Abstract
Individual differences in telomere length are associated with individual differences in behaviour in humans and birds. Within the human epidemiological literature this association is assumed to result from specific behaviour patterns causing changes in telomere dynamics. We argue that selective adoption—the hypothesis that individuals with short telomeres are more likely to adopt specific behaviours—is an alternative worthy of consideration. Selective adoption could occur either because telomere length directly affects behaviour or because behaviour and telomere length are both affected by a third variable, such as exposure to early-life adversity. We present differential predictions of the causation and selective adoption hypotheses and describe how these could be tested with longitudinal data on telomere length. Crucially, if behaviour is causal then it should be associated with differential rates of telomere attrition. Using smoking behaviour as an example, we show that the evidence that smoking accelerates the rate of telomere attrition within individuals is currently weak. We conclude that the selective adoption hypothesis for the association between behaviour and telomere length is both mechanistically plausible and, if anything, more compatible with existing empirical evidence than the hypothesis that behaviour is causal.
This article is part of the theme issue ‘Understanding diversity in telomere dynamics’.
Keywords: telomere length, telomere attrition, behaviour, biomarker, biological age, smoking
1. Introduction
There is mounting evidence for statistical associations between individual differences in telomere length and behaviour. In both humans and birds, individuals with shorter telomeres make different behavioural decisions as adults. For example, recent evidence suggests that there is an association between telomere length and impulsivity in both humans [1,2] and European starlings (Sturnus vulgaris) [3]. Adult humans with shorter leucocyte telomeres and adult European starlings with more developmental erythrocyte telomere attrition are both more impulsive in their choices, discounting rewards that are delayed in time more steeply than individuals with longer telomeres or less developmental attrition. Our aim in this paper is to attempt to answer the question of why such associations exist. In §2, we start by reviewing the evidence for associations between telomere length and behaviour in both humans and birds, with a view to describing the suite of behaviour patterns that are associated with relatively shorter telomeres. In §3, we present two, not necessarily mutually exclusive, hypotheses to explain the observed associations between telomere length and behaviour: first that behaviour is causal and second that the association is due to selective adoption, whereby individuals with shorter telomeres are more likely to adopt some behaviours. We develop a specific account of selective adoption, whereby exposure to early-life adversity causes correlated changes in both telomere length and subsequent behaviour. Distinguishing between causation and selective adoption is important because, if the causal hypothesis is correct, then telomere attrition can be used to identify those behaviours that are most harmful, whereas if the selective adoption hypothesis is correct, then we need to reinterpret relatively short telomeres as a biomarker of something else (e.g. exposure to early-life adversity) as opposed to as a dynamic consequence of current behaviour. In §4, we outline a strategy for using longitudinal data on telomere length to test between the causation hypothesis, the selective adoption hypothesis and a mixed hypothesis that assumes that both causation and selective adoption are operative. In §5, we present a review of the data relating to the association between smoking behaviour and telomere length as a test case of the predictions derived in §4. In §6, we provide a summary of our arguments and conclude that the selective adoption hypothesis is more compatible with currently available empirical data and theoretical thinking than the hypothesis that behaviour causes telomere attrition.
2. Evidence for associations between telomere length and behaviour
A large number of human correlational studies report evidence for associations between individual differences in telomere length and behaviour. Table 1 summarizes examples of the types of associations that have been found; this list is not intended to be a systematic review, but to give a sense of the positive results being reported in humans. In the majority of studies, the measure of telomere length is from leucocytes or other categories of white blood cells. In a few studies, the behaviour of subjects has been directly measured, such as choices in an economic game, activity levels via accelerometers and cortisol responses to an acute stressor. However, in most studies, the measures of behaviour are more indirect. For example, many behavioural variables are measured via self-report, including the type of physical activity undertaken, smoking behaviour and questionnaires designed to assess personality traits. We included studies on body mass index (BMI) and other indices of obesity based on findings showing that these measures are likely to imply variation in behavioural decisions regarding the amount or type of food consumed [16]. We also included studies that measured cortisol levels, either at baseline, or in response to an acute stressor, on the assumption that differences in cortisol levels are likely to translate into differences in behaviour. Table 1 shows that in humans, shorter adult leucocyte telomere length appears to be associated with a suite of differences in behaviour including: lower levels of physical activity, higher BMI (indicative of higher food consumption), higher impulsivity in choices between delayed rewards, higher propensity to take risky decisions, higher probability of smoking, higher alcohol consumption, higher stress reactivity, and more neurotic and pessimistic personality types. While some of these associations are based on single papers and may turn out not to be robust, others are based on meta-analyses of many published studies (e.g. for BMI, physical activity and smoking).
Table 1.
Examples of associations between behaviour and telomere length in humans.
| behaviour type | sample | behavioural measure | tissue | result | references |
|---|---|---|---|---|---|
| impulsivity (i.e. impatience, steeper time discounting) | Chinese undergraduate students | preference for smaller sooner versus larger later financial reward | leucocytes | greater impulsivity associated with shorter telomeres | [1] |
| children with ADHD | hyperactive–impulsive symptoms (assessed via structured interview of parents) | leucocytes | higher levels of hyperactivity–impulsivity associated with shorter telomeres | [2] | |
| risk-proneness (i.e. strength of preference for a riskier option) | Chinese undergraduate students | decision of how much to invest in an experimental stock | leucocytes | higher risk proneness associated with shorter telomeres | [1] |
| physical activity | systematic review of 37 studies | all forms of exercise | varied but mostly leucocytes | tendency for exercise to be associated with longer telomeres | [4] see also [5] |
| British white twins | physical activity in leisure time | leucocytes | less physical activity associated with shorter telomeres | [6] | |
| American men and women (NHANES) | running | leucocytes | meeting physical activity guidelines from running associated with longer telomeres | [7] | |
| less physically active white and African-American women | accelerometer-measured sedentary time | leucocytes | higher sedentary time associated with shorter telomeres | [8] | |
| eating (amount and/or frequency) | meta-analysis of 16 studies | body mass index (BMI) | leucocytes | higher BMI associated with short telomeres | [9] |
| meta-analysis | obesity (multiple measures) | mostly leucocytes | tendency for higher BMI/obesity to be associated with shorter telomeres | [4] | |
| smoking | systematic review of 84 studies and meta-analysis of 30 | ever versus never smokers and pack years smoked | varied but mostly blood | smoking associated with shorter telomeres. More pack years smoked associated with shorter telomeres | [10] |
| alcohol consumption | middle-aged, Finnish businessmen | past alcohol consumption | leucocytes | more alcohol consumption associated with shorter telomeres | [11] |
| stress reactivity | American kindergarten children (5–6 years) | heart rate, salivary cortisol, internalizing behaviours | buccal cells | higher heart rate, cortisol and internalizing behaviours associated with shorter telomeres | [12] |
| community sample of carer and non-carer American women | cortisol responses to acute stress (Trier Social Stress Test), overnight urinary-free cortisol, diurnal basal cortisol slope | PBMCs | higher cortisol responses to an acute stressor, higher overnight urinary-free cortisol and flatter daytime cortisol slopes associated with shorter telomeres | [13] | |
| neurotic personality type | population cohort study | neuroticism scale of Eysenck Personality Questionnaire | leucocytes | higher neuroticism associated with shorter telomeres | [14] |
| pessimistic personality | post-menopausal American women | questionnaire (Revised Life Orientation Test) | leucocytes | higher pessimism associated with shorter telomeres | [15] |
In our recent work on European starlings, we have found associations between developmental erythrocyte telomere attrition and various aspects of behaviour. For example, adult starlings with greater developmental telomere attrition were: more neophobic as measured by slower autoshaping to a lit key [17], more impulsive in the pursuit of food as measured by cognitive bias [18] and delay discounting tasks [3,17], less persistent in the pursuit of food as measured by progressive ratio and extinction tasks [17], and less risk-prone in a foraging choice task [19]. Starlings with greater developmental telomere attrition also displayed an attenuated response to the acute stressor of being caught and restrained in a bag [20].
Note that these starling studies used developmental telomere attrition (i.e. the extent to which telomeres shorten over the developmental period) rather than adult telomere length (as in the human studies in table 1). However, it is reasonable to assume that developmental telomere attrition and adult telomere length are related, because individuals whose telomeres shorten more during development will tend to have shorter adult telomere length [21]. In our starling studies, developmental telomere attrition and adult telomere length are often both associated with adult behaviour and the direction of the effects is the same [3,20]. It should be noted that developmental telomere attrition is not the only contributor to adult telomere length because there is also heritable variation in telomere length [22–24].
3. Hypotheses and causal pathways
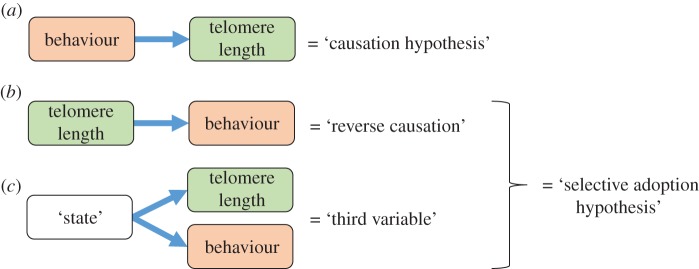
Logically, there are two possible explanations for the patterns of association between telomere length and behaviour described above. First, behaviour might directly cause changes in telomere length; we henceforth refer to this as the ‘causation hypothesis’. Second, individuals with short telomeres might be more likely to adopt certain patterns of behaviour, henceforth the ‘selective adoption hypothesis’. Selective adoption could result from two alternative causal pathways. Either short telomeres could directly cause changes in behaviour (henceforth ‘reverse causation’); or behaviour and telomere length could both be caused by a third variable (figure 1). We describe these three causal pathways in more detail below and discuss their mechanistic plausibility.
Figure 1.
Three possible causal pathways via which behaviour could be associated with telomere length: (a) behaviour causes telomere attrition, (b) telomere attrition causes changes in behaviour and (c) another variable, which we refer to as ‘state’, causes both telomere attrition and changes in behaviour. The two emerging hypotheses for the pattern in the observed data are named according to whether the difference in behaviour is assumed to be causal (causation hypothesis) or secondary (selective adoption hypothesis). (Online version in colour.)
(a). Causation: behaviour causes telomere attrition
There is evidence that the rate of telomere attrition is altered via a range of cellular mechanisms. Inflammation causes telomere attrition in blood by increasing the rate of leucocyte turnover and hence increasing the rate of replicative senescence. Oxidative stress involves the production of reactive oxygen species that can cause telomere attrition by damaging the vulnerable G triplets of the telomeric sequence [25]. Finally, although the activity of telomerase (the enzyme that repairs telomeres) is generally repressed in somatic cells, cortisol may further inhibit it and hence decrease telomere repair [26]. Cortisol also increases free radical production and interferes with antioxidant defences, thus increasing oxidative stress within the cell (reviewed in [27]). Thus, increased inflammation, oxidative stress and biological stress are all implicated in increased telomere attrition.
The way in which an individual behaves could plausibly change the levels of inflammation, oxidative stress and stress hormones within the body. Behaviour could acutely alter exposure to environmental conditions, or substances (either via foods or environmental toxins) that directly increase or reduce inflammation, oxidative stress or telomerase activity. For example, the toxins in tobacco smoke cause increased inflammation and oxidative stress [28,29], whereas eating a diet high in fresh fruit and vegetables boosts antioxidant defences, and some naturally occurring substances in food may boost telomerase activity [30]. Furthermore, habitually performing certain behaviours may cause chronic changes in inflammation, oxidative stress or biological stress. For example, obesity, the cumulative effect of overeating, is characterized by high oxidative stress and inflammation [31], and behaving impatiently or engaging in more risky behaviour might be associated with differences in exposure to biological stress [32]. By contrast, regular physical exercise causes a net reduction in inflammation, oxidative stress and stress hormones (despite individual bouts of exercise causing acute increases in all three), and has been associated with increases in telomerase activity in both humans and mice [33]. In humans, practising mindfulness meditation is also associated with decreased cortisol and inflammation and increased telomerase levels [34].
Thus, there are multiple plausible mechanisms via which behaviour could directly affect the cellular mechanisms responsible for telomere attrition and repair, and hence affect telomere length [35]. However, it is important to point out that many of these mechanisms have only been demonstrated in vitro, and it is unclear whether they also operate in vivo under biologically realistic physiological conditions. A recent study in jackdaws (Corvus monedula) found no evidence that oxidative stress shortens telomeres in vivo [36].
(b). Reverse causation: telomere length causes behaviour
Until recently, the function of telomeres was believed to be solely in protecting the chromosome ends and preventing activation of the DNA damage response. However, recent work has shown that telomeres also have a function in regulating gene expression. For some time, it has been known that genes proximal to telomeres (within approx. 100 kb) are silenced by the spreading of heterochromatin from the telomeres into the subtelomeric region, an effect known as the telomere position effect (TPE) [37]. However, more recent work has shown that telomeres can also affect gene expression over much longer distances by looping back onto chromosomes, a mechanism referred to as TPE over long distances (TPE-OLD). Robin et al. [38] identified more than 140 genes within 10 Mb of telomeres whose expression was affected by the telomere. These long-range interactions between telomeres and target genes are lost as telomeres shorten, providing a potential regulatory role of telomeres on these genes. This regulation occurs long before telomeres become short enough to produce a DNA damage response. Thus, telomere length could directly cause adaptive, age-related changes in physiology as an organism ages [38,39]. No TPE- or TPE-OLD-regulated genes known to be involved in behavioural differences have yet been identified, but it is at least theoretically plausible that they will be in the future. Thus, it is not possible to discount the hypothesis that shorter telomere length could have a direct causal effect on behaviour by changing the expression of genes involved in behaviour (see also [40] for a related argument: the life-history regulation hypothesis of telomere attrition).
(c). A third variable causes telomere length and behaviour
The final possibility that we consider is that both telomere length and behaviour are caused by a third variable. Although there are other possibilities, the specific hypothesis that we develop here is that exposure to early-life adversity causes both shorter telomeres and changes in adult behaviour. Living organisms are thought to be particularly vulnerable to being damaged by exposure to adverse experiences early in life, when the rate of developmental change is at its fastest [41]. In support of this ‘high initial damage load’ hypothesis, there is mounting evidence that adversity of various types, especially that experienced early in life, causes enduring changes in biological systems including both telomeres and the mechanisms that underlie behaviour.
In humans, there are numerous correlational studies linking early-life adversity with shorter adult telomeres [42–46]. Establishing causation in humans is difficult because experimental manipulation of early-life adversity is unethical, but longitudinal studies provide the best evidence available that early-life adversity causes telomere attrition. For example, childhood exposure to violence between 5 and 10 years of age is associated with significantly greater telomere attrition over the same time period [47]. In altricial birds it has been possible to conclusively demonstrate that early-life adversity causes accelerated telomere attrition via experimental studies in which early-life adversity is manipulated in various ways. For example, competitively disadvantaging a starling or jackdaw nestling within its brood, either by experimentally manipulating brood size [48,49] or by manipulating the relative size of the focal nestling's competitors [50], accelerates the rate of erythrocyte telomere attrition measured in the first few weeks of life. Similarly, stress induced by daily human handling of European shag (Phalacrocorax aristotelis) nestlings between days 10 and 30 post-hatch caused accelerated erythrocyte telomere shortening relative to unhandled controls [51]. In a recent hand-rearing study in which we experimentally dissociated the adversity caused by developmental food deprivation and the adversity caused by increased begging effort (both of which are likely to occur when there is greater competition within the brood), we showed that these two sources of adversity had additive effects on erythrocyte telomere attrition in nestling starlings [52].
There is also considerable evidence that experience of early-life adversity alters subsequent behaviour in humans and other species. In humans, childhood adversity is associated with a range of enduring changes in brain structure, stress physiology and behaviour [53,54]. For example, childhood adversity is associated with a greater probability of smoking, starting smoking at an earlier age, smoking more and being less likely to quit [55,56]. While the human evidence is correlational, evidence that these associations are due to early-life adversity causing changes in behaviour comes from experimental studies on animals. For example, European starlings subjected to the same developmental manipulations that accelerate telomere attrition showed a range of alterations in adult behaviour. Birds that were competitively disadvantaged as nestlings were fatter than their advantaged siblings as adults and showed altered foraging behaviour, investing more time in seeking information about food and eating more following food deprivation [57]. Similarly, birds reared in large broods were less discriminating in their food choices than their siblings reared in small broods, behaving as if acutely hungry despite equivalent levels of actual food deprivation [58]. The developmentally disadvantaged birds also showed altered escape flight performance indicative of a steeper trade-off between take off speed and take off angle [59]. Daily treatment of zebra finch (Taeniopygia guttata) nestlings with corticosterone to simulate the effects of early-life stress caused changes in the subsequent juvenile response to an acute stressor [60].
Given the evidence that exposure to early-life adversity causes both telomere attrition and changes in later behaviour, we therefore expect indirect correlations between telomere length and behaviour. Furthermore, the suite of behaviour patterns associated with short telomeres should be similar to that associated with experience of early-life adversity. In humans, this prediction appears to be broadly correct. In birds, there are currently too few data to assess to what extent the behaviours associated with short telomeres overlap with those caused by early-life adversity. In our recent studies with European starlings, we have found some behaviour patterns that are significantly predicted by the early-life adversity manipulation to which the birds have been subjected [57–59] and others that are significantly predicted by telomere attrition or length [3]. However, the statistical effects of telomere attrition/length and the developmental manipulation on behaviour are generally in the same direction [20]. Furthermore, because we know that our manipulations of early-life adversity cause changes in telomere length, it seems probable that any differences in whether behaviour is significantly predicted by early-life adversity or by telomere length are explained by our relatively small sample sizes.
In summary, we have reviewed evidence that early-life adversity damages the developing individual, causing enduring changes in its somatic state. Shorter telomere length is one of the symptoms of exposure to early-life adversity and hence a biomarker of this damage. The observed behavioural differences in individuals that have experienced early-life adversity are either further pathological symptoms of adversity, or alternatively, evidence of an adaptive response to an alteration in state [61]. For the purposes of understanding why there might be associations between telomere length and behaviour, it is not significant whether the behavioural change is pathological or adaptive; all that matters is that both telomere length and adult behaviour are affected by exposure to early-life adversity.
(d). The case for selective adoption
Within the biomedical and epidemiological literatures it is almost universally assumed that observed correlations between behaviour and telomere length are due to behaviour causing altered rates of telomere attrition. For example, many cross-sectional studies have found an association between smoking and shorter leucocyte telomeres in humans [62–71]. These data are widely interpreted as demonstrating that smoking reduces health by accelerating the rate of biological ageing [62,64]. In their discussion of one such study, Valdes et al. [62] conclude, ‘Our findings suggest that obesity and cigarette smoking accelerate human ageing…smoking a pack a day for 40 years corresponds to 7.4 years of ageing’. This leap from correlation to causation is probably explained by the existence of the many plausible mechanisms outlined in §3a via which smoking could alter telomere length. Thus, the possibility that there might exist plausible alternative hypotheses to explain the associations between behaviour and telomere length has not been seriously considered. Benetos et al. [21] simply dismiss the possibility that short telomeres might cause smoking as ‘unlikely’. In a recent study demonstrating a correlation between delay discounting and leucocyte telomere length in humans, Yim et al. [1] briefly discuss the possibility of a reverse causation explanation of their finding, but discard it as less plausible than the hypothesis that impatience causes telomere attrition.
By contrast, within the behavioural ecology literature there is a strong tradition of research into the effects of state on behaviour. State is used in a broad sense to refer to any variation within or between individuals (territory size, dominance status, fat reserves, age, etc.) that might have consequences for how it is optimal to behave in order to maximize Darwinian fitness. In the current context, we suggest that state could be conceived as referring to the degree of overall damage caused to the body by exposure to adversity. Under this conception, state is a measure of biological age, by which we mean here that it predicts future morbidity and mortality. Theoretical models show that biological age is a centrally important state variable in predicting life-history decisions [72,73]. Thus, there is strong theoretical support available within behavioural ecology for either reverse causation or a third variable pathway as outlined above. Either telomere length or some other, related state variable affected by early-life adversity might make an animal more likely to adopt (or perform more of) a particular type of behaviour.
The plausibility of the selective adoption hypothesis is strengthened by the timing of the period during which the fastest telomere attrition occurs. In humans, leucocyte telomere attrition is most rapid in the early years of life, with the rate of telomere loss being estimated at between 270 and 1000 bp yr−1 between birth and age 3–4 years with a sharp transition to a much slower adult rate of attrition of 30–50 bp yr−1 at around the age of 4 years [74–76]. In adulthood, the ranking of individuals by leucocyte telomere length has been shown to be largely stable over a period of 13 years [21]. Thus, most of the variation in adult leucocyte telomere length is due to inheritance and early-life experience as opposed to adult experience (see also [77]). This leaves rather little scope for behaviour to affect telomere attrition as assumed by the causation hypothesis. For smoking behaviour, most leucocyte telomere attrition occurs prior to the age at which children start smoking, making it relatively implausible that smoking could contribute significantly to between-individual variation in adult telomere length. Furthermore, in our studies of European starlings, our manipulations of early-life adversity cause changes in telomere length by two weeks of age, before the nestlings are foraging for themselves, whereas the differences in impulsivity are measured in adult birds a year later [3]. Again, therefore, the timing of the emergence of the differences in telomere length and behaviour make it relatively implausible that the behaviour could cause the observed differences in telomere length.
In summary, we believe that the assumption that behaviour causes the observed relationship between behaviour and telomere length is unjustified based on current evidence. There is theoretical and empirical support for alternative accounts, whereby changes in behaviour are the outcome of damage caused by exposure to adverse experiences in early life (see [78] for a related hypothesis). The selective adoption hypothesis, whereby individuals with shorter telomeres are more or less likely to adopt specific behaviours, therefore, deserves serious consideration.
4. Testing between causation and selective adoption
The causation and selective adoption hypotheses make different predictions regarding the patterns of telomere length that should be observed in individuals with different behaviour patterns. For simplicity, we develop these predictions for the simple case of two discrete behavioural categories: in our example, individuals with and without a specific behaviour pattern (smoking). However, the predictions that we develop could equally be applied to any binary classification of more continuously distributed behavioural variation, such as individuals who eat more or less, drink more than a certain number of units of alcohol per week, take more or less physical exercise or are more or less impatient. Our reason for choosing smoking as an example is that it is one of the most highly studied behaviour patterns in the context of human telomere length: many epidemiological studies include smoking status as a control variable, even if it is not the major focus of the study.
From the perspective of behavioural ecology, smoking might seem like an odd choice: it is an exclusively human behaviour for which it is difficult to provide an adaptive explanation. However, we argue that the fact that some people are more likely to start smoking than others, and also find it harder to quit, suggests that there is variation in anatomy or physiology between future smokers and non-smokers, for example, in the brain circuits responsible for emotional regulation, that is likely to have broader significance for other types of behaviour. Thus, although the behaviour pattern that we are studying is a comparatively recent human cultural innovation, we argue that it reflects differences in fundamental mechanisms of behaviour that are likely to be evolutionarily ancient and of broad significance in explaining behavioural variation in humans and animals.
(a). Assumptions regarding telomere dynamics
In order to generate testable predictions from the causation and selective adoption hypotheses, it is first necessary to explicitly state their assumptions. In smokers, we assume that from the point at which an individual starts smoking (t = 0) their telomere length, TL, in subsequent years (t = 1, 2, 3, etc.) can be modelled as a straight line with a positive intercept (cs) corresponding to the TL at the start of smoking, and a negative slope (ms) corresponding to the telomere attrition per year of smoking. Thus, for smokers, current TL is given by the following equation: 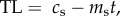 where t is the years of smoking. For non-smokers, we also assume that TL can be modelled as a straight line with a positive intercept and negative slope with values cn and mn, respectively, for individuals of the same age as smokers. Thus, for non-smokers, we can write the corresponding equation:
where t is the years of smoking. For non-smokers, we also assume that TL can be modelled as a straight line with a positive intercept and negative slope with values cn and mn, respectively, for individuals of the same age as smokers. Thus, for non-smokers, we can write the corresponding equation: 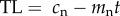 .
.
For the causation hypothesis, we assume that prior to commencing smoking there is no difference in the TL of future smokers and non-smokers (i.e.  ), but that following the start of smoking the rate of telomere attrition is higher for smokers than for non-smokers (
), but that following the start of smoking the rate of telomere attrition is higher for smokers than for non-smokers (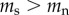 ). By contrast, for the selective adoption hypothesis, we assume that prior to commencing smoking, future smokers have shorter TL than future non-smokers (
). By contrast, for the selective adoption hypothesis, we assume that prior to commencing smoking, future smokers have shorter TL than future non-smokers (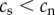 ), but that following the start of smoking the rate of telomere attrition is equal in smokers and non-smokers (
), but that following the start of smoking the rate of telomere attrition is equal in smokers and non-smokers ( ).
).
There is no reason to assume that the causation and selective adoption hypotheses are mutually exclusive. Both processes could operate within an individual. For completeness, therefore, we also consider a mixed hypothesis that assumes that both selective adoption and causation are in operation. For the mixed hypothesis it follows that prior to commencing smoking, future smokers have shorter telomeres than future non-smokers (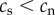 ), and that following the start of smoking, the rate of telomere attrition is greater in smokers than in non-smokers (
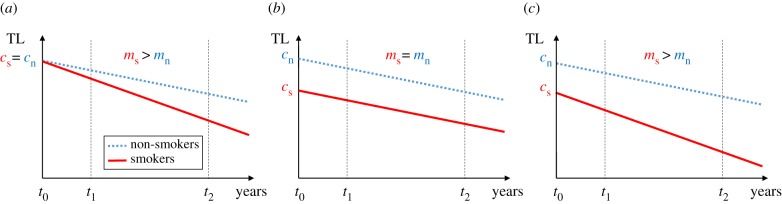
), and that following the start of smoking, the rate of telomere attrition is greater in smokers than in non-smokers (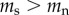 ). The telomere dynamics resulting from the causation, selective uptake and mixed hypotheses are depicted graphically in figure 2a, b and c, respectively.
). The telomere dynamics resulting from the causation, selective uptake and mixed hypotheses are depicted graphically in figure 2a, b and c, respectively.
Figure 2.
Changes in telomere length (TL) as a function of years since commencement of smoking predicted by three alternative models to explain the observed relationship between smoking and telomere length: (a) causation hypothesis, (b) selective adoption hypothesis and (c) mixed hypothesis (selective adoption + causation). We assume that smoking starts at t0 for smokers and continues thereafter, whereas non-smokers never smoke. The solid (red) line represents the change in telomere length for smokers and the dotted (blue) line for age-matched non-smokers. The dashed lines at t1 and t2 represent two telomere measurements made at different time points after the commencement of smoking; in a longitudinal study of TL these could represent baseline and follow-up measurements, respectively. cs and cn are TL at the time of commencement of smoking for smokers and non-smokers, respectively; ms and mn are the slopes of the lines describing how TL changes with time for smokers and non-smokers, respectively. See text for further details. (Online version in colour.)
The models presented here assume a linear decline of telomere length with age. Over the entire life course, the function is actually decelerating rather than linear in both humans and birds [52,74,79]. However, the greatest deceleration occurs very early in life [74–76] and across the span of adulthood a linear approximation is reasonable. Moreover, it would be possible to substitute any monotonic, nonlinear function and the general logic of the predictions we make below would still hold.
(b). Testable predictions regarding telomere dynamics
Table 2 summarizes some key predictions of the three hypotheses outlined above for the telomere dynamics that should be observed in smokers and non-smokers. Prediction 1 is included for completeness in order to emphasize the point that all the hypotheses predict that telomere length will be shorter in smokers than in non-smokers. Thus, no inference regarding causality can be made from a confirmation of prediction 1. Predictions 2 and 4 are unique to the causation hypothesis. A confirmation of either prediction 2 or 4 alone would provide strong support for the causation hypothesis. Prediction 3 is made by both the causation and mixed hypotheses. Thus, rejection of prediction 3 alone would provide strong support for selective adoption. The mixed hypothesis cannot be tested with a single prediction, but requires confirmation of prediction 3 combined with rejection of either prediction 2 or 4.
Table 2.
Positive predictions of the causal hypothesis.
| no. | prediction | hypothesis |
||
|---|---|---|---|---|
| causation | selective adoption | mixed | ||
| 1 | telomere length for smokers is shorter than telomere length for non-smokers at any time point following the adoption of smoking | yes | yes | yes |
| 2 | telomere length prior to adopting smoking is equal in future smokers and non-smokers | yes
|
no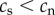
|
no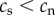
|
| 3 | rate of telomere attrition is greater in smokers than in non-smokers | yes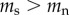
|
no
|
yes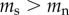
|
| 4 | the difference in rates of telomere attrition between smokers and non-smokers is sufficient to explain the difference in telomere length between smokers and non-smokers at any time point following the start of smoking | yes | no | no |
5. Empirical evidence on smoking and telomere dynamics
(a). Prediction 1: shorter telomeres in smokers
Prediction 1 states that telomere length for smokers is shorter than telomere length for non-smokers at any time point following the adoption of smoking. A test of prediction 1 requires measurement of telomere length for smokers and non-smokers at a single time point, and hence it can be tested with either cross-sectional or longitudinal data. Controlling for the age and sex of subjects is important, because telomere length decreases with age [80], females tend to have longer telomeres for their age than males [81] and rates of smoking often differ between males and females.
In support of prediction 1, many cross-sectional studies have found an association between smoking status and shorter leucocyte telomeres, and this association persists after controlling for age, sex and additional variables believed to cause telomere attrition [62–71,82–91]. Although some studies fail to find a cross-sectional association [92–94], these are in the minority and are often based on a relatively smaller number of subjects, suggesting lower power (table 3). The generality of the cross-sectional association between shorter telomeres and smoking has been confirmed by a recent meta-analysis [10]. Some studies additionally provide evidence that smoking more cigarettes per day is associated with shorter telomere length (interpreted as a dose-response [82,90]) and that a greater number of years since smoking cessation in former smokers is associated with longer current telomere length [82,87,90]. These findings are also supported by meta-analysis [10].
Table 3.
Longitudinal studies of human leucocyte telomere length (LTL) in smokers and non-smokers. BMI, body mass index; WHR, waist/hip ratio; RTLU, relative telomere length units; HDL, high-density lipoprotein.
| reference | cohort | no. subjects |
age at baseline (mean ± s.d.) (years) | follow-up interval (years) | TL methoda | change in TL yr−1 between baseline and follow-up (mean ± s.d.) | analysis of effects of smoking on telomere dynamics |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| covariates controlled forb | reported significant differences |
|||||||||||
| shorter LTL in smokers (prediction 1) |
higher LTL attrition in smokers (prediction 3) | |||||||||||
| baseline | follow-up | longitudinal analysis | baseline | follow-up | ||||||||
| Aviv et al. [83] | Bogalusa Heart Study: white and African-Americans | 635 | 635 | 635 | 31.4 ± 5.1 | 5.9 | TRF | −40.7 ± 46.0 bp (no test) | age, sex, BMI, race | yes | yes | no |
| Bendix et al. [88] | Danish MONICA1 and 10 surveys | 1763 | 1356 | 1356 | 44.7 ± 10.9 | 10.6 | qPCR | no summary statistics presented | age, sex, alcohol consumption | no | yes | yes |
| Ehrlenbach et al. [94] | population-based Bruneck Study, Italy | 510 | 510 | 510 | 62 (range: 53–71) | 10 | qPCR | −45.5 bp (no test) | age, sex | no | no | no |
| Farzaneh-Far et al. [93] | Heart and Soul Study, USA | 608 | 608 | ca 66 ± 10? | 5 | qPCR | −42.0 bp (significant loss) | age, sex, WHR | no | not tested | no | |
| Huzen et al. [89] | PREVEND study, The Netherlands | 8074 | 5886 | 48 (median, range: 39–60) | 6.6 | qPCR | −0.47 ± 0.16 RTLU (no test) | age, sex, glucose, WHR, HDL | yes | not tested | yes | |
| Muezzinler et al. [90] | population-based cohort of older adults, Germany | 3600 | 961 | range: 50–75 | 8 | qPCR | −13 ± 81 bp (no test) | age, sex | yes | not tested | no | |
| Révész et al. [91] | Netherlands Study of Anxiety and Depression | 2936 | 1860 | 41.8 ± 13.1 | 6 | qPCR | −8.8 bp (no test) | age, sex | yes | not tested | no | |
| Toupance et al. [95] | ERA study (France) | 154 | 58 ± 10 | 9.5 | TRF | −24.2 ± 16.0 bp (significant loss) | age, sex | not tested | not tested | no | ||
| Weischer et al. [96] | prospective Copenhagen City Heart Study | 4576 | 4576 | 4576 | range: 38–68 | 10 | qPCR | −19.3 bp (no test) | yes | no | no | |
aMethod used for measuring TL: TRF, Southern blot terminal restriction fragment; qPCR, quantitative PCR.
bAll studies additionally controlled for baseline LTL in their analysis of telomere attrition.
Importantly, none of the results described above provides proof for the causation hypothesis because it is equally plausible that individuals with shorter telomeres become heavier smokers and find it more difficult to quit than individuals with longer telomeres. Thus, although the majority of these studies interpret their findings as supporting a causal relationship between smoking and telomere attrition (for a rare exception, see [84]), this hypothesis cannot be separated from selective adoption, or indeed a mixed hypothesis, with cross-sectional data.
(b). Prediction 2: equal telomere length prior to smoking
Prediction 2 states that telomere length prior to adopting smoking is equal in future smokers and non-smokers. A test of prediction 2, therefore, requires measurement of telomere length prior to the start of smoking for future smokers and non-smokers. A single cross-sectional telomere length measurement in early childhood is sufficient to test prediction 2, but longitudinal follow-up is required to establish the future smoking status of each subject. It would be necessary to account for possible effects of passive smoking if parents are smokers. We are not aware of any studies that have attempted to test prediction 2, or of any existing datasets in which a test would be possible. A rejection of prediction 2 would provide strong evidence in support of the selective adoption hypothesis.
(c). Prediction 3: faster telomere attrition in smokers
Prediction 3 states that the rate of telomere attrition is greater in smokers than non-smokers. A test of prediction 3 requires longitudinal measurement of rates of telomere attrition in individual smokers and non-smokers, because telomere attrition can only be unambiguously measured within subjects. Therefore, longitudinal data, with at least two measurements per subject separated by a substantial follow-up interval, are required to test prediction 3.
We searched the literature for studies meeting the above criteria. Table 3 summarizes nine longitudinal studies of leucocyte telomere length that test the effect of smoking on telomere attrition. Two of these studies [83,95] measured telomere length using the Southern blot (TRF) method and the other seven used the quantitative polymerase chain reaction (qPCR) method. Given concerns over high measurement error with qPCR [97], we looked for an overall decline in telomere length between the baseline and follow-up measurements as a basic check of the validity of the data; the absence of telomere attrition with time would suggest unacceptably high levels of measurement error. Reassuringly, all studies that reported overall change in telomere length reported attrition with time (although only two studies provided a significance test in support of this difference [93,95]). One study [88] reported no statistics on overall change in telomere length, but a graph within the paper suggested that attrition was present.
In line with the evidence presented for prediction 1 above, the majority of the studies in table 3 (six of eight that report an effect) find a cross-sectional association between shorter telomeres and smoking at baseline and/or follow-up. However, only two studies out of nine [88,89] report faster telomere attrition in smokers (with one actually finding evidence for faster attrition in non-smokers [90]). Furthermore, these results should be interpreted with caution because the common practice of adjusting for baseline telomere length in multiple regression models of telomere attrition (all the studies in table 3 do this) may lead to overestimation of the effect of smoking on telomere attrition and a consequent increase in the probability of type 1 errors [98]. Thus, the evidence for prediction 3 is weak, and the lack of strong support for the causal and mixed hypotheses provides some support for the alternative selective adoption hypothesis. A quantitative meta-analysis will be required to establish whether there is any evidence that the rate of telomere attrition is higher in smokers than non-smokers, but this is not straightforward from the published literature because effect sizes are not reported in a standard way.
A number of explanations other than selective adoption have been suggested for the apparent lack of difference in the rates of telomere attrition observed in smokers and non-smokers. First, longitudinal analyses might simply have lower power due to being based on smaller numbers of subjects [90]. While this is true in two of the studies that report significant cross-sectional effects of smoking but no longitudinal effects [90,91], it is not true for the other two studies showing this pattern [83,96]. Second, smokers are more likely to be lost to follow-up, and if this loss is non-random with respect to telomere length, this could reduce the telomere attrition rates observed for smokers in longitudinal studies [90]. While this is likely to be correct, selective loss of smokers with short telomeres would also reduce cross-sectional associations between smoking and telomere length. Therefore, selective loss of subjects cannot explain the difference between cross-sectional and longitudinal findings. Third, the effect of smoking on telomere attrition could be nonlinear, occurring rapidly when smoking is first adopted, but then slowing down as telomeres shorten [83,94]. The basis for this theory is the strong association between longer baseline telomere length and faster attrition observed in most longitudinal studies. However, this relationship is now understood to be mainly attributable to a statistical artefact arising from regression to the mean, as opposed to a biological mechanism operating within individuals [99], meaning that a mechanistic basis for this theory is lacking. Fourth, smoking is often associated with cancer, and various cancers are associated with telomere elongation, potentially offsetting any attrition caused by smoking [90]. While it is true that telomere elongation has been reported within tumours, it is not clear that this effect extends to other tissues such as leucocytes. Indeed, some studies report shorter leucocyte telomere length in pre-diagnosis cancer patients [100]. In summary, the above explanations deserve further exploration, but we are currently unconvinced that any one of them can rescue the causal hypothesis. It is surprising that none of the studies failing to find a difference in telomere attrition rates between smokers and non-smokers suggests selective adoption as a possible alternative explanation for their findings.
(d). Prediction 4: differences in attrition should explain differences in length
Prediction 4 states that the difference in rates of telomere attrition between smokers and non-smokers is sufficient to explain the difference in telomere length between smokers and non-smokers at any time point following the start of smoking. A test of prediction 4 requires longitudinal data. The difference in the rates of attrition by smoking can then be used to calculate the number of years of smoking necessary to generate the difference in telomere length observed at any time point. If this number is incompatible with the age of the subjects at that time point (e.g. if the subjects are younger than the number of years of smoking required to explain the difference in telomere length), then prediction 4 can be rejected. Thus, a test of prediction 4 does not necessarily require an estimate of how long subjects have been smoking.
One study in table 3 reports the statistics necessary to make the above calculation [83]. Given the reported telomere attrition rates for smokers and non-smokers (42 and 40 bp yr−1, respectively), the number of years of smoking necessary to produce the observed difference in telomere length at baseline (7359 versus 7500 bp, respectively) is 70.5 years and at follow-up (7116 versus 7280, respectively) is 82 years. Therefore, the causal impact of smoking is insufficient to explain the difference in telomere length in this study, because the subjects were only 37.3 years old at follow-up. This result provides support for the selective adoption and mixed hypotheses, but further data are required to test its generality.
6. Discussion and conclusion
Our detailed review of the existing longitudinal data on smoking behaviour and telomere dynamics provides support for a cross-sectional association between smoking and shorter telomeres, but less support for accelerated leucocyte telomere attrition in smokers. Since accelerated telomere attrition in smokers is a critical prediction of the causal hypothesis, our review calls into question the causal role of smoking in telomere attrition. This conclusion is supported by a Mendelian randomization study that used a genetic polymorphism (CHRNA3 genotype) previously established to be strongly associated with tobacco consumption to provide a unidirectional, unbiased test of whether smoking causes short telomeres. This study also found no evidence for a causal association between smoking and short telomeres [84]. Another recent study found no evidence for any differences in the expression of genes related to telomere length regulation between smokers and non-smokers, which could be interpreted as further evidence against the causal hypothesis [101]. Thus, while it is possible that existing longitudinal analyses have underestimated the true effect of smoking on telomere attrition for some reason, or that the Mendelian randomization study was underpowered, it is perhaps more parsimonious to reject the causal hypothesis in favour of selective adoption.
Although we restricted our detailed review of the evidence to smoking behaviour, there is evidence that the difference in telomere dynamics between cross-sectional and longitudinal studies described above is not confined to smoking. A similar pattern of clear cross-sectional effects on telomere length but weak or absent longitudinal effects on telomere attrition has also been reported for several of the other behaviours listed in table 1, including physical activity [96,102], calorie intake [103], BMI [9,96] and alcohol intake [96]. These data start to support a more general case against the hypothesis that behaviour causes changes in telomere dynamics.
We have argued that the selective adoption hypothesis, whereby individuals with shorter telomeres are more likely to adopt specific behaviour patterns, is supported by a number of empirical observations. First, the majority of the variation in adult telomere length occurs very early in life, providing little opportunity for adult behaviour to substantially impact telomere length [3,21]. Second, the evidence that different behaviour patterns are associated with different rates of telomere attrition, as required by the causal hypothesis, is weak. Finally, in one case it has been possible to show that the small difference in telomere attrition between smokers and non-smokers was insufficient to explain the much larger cross-sectional differences in telomere length. We hope that this paper serves to motivate the studies necessary to conclusively test the predictions of the causal hypothesis outlined in this paper.
Selective adoption could arise via two different causal pathways: reverse causation, whereby short telomeres directly cause behavioural differences, and a third variable account, whereby both telomere length and behaviour are caused by a third variable. We have argued that a plausible third variable is exposure to early-life adversity based on evidence that the suite of behavioural differences associated with short telomeres is similar to the suite of behavioural differences associated with exposure to early-life adversity. These data suggest that early-life adversity could be a direct or indirect cause of both telomere length and behaviour. From an adaptive perspective it makes sense that individuals damaged by exposure to early-life adversity, with consequent reduced life expectancy, should reduce their future orientation, with consequences for the decisions that give rise to such outcomes as obesity, smoking and alcohol abuse [54].
As indicated earlier, the reverse causation and the third variable pathways make similar predictions in terms of observed telomere dynamics. Observing the temporal sequence of events is unlikely to be informative in separating the hypotheses, because a third variable could be causal but have its effects at very different time points, meaning that observing that telomere attrition precedes the adoption/emergence of specific behaviour patterns does not prove reverse causality. Separating reverse causation from a third variable conclusively requires an experimental approach in which telomere length is manipulated directly (for a discussion of this approach, see [104]). This could potentially be achieved in animal studies via the use of a telomerase activator such as TA-65 [105,106].
We conclude by re-emphasizing our earlier point that distinguishing between the causation and selective adoption hypotheses for the associations between telomere dynamics and behaviour is a worthwhile endeavour. The answer has consequences for how measures of telomere length are used in both human epidemiology and behavioural ecology. Under the currently prevailing view, that certain types of behaviour cause accelerated telomere attrition, measures of telomere length can be used to identify those behaviours that are most harmful. Changes in telomere dynamics could also potentially be used to monitor the somatic consequences of behaviour change (e.g. the positive effects of quitting smoking). However, if we are correct, and selective causation turns out to be the explanation for observed associations, then we need to reinterpret shorter telomeres as a relatively static biomarker of early-life adversity as opposed to as a dynamic consequence of current behaviour.
Acknowledgements
We thank members of the COMSTAR group at Newcastle University for their constructive input into discussions surrounding the ideas in this paper, and Patrick Bateson, Dan Eisenberg and two anonymous referees for useful comments on an earlier draft of the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
M.B. and D.N. contributed equally to the conception of this paper. M.B. wrote the first draft. Both authors edited and approved the final draft.
Competing interests
We have no competing interests. M.B. and D.N. have previously held a joint grant with one of the Guest Editors of this special issue (Pat Monaghan) and have co-authored several papers with her.
Funding
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement no. AdG 666669, COMSTAR).
References
- 1.Yim O-S, Zhang X, Shalev I, Monakhov M, Zhong S, Hsu M, Chew SH, Lai PS, Ebstein RP. 2016. Delay discounting, genetic sensitivity, and leukocyte telomere length. Proc. Natl Acad. Sci. USA 113, 201514351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Souza Costa D, Rosa DVF, Barros AGA, Romano-Silva MA, Malloy-Diniz LF, Mattos P, De Miranda DM. 2015. Telomere length is highly inherited and associated with hyperactivity-impulsivity in children with attention deficit/hyperactivity disorder. Front. Mol. Neurosci. 8, 1–8. ( 10.3389/fnmol.2015.00028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bateson M, Brilot BO, Gillespie R, Monaghan P, Nettle D. 2015. Developmental telomere attrition predicts impulsive decision-making in adult starlings. Proc. R. Soc. B 282, 20142140 ( 10.1098/rspb.2014.2140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mundstock E, et al. 2015. Effect of obesity on telomere length: systematic review and meta-analysis. Obesity 23, 2165–2174. ( 10.1002/oby.21183) [DOI] [PubMed] [Google Scholar]
- 5.Denham J, O'Brien BJ, Charchar FJ. 2016. Telomere length maintenance and cardio-metabolic disease prevention through exercise training. Sport Med. 46, 1213–1237. ( 10.1007/s40279-016-0482-4) [DOI] [PubMed] [Google Scholar]
- 6.Cherkas LF, et al. 2008. The association between physical activity in leisure time and leukocyte telomere length. Arch. Intern. Med. 168, 154–158. ( 10.1001/archinternmed.2007.39) [DOI] [PubMed] [Google Scholar]
- 7.Loprinzi PD, Sng E. 2016. Mode-specific physical activity and leukocyte telomere length among U.S. adults: implications of running on cellular aging. Prev. Med. 85, 17–19. ( 10.1016/j.ypmed.2016.01.002) [DOI] [PubMed] [Google Scholar]
- 8.Shadyab AH, et al. 2017. Associations of accelerometer-measured and self-reported sedentary time with leukocyte telomere length in older women. Am. J. Epidemiol. 11, 171–181. ( 10.1093/aje/kww196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Müezzinler A, Zaineddin AK, Brenner H. 2014. Body mass index and leukocyte telomere length in adults: a systematic review and meta-analysis. Obes. Rev. 15, 192–201. ( 10.1111/obr.12126) [DOI] [PubMed] [Google Scholar]
- 10.Astuti Y, Wardhana A, Watkins J, Wulaningsih W. 2017. Cigarette smoking and telomere length: a systematic review of 84 studies and meta-analysis. Environ. Res. 158, 480–489. ( 10.1016/j.envres.2017.06.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strandberg TE, Strandberg A, Saijonmaa O, Tilvis RS, Pitkälä KH, Fyhrquist F. 2012. Association between alcohol consumption in healthy midlife and telomere length in older men. The Helsinki businessmen study. Eur. J. Epidemiol. 27, 815–822. ( 10.1007/s10654-012-9728-0) [DOI] [PubMed] [Google Scholar]
- 12.Kroenke CH, Epel E, Adler N, Bush NR, Obradovic J, Lin J, Blackburn E, Stamperdahl JL, Boyce WT. 2011. Autonomic and adrenocortical reactivity and buccal cell telomere length in kindergarten children. Psychosom. Med. 73, 533–540. ( 10.1097/PSY.0b013e318229acfc) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomiyama AJ, et al. 2012. Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiol. Behav. 106, 40–45. ( 10.1016/j.physbeh.2011.11.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Ockenburg SL, de Jonge P, van der Harst P, Ormel J, Rosmalen JGM. 2014. Does neuroticism make you old? Prospective associations between neuroticism and leukocyte telomere length. Psychol. Med. 44, 723–729. ( 10.1017/S0033291713001657) [DOI] [PubMed] [Google Scholar]
- 15.O'Donovan A, Lin J, Dhabhar FS, Wolkowitz O, Tillie JM, Blackburn E, Epel E. 2009. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav. Immun. 23, 446–449. ( 10.1016/j.bbi.2008.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swinburn B, Sacks G, Ravussin E. 2009. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am. J. Clin. Nutr. 90, 1453–1456. ( 10.3945/ajcn.2009.28595) [DOI] [PubMed] [Google Scholar]
- 17.Nettle D, Andrews CP, Monaghan P, Brilot BO, Bedford T, Gillespie R, Bateson M. 2015. Developmental and familial predictors of adult cognitive traits in the European starling. Anim. Behav. 107, 239–248. ( 10.1016/j.anbehav.2015.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bateson M, Emmerson M, Ergün G, Monaghan P, Nettle D. 2015. Opposite effects of early-life competition and developmental telomere attrition on cognitive biases in juvenile European starlings. PLoS ONE 10, e0132602 ( 10.1371/journal.pone.0132602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews C, Nettle D, Bedford T, Reichert S, Monaghan P, Bateson M. 2018 A marker of biological ageing predicts adult risk preference in European starlings, Sturnus vulgaris. Behav. Ecol. in press. [DOI] [PMC free article] [PubMed]
- 20.Andrews C, et al. 2017. A marker of biological age explains individual variation in the strength of the adult stress response. R. Soc. Open Sci. 4, 171208 ( 10.1098/rsos.171208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benetos A, et al. 2013. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell 12, 615–621. ( 10.1111/acel.12086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slagboom PE, Droog S, Boomsma DI. 1994. Genetic determination of telomere size in humans: a twin study of three age groups. Am. J. Hum. Genet. 55, 876–882. [PMC free article] [PubMed] [Google Scholar]
- 23.Dugdale HL, Richardson DS. 2018. Heritability of telomere variation: it is all about the environment! Phil. Trans. R. Soc. B 373, 20160450 ( 10.1098/rstb.2016.0450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenberg DTA, Kuzawa CW. 2018. The paternal age at conception effect on offspring telomere length: mechanistic, comparative and adaptive perspectives. Phil. Trans. R. Soc. B 373, 20160442 ( 10.1098/rstb.2016.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Von Zglinicki T. 2002. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344. ( 10.1016/S0968-0004(02)02110-2) [DOI] [PubMed] [Google Scholar]
- 26.Choi J, Fauce SR, Effros RB. 2008. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav. Immun. 22, 600–605. ( 10.1016/j.bbi.2007.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haussmann MF, Marchetto NM. 2010. Telomeres: linking stress and survival, ecology and evolution. Curr. Zool. 56, 714–727. [Google Scholar]
- 28.Zuo L, He F, Sergakis GG, Koozehchian MS, Stimpfl JN, Rong Y, Diaz PT, Best TM. 2014. The interrelated role of cigarette-smoking, oxidative stress and immune response in COPD and corresponding treatments. Am. J. Physiol. Lung Cell. Mol. Physiol. 307, L205–L218. ( 10.1152/ajplung.00330.2013) [DOI] [PubMed] [Google Scholar]
- 29.Richards JM, Stipelman BA, Bornovalova MA, Daughters SB, Sinha R, Lejuez CW. 2011. Biological mechanisms underlying the relationship between stress and smoking: state of the science and directions for future work. Biol. Psychol. 88, 1–12. ( 10.1016/j.biopsycho.2011.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ait-Ghezala G, Hassan S, Tweed M, Paris D, Crynen G, Zakirova Z, Crynen S, Crawford F.. 2016. Identification of telomerase-activating blends from naturally occurring compounds. Altern. Ther. Health Med. 22, 6–14. [PubMed] [Google Scholar]
- 31.Furukawa S, et al. 2004. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114, 1752–1761. ( 10.1172/JCI21625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mischel W, Shoda Y, Rodriguez ML. 1989. Delay of gratification in children. Science 244, 933–938. ( 10.1126/science.2658056) [DOI] [PubMed] [Google Scholar]
- 33.Werner C, et al. 2009. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation 120, 2438–2447. ( 10.1161/CIRCULATIONAHA.109.861005) [DOI] [PubMed] [Google Scholar]
- 34.Schutte NS, Malouff JM. 2014. A meta-analytic review of the effects of mindfulness meditation on telomerase activity. Psychoneuroendocrinology 42, 45–48. ( 10.1016/j.psyneuen.2013.12.017) [DOI] [PubMed] [Google Scholar]
- 35.Lyon DE, Starkweather AR, Montpetit A, Menzies V, Jallo N. 2014. A biobehavioral perspective on telomere length and the exposome. Biol. Res. Nurs. 16, 448–455. ( 10.1177/1099800414522689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boonekamp JJ, Bauch C, Mulder E, Verhulst S. 2017. Does oxidative stress shorten telomeres? Biol. Lett. 13, 20170164 ( 10.1098/rsbl.2017.0164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottschling DE, Aparicio OM, Billington BL, Zakian VA. 1990. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell 63, 751–762. ( 10.1016/0092-8674(90)90141-Z) [DOI] [PubMed] [Google Scholar]
- 38.Robin JD, Ludlow AT, Batten K, Magdinier F, Stadler G, Wagner KR, Shay JW, Wright WE. 2014. Telomere position effect: regulation of gene expression with progressive telomere shortening over long distances. Genes Dev. 28, 2464–2476. ( 10.1101/gad.251041.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim W, Ludlow AT, Min J, Robin JD, Stadler G, Mender I, Lai T-P, Zhang N, Wright WE. 2016. Regulation of the human telomerase gene TERT by telomere position effect—over long distances (TPE-OLD): implications for aging and cancer. PLoS Biol. 14, 1–25. ( 10.1371/journal.pbio.2000016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young AJ. 2018. The role of telomeres in the mechanisms and evolution of life-history trade-offs and ageing. Phil. Trans. R. Soc. B 373, 20160452 ( 10.1098/rstb.2016.0452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gavrilov LA, Gavrilova NS. 2004. Early-life programming of aging and longevity: the idea of high initial damage load (the HIDL hypothesis). Ann. N. Y. Acad. Sci. 1019, 496–501. ( 10.1196/annals.1297.091) [DOI] [PubMed] [Google Scholar]
- 42.Drury SS, Mabile E, Brett ZH, Esteves K, Jones E, Shirtcliff EA, Theall KP. 2014. The association of telomere length with family violence and disruption. Pediatrics 134, e128–e137. ( 10.1542/peds.2013-3415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shalev I. 2012. Early life stress and telomere length: Investigating the connection and possible mechanisms: a critical survey of the evidence base, research methodology and basic biology. Bioessays 34, 943–952. ( 10.1002/bies.201200084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price LH, Kao H-T, Burgers DE, Carpenter LL, Tyrka AR. 2013. Telomeres and early-life stress: an overview. Biol. Psychiatry 73, 15–23. ( 10.1016/j.biopsych.2012.06.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tyrka AR, Price LH, Kao H, Porton B, Marsella SA, Carpenter LL. 2009. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol. Psychiatry 67, 531–534. ( 10.1016/j.biopsych.2009.08.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen SH, et al. 2014. Adverse childhood experiences and leukocyte telomere maintenance in depressed and healthy adults. J. Affect. Disord. 169, 86–90. ( 10.1016/j.jad.2014.07.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Mill J, Arseneault L, Caspi A. 2013. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol. Psychiatry 18, 576–581. ( 10.1038/mp.2012.32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nettle D, Monaghan P, Boner W, Gillespie R, Bateson M. 2013. Bottom of the heap: having heavier competitors accelerates early-life telomere loss in the European starling, Sturnus vulgaris. PLoS ONE 8, e83617 ( 10.1371/journal.pone.0083617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boonekamp JJ, Mulder GA, Salomons M, Dijkstra C, Verhulst S. 2014. Nestling telomere shortening, but not telomere length, reflects developmental stress and predicts survival in wild birds. Proc. R. Soc. B 281, 20133287 ( 10.1098/rspb.2013.3287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nettle D, Monaghan P, Gillespie R, Brilot B, Bedford T, Bateson M. 2015. An experimental demonstration that early-life competitive disadvantage accelerates telomere loss. Proc. R. Soc. B 282, 20141610 ( 10.1098/rspb.2014.1610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herborn KA, Heidinger BJ, Boner W, Noguera JC, Adam A, Daunt F, Monaghan P. 2014. Stress exposure in early post-natal life reduces telomere length: an experimental demonstration in a long-lived seabird. Proc. R. Soc. B 281, 20133151 ( 10.1098/rspb.2013.3151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nettle D, Andrews C, Reichert S, Bedford T, Kolenda C, Parker C, Martin-Ruiz C, Monaghan P, Bateson M. 2017. Early-life adversity accelerates cellular ageing and affects adult inflammation: experimental evidence from the European starling. Sci. Rep. 7, 40794 ( 10.1038/srep40794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH. 2006. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur. Arch. Psychiatry Clin. Neurosci. 256, 174–186. ( 10.1007/s00406-005-0624-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pepper G, Nettle D. 2017. The behavioural constellation of deprivation: causes and consequences. Behav. Brain Sci. 40, e314 ( 10.1017/S0140525X1600234X) [DOI] [PubMed] [Google Scholar]
- 55.Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF et al. . 1999. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA 282, 1652–1658. ( 10.1001/jama.282.17.1652) [DOI] [PubMed] [Google Scholar]
- 56.Smith PH, Saddleson ML, Homish GG, Mckee SA, Kozlowski LT, Giovino GA. 2015. The relationship between childhood physical and emotional abuse and smoking cessation among U.S. women and men. Psychol. Addict. Behav. 29, 338–346. ( 10.1037/adb0000033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrews C, Viviani J, Egan E, Bedford T, Brilot B, Nettle D, Bateson M. 2015. Early life adversity increases foraging and information gathering in European starlings. Anim. Behav. 109, 123–132. ( 10.1016/j.anbehav.2015.08.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bloxham L, Bateson M, Bedford T, Brilot B, Nettle D. 2014. The memory of hunger: developmental plasticity of dietary selectivity in the European starling, Sturnus vulgaris. Anim. Behav. 91, 33–40. ( 10.1016/j.anbehav.2014.02.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Hagan D, Andrews CP, Bedford T, Bateson M, Nettle D. 2015. Early life disadvantage strengthens flight performance trade-offs in European starlings, Sturnus vulgaris. Anim. Behav. 102, 141–148. ( 10.1016/j.anbehav.2015.01.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spencer KA, Evans NP, Monaghan P. 2009. Postnatal stress in birds: a novel model of glucocorticoid programming of the hypothalamic-pituitary-adrenal axis. Endocrinology 150, 1931–1934. ( 10.1210/en.2008-1471) [DOI] [PubMed] [Google Scholar]
- 61.Nettle D, Bateson M. 2015. Adaptive developmental plasticity: what is it, how can we recognize it and when can it evolve? Proc. R. Soc. B 282, 20151005 ( 10.1098/rspb.2015.1005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. 2005. Obesity, cigarette smoking, and telomere length in women. Lancet 366, 662–664. ( 10.1016/S0140-6736(05)66630-5) [DOI] [PubMed] [Google Scholar]
- 63.Verde Z, et al. 2015. Effects of cigarette smoking and nicotine metabolite ratio on leukocyte telomere length. Environ. Res. 140, 488–494. ( 10.1016/j.envres.2015.05.008) [DOI] [PubMed] [Google Scholar]
- 64.Morlá M, Busquets X, Pons J, Sauleda J, MacNee W, Agustí AGN. 2006. Telomere shortening in smokers with and without COPD. Eur. Respir. J. 27, 525–528. ( 10.1183/09031936.06.00087005) [DOI] [PubMed] [Google Scholar]
- 65.McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. 2007. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol. Biomarkers Prev. 16, 815–819. ( 10.1158/1055-9965.EPI-06-0961) [DOI] [PubMed] [Google Scholar]
- 66.O'Donnell CJ, et al. 2008. Leukocyte telomere length and carotid artery intimal medial thickness. The Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 28, 1165–1171. ( 10.1161/ATVBAHA.107.154849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weischer M, Bojesen SE, Cawthon RM, Freiberg JJ, Tybjærg-Hansen A, Nordestgaard BG. 2012. Short telomere length, myocardial infarction, ischemic heart disease, and early death. Arterioscler. Thromb. Vasc. Biol. 32, 822–829. ( 10.1161/ATVBAHA.111.237271) [DOI] [PubMed] [Google Scholar]
- 68.Pellatt AJ, Wolff RK, Lundgreen A, Cawthon R, Slattery ML. 2012. Genetic and lifestyle influence on telomere length and subsequent risk of colon cancer in a case control study. Int. J. Mol. Epidemiol. Genet. 3, 184–194. [PMC free article] [PubMed] [Google Scholar]
- 69.Needham BL, Adler N, Gregorich S, Rehkopf D, Lin J, Blackburn EH, Epel ES. 2013. Socioeconomic status, health behavior, and leukocyte telomere length in the national health and nutrition examination survey, 1999–2002. Soc. Sci. Med. 85, 1–8. ( 10.1016/j.socscimed.2013.02.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strandberg TE, Saijonmaa O, Tilvis RS, Pitkala KH, Strandberg AY, Miettinen TA, Fyhrquist F. 2011. Association of telomere length in older men with mortality and midlife body mass index and smoking. J. Gerontol. 66A, 815–820. ( 10.1093/gerona/glr064) [DOI] [PubMed] [Google Scholar]
- 71.Shalev I. et al 2014. Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Mol. Psychiatry 19, 1163–1170. ( 10.1038/mp.2013.183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McNamara JM, Houston AI. 1996. State-dependent life histories. Nature 380, 215–221. ( 10.1038/380215a0) [DOI] [PubMed] [Google Scholar]
- 73.Houston AI, McNamara JM.. 1999. Models of adaptive behaviour: an approach based on state. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 74.Frenck RW, Blackburn EH, Shannon KM. 1998. The rate of telomere sequence loss in human leukocytes varies with age. Proc. Natl Acad. Sci. USA 95, 5607–5610. ( 10.1073/pnas.95.10.5607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wojcicki JM, Shiboski S, Heyman MB, Elwan D, Lin J, Blackburn E, Epel E. 2016. Telomere length change plateaus at 4 years of age in Latino children: associations with baseline length and maternal change. Mol. Genet. Genomics 291, 1379–1389. ( 10.1007/s00438-016-1191-2) [DOI] [PubMed] [Google Scholar]
- 76.Zeichner SL, Palumbo P, Feng Y, Xiao X, Gee D, Sleasman J, Goodenow M, Biggar R, Dimitrov D. 1999. Rapid telomere shortening in children. Blood 93, 2824–2830. [PubMed] [Google Scholar]
- 77.Aviv A, Shay JW. 2018. Reflections on telomere dynamics and ageing-related diseases in humans. Phil. Trans. R. Soc. B 373, 20160436 ( 10.1098/rstb.2016.0436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Entringer S, de Punder K, Buss C, Wadhwa PD. 2018. The fetal programming of telomere biology hypothesis: an update. Phil. Trans. R. Soc. B 373, 20170151 ( 10.1098/rstb.2017.0151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Müezzinler A, Karina A, Brenner H. 2013. A systematic review of leukocyte telomere length and age in adults. Ageing Res. Rev. 12, 509–519. ( 10.1016/j.arr.2013.01.003) [DOI] [PubMed] [Google Scholar]
- 81.Gardner M, et al. 2014. Gender and telomere length: systematic review and meta-analysis. Exp. Gerontol. 51, 15–27. ( 10.1016/j.exger.2013.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao X, Mons U, Zhang Y, Breitling LP, Brenner H. 2016. DNA methylation changes in response to active smoking exposure are associated with leukocyte telomere length among older adults. Eur. J. Epidemiol. 31, 1–11. ( 10.1007/s10654-016-0210-2) [DOI] [PubMed] [Google Scholar]
- 83.Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Berenson GS. 2009. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am. J. Epidemiol. 169, 323–329. ( 10.1093/aje/kwn338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rode L, Bojesen SE, Weischer M, Nordestgaard BG. 2014. High tobacco consumption is causally associated with increased all-cause mortality in a general population sample of 55 568 individuals, but not with short telomeres: a Mendelian randomization study. Int. J. Epidemiol. 43, 1473–1483. ( 10.1093/ije/dyu119) [DOI] [PubMed] [Google Scholar]
- 85.Mirabello L, Huang WY, Wong JYY, Chatterjee N, Reding D, Crawford ED, De Vivo I, Hayes RB, Savage SA. 2009. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell 8, 405–413. ( 10.1111/j.1474-9726.2009.00485.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Latifovic L, Peacock SD, Massey TE, King WD. 2016. The influence of alcohol consumption, cigarette smoking, and physical activity on leukocyte telomere length. Cancer Epidemiol. Biomarkers Prev. 25, 374–380. ( 10.1158/1055-9965.EPI-14-1364) [DOI] [PubMed] [Google Scholar]
- 87.Wulaningsih W, Serrano FEC, Utarini A, Matsuguchi T, Watkins J, for PILAR Research Network. 2016. Smoking, second-hand smoke exposure and smoking cessation in relation to leukocyte telomere length and mortality. Oncotarget 7, 60 419–60 431. ( 10.18632/oncotarget.11051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bendix L, Thinggaard M, Fenger M, Kolvraa S, Avlund K, Linneberg A Osler M. 2014. Longitudinal changes in leukocyte telomere length and mortality in humans. J. Gerontol. 69A, 231–239. ( 10.1093/gerona/glt153) [DOI] [PubMed] [Google Scholar]
- 89.Huzen J, et al. 2014. Telomere length loss due to smoking and metabolic traits. J. Intern. Med. 275, 155–163. ( 10.1111/joim.12149) [DOI] [PubMed] [Google Scholar]
- 90.Müezzinler A, et al. 2015. Smoking habits and leukocyte telomere length dynamics among older adults: results from the ESTHER cohort. Exp. Gerontol. 70, 18–25. ( 10.1016/j.exger.2015.07.002) [DOI] [PubMed] [Google Scholar]
- 91.Révész D, Milaneschi Y, Terpstra EM, Penninx BWJH. 2016. Baseline biopsychosocial determinants of telomere length and 6-year attrition rate. Psychoneuroendocrinology 67, 153–162. ( 10.1016/j.psyneuen.2016.02.007) [DOI] [PubMed] [Google Scholar]
- 92.Cassidy A, De Vivo I, Liu Y, Han J, Prescott J, Hunter DJ, Rimm EB. 2010. Associations between diet, lifestyle factors, and telomere length in women. Am. J. Clin. Nutr. 91, 1273–1280. ( 10.3945/ajcn.2009.28947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley MA. 2010. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the Heart and Soul Study. PLoS ONE 5, e8612 ( 10.1371/journal.pone.0008612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ehrlenbach S, Willeit P, Kiechl S, Willeit J, Reindl M, Schanda K, Kronenberg F, Brandstätter A. 2009. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. Int. J. Epidemiol. 38, 1725–1734. ( 10.1093/ije/dyp273) [DOI] [PubMed] [Google Scholar]
- 95.Toupance S, Labat C, Temmar M, Rossignol P, Kimura M, Aviv A, Benetos A. 2017. Short telomeres, but not telomere attrition rates, are associated with carotid atherosclerosis. Hypertension 70, 420–426. ( 10.1161/HYPERTENSIONAHA.117.09354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weischer M, Bojesen SE, Nordestgaard BG. 2014. Telomere shortening unrelated to smoking, body weight, physical activity, and alcohol intake: 4,576 general population individuals with repeat measurements 10 years apart. PLoS Genet. 10, e1004191 ( 10.1371/journal.pgen.1004191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. 2011. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 39, 1–5. ( 10.1093/nar/gkr634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bateson M, Nettle D.. 2017. Controlling for baseline telomere length biases estimates of the effect of smoking on leukocyte telomere attrition in longitudinal studies. Zenodo. ( 10.5281/zenodo.1009086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Verhulst S, Aviv A, Benetos A, Berenson GS, Kark JD. 2013. Do leukocyte telomere length dynamics depend on baseline telomere length? An analysis that corrects for ‘regression to the mean’. Eur. J. Epidemiol. 28, 859–866. ( 10.1007/s10654-013-9845-4) [DOI] [PubMed] [Google Scholar]
- 100.Yang M, Prescott J, Poole EM, Rice MS, Kubzansky LD, Idahl A, Lundin E, De Vivo I, Tworoger SS. 2017. Prediagnosis leukocyte telomere length and risk of ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 26, 339–345. ( 10.1158/1055-9965.EPI-16-0466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patel CJ, Manrai AK, Corona E, Kohane IS. 2017. Systematic correlation of environmental exposure and physiological and self-reported behaviour factors with leukocyte telomere length. Int. J. Epidemiol. 46, 44–56. ( 10.1093/ije/dyw043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Soares-Miranda L, Imamura F, Siscovick D, Jenny NS, Fitzpatrick AL, Mozaffarian D. 2015. Physical activity, physical fitness, and leukocyte telomere length: the Cardiovascular Health Study. Med. Sci. Sport Exerc. 47, 2525–2534. ( 10.1249/MSS.0000000000000720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kark J, Goldberger N, Kimura M, Sinnreich R, Aviv A. 2012. Energy intake and leukocyte telomere length in young adults. Am. J. Clin. Nutr. 95, 479–487. ( 10.3945/ajcn.111.024521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Criscuolo F, Smith S, Zahn S, Heidinger BJ, Haussmann MF. 2018. Experimental manipulation of telomere length: does it reveal a corner-stone role for telomerase in the natural variability of individual fitness? Phil. Trans. R. Soc. B 373, 20160440 ( 10.1098/rstb.2016.0440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reichert S, Bize P, Arrivé M, Zahn S, Massemin S, Criscuolo F. 2014. Experimental increase in telomere length leads to faster feather regeneration. Exp. Gerontol. 52, 36–38. ( 10.1016/j.exger.2014.01.019) [DOI] [PubMed] [Google Scholar]
- 106.de Jesus BB, Schneeberger K, Vera E, Tejera A, Harley CB, Blasco MA. 2011. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell 10, 604–621. ( 10.1111/j.1474-9726.2011.00700.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.