R. Soc. B 276, 2451–2460. ( 10.1098/rspb.2009.0044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Starkey P, Cooper R. 1980. Perception of numbers by human infants. Science 210, 1033–1035. ( 10.1126/science.7434014) [DOI] [PubMed] [Google Scholar]
- 57.McCrink K, Wynn K. 2004. Large-number addition and subtraction by 9-month-old infants. Psychol. Sci. 15, 776–781. ( 10.1111/j.0956-7976.2004.00755.x) [DOI] [PubMed] [Google Scholar]
- 58.Rugani R, Cavazzana A, Regolin L, Vallortigara G. 2013. One, two, three, four, or is there something more? Numerical discrimination in day-old domestic chicks. Anim. Cogn. 16, 557–564. ( 10.1007/s10071-012-0593-8) [DOI] [PubMed] [Google Scholar]
- 59.Rugani R, Regolin L, Vallortigara G. 2011. Summation of large numerousness by newborn chicks. Front. Psychol. 2, 179 ( 10.3389/fpsyg.2011.00179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davis H, Bradford SA. 1986. Counting behavior by rats in a simulated natural environment. Ethology 73, 265–280. ( 10.1111/j.1439-0310.1986.tb00809.x) [DOI] [Google Scholar]
- 61.Suzuki K, Kobayashi T. 2000. Numerical competence in rats (Rattus norvegicus): Davis and Bradford (1986) extended. J. Comp. Psychol. 114, 73–85. ( 10.1037/0735-7036.114.1.73) [DOI] [PubMed] [Google Scholar]
- 62.Chittka L, Geiger K. 1995. Can honey bees count landmarks? Anim. Behav. 49, 159–164. ( 10.1016/0003-3472(95)80163-4) [DOI] [Google Scholar]
- 63.Skorupski P, MaBouDi HD, GalpayageDona HS, Chittka L. 2017. Counting insects. Phil. Trans. R. Soc. B 373, 20160513 ( 10.1098/rstb.2016.0513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dacke M, Srinivasan MV. 2008. Evidence for counting in insects. Anim. Cogn. 11, 683–689. ( 10.1007/s10071-008-0159-y) [DOI] [PubMed] [Google Scholar]
- 65.Rugani R, Kelly MD, Szelest I, Regolin L, Vallortigara G. 2010. It is only humans that count from left to right? Biol. Lett. 6, 290–292. ( 10.1098/rsbl.2009.0960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rugani R, Vallortigara G, Vallini B, Regolin L. 2011. Asymmetrical number-space mapping in the avian brain. Neurobiol. Learn. Mem. 95, 231–238. ( 10.1016/j.nlm.2010.11.012) [DOI] [PubMed] [Google Scholar]
- 67.Berkay D, Çavdaroğlu B, Balcı F. 2016. Probabilistic numerical discrimination in mice. Anim. Cogn. 19, 351–365. ( 10.1007/s10071-015-0938-1) [DOI] [PubMed] [Google Scholar]
- 68.Kawai N, Matsuzawa T. 2000. Numerical memory span in chimpanzee. Nature 403, 39–40. ( 10.1038/47405) [DOI] [PubMed] [Google Scholar]
- 69.Biro D, Matsuzawa T. 2001. Use of numerical symbols by the chimpanzee (Pan troglodytes): cardinals, ordinals and the introduction of zero. Anim. Cogn. 4, 193–199. ( 10.1007/s100710100086) [DOI] [PubMed] [Google Scholar]
- 70.Suanda S, Tompson W, Brannon EM. 2008. Changes in the ability to detect ordinal numerical relationships between 9 and 11 months of age. Infancy 13, 308–337. ( 10.1080/15250000802188800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burr DC, Anobile G, Arrighi R. 2017. Psychophysical evidence for the number sense. Phil. Trans. R. Soc. B 373, 20170045 ( 10.1098/rstb.2017.0045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Welford AT. 1960. The measurement of sensory-motor performance: survey and reappraisal of twelve years’ progress. Ergonomics 3, 189–230. ( 10.1080/00140136008930484) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.



