Abstract
Much telomere loss takes place during the period of most rapid growth when cell proliferation and potentially energy expenditure are high. Fast growth is linked to reduced longevity. Therefore, the effects of somatic cell proliferation on telomere loss and cell senescence might play a significant role in driving the growth-lifespan trade-off. While different species will have evolved a growth strategy that maximizes lifetime fitness, environmental conditions encountered during periods of growth will influence individual optima. In this review, we first discuss the routes by which altered cellular conditions could influence telomere loss in vertebrates, with a focus on oxidative stress in both in vitro and in vivo studies. We discuss the relationship between body growth and telomere length, and evaluate the empirical evidence that this relationship is generally negative. We further discuss the potentially conflicting hypotheses that arise when other factors are taken into account, and the further work that needs to be undertaken to disentangle confounding variables.
This article is part of the theme issue ‘Understanding diversity in telomere dynamics’.
Keywords: environment, body size, compensatory growth, oxidative stress, longevity, nutrition
1. Introduction
There is considerable evidence from diverse studies across a wide range of taxa, that animals can vary their growth rate, and that faster growth is associated with a lifespan reduction [1–3]. One possible factor that might contribute to this association is the effect of growth on telomere loss. The telomeric system of chromosome protection is highly conserved across eukaryotes, acting to maintain the integrity of the linear chromosomes. Vertebrate telomeres comprise tandem repeats of a short hexameric DNA sequence (TTAGGG) at the chromosome ends, with a single-stranded overhang that doubles back on itself and intrudes into the double-stranded section, forming the so-called ‘t-loop’ [4]. The telomere itself is protected by the shelterin proteins, which prevent it being accessed by cellular mechanisms that repair breaks in DNA and which could otherwise give rise to catastrophic end-to-end joining of chromosomes [4,5]. Telomeres also protect the coding sequences on the chromosomes from the loss that occurs as a consequence of the incomplete replication of the 3′ ends of DNA strands during cell division. In the absence of telomere restoration, the loss of telomere sequences during cell division results in progressive telomere shortening until a point is reached when the telomeres become dysfunctional and the genome unstable. This triggers cell senescence, often followed by apoptosis, and the rate at which this occurs has consequences for tissue and organism function [6]. There are various mechanisms whereby telomeres can be restored or even lengthened, including the recombination-based alternative telomere-lengthening pathways [7,8], but the most widespread restoration mechanism in normal cells is via the reverse transcriptase enzyme telomerase [4]. This enzyme is variably active in different species, cell types and life stages.
While this basic system remains essentially similar across the eukaryotes, with some notable exceptions (such as the Diptera [9]), the details of telomere length, loss and restoration vary within and among species, and among tissues. Given that telomere length and/or loss have been linked to health and longevity [5,10,11], telomere dynamics are expected to be under strong selection pressure. Species-specific telomere dynamics have evolved in tandem with the species life history, particularly in relation to the selection pressures that shape growth rate, body size and longevity, all of which influence the need for cell division [12]. In addition to differences among species, there is also considerable variation in telomere length and loss among individuals of the same species. Inter-individual differences in inherited telomere length are part of the picture [13], as is variation among conspecifics in cell division and turnover in tissues and at different life stages. However, much of the within-species variation is likely to be due to environmental factors that influence rates of telomere loss [13]. The amount of telomere loss per round of cell division that can be attributed to the end replication problem depends on how close to the chromosome end the distal primer can be placed during DNA replication; in cultured human cells, in which almost all of this work has been done, this loss is small (possibly as little as 10–20 base pairs [14]), but the observed loss rate is in often considerably greater [14,15]. Conditions within the cell are thought to play an important role here, and these conditions can obviously be influenced by the environment that the organism experiences. Environmental factors can act directly or indirectly (i.e. via parental effects) on the individual, inducing increased cell division rates, changing body size or creating intra-cellular conditions that accelerate telomere loss.
In this review, we consider the mechanisms whereby variation in growth rates might give rise to variation in telomere loss. We consider the effects of environmentally generated oxidative stress in particular. Recent reviews of other important environmental factors that can influence telomere loss such as exposure to stressors, inflammation and toxic chemicals are available elsewhere [16–20].We then discuss the evidence that somatic growth during post-natal life, when telomere restoration is more limited, is linked to increased telomere attrition, discuss why effects might differ among studies as well as among and within species, and identify where we lack important information [16–19].
2. Telomere length and loss
Both telomere length and the rate of telomere loss are likely to be important to organism health and longevity. There is variation among species in the age-specific telomere length [4]. Why such interspecific differences in telomere length have evolved, and what the functional significance of this might be, is unclear. In addition to the 3′ end replication problem, there are several other conserved mechanisms contributing to telomere shortening that have been reported in the literature. These include oxidative stress, reviewed in [21] and telomere trimming, reviewed in [22]. Recent evidence suggests that length is set during embryo development [23], and that any aberrantly long telomeres in embryonic stem cells are ‘trimmed’ back to the appropriate length by so-called TZAPs (telomeric zinc-finger associated proteins) [24]. Thereafter, telomere restoration in most somatic cells is limited [4]. Consequently, ‘starting’ length presumably determines the fate of cells, because this will determine the number of cell divisions that occur before a critically short telomere length triggers cell-replicative senescence. Studies using human cells have identified several additional factors that influence telomere loss [25,26]; these include errors during DNA replication (including problems with the unwinding of the telomeric structure during DNA replication), exonuclease activity and deletion of t-loops by homologous recombination damage and damage induced by exposure to oxidative stress, stress hormones, inflammation, UV radiation or toxic chemicals [18,19,27,28]. The importance and impact of these processes is likely to differ among cell types and potentially also at different life stages, and there may also be differences among species. However, little comparative data to examine this variation are available.
Since adverse environmental conditions can increase telomere loss, telomere loss rate can potentially give an indication of the state of the individual, reflecting the environmental challenge that it faces, or has faced, and the individual's capacity to deal with it [29,30]. The relationships among length, loss rate and fitness outcomes depend in part on whether short telomeres have a causal role in bringing about reduced health or longevity. If this is the case, then the same loss rate will have different consequences depending on the starting telomere length [29]. If, on the other hand, loss rate is simply a biomarker of health, then a relatively high loss rate indicates a poor state whatever the telomere length. Telomere loss is more difficult to measure than length. Repeated measures from the same individual are required to avoid results that are confounded by differential survival of individuals (see example in the next paragraph). Such repeat sampling is only feasible in a limited number of tissues where relatively non-invasive sampling is possible, such as via small blood samples or skin biopsies. It is therefore important that we know the extent to which telomere changes in these tissues reflect those in other tissues whose function is important to health and longevity. Studies of variation in telomere dynamics in tissues within individuals are limited, but there does appear to be an association across tissues [31–33]. In practice, individuals with high telomere loss rates are likely also to have relatively shorter age-specific telomere length, unless there is considerable inter-individual variation in initial telomere length. Variation in telomere length across individuals, environments or experimental treatments can therefore still provide us with valuable information.
Early in life, telomere length is unlikely to have a causal role in determining survival prospects over the short term, since sufficient telomere loss to compromise health is unlikely to have occurred at this life stage. However, since, as mentioned above, telomere loss rate itself may be indicative of exposure to poor conditions, length or loss may be correlated with survival even early in life [34,35] and loss rate can be a better predictor of juvenile survival than is telomere length [35]. In a long-term study of Soay sheep Ovis aries (a feral breed of domesticated sheep on an isolated island off the west coast of Scotland) individual telomere length was repeatedly measured from shortly after birth; individuals with longer telomeres survived better over the first 2 years of life, but not in later adulthood [36]. In addition to illustrating how telomere dynamics might be differentially related to individual state at different life stages, this study also shows how differential mortality with respect to telomere length can alter variation in telomere length in different age categories; individuals with the shortest telomeres will already have been eliminated before sexual maturation and thus be under-represented in older age classes.
3. Oxidative stress and telomere loss
Figure 1 summarizes the main routes described above whereby growth, telomere loss and cell senescence are linked involving changes to cell proliferation, oxidative damage and triggering of a persistent DNA damage response. In this review, we concentrate the effect of on oxidative stress on telomere loss, as this has been most widely studied, and there is good evidence that growth rates, a major focus of this review, can influence levels of oxidative stress.
Figure 1.
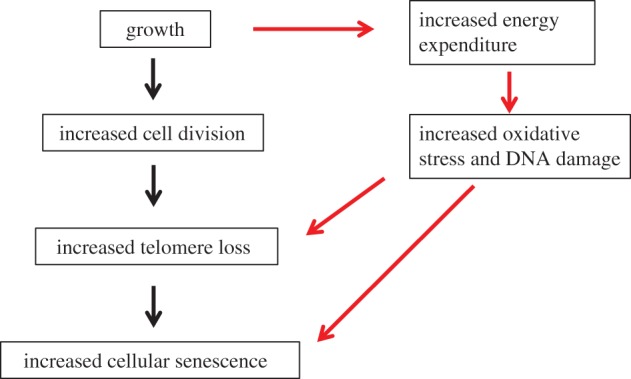
The routes whereby growth and telomere loss can be linked. The main route via increased cell division and the route via increased energy expenditure are shown. While normal growth will involve energy expenditure, organisms may have evolved strategies to minimize oxidative damage during this time. However, when circumstances favour more or faster growth, oxidative damage to DNA may occur as a result of the further increase in expenditure. Oxidative damage to telomeric DNA can increase the telomere loss per round of cell division, and increase the rate at which cells senesce. This oxidative damage may also trigger a persistent DNA damage response in the cell, triggering cell senescence directly in the absence of increased telomere loss. (Online version in colour.)
(a). Oxidative stress at the cellular level
Intense cellular stresses that induce high levels of double-stranded breaks to DNA can cause telomere shortening without DNA replication. However, under the less-catastrophic stresses more likely to occur in natural conditions, loss largely occurs during DNA replication [14,37]. Oxidative stress can damage DNA, and such damage may underlie the effects of many environmental factors on telomeres. Oxidative damage occurs primarily when the antioxidant defences cannot fully quench the reactive oxygen species (ROS) that are generated in the mitochondria. Telomeres are considered particularly sensitive to oxidative damage, possibly because of the increased vulnerability of their stacked guanine bases [14,38–41]. There is also evidence that the dynamics of damage repair differ in the telomeric region from elsewhere in the genome [27]. Oxidative lesions can also interfere with the shelterin proteins and result in telomeres becoming dysfunctional [42]. However, the oxidative lesions to the telomeric DNA itself, especially to the G-rich strand [42], are considered to be particularly important; a relatively high proportion of this damage remains unrepaired [43], increasing the amount of shortening at the next round of cell division [14,44]. Interestingly, it is also known that oxidative damage to telomeric regions can induce a persistent DNA damage response that gives rise to cell-replicative senescence irrespective of telomere length [45,46].
While the effect of oxidative stress on telomere length has been studied both in vivo and in vitro, most experimental studies have been done in cell culture, enabling specific pathways to be elucidated. Generation of oxidative stress in cultured cells has been shown to increase telomere shortening during cell division, and experimental reduction of the production of ROS in mitochondria shown to reduce telomere shortening [14,39,47,48]. An important caveat here [46] is that much of the in vitro work has been done using immortalized cell lines or cancer cells, so the relevance to normal cells is somewhat unclear. In addition, the doses of the pro-oxidants applied directly to cells in culture may sometimes be much higher than would be the case in vivo [18].
It has been argued that the increased cell-cycle arrest and cell death that follows persistent exposure to oxidative stress might arise largely from oxidative damage to the whole genome and to other macromolecules, rather than being triggered by telomere dysfunction [44]. That oxidative damage to the telomeric DNA is in itself of considerable importance in determining cell fates has been demonstrated via the experimental generation of oxidative damage only to the telomeres; as predicted, this led to more cell death [44]. Nonetheless, under natural conditions, the amount of unrepaired oxidative damage in the telomere is likely to be related to the genome-wide level of damage incurred. This observation has led to the suggestion that the sensitivity of telomeres to oxidative damage has functional significance, enabling telomeres to act as ‘sentinels’ of damaged cells, triggering their removal [14].
(b). Oxidative stress at the organismal level
The evidence that oxidative stress exposure has an important effect on telomere length studied at the individual levels is more mixed than the results from cell culture. Several correlative studies in whole animals show that individuals with increased exposure to oxidative stress show increased telomere loss [18]. However, these studies have generally not manipulated oxidative stress directly but have compared individuals found with different toxin levels or in different environmental conditions. For example, telomere loss in elderly humans over a 10 year period was found to be positively related to levels of persistent organic pollutants in their blood at the start of the study, including oxychlordane, a widespread pesticide [49]. However, the variation in pollutant levels might well be correlated with other lifestyle factors that have induced differences in telomere loss. Similarly, levels of oxychlordane circulating in the blood of a long-lived seabird, the kittiwake Rissa tridactyla, were found to be negatively related to red blood cell telomere length in female birds [50]. Particularly interesting in this kittiwake study is that no relationship was found in the male birds, despite the plasma levels of pesticides being similar in both sexes. As the authors point out, many factors might underlie this sex difference, such as differences in the resilience of males and females due to differences in antioxidant defences, antioxidant deployment priorities or in the ages of the male and female birds examined. It is difficult to control potentially confounding variables in the field, and this kind of inter-individual variation in behaviour and life-history priorities might explain the inconsistent results in correlative studies at the organism level, especially where they are done in the wild.
Rather than relating telomere length to pro-oxidant chemical exposure, some studies have examined the relationship between actual measures of oxidative stress and telomere length or loss. For example, a positive association between oxidative damage (measured by circulating levels of hydrogen peroxides (the d-ROMS test) and telomere loss in red blood cells has been reported in king penguin chicks Aptenodytes patagonicus [51]. However, in a similar study on jackdaw Corvus monedula chicks, using a number of markers including d-ROMs, found no relationship with telomere loss [52]. The difference between these two avian studies, both of which involved telomere measurement in red blood cells of growing chicks and oxidative stress markers in plasma, might relate to species differences in the level of telomere restoration, in antioxidant defences, or in the way, and time points at which, the markers were measured. Neither study involved any experimental manipulation of environmental conditions; both used naturally generated variation in oxidative stress, which might covary with many other individual differences.
More detailed experimental studies in rats involving maternal dietary manipulation during pregnancy have also related measures of oxidative damage to telomere loss. Maternal protein restriction during pregnancy followed by accelerated pup post-natal growth during the lactation period has been associated with shorter telomere length (table 1) and indicators of oxidative stress in a wide range of tissues in the offspring including pancreatic islets [60], the heart [59], aorta [82], kidney [62], uterine tract [58] and skeletal muscle [56]. These studies demonstrate that telomere shortening is accompanied by oxidative stress as a consequence of a suboptimal early environment. They do not, however, give insight as to whether there is a causal relationship.
Table 1.
Examples of studies of the relationship between post-natal growth parameters and telomere length and/or loss in a range of vertebrate taxa. GH, growth hormone; SDS, standard deviation score; d, day; PCA, principal component analysis; cort, corticosterone.
| species | field/lab | exp/corr | growth manipulation | growth measurements | telomere measurement points | tissue | telo method | telo length or change | relationship between growth and telomere measurements | reference |
|---|---|---|---|---|---|---|---|---|---|---|
| mammal Homo sapiens |
n.a. | corr + exp | GH treatment in childhood | birth length and weight SDS + adult height and weight SDS | 17 and 24 years | leucocytes | qPCR | length | not significant for birth + adult measurements and GH treatment | Smeets et al. [53] |
| mammal Homo sapiens |
n.a. | corr | n.a. | mass and length at birth and 11 years | Ca 60 and 70 years | leucocytes | qPCR | length and change over 10 years in late life | weight gain in first 12 months: −ve TL and + with loss between 60 and 70 years | Guzzardi et al. [54] |
| mammal Ovis aries |
field | corr | n.a. | horn length in males at 4 months | 4 months | leucocytes | qPCR | length | −ve between horn length and TL at 4 months | Watson et al. [55] |
| mammal Rattus norvegicus Wistar |
laboratory | exp | maternal diet | 3, 7, 14, 21 d and 12 months | 12 months | skeletal muscle | TRF Southern blot | length | −ve for growth 3–21 d | Tarry-Adkins et al. [56] |
| mammal Eliomys quercinus | laboratory | corr | n.a. | weekly mass 6–10 weeks | 6 and 10 weeks | buccal swab | qPCR | change | not significant | Giroud et al. [57] |
| mammal Rattus norvegicus Wistar |
laboratory | exp | maternal diet | 3, 7, 14 d, 3 months, 6 months | 3 and 6 months | oviduct | Southern blot | length | −ve for growth 3–14 d | Aiken et al. [58] |
| mammal Rattus norvegicus Wistar |
laboratory | exp | maternal diet | 3, 7, 14, 21 d, 3 and 12 months | 3 months and 12 months | heart | Southern blot | length | −ve for growth 3–21 d | Tarry-Adkins et al. [59] |
| mammal Rattus norvegicus Wistar |
laboratory | exp | maternal diet | 3, 7, 14, 21 d, and 3 months | 3 months | pancreatic islets | Southern blot | length | −ve for growth 3–21 d | Tarry-Adkins et al. [60] |
| mammal Rattus norvegicus Wistar |
laboratory | exp | maternal diet | 3, 7, 14, 21 d, and 12 months | 12 months | aorta | Southern blot | length | −ve for growth 3–21 d | Tarry-Adkins et al. [61] |
| mammal Rattus norvegicus Wistar |
laboratory | exp | maternal diet | 3, 7, 14 and 21 d | 13 months | kidney | Southern blot | length | −ve for growth 3–21 d | Jennings et al. [62] |
| bird Rissa tridactyla |
field | exp | brood size increase and food supp | 9 and 25 d, g/d mass mm/d tarsus and wing | 9 and 25 d post-hatch | RBC | TRF in-gel | proportional change in TL | +ve for wing growth | Young et al. [24] |
| bird Parus major |
field | corr | n.a. | mass and size (PCA on wing, tarsus and head) 2 d intervals hatching to fledging at 17 d | 7 and 16 d post-hatch | RBC | qPCR | change 7–16 d | −ve for body size in last hatched nestlings but not first; NS for body mass | Stier et al.
[63] |
| bird Hirundo rustica |
field | corr + exp | brood size increased and reduced | 12 d mass and tarsus | 12 d | RBC | qPCR | length | not significant for treatment, mass or tarsus | Costanzo et al. [64] |
| bird Sterna hirundo |
field | corr | n.a. | 3 and 18–22 d mass | 3 and 18–22 d | RBC | TRF in-gel | length + change 5–20 d | not significant | Vedder et al. [65] |
| bird Hirundo rustica |
field | corr | n.a. | 7 and 16 d tarsus length and mass; 16 d wing and tail length | 7 and 16 d | RBC | qPCR | length | not significant for tarsus at 7 and 16 d; NS for mass at 16 d; +ve for wing and tail length at 16 d | Parolini et al. [66] |
| bird Passer domesticus |
field | corr | n.a. | tarsus, bill, wing length, mass at 9 d | length | RBC | qPCR | length | not significant | Meillere et al. [67] |
| bird Taeniopygia guttata |
laboratory | exp | brood size increased and reduced | daily mass 0–30 d | 10 and 30 d | RBC | qPCR | change | treatment effect but mass not related to telomere length | Reichert et al. [68] |
| bird Taeniopygia guttata |
laboratory | exp/corr | dietary antioxidants | mass at hatching 20 and 40 d | 20 and 40 d | RBC | qPCR | length and change | no treatment effect of growth or TL; −ve between TL at 40 d and mass | Noguera et al. [69] |
| bird Sturnus vulgaris |
field | exp/corr | position in brood hierarchy | 3, 4, 7 and 12 d mass | 3 and 12 d | RBC | qPCR | length | no treatment effect on growth; not significant bet mass growth and TL | Nettle et al. [70] |
| bird Corvus monedula |
field | exp | brood size increased and reduced | fledging mass | 5 and 30 d | RBC | TRF-in-gel | length and change | not significant in reduced broods; −ve bet TL change and fleding mass in enlarged brood | Boonekamp et al. [35] |
| bird Phalacro-corax aristotelis |
field | exp/corr | cort treatment daily 10–29 d | daily mass gain 10–30 d | 10 and 30 d | RBC | qPCR | length and change | no treatment effect on growth; −ve between growth rate and 30 d TL | Herborn et al. [71] |
| bird Taeniopygia guttata |
laboratory | exp | maternal treatment with oestradiol pre and during laying | mass at hatching, 10, 20 and 30 d | 10, 20 and 30 d | RBC | qPCR | change | treatment increased growth in male chicks; no effect on telomere change | Tissier et al. [72] |
| bird Ficedula albicollis |
field | exp | brood size increased and reduced | mass and tarsus | 12 d | 12 d | qPCR | length | heavier nestlings in reduced broods; NS effect on tarsus or TL | Voillemot et al. [73] |
| bird Phalacro-corax aristotelis |
field | corr | n.a. | mass | ca 15 days | RBS | TRF- Southern blot | change | +ve relationship between growth rate and loss | Hall et al. [74] |
| amphibian Delobates cultripes |
laboratory | exp | pond drying and predator exposure in tadpoles from 2 months to metamorphosis | average mass gain per family during treatment | at metamorphosis | leg muscle | qPCR | length | pond drying reduced growth, inc predatory exposure inc growth; weak –ve corr bet growth rate and TL | Burraco et al. [75] |
| fish Pungitius pungitius |
laboratory | exp | temperature during growth | length weekly from 17 to 115 d | 122 d | brain | qPCR | length | no temperature of length effect on TL | Noreikiene et al. [76] |
| fish Salmo salar |
field | corr + exp | harshness of post-natal growth environment | mass at fry stage | fry stage at ca 5 months | whole body | qPCR | length | −ve for mass at 5 months in both groups; exp—shorter TL when growing in harsher enviros | McLennan et al. [77] |
| fish Salmo trutta |
field | exp and corr | at 1 year food deprived for ca three weeks to induce compensatory growth | mass and length at start of treatment 1 year and at 2 years | 1 and 2 years | pelvic Fin TL | qPCR | change 1–2yrs | exp—not significant treatment effect; corr - +ve for mass-specific growth 1–2 years | Naslund et al. [78] |
| fish Oncorhyn-chus kisutch |
laboratory | exp | transgenic—manipulation of GH to give fish 54× heavier and 7× longer than wild-type | mass and fork length at 7 and 10 months | 7 and 10 months | peliv fin | qPCR | length and change | transgenics had longer length but lost more during growth; wild-type showed no change | Pauliny et al. [79] |
| fish Cyprinus carpio |
field | n.a. | mass and fork length at capture | at capture | muscle and caudal fin | qPCR | length | +ve bet TL in muscle and fork length; NS for fin | Izzo et al. [80] | |
| fish Oryzias latipes |
laboratory | corr | n.a. | 0 and 7 months body length | repeated | whole body and other tissues | TRF Southern blot | change | faster loss during period of rapid growth | Hatakeyama et al. [81] |
(c). Effects of antioxidants
If oxidative stress is an important contributor to telomere loss, then improving antioxidant capacity should reduce telomere loss and thereby help address causality. Administration of antioxidants to cultured cells does reduce telomere loss [14,15,28]. Similarly, antioxidant capacity in whole organisms has been linked to reduced telomere loss in both correlative and experimental studies [18,28]. However, conflicting results have also been reported. For example, Badas et al. [83] gave wild adult blue tits Cyanistes caeruleus an antioxidant supplement (vitamin E and methionine) while they were rearing their chicks in 2012. The birds were then recaptured in 2013, again during chick rearing. All birds showed telomere loss between 2012 and 2013, but the decline was less in the birds that had the antioxidant supplement during breeding in the previous year. By contrast, Noguera et al. [69] found no difference in telomere loss between chicks of captive zebra finches Taeniopygia guttata growing on high and low antioxidant diets. The difference between these studies may be related to species differences, differences between adults and chicks, variation in background dietary antioxidants, whether or not the supplement actually increases antioxidant capacity, prenatal levels or stored levels of antioxidants, the relative importance of endogenous versus exogenous antioxidants at different life stages and so on. In rats, post-weaning studies involving dietary supplementation with Coenzyme Q (ubiquinone, one of the most abundant antioxidants in vivo, present in the inner mitochondrial membrane) have demonstrated that supplementation prevented the early nutrition-induced changes in telomere length in both the heart and the aorta [59]. An alternative, but complementary, approach to studying oxidative stress and telomere dynamics examined variation in telomere length in relation to polymorphisms in genes known to be linked to oxidative stress and biomarkers of ageing [84]. While this involved a group of 79-year-old humans, which may in itself represent a biased group, the study found an association in the expected direction, and provides supporting evidence that cellular redox status has an important effect on telomere loss.
Differences in the deployment of antioxidants among individuals are also likely to be very important in organismal-level studies. For example, Noguera et al. found that antioxidant supplementation reduced telomere loss during sexual maturation [69] in females but not in males. This probably reflects a preferred allocation of these antioxidants to sexual coloration rather than oxidative defence in males. Kim & Velando [85] found that antioxidants can offset the increased telomere loss found in ‘bolder’ gull chicks in the wild, and suggested that this occurred because these chicks are exposed to more oxidative stress as a result of differences in their behaviour relative to the less-bold chicks.
The problem with all of the above studies at the organismal level is that, even when individuals are randomly allocated to treatment groups, it is very difficult to manipulate oxidative stress exposure without also affecting other factors. Multiple systems can be affected when individuals are exposed to oxidative stress, and compensatory effects triggered that are likely to protect some systems at the expense of others. How these multifaceted effects work is likely to vary with species, individual experience and life-history stages, and it is very difficult to design experiments at the organismal level that tease these effects apart. Further, these complex physiological and molecular interactions mean that studies in cell culture might not give the same results as studies in whole organisms. Both are required for pathways to be identified and outcomes understood. Mitochondrial functioning is likely to be very important, and ROS generation could potentially be increased or decreased at the organismal level using manipulations such as genetic interventions, and administration of compounds that affect uncoupling proteins [86], but potential co-lateral toxicity effects of these compounds need to be evaluated.
More studies are needed to help clarify whether, and under what circumstances, what we see when oxidative stress is generated in cultured cells actually mirrors what occurs at the organismal level. Furthermore, while the effect of oxidative stress on telomere loss is the most studied, and clearly an important, route of environmentally generated damage, but we should not expect that all environmental stressors act on telomere length via oxidative stress. The nature of the stressor might also matter. For example, an experimental study in which individuals were or were not exposed to social stress by altering their position in the brood hierarchy found that chicks of wild starlings Sturnus vulgaris placed in a subordinate position in a foster brood showed more telomere loss than their siblings that were placed in dominant positions in foster broods [70]. However, there was no difference in oxidative damage between groups (measured in this case via lipid peroxidation). This does not tell us that oxidative stress is not involved in telomere loss, but rather that the source of the experimentally generated telomere difference, which related to a manipulation of social stress, was not via experimentally generated differences in oxidative stress.
4. Growth and telomere dynamics
(a). Telomeres and trade-offs
Most organisms appear to be capable of growing at a much faster rate than they generally do, and growth is expected to be optimized via a number of life-history trade-offs [1,87]. In life-history theory, trade-offs are most often viewed in the context of the allocation of limited resources to competing traits. So, resources allocated to growth might be at the expense of resources allocated to self-maintenance and thereby longevity. This might involve energy allocation to cell proliferation versus energy allocated to telomere maintenance, restoration or protection from oxidative damage. We know little about the resource costs of telomere maintenance. However, resource independent trade-offs can also occur. For example, inevitable downstream or co-lateral consequences of a particular process during growth could affect longevity. With respect to telomere loss, a trade-off could occur between, for example, high cell proliferation levels needed to grow to a particular size, and the downstream consequences for cell (and organism) senescence of the resultant pace of telomere loss, which would occur irrespective of resource availability. This non-resource dependent trade-off may well be the route by which telomeres are involved in a growth-lifespan trade-off. If so, we would expect to see such a positive relationship between growth and telomere loss (or negative relationship with telomere length) even in correlative studies since experimental deflection of individuals from their expected resource allocations is not required.
(b). Growth and telomeres
All individuals produced by sexual reproduction start life as a single cell. Growth then occurs via increases in cell size and/or cell number [88,89]. In general, homeostatic mechanisms maintain cell number and size within individuals in adulthood, thereby preserving organ size and function [89]. Variation in cell size among species, individuals and tissue types within individuals, has all been reported [90]. However, cell size does not vary to a sufficient extent to account for the large variations that we see among species in body size; bigger bodies in principle mean more cell division. This need not translate into more telomere loss, however, since this will depend on restoration processes, which may be driven by other factors such as tumour risk [91]. There has so far been little attempt to examine cell proliferation rates in relation to telomere loss in vivo. During the period when most body growth is taking place, cell division rates tend to be higher than at other life stages. This could select for longer initial telomere length, but there may be costs associated with this, such as slowing of the cell cycle and/or increased risk of telomere damage. There is evidence that loss rate is higher in longer chromosomes [92], possibly due to their presenting a bigger target for damage to occur [46]. Little is known about telomere length regulation during embryonic stages; it appears that telomere length is shorter in oocytes but, following fertilization, lengthens during early cleavage, after which a ‘set point’ is established [23,24]. However, it is also clear that telomere length at birth is influenced by environmental conditions during development [93–95], and much more work is needed to understand the processes involved.
There are at least two routes whereby more or faster post-natal growth could lead to shorter telomere length—increased cell division required to attain larger size, or increased loss per round of cell division as a consequence of the conditions required to sustain fast growth, or created by it. These two routes are not mutually exclusive and indeed could act in concert; the increased cell division rate could give rise to increased oxidative stress due to the higher metabolic activity needed to generate more ATP to fuel this growth. A number of correlative and experimental studies have found that relatively fast growth is associated with higher levels of oxidative stress markers in both laboratory and field studies [61,96,97], and a recent meta-analysis has demonstrated that there is good evidence that faster growth incurs increased oxidative damage, and that this may constrain growth strategies [98]. The context in which growth takes place will therefore be expected to influence the level of oxidative stress that occurs. Thus body size, growth rate and environmental conditions are likely to matter in the context of telomere dynamics, and we consider these further below.
An additional complexity is brought by the fact that the pattern of growth can vary considerably among taxa, most notably between determinate and indeterminate growers which relates to the degree of genetic determination of growth [99]. The typical growth pattern of determinate growers involves growth to an asymptote with limited environmental input to final size [99]. Indeterminate growth generally involves a high environmental input, and considerable variation in body size, and in many cases growth throughout life. The life history of determinate growers, such as birds and most mammals, is that growth to a final body size takes place relatively early in life and prior to sexual maturity, after which relatively little growth takes place. There are important differences among the typical avian and mammalian pattern in that in birds, growth as a nestling is generally very rapid and final body size is achieved by fledging or fairly soon afterwards. There will then be a variable period before reproduction occurs, which in some species, such as the seabirds, can stretch into several years. In mammals on the other hand, growth usually continues till sexual maturation, and there can be a series of further growth ‘spurts’ during adolescence; whether these growth spurts affect telomere dynamics has not been studied.
Significant variation in growth rate occurs within species because of genetic and environmental variation (e.g. factors such as conditions during embryonic growth, birth or hatching order, time of season, temperature and resource availability), whereas variation in body size is often more limited. There is good evidence from a fairly wide range of species that the rate of telomere loss is greatest during early life [33,100] and correlative studies in birds [51,69,71,74] and fish [101] suggest that faster growth is associated with reduced telomere length measured either during growth itself or in adulthood. However, not all studies find such a relationship [24]. There is as yet no real consistency in how studies of the relationship between growth and telomere length have been carried out, and growth rate and final size are not often teased apart. This is in part because the effect of growth on telomere loss is often a secondary consideration in studies that have been designed to examine the effects of other factors. Table 1 provides a summary of vertebrate studies in which post-natal growth and telomere loss have been examined. While not completely exhaustive, table 1 gives a good indication of what has been done and the approaches used in vertebrates so far. Of the 31 studies listed, most have been in birds (14 studies, 10 species, 11 in the field) and mammals (10 studies, four species, one in the field); thus the taxonomic coverage is relatively poor, with few studies of indeterminate growers (seven studies involving one amphibian and six species of fish). Ideally, studies of the relationship between growth and telomere loss should involve measurements of telomere change within individuals over the most rapid growth period. However, since we might expect that growth rate to have evolved to minimize detrimental effects later in life, we also need experimental manipulations of growth that induce individuals to grow at different rates to the same final body size, and do not involve inducing other factors known to accelerate telomere loss such as stress exposure. In some studies single measures of telomere length are taken, perhaps involving comparisons across stages or treatment groups. In the case of the two human studies, the telomere data have been collected many years after the main growth period, and thus do not relate to the period of most rapid growth. Many of the non-human studies are correlational, and thus will involve a number of confounding factors. Experimental studies have been carried out notably with laboratory rats, and in several bird species and some fish. With respect to the birds, in which most studies have been done so far, the results are mixed. Of the 14 studies listed, over half find non-significant effect, while three studies find positive and three negative relationships between telomere length or loss and growth measurements. In practice, it is very difficult to manipulate growth rate without affecting other processes. A commonly used experimental procedure in birds has involved manipulation of brood size, with chicks growing in enlarged broods being expected to grow more slowly, which is generally found to be the case. However, chicks in enlarged broods are in a more competitive situation, which in itself is known to increase telomere loss even when growth is not affected [70]. The extent to which stress exposure over-rides the effect of growth may underlie the inconsistences. Genetic and hormonal manipulations to date have been limited, and there is more scope for undertaking such studies, provided the effect of body size can be teased apart from growth rate. More studies of indeterminate growers are needed, particularly given that environmental temperature can be used to induce different growth rates. To date the most comprehensive studies have been undertake in laboratory rats, in which experimental manipulations of maternal diet have been used to induce variations in growth rate in offspring, with clearly demonstrated effect of telomere length. We discuss these and aspects other studies in more detail below.
(c). Body size
In practice, it is difficult to tease apart body size and growth rate, since the two are generally interlinked. In cross species comparisons, large-bodied animals tend to live longer than smaller bodied ones, but within species the opposite is the case, with smaller bodied individuals generally living longer than their larger bodied counterparts [2,102,103]. However, large species generally grow more slowly than small-bodied species, which could mean less oxidative stress during growth. Accordingly, we may not see the expected relationship between body size and telomere loss during growth when looking across species. By contrast, larger individuals of the same species appear to grow faster than their smaller conspecifics [2,104,105]. Positive effects of slow, and negative effects of fast, growth on the rate of ageing might in part explain the different relationship between body size and longevity seen in the among and within-species comparisons. Furthermore, dietary differences among species are also likely to affect outcomes since these could affect metabolism and antioxidant status. There have so far been no comparative studies that examine variation in species growth rates and telomere loss, and how these link to body size and life histories. Thus, there is considerable scope for further work in this area.
Within species, we would expect the larger, faster growing individuals to have shorter telomeres. In species where males are larger than females, the males often have shorter telomeres and shorter lives, while there is no sex difference in telomere length in monomorphic species; however, factors other than body size may drive this sex difference [106,107]. Understanding the relationship between body size and telomere dynamics within species is complicated by the fact that individuals may be small due to poor nutritional or social conditions during growth [108]; both of these factors can accelerate telomere loss but not necessarily via generating oxidative stress [16,70,71,109].
An experimental study in which artificial selection for body size was imposed on a wild population of house sparrows Passer domesticus suggested that, within species, the relationship between size and telomere loss goes in the predicted direction [110]. However, since no detailed information on post-natal growth rate was collected during this study, it is not known how growth rate and body size were linked, or whether the observed effect on telomeres persisted beyond the nestling phase. Nonetheless, this study does provide a platform on which to base further studies of the relationship between size, growth and telomere dynamics, and the underlying genetic relationships among these traits.
(d). Experimental studies in rats
There is strong evidence from a range of taxa to suggest that changes in growth and nutrition during critical periods of development can impact on the long-term health of an organism including humans. This has been termed the developmental origins of health and disease [2]. It has been demonstrated in both correlative and experimental studies that this is linked to growth rate and that growth acceleration to compensate for an episode of reduced growth either pre or post-natally is associated with reduced lifespan [1,111]. Detailed studies of the effect of accelerated growth in rats have been undertaken from a biomedical perspective, in order to shed light on the processes whereby early life growth and nutrition might influence long-term heath. These studies have shown that low birth weight, especially when followed by accelerated neonatal growth, is associated with increased risk of traditionally adult-onset diseases such as type 2 diabetes and cardiovascular disease [112,113]. By contrast, slow growth during the lactation period is associated with protection from these conditions [114]. Reduced nutrition and/or growth during these critical periods is also associated with permanent differences in body size and composition [115]. Accelerated early post-natal growth with or without low birth weight is associated with increased body weight and adiposity whereas slow growth during this time period is associated with a permanent reduction in body weight and reduced adiposity [116]. Both rats and mice that are exposed to maternal protein restriction during foetal life have a low birth weight and undergo rapid catch-up growth if suckled by a normally fed mother; these animals have a significant reduced lifespan compared to offspring of mothers fed a control diet during pregnancy and lactation. By contrast, pups born with a normal birth weight but suckled by a low protein fed mother, grow slowly during lactation and never catch up in body weight even when weaned onto standard chow fed ad libitum [62,117,118]. These differences in lifespan have been associated with differences in telomere length (table 1) [56,60,61,119]. As mentioned earlier, the pups that have undergone faster growth display reduced telomere length compared to controls in many tissues. Interestingly, the timing of the presence of shortened telomeres differs between different tissues, with differences in telomere lengths in pancreatic islets and the reproductive tract being present in young adult life and other tissues such as the aorta not displaying accelerated shortening until later in life. The different time courses observed between tissues in terms of maternal diet-induced telomere shortening may relate to differences in the number of rounds of cell division that different tissues undergo post-natally and/or differences in levels of oxidative stress. Pancreatic islets are known to have a low antioxidant defence capacity that thus may explain their particular vulnerability to maternal diet effects on telomere length. By contrast, pups exposed to the low protein diet during lactation display increase telomere length compared to controls, especially in their kidneys, of note since kidney disease is thought to a common cause of death in laboratory rodents. Recent studies have demonstrated that it is not just exposure to suboptimal nutrition during foetal life that can impact on telomere length. Exposure to hypoxia during foetal life also led to accelerated telomere shortening [120]. These detailed experimental studies illustrate the complexity of the relationship between growth, long-term health and telomere dynamics and emphasize the fact that there may be tissue-specific responses.
(e). The context in which growth occurs
A further problem is that, in correlative studies where telomere length and loss are compared in individuals observed to be growing at different rates, the outcome may be confounded by differences in the environmental conditions they are experiencing, including the social environment as well as nutrition and stress exposure. Depending on the importance of these factors in generating adverse environmental conditions, two different predictions are possible here—(i) that faster growing individuals will have relatively shorter telomeres as a result of more cell division and/or oxidative stress exposure or (ii) that faster growing individuals will have longer telomeres since the faster growth indicates better environmental conditions and less exposure to hormonal or oxidative stress. Where animals in naturally occurring broods are used, these differences will be very important since brood size will be positively related to environmental conditions and parental quality. But even in experimental studies where food is ad libitum, social conditions can generate adversity for at least some individuals. This complexity is illustrated in the study by Reichert et al. (table 1) who found that the chicks in experimentally reduced broods of captive zebra finches grew faster. However, these showed less oxidative damage and had longer telomeres at the end of the growth period compared to those in enlarged broods; the latter grew more slowly, but the increased provisioning burden on their parents meant that the growth conditions more stressful. In the field study on great tits Parus major, the relationship between growth and telomere length was found to be negative in the last hatched chicks in broods, but there was no relationship in the first hatched chicks (table 1). The latter generally experience better conditions and may also have hatched from higher quality eggs. In the experimental study in the wild by McLennan et al. (table 1), early stage Atlantic salmon Salmo salar eggs from the same families were released into relatively benign and harsh growth conditions [77]. All fish in this study showed a negative relationship between telomere length and growth; fish growing in the harsh streams grew more slowly, but, for the same amount of body growth, showed a higher telomere loss than the fish from the same families that had been released into the benign streams. These studies clearly show that, as expected, the conditions under which growth occurs have important consequences for the effect of growth rate on telomere loss.
(f). Might telomere restoration during growth mitigate longer term effects?
The elephant in the room in all of the above studies is that we know little about telomere restoration during growth, and this is also likely to vary across taxa and with environmental conditions. Telomerase activity has been found to increase under chronic stress in rats for example [121]. However, this has been little studied in the context of growth conditions. There is also evidence that the degree of somatic telomerase activity differs between endothermic and ectothermic vertebrates, and probably also among other taxa [4]. Many ectotherms continue to grow throughout life, and also show somatic telomerase activity throughout life. This may explain why in red-sided garter snakes Thamnophis sirtalis for example [122], no relationship between telomere length and age has been found in either sex, and no difference between young and adult animals in leather-backed turtles Dermochelys coriacea [123]. In zebra fish Danio rerio, there is also apparently no telomere loss with age [124], but in other fish species such as the Atlantic salmon, age-related loss does occur [77] presumably because the telomerase activity cannot fully compensate for the telomere loss. A recent analysis of the literature of telomere changes in fish found that only around half of the studies so far have reported age-related declines in telomere length [125]. In some species, telomerase activity appears to vary at different life stages. An interesting illustration of this is provided in a study on growth and telomere dynamics in a small fish species, the medaka Oryzias latipes [126]. In this species, growth is at its maximal rate for the first seven months of life; telomeres decline during this time and telomerase activity is also low. Then, during adolescence, from seven months to 1 year, growth slows, telomerase activity increases and telomere length increases. After 1 year, little further growth occurs, telomerase activity drops and telomere length declines. As is the case with most studies on small-bodied animals, these measurements are based on whole body measures of telomere length, and are therefore cross sectional. It would be interesting to see if the same pattern holds within individuals. Whether such variation in telomerase activity occurs in other taxa is currently unknown.
5. Conclusion
What happens during the foetal and post-natal growth period can set the stage for later life telomere length, and thereby influence health and longevity. More experimental and correlative studies on the relationship between growth rate, body size, telomere dynamics and exposure to different environmental conditions, in a broad range of taxa, are needed. We still know little about the effect of key factors such as cell proliferation rates on telomere loss, and how these effects vary among species, tissues and life stages. Much more work is needed on variation in telomerase activity at every level, and this would be particularly useful in taxa such as birds where a great deal of work has recently been done on telomere length and loss, but little on telomerase and telomere restoration. It is not surprising that there are inconsistences among studies in the nature of the link between growth and telomere loss given the number of potentially confounding variables, the differences in priorities among species with different lifespan potentials, and the differences in the pattern of growth. There are considerable challenges associated with studying telomere dynamics in non-model organisms where the toolkit available is much reduced and conditions in the field and laboratory more difficult to control. However, many of the pathways involved in the vertebrates are highly conserved, and their operation is likely to vary in a predictable way with species life histories. Therefore, combining studies in more tractable species and in cell culture with targeted studies in other taxa has the potential to yield considerable insights.
Acknowledgements
We thank Neil Metcalfe, two referees and the handling editor for comments on an earlier draft of this paper.
Data accessibility
This article has no additional data.
Authors' contributions
Both authors were involved in the planning and writing this manuscript. P.M. took the lead in the more life-history–oriented studies, and S.E.O. in the more biomedical-oriented studies.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260. ( 10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 2.Metcalfe NB, Monaghan P. 2003. Growth versus lifespan: perspectives from evolutionary ecology. Exp. Gerontol. 38, 935–940. ( 10.1016/S0531-5565(03)00159-1) [DOI] [PubMed] [Google Scholar]
- 3.Hector KL, Nakagawa S. 2012. Quantitative analysis of compensatory and catch-up growth in diverse taxa. J. Anim. Ecol. 81, 583–593. ( 10.1111/j.1365-2656.2011.01942.x) [DOI] [PubMed] [Google Scholar]
- 4.Gomes NM V, Shay JW, Wright WE. 2010. Telomeres and telomerase. In The comparative biology of aging (ed. Wolf NS.), pp. 227–258. New York, NY: Springer. [Google Scholar]
- 5.Aubert G, Lansdorp PM. 2008. Telomeres and aging. Physiol. Rev. 88, 557–579. ( 10.1152/physrev.00026.2007) [DOI] [PubMed] [Google Scholar]
- 6.van Deursen JM. 2014. The role of senescent cells in ageing. Nature 509, 439–446. ( 10.1038/nature13193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickett HA, Reddel RR. 2009. Alternative Lengthening of Telomeres in Human Cells, pp. 127–148.
- 8.Apte MS, Cooper JP. 2017. Life and cancer without telomerase: ALT and other strategies for making sure ends (don't) meet. Crit. Rev. Biochem. Mol. Biol. 52, 57–73. ( 10.1080/10409238.2016.1260090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardue M-L, DeBaryshe PG. 2011. Retrotransposons that maintain chromosome ends. Proc. Natl Acad. Sci. USA 108, 20 317–20 324. ( 10.1073/pnas.1100278108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tricola GM, et al. 2018. The rate of telomere loss is related to maximum lifespan in birds. Phil. Trans. R. Soc. B 373, 20160445 ( 10.1098/rstb.2016.0445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilbourn RV, Moatt JP, Froy H, Walling CA, Nussey DH, Boonekamp JJ. 2018. The relationship between telomere length and mortality risk in non-model vertebrate systems: a meta-analysis. Phil. Trans. R. Soc. B 373, 20160447 ( 10.1098/rstb.2016.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young AJ. 2018. The role of telomeres in the mechanisms and evolution of life-history trade-offs and ageing. Phil. Trans. R. Soc. B 373, 20160452 ( 10.1098/rstb.2016.0452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dugdale HL, Richardson DS. 2018. Heritability of telomere variation: it is all about the environment! Phil. Trans. R. Soc. B 373, 20160450 ( 10.1098/rstb.2016.0450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Zglinicki T. 2002. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344. ( 10.1016/s0968-0004(02)02110-2) [DOI] [PubMed] [Google Scholar]
- 15.Houben JMJ, Moonen HJJ, van Schooten FJ, Hageman GJ. 2008. Telomere length assessment: biomarker of chronic oxidative stress? Free Radical Biol. Med. 44, 235–246. ( 10.1016/j.freeradbiomed.2007.10.001) [DOI] [PubMed] [Google Scholar]
- 16.Haussmann MF, Marchetto NM. 2010. Telomeres: Linking stress and survival, ecology and evolution. Curr. Zool. 56, 703–713. [Google Scholar]
- 17.Monaghan P. 2014. Organismal stress, telomeres and life histories. J. Exp. Biol. 217, 57–66. ( 10.1242/jeb.090043) [DOI] [PubMed] [Google Scholar]
- 18.Glade MJ, Meguid MM. 2015. A glance at … telomeres, oxidative stress, antioxidants, and biological aging. Nutrition 31, 1447–1451. ( 10.1016/j.nut.2015.05.018) [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, et al. 2016. Ageing and the telomere connection: an intimate relationship with inflammation. Ageing Res. Rev. 25, 55–69. ( 10.1016/j.arr.2015.11.006) [DOI] [PubMed] [Google Scholar]
- 20.Angelier F, Costantini D, Blevin P, Chastel O. 2017. Do glucocorticoids mediate the link between environmental conditions and telomere dynamics in wild vertebrates? A review. Gen. Comp. Endocrinol. 26, 3572–3584. ( 10.1016/j.ygcen.2017.07.007) [DOI] [PubMed] [Google Scholar]
- 21.von Zglinicki T. 2000. Role of oxidative stress in telomere length regulation and replicative senescence. Mol. Cell. Gerontol. 908, 99–110. [DOI] [PubMed] [Google Scholar]
- 22.Pickett HA, Reddel RR. 2012. The role of telomere trimming in normal telomere length dynamics. Cell Cycle 11, 1309–1315. ( 10.4161/cc.11.7.19632) [DOI] [PubMed] [Google Scholar]
- 23.Liu L, et al. 2007. Telomere lengthening early in development. Nat. Cell Biol. 9, 1436 ( 10.1038/ncb1664) [DOI] [PubMed] [Google Scholar]
- 24.Young RC, Welcker J, Barger CP, Hatch SA, Merkling T, Kitaiskaia EV, Haussmann MF, Kitaysky AS. 2017. Effects of developmental conditions on growth, stress and telomeres in black-legged kittiwake chicks. Mol. Ecol. ( 10.1111/mec.14121) [DOI] [PubMed] [Google Scholar]
- 25.Lansdorp PM. 2005. Major cutbacks at chromosome ends. Trends Biochem. Sci. 30, 388–395. ( 10.1016/j.tibs.2005.05.004) [DOI] [PubMed] [Google Scholar]
- 26.Makarov VL, Hirose Y, Langmore JP. 1997. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell 88, 657–666. ( 10.1016/s0092-8674(00)81908-x) [DOI] [PubMed] [Google Scholar]
- 27.Jia P, Her C, Chai W. 2015. DNA excision repair at telomeres. DNA Repair 36, 137–145. ( 10.1016/j.dnarep.2015.09.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasad KN, Wu M, Bondy SC. 2017. Telomere shortening during aging: attenuation by antioxidants and anti-inflammatory agents. Mech. Ageing Dev. 164, 61–66. ( 10.1016/j.mad.2017.04.004) [DOI] [PubMed] [Google Scholar]
- 29.Monaghan P. 2010. Telomeres and life histories: the long and the short of it. Ann. NY Acad. Sci. 1206, 130–142. ( 10.1111/j.1749-6632.2010.05705.x) [DOI] [PubMed] [Google Scholar]
- 30.Bateson M. 2016. Cumulative stress in research animals: telomere attrition as a biomarker in a welfare context? Bioessays 38, 201–212. ( 10.1002/bies.201500127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benetos A, et al. 2013. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell 12, 615–621. ( 10.1111/acel.12086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichert S, Criscuolo F, Verinaud E, Zahn S, Massemin S. 2013. Telomere length correlations among somatic tissues in adult zebra finches. PLoS ONE 8, e0081496 ( 10.1371/journal.pone.0081496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, Desai K, Granick M, Aviv A. 2013. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat. Commun. 4, 1597 ( 10.1038/ncomms2602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson H, Bolton M, Monaghan P. 2015. Variation in early-life telomere dynamics in a long-lived bird: links to environmental conditions and survival. J. Exp. Biol. 218, 668–674. ( 10.1242/jeb.104265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boonekamp JJ, Mulder GA, Salomons HM, Dijkstra C, Verhulst S. 2014. Nestling telomere shortening, but not telomere length, reflects developmental stress and predicts survival in wild birds. Proc. R. Soc. B 281, 20133287 ( 10.1098/rspb.2013.3287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fairlie J, Holland R, Pilkington JG, Pemberton JM, Harrington L, Nussey DH. 2016. Lifelong leukocyte telomere dynamics and survival in a free-living mammal. Aging Cell 15, 140–148. ( 10.1111/acel.12417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allsopp RC, Chang E, Kashefiaazam M, Rogaev EI, Piatyszek MA, Shay JW, Harley CB. 1995. Telomere shortening is associated with cell division in-vitro and in-vivo. Exp. Cell Res. 220, 194–200. ( 10.1006/excr.1995.1306) [DOI] [PubMed] [Google Scholar]
- 38.Petersen S, Saretzki G, von Zglinicki T. 1998. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp. Cell Res. 239, 152–160. ( 10.1006/excr.1997.3893) [DOI] [PubMed] [Google Scholar]
- 39.Passos JF, Nelson G, von Zglinicki T. 2008. Telomeres, senescence, oxidative stress, and heterogeneity. In Telomeres and telomerase in ageing, disease, and cancer (ed. Rudolph KL.), pp. 43–56. Berlin, Germany: Springer. [Google Scholar]
- 40.Ludlow AT, Spangenburg EE, Chin ER, Cheng W-H, Roth SM. 2014. Telomeres shorten in response to oxidative stress in mouse skeletal muscle fibers. J. Gerontol. Ser A. 69, 821–830. ( 10.1093/gerona/glt211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarkar J, Liu Y. 2016. The origin of oxidized guanine resolves the puzzle of oxidation-induced telomere-length alterations. Nat. Struct. Mol. Biol. 23, 1070–1071. ( 10.1038/nsmb.3332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fouquerel E, Opresko PL. 2017. Convergence of the nobel fields of telomere biology and DNA repair. Photochem. Photobiol. 93, 229–237. ( 10.1111/php.12672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hewitt G, et al. 2012. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 3, 708 ( 10.1038/ncomms1708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun L, et al. 2015. Targeted DNA damage at individual telomeres disrupts their integrity and triggers cell death. Nucleic Acids Res. 43, 6334–6347. ( 10.1093/nar/gkv598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fumagalli M, et al. 2012. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol. 14, 355 ( 10.1038/ncb2466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Victorelli S, Passos JF. 2017. Telomeres and cell senescence - size matters not. EBioMedicine 21, 14–20. ( 10.1016/j.ebiom.2017.03.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saretzki G, Murphy MP, von Zglinicki T. 2003. MitoQ counteracts telomere shortening and elongates lifespan of fibroblasts under mild oxidative stress. Aging Cell 2, 141–143. ( 10.1046/j.1474-9728.2003.00040.x) [DOI] [PubMed] [Google Scholar]
- 48.Kawanishi S, Oikawa S. 2004. Mechanism of telomere shortening by oxidative stress. In Strategies for engineered negligible senescence: why genuine control of aging may be foreseeable (ed. DeGrey ADN.), pp. 278–284. [DOI] [PubMed] [Google Scholar]
- 49.Guzzardi MA, Iozzo P, Salonen MK, Kajantie E, Airaksinen R, Kiviranta H, Rantakokko P, Eriksson JG. 2016. Exposure to persistent organic pollutants predicts telomere length in older age: results from the Helsinki birth cohort study. Aging Dis. 7, 540–552. ( 10.14336/ad.2016.0209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blevin P, et al. 2016. Exposure to oxychlordane is associated with shorter telomeres in arctic breeding kittiwakes. Sci. Total Environ. 563, 125–130. ( 10.1016/j.scitotenv.2016.04.096) [DOI] [PubMed] [Google Scholar]
- 51.Geiger S, Le Vaillant M, Lebard T, Reichert S, Stier A, Le Maho Y, Criscuolo F. 2012. Catching-up but telomere loss: half-opening the black box of growth and ageing trade-off in wild king penguin chicks. Mol. Ecol. 21, 1500–1510. ( 10.1111/j.1365-294X.2011.05331.x) [DOI] [PubMed] [Google Scholar]
- 52.Boonekamp JJ, Bauch C, Mulder E, Verhulst S. 2017. Does oxidative stress shorten telomeres? Biol. Lett. 13, 20170164 ( 10.1098/rsbl.2017.0164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smeets CCJ, Codd V, Denniff M, Samani NJ, Hokken-Koelega ACS. 2017. Effects of size at birth, childhood growth patterns and growth hormone treatment on leukocyte telomere length. PLoS ONE 12, e0171825 ( 10.1371/journal.pone.0171825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guzzardi MA, Iozzo P, Salonen MK, Kajantie E, Eriksson JG. 2016. Maternal adiposity and infancy growth predict later telomere length: a longitudinal cohort study. Int. J. Obesity 40, 1063–1069. ( 10.1038/ijo.2016.58) [DOI] [PubMed] [Google Scholar]
- 55.Watson RL, et al. 2017. Sex differences in leucocyte telomere length in a free-living mammal. Mol. Ecol. 26, 3230–3240. ( 10.1111/mec.13992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tarry-Adkins JL, Fernandez-Twinn DS, Chen JH, Hargreaves IP, Neergheen V, Aiken CE, Ozanne SE. 2016. Poor maternal nutrition and accelerated postnatal growth induces an accelerated aging phenotype and oxidative stress in skeletal muscle of male rats. Dis. Models Mech. 9, 1221–1229. ( 10.1242/dmm.026591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giroud S, Zahn S, Criscuolo FO, Chery I, Blanc S, Turbill C, Ruf T. 2014. Late-born intermittently fasted juvenile garden dormice use torpor to grow and fatten prior to hibernation: consequences for ageing processes. Proc. R. Soc. B 281, 20141131 ( 10.1098/rspb.2014.1131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aiken CE, Tarry-Adkins JL, Ozanne SE. 2013. Suboptimal nutrition in utero causes DNA damage and accelerated aging of the female reproductive tract. FASEB J. 27, 3959–3965. ( 10.1096/fj.13-234484) [DOI] [PubMed] [Google Scholar]
- 59.Tarry-Adkins JL, Martin-Gronert MS, Fernandez-Twinn DS, Hargreaves I, Alfaradhi MZ, Land JM, Aiken CE, Ozanne SE. 2013. Poor maternal nutrition followed by accelerated postnatal growth leads to alterations in DNA damage and repair, oxidative and nitrosative stress, and oxidative defense capacity in rat heart. FASEB J. 27, 379–390. ( 10.1096/fj.12-218685) [DOI] [PubMed] [Google Scholar]
- 60.Tarry-Adkins JL, Chen JH, Smith NS, Jones RH, Cherif H, Ozanne SE. 2009. Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB J. 23, 1521–1528. ( 10.1096/fj.08-122796) [DOI] [PubMed] [Google Scholar]
- 61.Tarry-Adkins JL, Martin-Gronert MS, Chen JH, Cripps RL, Ozanne SE. 2008. Maternal diet influences DNA damage, aortic telomere length, oxidative stress, and antioxidant defense capacity in rats. FASEB J. 22, 2037–2044. ( 10.1096/fj.07-099523) [DOI] [PubMed] [Google Scholar]
- 62.Jennings BJ, Ozanne SE, Dorling MW, Hales CN. 1999. Early growth determines longevity in male rats and may be related to telomere shortening in the kidney. FEBS Lett. 448, 4–8. ( 10.1016/S0014-5793(99)00336-1) [DOI] [PubMed] [Google Scholar]
- 63.Stier A, Massemin S, Zahn S, Tissier ML, Criscuolo F. 2015. Starting with a handicap: effects of asynchronous hatching on growth rate, oxidative stress and telomere dynamics in free-living great tits. Oecologia 179, 999–1010. ( 10.1007/s00442-015-3429-9) [DOI] [PubMed] [Google Scholar]
- 64.Costanzo A, et al. 2017. Brood size, telomere length, and parent-offspring color signaling in barn swallows. Behav. Ecol. 28, 204–211. ( 10.1093/beheco/arw147) [DOI] [Google Scholar]
- 65.Vedder O, Verhulst S, Bauch C, Bouwhuis S. 2017. Telomere attrition and growth: a life-history framework and case study in common terns. J. Evol. Biol. 30, 1409–1419. ( 10.1111/jeb.13119) [DOI] [PubMed] [Google Scholar]
- 66.Parolini M, Romano A, Khoriauli L, Nergadze SG, Caprioli M, Rubolini D, Santagostino M, Saino N, Giulotto E. 2015. Early-life telomere dynamics differ between the sexes and predict growth in the barn swallow (Hirundo rustica). PLoS ONE 10, e0142530 ( 10.1371/journal.pone.0142530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meillere A, Brischoux F, Ribout C, Angelier F. 2015. Traffic noise exposure affects telomere length in nestling house sparrows. Biol. Lett. 11, 20150559 ( 10.1098/rsbl.2015.0559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reichert S, Criscuolo F, Zahn S, Arrive M, Bize P, Massemin S. 2015. Immediate and delayed effects of growth conditions on ageing parameters in nestling zebra finches. J. Exp. Biol. 218, 491–499. ( 10.1242/jeb.109942) [DOI] [PubMed] [Google Scholar]
- 69.Noguera JC, Metcalfe NB, Boner W, Monaghan P. 2015. Sex-dependent effects of nutrition on telomere dynamics in zebra finches (Taeniopygia guttata). Biol. Lett. 11, 20140938 ( 10.1098/rsbl.2014.0938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nettle D, Monaghan P, Gillespie R, Brilot B, Bedford T, Bateson M. 2015. An experimental demonstration that early-life competitive disadvantage accelerates telomere loss. Proc. R. Soc. B 282, 20141610 ( 10.1098/rspb.2014.1610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herborn KA, Heidinger BJ, Boner W, Noguera JC, Adam A, Daunt F, Monaghan P. 2014. Stress exposure in early post-natal life reduces telomere length: an experimental demonstration in a long-lived seabird. Proc. R. Soc. B 281, 20133151 ( 10.1098/rspb.2013.3151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tissier ML, Williams TD, Criscuolo F. 2014. Maternal effects underlie ageing costs of growth in the zebra finch (Taeniopygia guttata). PLoS ONE 9, e97705 ( 10.1371/journal.pone.0097705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Voillemot M, Hine K, Zahn S, Criscuolo F, Gustafsson L, Doligez B, Bize P. 2012. Effects of brood size manipulation and common origin on phenotype and telomere length in nestling collared flycatchers. BMC Ecol. 12, 17 ( 10.1186/1472-6785-12-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hall ME, Nasir L, Daunt F, Gault EA, Croxall JP, Wanless S, Monaghan P. 2004. Telomere loss in relation to age and early environment in long-lived birds. Proc. R. Soc. Lond. B 271, 1571–1576. ( 10.1098/rspb.2004.2768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burraco P, Diaz-Paniagua C, Gomez-Mestre I. 2017. Different effects of accelerated development and enhanced growth on oxidative stress and telomere shortening in amphibian larvae. Sci. Rep. 7, Article number: 7494 ( 10.1038/s41598-017-07201-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Noreikiene K, Kuparinen A, Merilae J. 2017. Age at maturation has sex- and temperature-specific effects on telomere length in a fish. Oecologia 184, 767–777. ( 10.1007/s00442-017-3913-5) [DOI] [PubMed] [Google Scholar]
- 77.McLennan D, Armstrong JD, Stewart DC, McKelvey S, Boner W, Monaghan P, Metcalfe NB. 2016. Interactions between parental traits, environmental harshness and growth rate in determining telomere length in wild juvenile salmon. Mol. Ecol. 25, 5425–5438. ( 10.1111/mec.13857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Naslund J, Pauliny A, Blomqvist D, Johnsson JI. 2015. Telomere dynamics in wild brown trout: effects of compensatory growth and early growth investment. Oecologia 177, 1221–1230. ( 10.1007/s00442-015-3263-0) [DOI] [PubMed] [Google Scholar]
- 79.Pauliny A, Devlin RH, Johnsson JI, Blomqvist D. 2015. Rapid growth accelerates telomere attrition in a transgenic fish. BMC Evol. Biol. 15, 159 ( 10.1186/s12862-015-0436-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Izzo C, Bertozzi T, Gillanders BM, Donnellan SC. 2014. Variation in telomere length of the common carp, Cyprinus carpio (Cyprinidae), in relation to body length. Copeia 2014, 87–94. ( 10.1643/ci-11-162) [DOI] [Google Scholar]
- 81.Hatakeyama H, Nakamura K-I, Izumiyama-Shimorriura N, Ishii A, Tsuchida S, Takubo K, Ishikawa N. 2008. The teleost Oryzias latipes shows telomere shortening with age despite considerable telomerase activity throughout life (vol 129, pg 550, 2008). Mech. Ageing Dev. 129, 692 ( 10.1016/j.mad.2008.09.014) [DOI] [PubMed] [Google Scholar]
- 82.Tarry-Adkins JL, Ozanne SE. 2014. The impact of early nutrition on the ageing trajectory. Proc. Nutr. Soc. 73, 289–301. ( 10.1017/s002966511300387x) [DOI] [PubMed] [Google Scholar]
- 83.Badas EP, Martinez J, de Aguilar Cachafeiro JR, Miranda F, Figuerola J, Merino S. 2015. Ageing and reproduction: antioxidant supplementation alleviates telomere loss in wild birds. J. Evol. Biol. 28, 896–905. ( 10.1111/jeb.12615) [DOI] [PubMed] [Google Scholar]
- 84.Starr JM, Shiels PG, Harris SE, Pattie A, Pearce MS, Relton CL, Deary IJ. 2008. Oxidative stress, telomere length and biomarkers of physical aging in a cohort aged 79 years from the 1932 Scottish Mental Survey. Mech. Ageing Dev. 129, 745–751. ( 10.1016/j.mad.2008.09.020) [DOI] [PubMed] [Google Scholar]
- 85.Kim S-Y, Velando A. 2015. Antioxidants safeguard telomeres in bold chicks. Biol. Lett. 11, 20150211 ( 10.1098/rsbl.2015.0211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Passos JF, et al. 2007. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 5, 1138–1151. ( 10.1371/journal.pbio.0050110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arendt JD, Wilson DS. 1997. Optimistic growth: competition and an ontogenetic niche-shift select for rapid growth in pumpkinseed sunfish (Lepomis gibbosus). Evolution 51, 1946–1954. [DOI] [PubMed] [Google Scholar]
- 88.Arendt J. 2007. Ecological correlates of body size in relation to cell size and cell number: patterns in flies, fish, fruits and foliage. Biol. Rev. 82, 241–256. ( 10.1111/j.1469-185X.2007.00013.x) [DOI] [PubMed] [Google Scholar]
- 89.Lloyd AC. 2013. The regulation of cell size. Cell 154, 1194–1205. ( 10.1016/j.cell.2013.08.053) [DOI] [PubMed] [Google Scholar]
- 90.Falconer DS, Gauld IK, Roberts RC. 1978. Cell numbers and cell sizes in organs of mice selected for large and small body size. Genet. Res. 31, 287–301. ( 10.1017/S0016672300018061) [DOI] [PubMed] [Google Scholar]
- 91.Gorbunova V, Seluanov A, Zhang Z, Gladyshev VN, Vijg J. 2014. Comparative genetics of longevity and cancer: insights from long-lived rodents. Nat. Rev. Genet. 15, 531–540. ( 10.1038/nrg3728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Verhulst S, Aviv A, Benetos A, Berenson GS, Kark JD. 2013. Do leukocyte telomere length dynamics depend on baseline telomere length? An analysis that corrects for ‘regression to the mean’. Eur. J. Epidemiol. 28, 859–866. ( 10.1007/s10654-013-9845-4) [DOI] [PubMed] [Google Scholar]
- 93.Entringer S, Buss C, Wadhwa PD. 2012. Prenatal stress, telomere biology, and fetal programming of health and disease risk. Sci. Signal. 5, 12 ( 10.1126/scisignal.2003580) [DOI] [PubMed] [Google Scholar]
- 94.Noguera JC, Metcalfe NB, Reichert S, Monaghan P. 2016. Embryonic and postnatal telomere length decrease with ovulation order within clutches. Sci. Rep. 6, 25915 ( 10.1038/srep25915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Entringer S, de Punder K, Buss C, Wadhwa PD. 2018. The fetal programming of telomere biology hypothesis: an update. Phil. Trans. R. Soc. B 373, 20170151 ( 10.1098/rstb.2017.0151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alonso-Alvarez C, Bertrand S, Faivre B, Sorci G. 2007. Increased susceptibility to oxidative damage as a cost of accelerated somatic growth in zebra finches. Funct. Ecol. 21, 873–879. ( 10.1111/j.1365-2435.2007.01300.x) [DOI] [Google Scholar]
- 97.Kim SY, Noguera JC, Morales J, Velando A. 2011. Quantitative genetic evidence for trade-off between growth and resistance to oxidative stress in a wild bird. Evol. Ecol. 25, 461–472. ( 10.1007/s10682-010-9426-x) [DOI] [Google Scholar]
- 98.Smith SM, Nager RG, Costantini D. 2016. Meta-analysis indicates that oxidative stress is both a constraint on and a cost of growth. Ecol. Evol. 6, 2833–2842. ( 10.1002/ece3.2080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sebens KP. 1987. The ecologuy of indeterminate growth in animals. Ann. Rev. Ecol. Syst. 18, 371–407. ( 10.1146/annurev.ecolsys.18.1.371) [DOI] [Google Scholar]
- 100.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Debes PV, Visse M, Panda B, Ilmonen P, Vasemagi A. 2016. Is telomere length a molecular marker of past thermal stress in wild fish?. Mol. Ecol. 25, 5412–5424. ( 10.1111/mec.13856) [DOI] [PubMed] [Google Scholar]
- 102.Rollo CD. 2002. Growth negatively impacts the life span of mammals. Evol. Dev. 4, 55–61. ( 10.1046/j.1525-142x.2002.01053.x) [DOI] [PubMed] [Google Scholar]
- 103.de Magalhaes JP, Faragher RG.A. 2008. Cell divisions and mammalian aging: integrative biology insights from genes that regulate longevity. Bioessays 30, 567–578. ( 10.1002/bies.20760) [DOI] [PubMed] [Google Scholar]
- 104.Blagosklonny MV. 2013. Big mice die young but large animals live longer. Aging-Us 5, 227–233. ( 10.18632/aging.100551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kraus C, Pavard S, Promislow DE.L. 2013. The size-life span trade-off decomposed: why large dogs die young. Am. Nat. 181, 492–505. ( 10.1086/669665) [DOI] [PubMed] [Google Scholar]
- 106.Stindl R. 2004. Tying it all together: telomeres, sexual size dimorphism and the gender gap in life expectancy. Med. Hypotheses 62, 151–154. ( 10.1016/S0306-9877(03)00316-5) [DOI] [PubMed] [Google Scholar]
- 107.Barrett EL, Richardson DS. 2011. Sex differences in telomeres and lifespan. Aging Cell 10, 913–921. ( 10.1111/j.1474-9726.2011.00741.x) [DOI] [PubMed] [Google Scholar]
- 108.Angelier F, Vleck CM, Holberton RL, Marra PP. 2015. Bill size correlates with telomere length in male American Redstarts. J. Ornithol. 156, 525–531. ( 10.1007/s10336-015-1158-9) [DOI] [Google Scholar]
- 109.Epel ES. 2009. Telomeres in a life-span perspective: a new ‘psychobiomarker’ ? Curr. Dir. Psychol. Sci. 18, 6–10. ( 10.1111/j.1467-8721.2009.01596.x) [DOI] [Google Scholar]
- 110.Ringsby TH, et al. 2015. On being the right size: increased body size is associated with reduced telomere length under natural conditions. Proc. R. Soc. B 282, 20152331 ( 10.1098/rspb.2015.2331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee W-S, Monaghan P, Metcalfe NB. 2013. Experimental demonstration of the growth rate - lifespan trade-off. Proc. R. Soc. B 280, 20122370 ( 10.1098/rspb.2012.2370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hales CN, Barker DJP, Clark PMS, Cox LJ, Fall C, Osmond C, Winter PD. 1991. Fetal and infant growth and impaired glucose tolerance at age 64. Br. Med. J. 303, 1019–1022. ( 10.1136/bmj.303.6809.1019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Crowther NJ, Cameron N, Trusler J, Gray IP. 1998. Association between poor glucose tolerance and rapid post natal weight gain in seven-year-old children. Diabetologia 41, 1163–1167. ( 10.1007/s001250051046) [DOI] [PubMed] [Google Scholar]
- 114.Singhal A. 2010. Does Early Growth Affect Long-Term Risk Factors for Cardiovascular Disease?. Imp. Growth Health Dev. 65, 55–69. ( 10.1159/000281145) [DOI] [PubMed] [Google Scholar]
- 115.Widdowson EM, McCance RA. 1963. Effect of finite periods of undernutrition at different ages on composition and subsequent development of rat. Proc. R. Soc. Lond. B 158, 329–342. ( 10.1098/rspb.1963.0051) [DOI] [PubMed] [Google Scholar]
- 116.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. 2005. Being big or growing fast: systematic review of size and growth in infancy and later obesity. Br. Med. J. 331, 929–931. ( 10.1136/bmj.38586.411273.E0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ozanne SE, Hales CN. 2004. Lifespan - catch-up growth and obesity in male mice. Nature 427, 411–412. ( 10.1038/427411b) [DOI] [PubMed] [Google Scholar]
- 118.Hales CN, Desai M, Ozanne SE, Crowther NJ. 1996. Fishing in the stream of diabetes: From measuring insulin to the control of fetal organogenesis. Biochem. Soc. Trans. 24, 341–350. ( 10.1042/bst0240341) [DOI] [PubMed] [Google Scholar]
- 119.Tarry-Adkins JL, Blackmore HL, Martin-Gronert MS, Fernandez-Twinn DS, McConnell JM, Hargreaves IP, Giussani DA, Ozanne SE. 2013. Coenzyme Q(10) prevents accelerated cardiac aging in a rat model of poor maternal nutrition and accelerated postnatal growth. Mol. Metabol. 2, 480–490. ( 10.1016/j.molmet.2013.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Allison BJ, et al. 2016. Divergence of mechanistic pathways mediating cardiovascular aging and developmental programming of cardiovascular disease. FASEB J. 30, 1968–1975. ( 10.1096/fj.201500057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beery AK, Lin J, Biddle JS, Francis DD, Blackburn EH, Epel ES. 2012. Chronic stress elevates telomerase activity in rats. Biol. Lett. 8, 1063–1066. ( 10.1098/rsbl.2012.0747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rollings N, Uhrig EJ, Krohmer RW, Waye HL, Mason RT, Olsson M, Whittington CM, Friesen CR. 2017. Age-related sex differences in body condition and telomere dynamics of red-sided garter snakes. Proc. R. Soc. B 284, 20162146 ( 10.1098/rspb.2016.2146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Plot V, Criscuolo F, Zahn S, Georges JY. 2012. Telomeres, age and reproduction in a long-lived reptile. PLoS ONE 7, e40855 ( 10.1371/journal.pone.0040855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lund TC, Glass TJ, Tolar J, Blazar BR. 2009. Expression of telomerase and telomere length are unaffected by either age or limb regeneration in Danio rerio. PLoS ONE 4, e7688 ( 10.1371/journal.pone.0007688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Simide R, Angelier F, Gaillard S, Stier A. 2016. Age and heat stress as determinants of telomere length in a long-lived fish, the Siberian sturgeon. Physiol. Biochem. Zool. 89, 441–447. ( 10.1086/687378) [DOI] [PubMed] [Google Scholar]
- 126.Hatakeyama H, et al. 2016. Telomere attrition and restoration in the normal teleost Oryzias latipes are linked to growth rate and telomerase activity at each life stage. Aging-Us 8, 62–76. ( 10.18632/aging.100873) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.


