Abstract
Evidence is discussed about the use of geometric information for spatial orientation and the association between space and numbers in non-human animals. A variety of vertebrate species can reorient using simple Euclidian geometry of the environmental surface layout, i.e. in accord with metric and sense (right/left) relationships among extended surfaces. There seems to be a primacy of geometric over non-geometric information in spatial reorientation and, possibly, innate encoding of the sense of direction. The hippocampal formation plays a key role in geometry-based reorientation in mammals, birds, amphibians and fish. Although some invertebrate species show similar behaviours, it is unclear whether the underlying mechanisms are the same as in vertebrates. As to the links between space and number representations, a disposition to associate numerical magnitudes onto a left-to-right-oriented mental number line appears to exist independently of socio-cultural factors, and can be observed in animals with very little numerical experience, such as newborn chicks and human infants. Such evidence supports a nativistic foundation of number–space association. Some speculation about the possible underlying mechanisms is provided together with consideration on the difficulties inherent to any comparison among species of different taxonomic groups.
This article is part of a discussion meeting issue ‘The origins of numerical abilities'.
Keywords: number, space, geometry, mental number line, chicks, zebrafish
1. Introduction
In the past few decades, data have emerged suggesting that several animal species of different taxonomic groups are capable of representing quantity [1,2], which allows the estimation of set sizes (for reviews of comparative literature, see [3–6]). One open issue is to what extent quantities (such as spatial distances, time durations or numbers) are encoded in the nervous systems by similar mechanisms. To be more precise: to what extent do similarities in behavioural performance correspond to similarity of mechanisms, or even to a common evolutionary origin? Although any definite answer to this question is difficult, I would like to discuss two cases in point: one is related to the encoding of some basic Euclidian geometric properties of space, such as distances and sense, the other is related to the so-called ‘mental number line’, which underscores the important fact that number and space are intimately related concepts.
2. The case of geometry
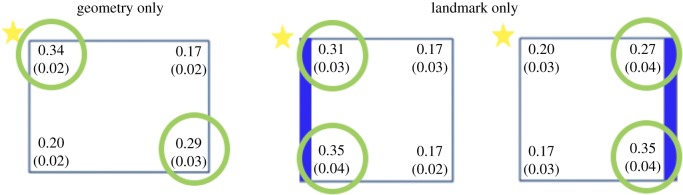
Geometry cognition has been much investigated in the past few decades using a task developed originally by Cheng & Gallistel [7–9]. Basically, in the task, animals should reorient in a rectangular environment in accord with its metrical and sense (right/left) relationship. It was noted that during initial, spontaneous reorientation (i.e. a working memory task) animals often ignore other types of cues, such as visual patterns and distinctive odours. With extended reinforcement training (i.e. in a reference memory task), animals can learn to use these other non-geometric cues, as shown by their ability to disentangle among geometrically equivalent locations; however, these non-geometric cues are merely used as local markers, or beacons, and animals do not appear to be able to learn sense relationships between them. An example is shown in figure 1, depicting some work we did with zebrafish (Danio rerio) [10]. The results show proportions of choice for the four corners of the rectangular enclosure (means on top with s.e.m. below) when only geometric cues were available (leftmost panel) and when a prominent visual cue (a blue coloured wall) was provided located either close to or far away from the correct corner. As can be seen, zebrafish chose the correct corner and its geometric equivalent in the ‘geometry only’ condition; in the ‘landmark only’ condition, by contrast, zebrafish oriented towards the visual cue provided by the blue wall but were unable to distinguish between the two blue-marked corners using sense (left–right) relationships.
Figure 1.
Zebrafish were presented with a companion visible in a corner and, after spatial disorientation in the dark, they were allowed to choose among the four corners when the companion was no longer visible. They reoriented correctly in the geometry only condition (i.e. in a rectangular enclosure in the absence of non-geometric cues). In the landmark only condition (i.e. in a square-shaped enclosure with a single blue wall), they oriented towards the blue wall but were unable to encode the relative positions of the corners using sense (left/right) information. (Online version in colour.)
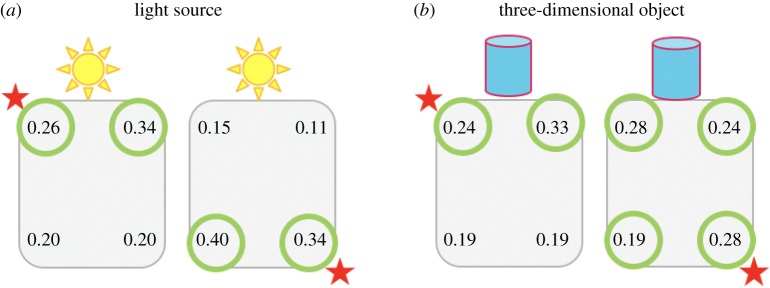
Even when using a more ecological cue (such as a light source; figure 2a) or a prominent three-dimensional object (figure 2b), fish appeared unable to discriminate the relative positions of these cues.
Figure 2.
Even when tested with a light source or a conspicuous three-dimensional object that may have provided a more ecological cue than a coloured wall (figure 2), fish appeared unable to discriminate the relative positions of the corners. (Online version in colour.)
It is thus apparent that the brain organizes spatial information into two basically different classes of environmental cues: extended boundaries and featural (non-geometric) cues (see for reviews [11,12]). The two types of cues differentially influence navigation and spatial mapping. Organisms seem to spontaneously compute relative spatial locations and directions with respect to extended boundaries such as walls (e.g. ‘the corner to the southeast of the wall’) but are more likely to use featural cues such as two-dimensional surface markings as a direct beacon to location (e.g. ‘the corner near the red wall’) rather than a relative spatial cue (e.g. ‘the corner to the east of the red wall’).
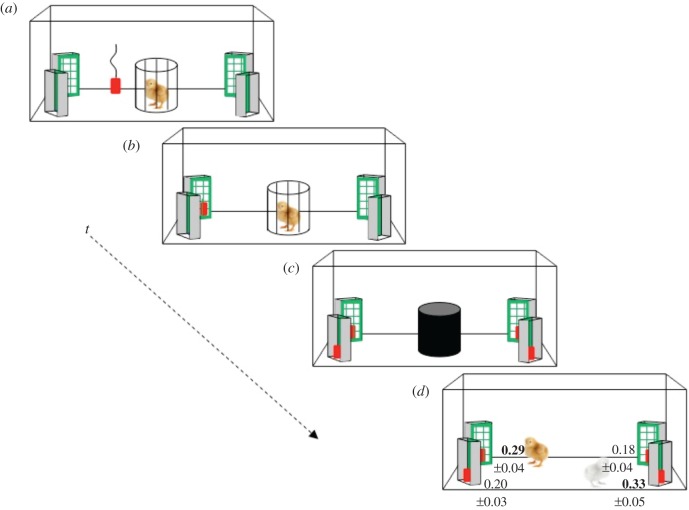
One may wonder what is the developmental origin of the ability to deal with extended boundaries. Evidence from single-cell studies in young rats [13,14] and controlled-rearing studies in newly hatched chicks [15–17] suggests that the ability to deal with geometric information is mostly predisposed in the brain, with no need for specific experience. A striking example is shown in figure 3. Here, newly hatched chicks maintained in the dark were located for the first time in a rectangular enclosure and exposed to a conspicuous object that slowly moved behind one of the four grid-panels in the corners [18].
Figure 3.
Chicks were located in a rectangular enclosure immediately after hatching in darkness, and presented (t indicates time) with an object moving (a) and hiding behind a grid placed in a corner (b). Chicks were then spatially disoriented by slowly rotating them in darkness and four identical replicas of the object were located in each corner (c) partly visible behind the grids. At test, chicks oriented by using the metric arrangement of the surfaces together with the left–right directional sense, choosing the two geometrically correct corners (yellow (dark grey in print) and grey chicks in (d) (see [18]). (Online version in colour.)
Then the chick’s vision was blocked by an opaque partition, and the chick was slowly rotated clockwise and anticlockwise to disrupt inertial orientation. The enclosure was also rotated and four identical replicas of the object were located in the corners. Finally, the chick was allowed to choose the object. As can be seen (figure 3), the animals did not return to random choice but rather searched more in the two geometrically correct corners (the correct one and its geometrical equivalent) than in the two geometrically incorrect ones.
Convergent evidence in mammals [19], birds [20], amphibians [21] and fish [22] suggests that the hippocampal formation plays a crucial role in geometry representation. There are of course also species differences. These are expected, given that the last common ancestor of all these taxonomic groups dates back some 500 Ma. For instance, in ray-finned (actinopterygian) fishes, as a result of a major divergence during early embryogenesis, the telencephalon develops by a process of eversion (bending outward of the embryonic prosencephalic plate) instead of evagination, as it does in all others vertebrate groups [23]. As a consequence, the medial-to-lateral topography of the pallial areas (which refer to the upper surface of the cerebrum) is reversed in teleost fishes compared with that of other vertebrates. Accordingly, in these species of fishes, the region considered homologous to the hippocampus is located laterally rather than medially in the telencephalon. Consistent with that, it has been found that goldfish with telencephalic lesions in the lateral pallium (but not in the medial pallium) appeared to be insensitive to geometric information [22].
Several other pieces of neurobiological evidence converge to a similar role of the hippocampal formation in different taxonomic groups. For instance, metric information seems to be encoded in the geometric task of Cheng & Gallistel in the form of distances among the walls of the enclosures, rather than in the form of lengths of the walls [24]. Chicks lesioned in the hippocampal information appear unable to find a goal using the distances between the walls of the enclosure, even though they can orient on the basis of non-geometric information [25]. Recently, Keinath et al. [26] have shown that hippocampal place cells in mice encode global shape aspects of an enclosure while being insensitive to salient non-geometric landmarks. When spatially disoriented mice reoriented in a rectangular enclosure, a given place cell could fire both when the mouse was at the place where that cell should fire and when the mouse was at the rotationally equivalent location. The cell rotational error occurred in spite of the fact that one wall was painted with white and black stripes that would have provided an unambiguous cue to distinguish between rotationally equivalent locations. These electrophysiological results match perfectly the results of (many) behavioural experiments revealing the use of purely geometric information in spatial reorientation (see also [27] for a theoretical commentary).
Thus, a strong case can be made for a common, shared and homologous mechanism in the hippocampal formation underlying spatial reorientation in vertebrates that computes distance and direction to allow an organism's heading. A challenge to this view, however, might come from research on insect orientation.
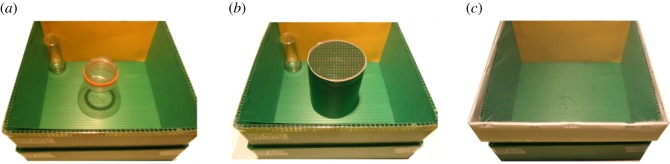
At more or less the same time as Cheng & Gallistel [7] formulated their theory of vertebrate navigation, Cartwright & Collet [28] put forward a theory of a view-based image-matching process (snapshot memories) in insects. According to this hypothesis, an animal would actively move in the environment to reduce the discrepancy between a snapshot representation of the visual scene stored in memory and the scene currently perceived. Although alternative hypotheses based on mapping-like mechanisms for insect navigation have been formulated (e.g. [29]), experiments carried out with ants and bumblebees in the specific domain of spatial reorientation suggest that these animals, like vertebrates, make rotational errors between two geometrically identically corners in the rectangular arena task [30–33]. It is of course an attractive idea that vertebrate navigation may be explained by simpler processes available also to insects. However, a major limitation of this hypothesis is that the aforementioned reorientation tests in insects were all based on a reinforcement learning paradigm. A snapshot or view-image account of reorientation may perhaps work for the cases in which it is possible for the animals to have a period of extended learning to integrate featural and geometric information (e.g. [34]), but it appears untenable for spontaneous, immediate reorientation. According to the snapshot hypothesis, the retinal salience would be the crucial factor in animals' reorientation. However, it has been shown that chicks can spontaneously reorient using geometric relationships of subtle three-dimensional terrain cues, but not by using salient two-dimensional brightness contours of surfaces or columns [35]. Direct evidence against the snapshot theory also arises from work on non-rewarded, spontaneous reorientation tasks in insects. We observed bumblebees' spatial navigation in response to the observed location of a conspecific ([36], figure 4).
Figure 4.
(a) Bumblebees were first allowed to observe the arena from a central transparent cylinder, with a conspecific visible in a small glass vial in one corner of the arena. (b) Bumblebees were then covered with an opaque cup and rotated for disorientation. (c) Bumblebees were released into an empty arena with a netted cover, and their first approaches to the four corners of the arena and their total approaches in 30 s were recorded (see for details [36]). (Online version in colour.)
When tested in a rectangular enclosure without non-geometric cues, bumblebees oriented using geometry (choosing the two geometrically correct locations over the two geometrically incorrect ones). However, when tested in a square-shaped enclosure with a coloured wall providing featural information (figure 4), bumblebees only used the feature to distinguish the corners near the feature from those distal from the feature, without discriminating the mirror-image corners from one another (figure 5). These results showed that no simple snapshot representations of corners (orange–green versus green–orange; figure 4) guided the bumblebees’ place coding; rather, the orange feature seems to have been used more like a beacon or as an associative near/far cue to location. Thus, the snapshot theory appears inadequate for both vertebrate and invertebrate navigation.
Figure 5.
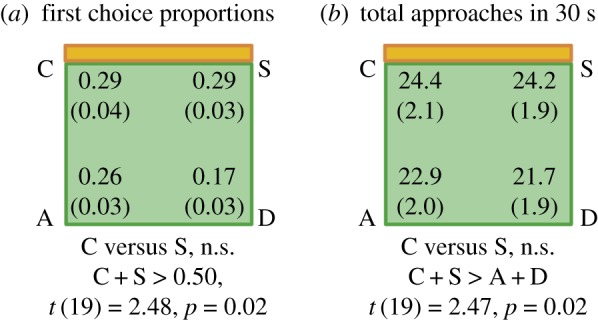
(a,b) When tested in a square-shaped enclosure with an orange coloured wall (the others being uniformly green), bumblebees oriented correctly towards the orange coloured wall but were unable to discriminate between its two corners (C and S) on the basis of sense information. See [36] for details. (Online version in colour.)
3. The case of the mental number line
A natural disposition to map numbers into space has been documented since Galton's [37] classical study, in which human adults were asked to draw how they visualize numbers. People seem to represent numbers as different visuo-spatial forms, typically organized along horizontal continua oriented from left (smaller numbers) to right (larger numbers), the so-called ‘mental number line’ [37]. The phenomenon was then confirmed in a more experimental setting. Dehaene et al. [38] found that responses to relatively smaller numbers are faster for the left hand and those to relatively larger numbers are faster for the right hand (Spatial Numerical Association of Response Codes, or SNARC effect). The SNARC effect does not reverse in left-handers and in right-handers tested while they cross their hands, which suggests that the spatial correspondence between the location of the number in the mental number line and the location in which the response is executed is the crucial factor for the occurrence of a SNARC effect.
Although traditionally the left-to-right orientation of the mental number line has been accounted for in terms of reading and writing habits [39] or more generally in terms of early directional experiences (review in [40]), there is reason to believe that biological factors can also play a role. First, evidence for a left-to-right bias has been found in pre-verbal infants. When presented with increasing and decreasing left-to-right sequences of numerosities, seven-month-old infants showed a preference for the increasing left-to-right oriented sequence [41]. Moreover, eight-month-old infants tended to orient attention to the left after observing a small number, and towards the right after observing a large number [42]. Second, evidence for a privileged left-to-right direction of number–space association has been reported in non-human animals, i.e. in two species of birds (domestic chicks and Clark nutcrackers [43–45]), in monkeys [46] and in one chimpanzee [47].
Probably the most convincing evidence of a phenomenon resembling the human mental line of number has been documented in newborn domestic chicks [48]. Three-day-old chicks were first familiarized with a target number (e.g. ‘5’ in figure 6) by turning around a panel with the depicted target number to obtain a reward. When they were then presented with pairs of panels depicting different numerosities, they tended to turn preferentially to the left with a smaller number (e.g. ‘2’) and to the right with a larger number (e.g. ‘8’) [48]. It is worth noting that a same number (for instance ‘8’) was associated by chicks with the right space when the target number was, for instance, ‘5’, but it was associated with the left space when the target number was, for instance, ‘20’. Thus, chicks seem to associate relatively smaller numbers with the left space and relatively larger numbers with the right space, most as humans do (see for a discussion [50]). Preliminary results obtained with human newborns [51] and zebrafish ([52,53]) using a similar task suggest that the phenomenon can be quite general, though in the case of zebrafish, it is unclear whether they use discrete or continuous quantities (or both) for the association with space.
Figure 6.
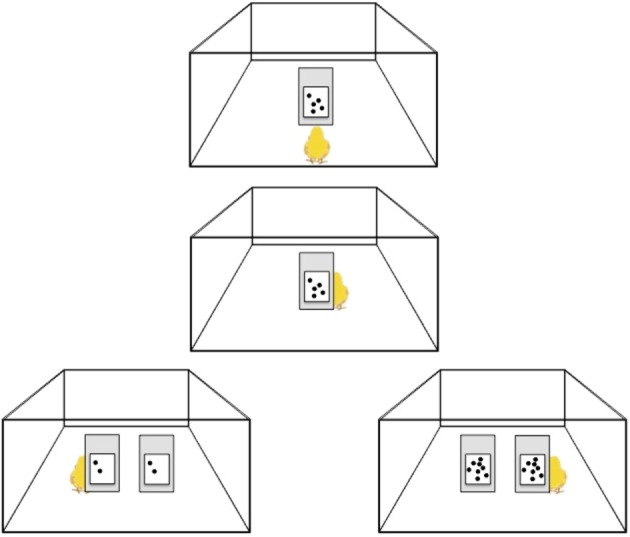
Chicks were trained to turn around a panel with five elements on it to find a food reward (upper central panels). At test, chicks were presented with two panels, one on the left and one on the right, depicting the same number of elements. When the elements were smaller than the target number (leftmost panel), chicks chose the left panel; when the elements were larger (rightmost panel), they chose the right panel (see [48] for a full description of the experiments including controls for continuous physical variables; and see also [49]). (Online version in colour.)
Clearly, the limited, if any, amount of experience available to newborn chicks and newborn humans with different numerosities before testing strongly supports an innately predisposed mechanism for the association between space and number. This would fit in well with a crucial argument that has been put forward by Gallistel [1,2] concerning the need for a common currency in the brain to represent different kinds of magnitudes (discrete as numerosities or continuous as spatial distances) in order to perform computations like ratio, which has been proved in fact to be available to both human infants [54], adult monkeys [55] and newborn chicks [56].
However, despite the behavioural resemblances, and differently from in the case of geometrical cognition, it is hard to say whether we are here facing common, homologous brain mechanisms, or evolutionary convergence, or mere resemblances reflecting completely different underlying mechanisms.
Let us start by considering current evidence for the underlying mechanisms of approximate number representation. We know that humans and other vertebrate animals share an evolutionarily old quantity representation system that allows the estimation of set sizes. There is convincing evidence that this system may be sustained by a shared homologous mechanism among mammals: single cell recording studies in monkeys [57,58] and functional magnetic resonance imaging studies in humans [59] converge in identifying a parieto-frontal cortical network in which individual neurons appear to be selectively tuned to the number of items. The physiological properties of these neurons can explain the typical signatures of numerosity judgements, such as the distance and the size effects (review in [60]). Parietal neurons represent not only discrete quantity (numerosity), but also continuous quantity (extent) and relations between quantities (proportions [61]), thus supporting the idea of a generalized magnitude system in the brain [2,62,63].
Recently, neurons have been found within the nidopallium caudolaterale, a forebrain avian structure, that selectively respond to specific numerical values. As in the monkey brain, their firing rate decreases as the numerosities shown to the animal get farther away from the preferred numerical value [64]. Although it has been proposed that the nidopallium caudolaterale could be homologous with the mammalian prefrontal cortex, a major difficulty with this view is that this structure mainly receives input from the collothalamic pathway (i.e. the collicular rather than the thalamic pathway—both routes being present and homologous in the two classes) and it seems to have no connections with the hippocampal formation (while the mammalian prefrontal cortex has). It has thus been suggested, as an alternative hypothesis, that the dorsolateral corticoid area, which is connected to the hippocampal formation, might be homologous with the mammalian prefrontal cortex [65]. If so, collopallial parietal and temporal associative cortices might be homologous with the nidopallium caudolaterale [66].
Nonetheless, even if common and shared structures could exist for birds and mammals, we know nothing about other vertebrate taxonomic groups (e.g. fish and amphibians, in spite of the evidence of their possession of an approximate number system, e.g. [67,68]). I do not discuss invertebrates here because, in my view, it is currently unclear whether they do possess an approximate number system that obeys Weber's law or if evidence for counting in animals like honeybees is limited to small numerosities (less than 3–4)—e.g. [69,70]—and would thus reflect the operating of some working memory system rather than a true quantity system (but see for a possible exception [71]).
As to the mechanisms underlying the association between number and space, things are even more complicated. One possibility is that continuous and numerical quantities are topographically represented in the brain. There is evidence for this in the human parietal cortex [72,73], at least for relatively small numerosities (less than 7) in the case of discrete quantities. Intriguingly, the topographical map of number in humans seems to be limited to the right hemisphere, for no clear topographical organization is apparent in the left hemisphere [72]. However, there is no evidence of this in other species and, more importantly, it is unclear how such topography, which maps increasing magnitudes from the medial to lateral portion of the parietal cortex, would produce the pattern of response that we observe in behaviour, with small numbers being more likely to produce a response to the left and large ones to the right. One can speculate on mirror organizations of the topography in the two hemispheres, but this seems not to be the case (in fact, there is no topographical organization at all in the left hemisphere; see above) and, besides, the functional reason for such mirror-image inversion, if existing, would be entirely mysterious.
Another possibility is that numerical and spatial cognition rely on common neural circuits [74,75], for the lateral and ventral intraparietal areas seem to play a role in both number and spatial processing in primates. Drucker & Brannon [75] argued that activation of these regions by numerical information could therefore lead to shifts in spatial attention, e.g. in left and right hemispace. However, it remains unspecified in this hypothesis why the mental line of numbers has the particular orientation it seems to have, from left to right.
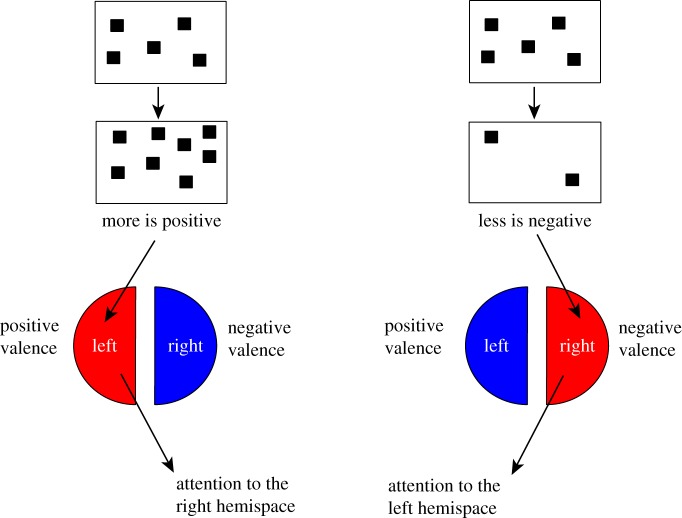
Here, I would like to propose a different hypothesis, which has the advantage of being amenable to empirical testing (figure 7). It has been argued that there exists a fundamental brain asymmetry in the control of functions related to motivation and emotion, sometimes referred to as the ‘valence hypothesis’ [76]. For example, it appears that the anterior regions of the left and right hemispheres are specialized for approach and withdrawal processes, respectively, and thus associated with positive and negative emotions. Although the valence theory was mainly developed in the context of human neuropsychology, approach and withdrawal are fundamental motivational dimensions that may be found at any level of phylogeny, and in fact there is also evidence supporting the theory in non-human species (e.g. [77]; see reviews in [78,79]). Now, let us imagine that, even if not explicitly trained for such an association, animals establish (other conditions being equal) that for appetitive stimuli, larger magnitudes are intrinsically better (and approachable) than smaller magnitudes. Then, chicks in Rugani et al.'s [48] experiments were faced with either abrupt increase or abrupt decrease in numerosities that would accordingly have evoked preferential activation of, respectively, the left hemisphere (positive emotion, approach) or the right hemisphere (negative emotion, withdrawal). This, in turn, would have promoted turning to the contralateral side of the activated hemisphere, i.e. to the left for smaller numerosities and to the right for larger numerosities (figure 7).
Figure 7.
According to the valence theories, the two hemispheres make qualitatively different contributions, e.g. negative versus positive valence or approach versus avoidance/withdrawal tendencies (review in [76]). Assuming that changes in numerosities towards larger or smaller magnitudes are associated with prevalent activation of, respectively, the left (positive valence) and right (negative valence) hemisphere, then attention to contralateral hemispace can be expected. This would nicely fit with choice for the left side with smaller magnitudes and for the right side with larger ones. (Online version in colour.)
As I said, this ‘motivational’ hypothesis is amenable to empirical investigation using, for instance, a specific association (acquired by training) between magnitudes and an aversive (rather than an appetitive) stimulus. I would predict a reduction if not an inversion of the direction of the space–number association in this case. Note also that although we found that a number–space association can be obtained for discrete numerosities when they covary with continuous physical quantities (confirming that number is salient relative to other object properties [80]), we can expect similar associations to occur for continuous physical variables as well when tested in isolation (e.g. that a change from a large-size stimulus to a small-size one would induce, via right hemispheric activation, preferential allocation of attention in the left hemispace).
Recent work in human neuropsychology by Brookshire & Casasanto [81] suggests that neural circuits for affective motivation may re-use circuits evolved for performing motor actions, and reports that individuals with strong motor asymmetries in a particular direction can actually show reversal of direction of left–right biases associated with motivation. Testing with animals artificially selected for lateralization in a particular direction may prove revealing (see, for instance, for fish [82,83]). Finally, whether this idea would also apply to the several other associations that humans seem to form between space and number and time and other quantities—the so-called ‘universal mental quantity line’ [84]—is open to empirical test.
In conclusion, coming back to the original question we asked in this paper, it is apparent in my view that the road from behaviour to the underlying mechanisms in different species is a tortuous one. We should be very cautious and attentive to the possibility of resemblances in behaviour that reflect true homologies and others that may be the outcome of homoplasic systems or that may be associated with completely different functions and underlying structures in different tasks and taxa.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Gallistel CR. 1989. Animal cognition: the representation of space, time and number. Annu. Rev. Psychol. 40, 155–189. ( 10.1146/annurev.ps.40.020189.001103) [DOI] [PubMed] [Google Scholar]
- 2.Gallistel CR. 2011. Mental magnitudes. In Space, time and number in the brain: searching for the foundations of mathematical thought (eds Dehaene S, Brannon E), pp. 3–12. New York, NY: Elsevier. [Google Scholar]
- 3.Vallortigara G, Regolin L, Chiandetti C, Rugani R. 2010. Rudiments of mind: insights through the chick model on number and space cognition in animals. Comp. Cogn. Behav. Rev. 5, 78–99. ( 10.3819/ccbr.2010.50004) [DOI] [Google Scholar]
- 4.Vallortigara G. 2014. Foundations of number and space representations in non-human species. In Evolutionary origins and early development of number processing (eds Geary DC, Bearch DB, Mann Koepke K), pp. 35–66. New York, NY: Elsevier. [Google Scholar]
- 5.Vallortigara G. 2017. An animal's sense of number. In The nature and development of mathematics: cross disciplinary perspective on cognition, learning and culture (eds Adams J, Barmby P, Mesoudi A), pp. 43–65. Oxford, UK: Routledge. [Google Scholar]
- 6.Versace E, Vallortigara G. 2015. Origins of knowledge: insights from precocial species. Front. Behav. Neurosci. 9, 338 ( 10.3389/fnbeh.2015.00338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng K, Gallistel CR. 1984. Testing the geometric power of an animal's spatial representation. In Animal cognition (eds Roitblat H, Bever TG, Terrace H), pp. 409–423. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- 8.Cheng K. 1986. A purely geometric module in the rat's spatial representation. Cognition 23, 149–178. ( 10.1016/0010-0277(86)90041-7) [DOI] [PubMed] [Google Scholar]
- 9.Margules J, Gallistel CR. 1988. Heading in the rat: determination by environmental shape. Anim. Learn. Behav. R16, 404–410. ( 10.3758/BF03209379) [DOI] [Google Scholar]
- 10.Lee SA, Ferrari A, Vallortigara G, Sovrano VA. 2015. Boundary primacy in spatial mapping: evidence from zebrafish (Danio rerio). Behav. Processes 119, 116–122. ( 10.1016/j.beproc.2015.07.012) [DOI] [PubMed] [Google Scholar]
- 11.Vallortigara G. 2009. Animals as natural geometers. In Cognitive biology: evolutionary and developmental perspectives on mind, brain and behavior (eds Tommasi L, Nadel L, Peterson M), pp. 83–104. Cambridge, MA: MIT Press. [Google Scholar]
- 12.Tommasi L, Chiandetti C, Pecchia T, Sovrano VA, Vallortigara G. 2012. From natural geometry to spatial cognition. Neurosci. Biobehav. Rev. 36, 799–824. ( 10.1016/j.neubiorev.2011.12.007) [DOI] [PubMed] [Google Scholar]
- 13.Langston RF, Ainge J, Couey JJ, Canto CB, Bjerknes TL, Witter MP, Moser E, Moser M-B. 2010. Development of the spatial representation system in the rat. Science 328, 1576–1580. ( 10.1126/science.1188210) [DOI] [PubMed] [Google Scholar]
- 14.Wills TJ, Cacucci F, Burgess N, O'Keefe J. 2010. Development of the hippocampal cognitive map in preweanling rats. Science 328, 1573–1576. ( 10.1126/science.1188224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiandetti C, Vallortigara G. 2008. Is there an innate geometric module? Effects of experience with angular geometric cues on spatial reorientation based on the shape of the environment. Anim. Cogn. 11, 139–146. ( 10.1007/s10071-007-0099-y) [DOI] [PubMed] [Google Scholar]
- 16.Chiandetti C, Vallortigara G. 2010. Experience and geometry: controlled-rearing studies with chicks. Anim. Cogn. 13, 463–470. ( 10.1007/s10071-009-0297-x) [DOI] [PubMed] [Google Scholar]
- 17.Vallortigara G, Sovrano VA, Chiandetti C. 2009. Doing Socrates' experiment right: controlled-rearing studies of geometrical knowledge in animals. Curr. Opin. Neurobiol. 19, 20–26. ( 10.1016/j.conb.2009.02.002) [DOI] [PubMed] [Google Scholar]
- 18.Chiandetti C, Spelke ES, Vallortigara G. 2015. Inexperienced newborn chicks use geometry to spontaneously reorient to an artificial social partner. Dev. Sci. 18, 972–978. ( 10.1111/desc.12277) [DOI] [PubMed] [Google Scholar]
- 19.McGregor A, Hayward AJ, Pearce JM, Good MA. 2004. Hippocampal lesions disrupt navigation based on the shape of the environment. Behav. Neurosci. 118, 1011–1021. ( 10.1037/0735-7044.118.5.1011) [DOI] [PubMed] [Google Scholar]
- 20.Mayer U, Pecchia T, Bingman V, Vallortigara G. 2016. Hippocampus and medial striatum dissociation during goal navigation by geometry or features in the domestic chick: an immediate early gene study. Hippocampus 26, 27–40. ( 10.1002/hipo.22486) [DOI] [PubMed] [Google Scholar]
- 21.Sotelo MI, Daneri MF, Bingman VP, Muzio RN. 2016. Telencephalic neuronal activation associated with spatial memory in the terrestrial Toad Rhinella arenarum: participation of the medial pallium during navigation by geometry. Brain Behav. Evol. 88, 149–160. ( 10.1159/000447441) [DOI] [PubMed] [Google Scholar]
- 22.Vargas JP, Bingman VP, Portavella M, López JC. 2006. Telencephalon and geometric space in goldfish. Eur. J. Neurosci. 24, 2870–2878. ( 10.1111/j.1460-9568.2006.05174.x) [DOI] [PubMed] [Google Scholar]
- 23.Broglio C, Martín-Monzón I, Ocaña FM, Gómez A, Durán E, Salas C, Rodríguez F. 2015. Hippocampal pallium and map-like memories through vertebrate evolution. J. Behav. Brain Sci. 5, 109–120. ( 10.4236/jbbs.2015.53011) [DOI] [Google Scholar]
- 24.Lee SA, Vallortigara G, Fiore M, Sovrano VA. 2013. Navigation by environmental geometry: the use of zebrafish as a model. J. Exp. Biol. 216, 3693–3699. ( 10.1242/jeb.088625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tommasi L, Gagliardo A, Andrew RJ, Vallortigara G. 2003. Separate processing mechanisms for encoding geometric and landmark information in the avian hippocampus. Eur. J. Neurosci. 17, 1695–1702. ( 10.1046/j.1460-9568.2003.02593.x) [DOI] [PubMed] [Google Scholar]
- 26.Keinath AT, Julian JB, Epstein RA, Muzzio IA. 2017. Environmental geometry aligns the hippocampal map during spatial reorientation. Curr. Biol. 27, 309–317. ( 10.1016/j.cub.2016.11.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallistel CR. 2017. Navigation: whence our sense of direction? Curr. Biol. 27, R103–R122. ( 10.1016/j.cub.2016.11.044) [DOI] [PubMed] [Google Scholar]
- 28.Cartwright BA, Collet TS. 1983. Landmark learning in bees. J. Comp. Physiol. A 151, 521543 ( 10.1007/BF00605469) [DOI] [Google Scholar]
- 29.Menzel R, et al. 2005. Honeybees navigate according to a map-like spatial memory. Proc. Natl Acad. Sci. USA 102, 3040–3045. ( 10.1073/pnas.0408550102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wystrach A, Beugnon G. 2009. Ants learn geometry and features. Curr. Biol. 19, 61–66. ( 10.1016/j.cub.2008.11.054) [DOI] [PubMed] [Google Scholar]
- 31.Wystrach A, Cheng K, Sosa S, Beugnon G. 2011. Geometry, features, and panoramic views: ants in rectangular arenas. J. Exp. Psychol. Anim. Behav. Proc. 37, 420–435. ( 10.1037/a0023886) [DOI] [PubMed] [Google Scholar]
- 32.Sovrano VA, Rigosi E, Vallortigara G. 2012. Spatial reorientation by geometry in bumblebees. PLoS ONE 7, e37449 ( 10.1371/journal.pone.0037449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sovrano VA, Potrich D, Vallortigara G. 2013. Learning of geometry and features in bumblebees (Bombus terrestris). J. Comp. Psychol. 127, 312–318. ( 10.1037/a0032040) [DOI] [PubMed] [Google Scholar]
- 34.Pecchia T, Vallortigara G. 2012. Spatial reorientation by geometry with freestanding objects and extended surfaces: a unifying view. Proc. R. Soc. B 279, 2228–2236. ( 10.1098/rspb.2011.2522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SA, Spelke ES, Vallortigara G. 2012. Chicks, like children, spontaneously reorient by three-dimensional environmental geometry, not by image matching. Biol. Lett. 8, 492–494. ( 10.1098/rsbl.2012.0067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SA, Vallortigara G. 2015. Bumblebees spontaneously map location of conspecific using geometry and features. Learn. Motiv. 50, 32–38. ( 10.1016/j.lmot.2014.10.004) [DOI] [Google Scholar]
- 37.Galton F. 1880. Visualised numerals. Nature 21, 252–256. ( 10.1038/021252a0) [DOI] [Google Scholar]
- 38.Dehaene S, Bossini S, Giraux P. 1993. The mental representation of parity and number magnitude. J. Exp. Psychol. Gen. 122, 371–396. ( 10.1037/0096-3445.122.3.371) [DOI] [Google Scholar]
- 39.Shaki S, Fischer MH, Petrusic WM. 2009. Reading habits for both words and numbers contribute to the SNARC effect. Psychon. Bull. Rev. 16, 328–331. ( 10.3758/PBR.16.2.328) [DOI] [PubMed] [Google Scholar]
- 40.Patro K, Nuerk H-C, Cress U. 2016. Mental number line in the preliterate brain: the role of early directional experiences. Child Dev. Perspect. 10, 172–177. ( 10.1111/cdep.12179) [DOI] [Google Scholar]
- 41.de Hevia MD, Girelli L, Addabbo M, Cassia VM. 2014. Human infants' preference for left-to-right oriented increasing numerical sequences. PLoS ONE 9, e96412 ( 10.1371/journal.pone.0096412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bulf H, de Hevia MD, Macchi-Cassia V. 2015. Small on the left, large on the right: numbers orient preverbal infants’ visual attention onto space. Dev. Sci. 19, 394–401. ( 10.1111/desc.12315) [DOI] [PubMed] [Google Scholar]
- 43.Rugani R, Regolin L, Vallortigara G. 2007. Rudimental numerical competence in 5-day-old domestic chicks: identification of ordinal position. J. Exp. Psychol. Anim. Behav. Processes 33, 21–31. ( 10.1037/0097-7403.33.1.21) [DOI] [PubMed] [Google Scholar]
- 44.Rugani R, Kelly DM, Szelest I, Regolin L, Vallortigara G. 2010. Is it only humans that count from left to right? Biol. Lett. 6, 290–292. ( 10.1098/rsbl.2009.0960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rugani R, Vallortigara G, Vallini B, Regolin L. 2011. Asymmetrical number-space mapping in the avian brain. Neurobiol. Learn. Mem. 95, 231–238. ( 10.1016/j.nlm.2010.11.012) [DOI] [PubMed] [Google Scholar]
- 46.Drucker CB, Brannon EM. 2014. Rhesus monkeys (Macaca mulatta) map number onto space. Cognition 132, 57–67. ( 10.1016/j.cognition.2014.03.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adachi I. 2014. Spontaneous spatial mapping of learned sequence in chimpanzees: evidence for a SNARC-like effect. PLoS ONE 9, e90373 ( 10.1371/journal.pone.0090373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rugani R, Vallortigara G, Priftis K, Regolin L. 2015. Number-space mapping in the newborn chick resembles humans' mental number line. Science 347, 534–536. ( 10.1126/science.aaa1379) [DOI] [PubMed] [Google Scholar]
- 49.Vallortigara G, Chiandetti C. 2017. Objects and space in an avian brain. In Avian cognition (eds ten Cate C, Healy SD), pp. 141–162. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 50.Brugger P. 2015. Chicks with a number sense. Science 347, 477–478. ( 10.1126/science.aaa4854) [DOI] [PubMed] [Google Scholar]
- 51.Rugani R, Lunghi M, Di Giorgio E, Regolin L, Vallortigara G, Simion F. 2017. A mental number line in human newborns. bioRxiv 159335 ( 10.1101/159335) [DOI] [PubMed] [Google Scholar]
- 52.Potrich D, Rugani R, Sovrano VA, Regolin L, Vallortigara G.2016. Mapping numerosities into space by zebrafish (Danio rerio). CogEvo—Rovereto Workshop on Cognition and Evolution, pp. 116, 6–9 July 2016. [DOI] [PMC free article] [PubMed]
- 53. Vallortigara G. 2016 Space and number in fish. Paper presented at ECCN 2016, 8th European Conference on Comparative Neurobiology, Munich, Germany, 7–9 April 2016. Abstract Book. Munich, Germany: University of Munich.
- 54.McCrink K, Wynn K. 2007. Ratio abstraction by 6-month-old infants. Psychol. Sci. 18, 740–745. ( 10.1111/j.1467-9280.2007.01969.x) [DOI] [PubMed] [Google Scholar]
- 55.Drucker CB, Rossa MA, Brannon EM. 2015. Comparison of discrete ratios by rhesus macaques (Macaca mulatta). Anim. Cogn. 19, 75–89. ( 10.1007/s10071-015-0914-9) [DOI] [PubMed] [Google Scholar]
- 56.Rugani R, McCrink K, de Hevia M-D, Vallortigara G, Regolin L. 2016. Ratio abstraction over discrete magnitudes by newly hatched domestic chicks (Gallus gallus). Sci. Rep. 6, 30114 ( 10.1038/srep30114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nieder A, Freedman DJ, Miller EK. 2002. Representation of the quantity of visual items in the primate prefrontal cortex. Science 297, 1708–1711. ( 10.1126/science.1072493) [DOI] [PubMed] [Google Scholar]
- 58.Roitman J, Brannon EM, Platt ML. 2007. Monotonic coding of numerosity in macaque lateral intraparietal area. PLoS Biol. 5, e208 ( 10.1371/journal.pbio.0050208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. 1999. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science 284, 970–974. ( 10.1126/science.284.5416.970) [DOI] [PubMed] [Google Scholar]
- 60.Nieder A. 2016. The neuronal code for number. Nat. Rev. Neurosci. 17, 366–382. ( 10.1038/nrn.2016.40) [DOI] [PubMed] [Google Scholar]
- 61.Vallentin D, Nieder A. 2008. Behavioural and prefrontal representation of spatial proportions in the monkey. Curr. Biol. 18, 1420–1425. ( 10.1016/j.cub.2008.08.042) [DOI] [PubMed] [Google Scholar]
- 62.Gallistel CR. 1990. The organization of learning. Cambridge, MA: MIT Press. [Google Scholar]
- 63.Walsh V. 2003. A theory of magnitude: common cortical metrics of time, space and quantity. Trends Cogn. Sci. 7, 483–488. ( 10.1016/j.tics.2003.09.002) [DOI] [PubMed] [Google Scholar]
- 64.Ditz HM, Nieder A. 2015. Neurons selective to the number of visual items in the corvid songbird endbrain. Proc. Natl Acad. Sci. USA 112, 7827–7832. ( 10.1073/pnas.1504245112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Montagnese CM, Mezey SE, Cisllag A. 2003. Efferent connections of the dorsomedial thalamic nuclei of the domestic chick (Gallus domesticus). J. Comp. Neurol. 459, 301–326. ( 10.1002/cne.10612) [DOI] [PubMed] [Google Scholar]
- 66.Butler AB, Manger PR, Lindhal BI, Arhem P. 2005. Evolution of the neural basis of consciousness: a bird–mammal comparison. Bioessays 27, 923–936. ( 10.1002/bies.20280) [DOI] [PubMed] [Google Scholar]
- 67.Potrich D, Sovrano VA, Stancher G, Vallortigara G. 2015. Quantity discrimination by zebrafish (Danio rerio). J. Comp. Psychol. 29, 388–393. ( 10.1037/com0000012) [DOI] [PubMed] [Google Scholar]
- 68.Stancher G, Rugani R, Regolin L, Vallortigara G. 2015. Numerical discrimination by frogs (Bombina orientalis). Anim. Cogn. 18, 219–229. ( 10.1007/s10071-014-0791-7) [DOI] [PubMed] [Google Scholar]
- 69.Dacke M, Srinivasan MV. 2008. Evidence for counting in insects. Anim. Cogn. 11, 683–689. ( 10.1007/s10071-008-0159-y) [DOI] [PubMed] [Google Scholar]
- 70.Gross HJ, Pahl M, Si A, Zhu H, Tautz J, Zhang S. 2009. Number-based visual generalisation in the honeybee. PLoS ONE 4, e4263 ( 10.1371/journal.pone.0004263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wittlinger M, Wehner R, Wolf H. 2006. The ant odometer: stepping on stilts and stumps. Science 312, 1965–1967. ( 10.1126/science.1126912) [DOI] [PubMed] [Google Scholar]
- 72.Harvey BM, Klein BP, Petridou N, Dumoulin SO. 2013. Topographic representation of numerosity in the human parietal cortex. Science 341, 1123–1126. ( 10.1126/science.1239052) [DOI] [PubMed] [Google Scholar]
- 73.Harvey BM, Fracasso A, Petridou N, Dumoulin SO. 2015. Topographic representations of object size and relationships with numerosity reveal generalized quantity processing in human parietal cortex. Proc. Natl Acad. Sci. USA 112, 13 525–13 530. ( 10.1073/pnas) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hubbard E, Piazza M, Pinel P, Dehaene S. 2005. Interactions between number and space in parietal cortex. Nat. Rev. Neurosci. 6, 435–448. ( 10.1038/nrn1684) [DOI] [PubMed] [Google Scholar]
- 75.Drucker CB, Brannon EM. 2015. Commentary on: Number space mapping in the newborn chick resembles humans’ mental number line. Front. Psychol. 6, 352 ( 10.3389/fpsyg) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davidson RJ. 2004. Well-being and affective style: neural substrates and biobehavioural correlates. Phil. Trans. R. Soc. Lond. B 359, 1395–1411. ( 10.1098/rstb.2004.1510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quaranta A, Siniscalchi M, Vallortigara G. 2007. Asymmetric tail-wagging responses by dogs to different emotive stimuli. Curr. Biol. 17, 199–201. ( 10.1016/j.cub.2007.02.008) [DOI] [PubMed] [Google Scholar]
- 78.Rogers LJ, Vallortigara G, Andrew RJ. 2013. Divided brains. New York, NY: Cambridge University Press. [Google Scholar]
- 79.Vallortigara G, Versace E. 2017. Laterality at the neural, cognitive, and behavioral levels. In APA handbook of comparative psychology: vol. 1. Basic concepts, methods, neural substrate, and behavior (ed. Call J.), pp. 557–577. Washington, DC: American Psychological Association. [Google Scholar]
- 80.Cantlon JF, Brannon EM. 2007. How much does number matter to a monkey? J. Exp. Psychol. Anim. Behav. Processes. 33, 32–41. ( 10.1037/0097-7403.33.1.32) [DOI] [PubMed] [Google Scholar]
- 81.Brookshire G, Casasanto D. 2012. Motivation and motor control: hemispheric specialization for approach motivation reverses with handedness. PLoS ONE 7, e36036 ( 10.1371/journal.pone.0036036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bisazza A, Facchin L, Vallortigara G. 2000. Heritability of lateralization in fish: concordance of right–left asymmetry between parents and offspring. Neuropsychologia 38, 907–912. ( 10.1016/S0028-3932(00)00018-X) [DOI] [PubMed] [Google Scholar]
- 83.Bisazza A, Sovrano VA, Vallortigara G. 2001. Consistency among different tasks of left–right asymmetries in lines of fish originally selected for opposite direction of lateralization in a detour task. Neuropsychologia 39, 1077–1085. ( 10.1016/S0028-3932(01)00034-3) [DOI] [PubMed] [Google Scholar]
- 84.Kiriakovski A, Utsuki N. 2012. From SNARC to SQUARC: universal mental quantity line? Int. J. Psychol. Stud. 4, 217–227. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.