Abstract
Telomere length (TL) has become a biomarker of increasing interest within ecology and evolutionary biology, and has been found to predict subsequent survival in some recent avian studies but not others. Here, we undertake the first formal meta-analysis to test whether there is an overall association between TL and subsequent mortality risk in vertebrates other than humans and model laboratory rodents. We identified 27 suitable studies and obtained standardized estimates of the hazard ratio associated with TL from each. We performed a meta-analysis on these estimates and found an overall significant negative association implying that short telomeres are associated with increased mortality risk, which was robust to evident publication bias. While we found that heterogeneity in the hazard ratios was not explained by sex, follow-up period, maximum lifespan or the age group of the study animals, the TL–mortality risk association was stronger in studies using qPCR compared to terminal restriction fragment methodologies. Our results provide support for a consistent association between short telomeres and increased mortality risk in birds, but also highlight the need for more research into non-avian vertebrates and the reasons why different telomere measurement methods may yield different results.
This article is part of the theme issue ‘Understanding diversity in telomere dynamics’.
Keywords: survival, longevity, systematic review, wild, publication bias
1. Introduction
Telomeres are highly repetitive sections of DNA that cap the ends of chromosomes in most eukaryote species, forming complexes with so-called ‘shelterin’ proteins that are essential to the maintenance of genomic integrity of linear chromosomes [1,2]. Telomeres shorten with each cell division due to the ‘end replication problem’ and in response to cellular stressors including oxidative stress, and induce cellular senescence when they shorten below a critical threshold [1,3,4]. Telomeres can be restored via several mechanisms, the most widely studied being the action of the enzyme telomerase [1,3]. Telomerase expression appears to be suppressed in adult somatic tissue in many large-bodied endothermic vertebrates, including humans [5]. Telomere attrition has been identified as one of nine ‘hallmarks of ageing’ [6] and while the role of telomere shortening in cellular senescence is beyond doubt, the evidence that it plays a causal role in senescence in otherwise healthy animals is currently weak [7]. However, there is mounting evidence in humans that average telomere length (TL), typically measured in blood cells, represents an important biomarker of health and ageing [3]. Leucocyte TL declines with age in humans [8] and meta-analyses have recently shown that in adult humans shorter average TL is associated with increased risk of type 2 diabetes, cardiovascular disease, cancer and follow-up mortality [9–12]. Although the majority of non-human research into telomere biology has been performed in laboratory rodents, studies beyond model organisms are crucial if we are to understand the evolutionary and environmental factors responsible for the diversity of TLs and levels of telomerase expression observed among species [13,14].
There is a rapidly growing literature exploring telomere dynamics and their significance for organismal function and fitness in non-human vertebrates and, in particular, in wild bird systems [15–17]. TL has been proposed as an important biomarker within evolutionary ecology and animal welfare because it may reflect an individual's cumulative experience of environmental stress and investment in growth or reproduction [17–19]. This leads to the expectation that shorter TL will predict raised subsequent mortality risk, without telomeres necessarily being causally involved in death, due to increased somatic damage associated with environmental stress and reduced investment in somatic repair [17,19]. In humans, evidence is also emerging that TL is both highly repeatable over time within individuals and highly heritable [20,21]. This raises the further possibility that individual differences in TL set at birth are maintained throughout life and are associated with consistent differences in physiological function or state and organismal lifespan. Although determining the relative importance of TL at birth and TL shortening over life for organismal health and fitness remains a major outstanding challenging within telomere biology [17,20], a crucial first step towards this goal is to establish whether an overall association between TL and mortality risk is evident in non-human species and how and why such an association might vary across species.
Several studies have reported significant associations between average blood cell TL and the risk of subsequent mortality in both wild and captive populations [22–25]. However, this emerging literature also contains numerous examples of studies that test for but do not find evidence to support a relationship between TL and mortality risk [26–28]. Thus, the generality of the relationship between TL and mortality is currently unclear outside of studies of humans and laboratory rodents. A number of factors may contribute to the variation observed in the relationship between TL and mortality in these non-model organisms. First, studies invariably apply one of two methodologies—quantitative PCR (qPCR) or terminal restriction fragment analysis (TRF)—which differ in accuracy and throughput, with the former providing the average amount of telomeric DNA within a sample on a relative and non-comparable scale and the latter providing information on the range of TLs within a sample in kilobase units [29,30]. The life history of the species in question, in particular its life expectancy under natural conditions, is also expected to play an important role in shaping the evolution of telomere dynamics [13,14,19]. Ecological studies of TL also vary considerably in the duration of the study follow-up time, from weeks [31] to over a decade [22], and typically involve investigating TL and survival in either young animals or adults rather than both. Furthermore, while sex differences in TL observed in humans and laboratory rodents have been proposed to underpin sex differences in longevity, the effect of sex on the relationships between TL and mortality has rarely been investigated [32,33]. Here, we undertake the first formal literature search and meta-analysis to test whether there is a consistent association between short TL and increased subsequent mortality risk in vertebrates other than humans and model laboratory rodents. In addition, we use meta-regression analyses to investigate potential sources of variation in this association across studies including methodology, life stage at sampling, follow-up period and sex.
2. Methods
(a). Literature search
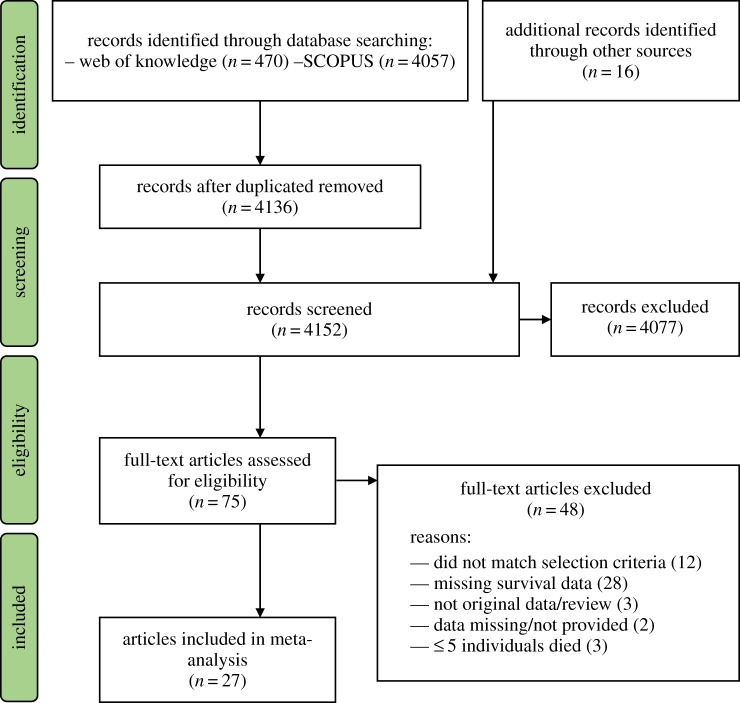
Data for our meta-analysis were collected using ISI Web of Science and SCOPUS databases with the following search string: ‘telom* AND surviv* OR longevity/lifespan/life span/life expectancy/mortality/fitness'. Additional papers were identified in two ways: (i) backward and forward searching was carried out on citations of the first paper showing an association between TL and survival in a non-model vertebrate [23]; (ii) screening the authors' own reference list, created from Google Scholar email alerts containing the keyword ’telomere’, for relevant papers. The last database search was carried out on 6 February 2017, although Google Scholar alerts were continuously checked and papers published up until May 2017 were included. We included studies published as part of PhD theses that were available online, but otherwise excluded studies that had not yet been published in peer-reviewed journals. Overall, these searches identified 4152 papers for potential inclusion in our meta-analysis (figure 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for identification and inclusion of studies in the meta-analysis. We present the number of papers identified through key word database searching in addition to records identified through other sources. Papers were excluded during initial screening phases and reasons for exclusion provided for those papers that reached the final full-text eligibility screening. (Online version in colour.)
Since our focus was on non-model vertebrate systems, we excluded studies involving human subjects, genetically modified or inbred laboratory strains of mice and non-vertebrate species. We excluded studies that did not report original empirical data (i.e. reviews or computer simulations) and those in which an association between TL and mortality or longevity was not reported. Our initial screening based on titles and abstracts of papers in the database led to the exclusion of 4077 papers (mostly studies of humans and model laboratory organisms), with 75 papers retained for more detailed interrogation of eligibility (figure 1; electronic supplementary material, table S1). The full text of these papers were downloaded and a further 46 excluded. This left 29 studies that were suitable for inclusion in our meta-analysis, and we were able to obtain data from 27 of these studies (see electronic supplementary material, table S1 for full details of reasons for exclusion). Although the majority of papers read in full measured TL in blood cells, several did measure TL in other tissues but none of those studies provided suitable data or analyses of lifespan or survival for inclusion our meta-analyses. Indeed, most studies read in full were excluded because suitable data on survival of individuals were lacking, but we also excluded three studies that reported an association between TL and survival but in which fewer than five individuals died (<10% of study population), as power to detect TL–mortality risk relationships would be extremely limited in such cases (see electronic supplementary material, table S1).
During our search, we noted a great deal of heterogeneity in the manner in which analyses of TL–mortality risk relationships were conducted and reported, as well as in the way TL was measured (qPCR or TRF methodologies). To maximize our ability to detect an overall association between TL and survival across studies, and to identify the factors responsible for variation in this association among studies, we decided to obtain raw data for each study and analyse the TL–mortality relationship in a standardized way. For studies in which raw data were not available online, we contacted the corresponding authors requesting either that they provided us with the raw data used in the relevant analyses, or that they performed standardized analyses using an R script that we provided (electronic supplementary material, file S1). We were able to obtain raw data or standardized measures of TL–mortality risk associations from 27 studies identified from 20 different species: 17 bird species, three reptiles and one mammal (table 1).
Table 1.
List of studies included in the meta-analysis with samples sizes (N), effect sizes, expressed as the natural logarithm of the hazard ratio of TL and associated standard error (s.e.) alongside information on moderator variables tested (see §2 for details).
| study | ref. | class | order | species | N | ln hazard ratio | s.e. | follow-up | TL method | age group | log lifespan |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Angelier (2013) | [34] | Aves | Passeriformes | Setophaga ruticilla | 36 | −1.100 | 0.460 | 1 | qPCR | adult | 2.312535 |
| Asghar (2015) | [35] | Aves | Passeriformes | Acrocephalus arundinaceus | 100 | −0.293 | 0.113 | 23 | qPCR | juvenile | 2.312535 |
| Barrett (2013)a | [22] | Aves | Passeriformes | Acrocephalus sechellensis | 203 | 0.064 | 0.071 | 15 | qPCR | adult | 2.833213 |
| Barrett (2013)a | [22] | Aves | Passeriformes | Acrocephalus sechellensis | 203 | −0.414 | 0.192 | 1 | qPCR | adult | 2.833213 |
| Bauch (2014) | [36] | Aves | Charadriiformes | Sterna hirundo | 181 | −0.140 | 0.113 | 4 | TRF | adult | 3.496508 |
| Beaulieu (2011) | [26] | Aves | Sphenisciformes | Pygoscelis adeliae | 72 | 0.036 | 0.313 | 3 | qPCR | adult | 2.772589 |
| Belmaker (2016) | [37] | Aves | Passeriformes | Tachycineta bicolor | 107 | −0.054 | 0.124 | 1 | TRF | adult | 2.493205 |
| Bize (2009) | [24] | Aves | Apodiformes | Apus melba | 96 | −0.348 | 0.126 | 6 | qPCR | adult | 3.258097 |
| Boonekamp (2014) | [38] | Aves | Passeriformes | Corvus monedula | 241 | −0.023 | 0.149 | 8 | TRF | juvenile | 3.010621 |
| Caprioli (2013) | [39] | Aves | Passeriformes | Hirundo rustico | 60 | −0.014 | 0.136 | 11 | TRF | juvenile | 2.772589 |
| Fairlie (2016)a | [40] | Mammalia | Artiodactyla | Ovis aries | 87 | −0.262 | 0.315 | 12 | qPCR | adult | 3.126761 |
| Fairlie (2016)a | [40] | Mammalia | Artiodactyla | Ovis aries | 116 | −0.405 | 0.206 | 1 | qPCR | juvenile | 3.126761 |
| Foote (2009) | [41] | Aves | Procellariiformes | Macronectes halli | 36 | −0.060 | 0.249 | 8 | TRF | adult | 3.688879 |
| Foote (2011) | [42] | Aves | Procellariiformes | Macronectes giganteus | 47 | −0.327 | 0.195 | 8 | TRF | adult | 3.688879 |
| Reichert (2017) | [43] | Aves | Procellariiformes | Diomedea exulans | 56 | −0.227 | 0.224 | 12 | qPCR | adult | 3.912023 |
| Geiger (2012) | [44] | Aves | Sphenisciformes | Aptenodytes patagonicus | 36 | −2.198 | 0.524 | 1 | qPCR | juvenile | 3.258097 |
| Haussmann (2005) | [23] | Aves | Passeriformes | Tachycineta bicolor | 22 | −0.741 | 0.308 | 4 | TRF | juvenile | 2.493205 |
| Heidinger (2012) | [25] | Aves | Passeriformes | Taeniopygia guttata | 99 | −0.420 | 0.108 | 8.7 | qPCR | juvenile | 2.484907 |
| Olsson (2011) | [33] | Reptilia | Squamata | Lacerta agilis | 126 | −0.071 | 0.084 | 25 | TRF | adult | 2.079442 |
| Ouyang (2016) | [45] | Aves | Passeriformes | Tachycineta bicolor | 74 | −0.034 | 0.127 | 3 | TRF | adult | 2.493205 |
| Reichert (2014) | [27] | Aves | Passeriformes | Taeniopygia guttata | 50 | −0.161 | 0.227 | 1 | qPCR | adult | 2.484907 |
| Reichert (2015) | [46] | Aves | Passeriformes | Taeniopygia guttata | 65 | −0.624 | 0.296 | 1 | qPCR | juvenile | 2.484907 |
| Salomons (2009) | [47] | Aves | Passeriformes | Corvus monedula | 48 | −0.076 | 0.219 | 4 | TRF | adult | 3.010621 |
| Stier (2014) | [48] | Aves | Sphenisciformes | Aptenodytes patagonicus | 82 | −0.352 | 0.190 | 1 | qPCR | juvenile | 3.258097 |
| Sudyka (2014) | [28] | Aves | Passeriformes | Cyanistes caeruleus | 56 | −0.036 | 0.145 | 2 | qPCR | adult | 2.681022 |
| Taff (2017) | [49] | Aves | Passeriformes | Geothlypis trichas | 89 | −0.296 | 0.167 | 4 | qPCR | adult | 2.442347 |
| Ujvari (2009)a | [50] | Reptilia | Squamata | Liasis fuscus | 50 | 0.477 | 0.182 | 10 | TRF | juvenile | 3.288402 |
| Ujvari (2009)a | [50] | Reptilia | Squamata | Liasis fuscus | 20 | 0.117 | 0.278 | 3 | TRF | adult | 3.288402 |
| Ujvari (2016) | [51] | Reptilia | Squamata | Chlamydosaurus kingii | 72 | −0.496 | 0.254 | 2 | qPCR | adult | 2.292535 |
| Watson (2015) | [31] | Aves | Procellariiformes | Hydrobates pelagicus | 59 | −1.260 | 0.430 | 0.2 | qPCR | juvenile | 3.520461 |
aMultiple estimates associated with different age groups or follow-up times in our analysis.
(b). Effect size extraction
For each of the 27 included studies, the following data were available: individual identity, age at blood sampling, sex, TL at sampling, sampling date, final date where survival was determined and survival status (survived = 0, dead = 1). Within each study, TL was mean centred and standardized to unit standard deviation prior to inclusion in analyses to create similarly scaled TL distributions among studies. We then applied Cox proportional hazard regression analysis in R using the package survival [52] including TL as an explanatory variable. We defined start time as the time of TL sampling and end time as the follow-up time at which survival was determined. This information was used to determine the hazard ratio of TL relative to baseline mortality. Final effect sizes were expressed as the natural logarithm of the hazard ratio for mortality (ln HR). As ln HR provides a measure of risk of death, a negative effect indicates that individuals with long TL on average are less likely to die in comparison to individuals with short TL. Hazard ratio estimates and associated standard errors were extracted, either by ourselves or the authors (using the R script in electronic supplementary material, file S1).
(c). Meta-analysis
We conducted our meta-analysis using the metafor package [53] in R, to investigate the relationship between TL and survival. We used a random-effects design fitted with restricted maximum log likelihood and used 1/s.e.2 as weighting factor [54], where s.e. was the standard error associated with the ln hazard ratio from the Cox regression model. We tested for evidence of publication bias using Kendall's tau test-statistics and through visual inspection of funnel plots. We used Q-tests to evaluate study heterogeneity.
We subsequently investigated potential sources of heterogeneity by including moderator variables in the meta-analysis. We extracted the following moderator variables for each study: TL measurement method (TRF or qPCR), the age group of the study animals (categorized as ‘young’ if ≤1 year and ‘adult’ if >1 year), the length of the follow-up period in years after TL measurement, and the log transformation of each species' maximum recorded lifespan (from the AnAge database: http://genomics.senescence.info/species/). To crudely test for a phylogenetic signal, we tested class, order and species separately as moderators (20 species, six orders, three classes; table 1). Two of the studies reported separate associations between TL and survival in both age classes [40,50], while one reported associations based on two different follow-up periods [22]. We generated and included two estimates for each of these studies, resulting in a total of 30 hazard ratio estimates in the meta-analysis (table 1). Although we categorized studies based on wild or captive animals, only three were based on captive populations and so we did not investigate this moderator further [25,27,46]. Although not all papers specifically reported on sex differences in the association between TL and survival, 25 out of 27 provided complete data on the sex of individuals. Of these, three studies included only females [23,31,40], two included only males [34,49] and the remaining 20 included both sexes. To assess the effect of sex on the association between TL and survival, we re-ran Cox regression models for these latter 20 studies separately for each sex. This generated a total of 46 sex-specific hazard ratio estimates, allowing us to test sex as a moderator variable. Individual moderator effects were evaluated using either Q-tests for effects of class, order or species and z-tests for all other moderator variables.
3. Results
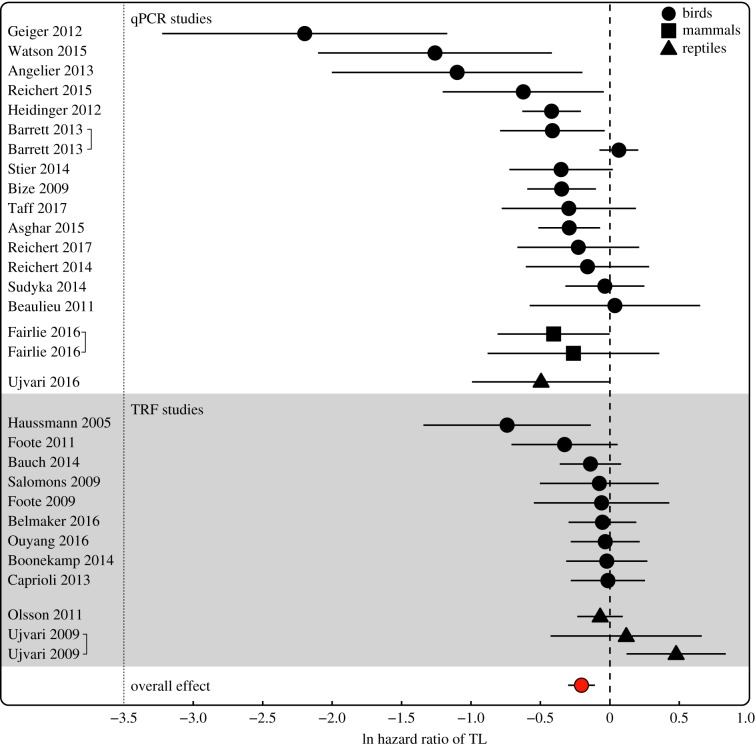
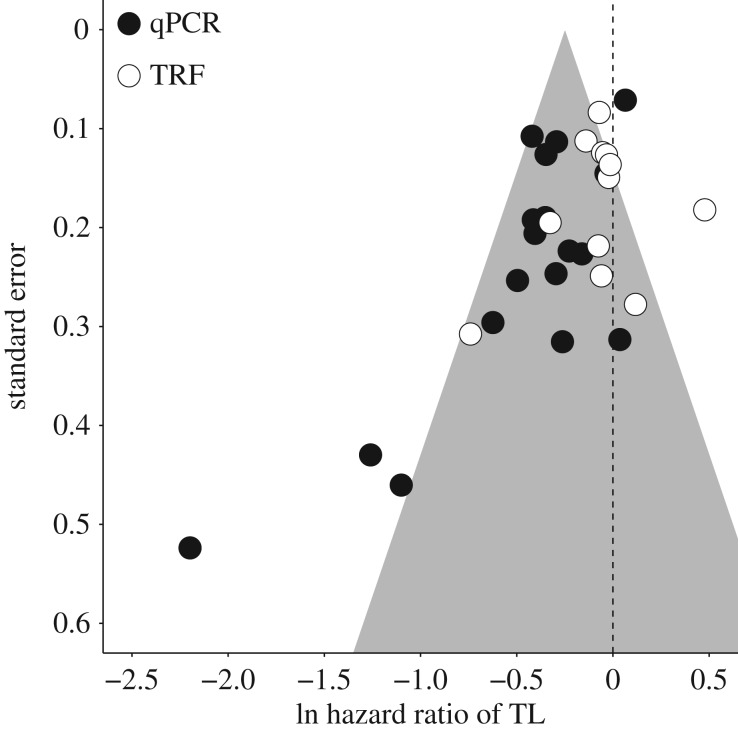
Overall, the hazard ratio associated with TL was significantly negative, supporting a decreased mortality risk with increasing TL across studies (mean ln HR = −0.205 ± 0.049 s.e., p < 0.001, figure 2). However, there was evidence for publication bias (Kendall's tau = −0.310; p = 0.016; figure 3). Visual inspection of a funnel plot relating effect size to s.e. (figure 3) revealed that this bias was primarily driven by three qPCR-based studies with small sample sizes with strongly negative hazard ratios (ln HR > −1: [31,34,44]). To establish whether this bias influenced the overall association between TL and mortality risk, we re-ran the models without these three studies; the overall association remained significant (−0.162 ± 0.044; p < 0.001) and the Kendall's tau statistic became non-significant (−0.134; p = 0.341). We also applied the ‘trim and fill’ method [55] to examine the sensitivity of the results to publication bias and found that the overall association became substantially weaker and remained marginally significant (−0.108 ± 0.062 s.e.; p = 0.083).
Figure 2.
Forest plot of effect sizes (natural logarithm of the hazard ratio for standardized telomere length) and associated 95% confidence intervals. The overall effect size is shown in red, with estimates grouped by measurement method and with vertebrate class indicated by symbol shape (circle: birds, square: mammals, triangle: reptiles). (Online version in colour.)
Figure 3.
Funnel plot relating the study standard error to effect size. Open circles denote qPCR-based studies, filled circles denote TRF-based studies.
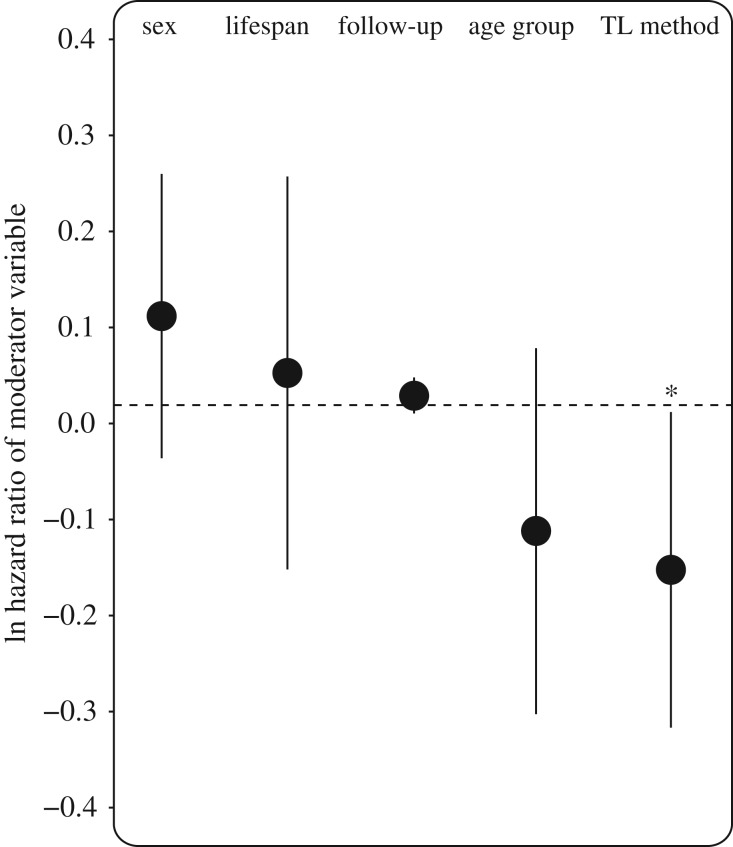
There was significant heterogeneity among study effect sizes (Q(d.f. = 29) = 77.77; p < 0.001) indicating substantial variation in TL–mortality risk associations among studies. We investigated the extent to which phylogeny, study follow-up period, sex, TL measurement method and age group at sampling reduced the observed study heterogeneity. We tested species, order and class as phylogenetic moderators in separate models and, although none was significant overall (QM(d.f. = 19) = 20.88; p = 0.405 and QM(5) = 6.035; p = 0.419 and QM(2) = 3.89, p = 0.143, respectively), post hoc comparisons within the class model suggested that the strength of the association was marginally weaker in reptiles than birds (difference bird–reptile: 0.255 ± 0.139 s.e., p = 0.066). The fact that there were only three reptile studies in our meta-analyses meant there was limited power to dissect this trend further, but visual inspection of figure 2 suggests it could be driven by a positive TL–mortality risk association from a TRF-based study of water pythons (Liasis fuscus) [50]. We did not detect a significant difference between the sexes (0.093 ± 0.076; p = 0.22) and there was no significant relationship with maximum lifespan (0.034 ± 0.104; p = 0.75), follow-up period (0.010 ± 0.006; p = 0.118) or age at sampling (0.131 ± 0.097; p = 0.177; figure 4). However, telomere measurement method explained a significant portion of the observed study heterogeneity (11.2%). The negative association between TL and mortality risk was significantly stronger in studies using qPCR relative to TRF methods (difference TRF–qPCR: −0.260 ± 0.090; p = 0.004; figure 4).
Figure 4.
Natural logarithm of hazard ratio of moderator variables. Grouping differences are expressed as follows: Sex: male–female; TL method: TRF–qPCR; age group: adult–juvenile. Follow-up and the log of lifespan were tested as continuous variable in years. Bars indicate the 95% confidence intervals.
To explore this further, we split the data by method and ran separate models without moderators. Within the qPCR studies, the TL–mortality risk association was highly significantly negative with significant heterogeneity among studies (−0.331 ± 0.068, p < 0.001; Q = 52.41, p < 0.001), while there was no significant overall association or evidence for heterogeneity in TRF studies (−0.056 ± 0.042, p = 0.183, Q = 16.62, p = 0.120). Since we detected a non-significant trend for a weaker TL–mortality risk association in reptiles, but have very limited power to differentiate effect sizes in non-avian classes (table 1), we re-ran our analyses including only bird studies. We found a similarly negative and significant overall effect (−0.224 ± 0.050; p < 0.001), but the method moderator effect became weaker and marginally non-significant in this dataset (difference TRF–qPCR: −0.184 ± 0.099; p = 0.063), suggesting that the strongly positive TL–mortality risk estimate from the python study was at least in part responsible for the method effect we observed.
4. Discussion
We found that short TL was associated with increased risk of mortality, and that this result is robust to correction for evident publication bias. While many recent papers have cited a handful of salient examples as evidence for such a general pattern, here we provide the first formal test to support a TL–mortality association across studies of non-human vertebrates. While our results provide important overarching support for the importance of TL as a biomarker within ecological and evolutionary studies, they also highlight several important issues for the rapidly emerging literature on telomere dynamics in non-model vertebrate systems. First, the overall effect size was small and showed considerable heterogeneity among studies. Evidence of publication bias in our analyses argues that particular effort should be directed at supporting the unbiased publication of both non-confirmative and confirmative findings in future research in this area. Second, the lack of suitable studies in mammals and ectothermic vertebrates means that we cannot currently generalize the overall TL–mortality risk association beyond birds, and more research effort into the links between TL and fitness is clearly required in non-avian vertebrate species. Finally, the presence of an unexpected effect of telomere measurement method on the strength of the TL–mortality risk association highlights the recurrent issue within this field posed by the application of differing methodologies across studies. More studies which apply both qPCR and TRF methods side-by-side within single studies are required to help understand the reasons for these method effects.
Our meta-analysis provides strong support for the proposition that short TL predicts increased mortality risk in birds, but the generality of this pattern across all vertebrate species remains an important and open question. Although telomeres perform a crucial conserved function across eukaryotes, telomere dynamics and levels of expression of telomerase in somatic tissues vary widely among taxa [5]. In ectothermic vertebrates, telomerase expression is frequently observed in somatic tissues and this is thought to be due to the indeterminate growth of many of these taxa [5]. There is evidence for complex telomere dynamics with age in ectotherms, with studies of wild reptiles demonstrating increases in average blood cell TL through early life followed by a plateau or decline [50,51]. Recent studies of laboratory fish suggest that somatic telomerase expression can be detected throughout life, and that TL and telomerase expression can increase during development and adolescence before plateauing or declining in later adulthood [56,57]. In mammals, variation in telomerase expression has been attributed to body size and cancer prevention: telomerase is repressed in somatic cells in larger-bodied species but not in smaller ones [13,14]. In birds, although variation in somatic telomerase expression has been observed [58], it seems to be widely accepted that somatic telomerase expression is limited and the situation resembles that in large-bodied mammals [5]. These differences are further complicated by the fact that mammals have enucleated red bloods cells and so blood cell TL is measured in leucocytes, while in other vertebrate classes it is measured very predominantly in erythrocytes. In our analyses, we found a suggestive trend for weaker TL–mortality risk associations in reptiles compared to birds that may have been driven by a single outlying study. That study, of water pythons, found that in adults long, rather than short, TL was significantly associated with increased mortality risk [50], suggesting that an inversion of the relationship we observed more widely in birds may occur in some ectothermic species. It is worth noting that the observed negative relationship in pythons could be driven by age differences in TL among recaptured and non-recaptured adults, if younger adults have both longer telomeres and are less likely to survive to recapture than older adults. However, a very recent study—published after our literature search was completed—showed a similar effect in wild Atlantic salmon: juveniles with short TL were more likely to survive to recapture at return migration to natal rivers in adults [59]. Studies from a wider range of mammalian and ectothermic vertebrate species relating TL to fitness will help understand when and why such positive associations might occur, and it may prove that null or positive association is the norm in small mammals and ectotherms, in which somatic telomerase expression may counteract any signal of cumulative stress or past life history on TL shortening.
We found that studies using qPCR methods detected a stronger overall association between TL and mortality risk, and greater heterogeneity in this relationship compared to TRF studies. This method difference in the overall association is surprising given that the qPCR method has been demonstrated to be less technically repeatable than TRF [60]. However, we note that technical variation in telomere assays is likely to vary greatly across laboratories and is rarely reported in a consistent enough way to make accounting for it possible in meta-analysis. As in the human literature, the qPCR methodology is progressively becoming the dominant method in non-model vertebrate studies, presumably because it is higher throughput and less expensive [29,30]. One possible driver of the stronger overall effect in qPCR studies could be differential publication bias among studies using this method, which is suggested by the presence of three qPCR-based studies outside the lower left end of the funnel plot (figure 3). The risk of publication bias could be expected to be stronger within qPCR studies, which are generally easier to set up and quicker and cheaper to run, compared to TRF studies [29,30]. TRF data are not just harder won in the laboratory, but also more informative as they measure the variation in TL within a sample, allowing a wider range of questions to be addressed [29,30]. Thus, researchers using TRF may be more inclined and readily able to publish non-confirmatory and opposing findings. However, it is also important to keep in mind that the two methods measure slightly different things: TRF quantifies the mean length of telomere sequence in the sample, qPCR the total quantity of telomere sequence present. It is possible that, because TL distributions within samples may be highly skewed and this will affect TRF measures more than qPCR measures, the latter method may provide estimates of TL that are better predictors of organismal health and fitness. Finally, we found hints that the method effect could be driven by taxonomic bias in our estimates. We found suggestive evidence that the effect was in part driven by a TRF-based reptile study that documented a significant positive association between TL and mortality risk (figure 2). However, without more studies of the TL–mortality risk association in ectothermic vertebrates using different methods it is impossible to dissect this suggestion further. The presence of an unexpected methodological difference in the association between TL and mortality risk highlights the need for more studies that apply both qPCR and TRF techniques to the same samples to understand how and why results might differ with methodology.
We found no evidence for effects of sex, age group or follow-up time on the association between TL and mortality risk. In humans and a handful of other mammals investigated to date, a general trend of longer TL in females than males has been observed and related to sex differences in lifespan commonly documented in polygynous species [61–63] (although see [64]). The vast majority of the studies included in our meta-analysis came from bird species, which tend to be monogamous and in which there remains limited evidence for sex differences in TL and lifespan [32]. To our knowledge, only one study to date has reported sex differences in the relationship between TL and lifetime reproductive fitness in any wild vertebrate and this study was in a polygynous reptile [33]. Although our analyses support the lack of a sex effect on the TL–mortality risk association in birds, further investigation of sex differences in systems exhibiting polygynous mating systems and sexual dimorphism is required before drawing any conclusions about the phylogenetic generality of this pattern. A previous meta-analysis found that the TL–mortality risk association declines with age within studies of healthy adult humans [9]. This result was interpreted as support for TL representing a better marker of the failure of somatic redundancy mechanisms rather than of biological ageing [9]. The fact that we found similar TL–mortality risk associations in both studies of juvenile and adults would support the idea that TL is not necessarily a biomarker of biological ageing. Furthermore, the lack of any association between TL–mortality risk association and species' maximum recorded lifespan suggests the observed association is not specific to particularly long- or short-lived bird species in our sample. Finally, the lack of any effect of follow-up period between TL measurement and assessment of mortality or recapture in our selected studies implies that TL predicts mortality just as well over short periods (e.g. to the subsequent year or breeding season) as it does over multiple years.
Our results provide support for the prediction that shorter TLs are associated with increased mortality risk in birds, but an important further question raised by this is what processes are responsible for this pattern. The association could be the result of individual differences, associated with genetic, epigenetic or developmental variation, which generate consistent differences in both TL and mortality risk across the lifetimes of individuals. In addition, cumulative experience of environmental stress or investment in growth and reproduction could simultaneously drive telomere shortening and increase mortality risk. The importance of among-individual differences in TL versus telomere shortening as predictors of mortality risk, while not mutually exclusive, remains a major question for researchers interested in telomere dynamics at the whole organism level. The human literature reveals TL to be moderately to highly heritable [21] and that self-reported experience of stressful events is associated with shorter TL [65]. One longitudinal study of different populations reported extremely high repeatability of TL within individuals across a period of a decade or so and argued that most of the variation in TL could therefore be attributed to genetic or early life factors [20]. The literature on non-model vertebrates, again very predominantly from birds, does offer evidence that rapid growth and physiological stress are associated with shorter TL [44,66]. However, estimates of the heritability of TL are very variable and often associated with very large confidence intervals, suggesting issues with power [67]. Furthermore, while some studies have identified telomere shortening as a predictor of survival [38,47], there is also evidence for associations between an individual's average TL and their lifespan [22,40,47]. Longitudinal studies capable of testing the degree to which survival and longevity are predicted by an individual's lifetime average TL or their rate of telomere attrition are now required to address this important question. Such studies can also help to establish whether TL measured in early life represents a better predictor of subsequent lifespan than later TL, as recently found in captive zebra finches (Taeniopygia guttata) [25]. In due course, the application of meta-analytic methods to the results of such longitudinal studies can provide consensus regarding when and how variation in TL predicts key components of organismal fitness.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
This meta-analysis would not have been possible without the tremendous effort and support of all the authors of the papers included in this study, who were willing to provide either the raw data or coxph model outputs. Along with the many co-authors involved in these studies, we would like to specifically thank the following, in no particular order, for providing data or model outputs: Frederic Angelier, Christina Bauch, Michael Beaulieu, Staffan Bensch, Amos Belmaker, Winnie Boner, Francois Criscuolo, Mark Haussmann, Britt Heindinger, Thomas Madsen, Pat Monaghan, Mats Olsson, Jenny Ouyang, Sophie Reichert, Simon Verhulst, Martijn Salomons, Antoine Stier, Joanna Sudyka, Beat Ujvari, Hannah Watson and David Winkler. We also would like to thank Sebastiano Manrico, Joanna Sudyka and Dustin Penn for providing data that were not able to include in our meta-analyses, as well as the authors who published their data online [22,39,49,51]. Finally, we thank all attendees at the Leverhume Trust-funded ‘Diversity in telomere dynamics' meetings for the many discussions that inspired this piece of work.
Data accessibility
Data used in the meta-analyses are published as the electronic supplementary material.
Authors' contributions
All authors participated in the conception and design of the study. R.V.W. conducted the initial literature searches, and R.V.W. and J.P.M. screened the literature for eligibility. R.V.W., J.J.B. and D.H.N. collated the data and performed the standardized analyses on raw data. R.V.W., J.J.B., H.F. and D.H.N. performed the analyses and wrote the first draft of the paper. All authors commented on the first draft and contributed to the final draft.
Competing interests
We have no competing interests.
Funding
This work was inspired and supported by meetings and participants in a Leverhulme Trust-funded International Network. R.V.W., H.F. and D.H.N. were supported by a BBSRC responsive mode grant (BB/L020769/1). J.P.M. was funded by the BBSRC through the EASTBIO Doctoral Training Partnership (BB/J01446X/1).
References
- 1.Blackburn EH. 1991. Structure and function of telomeres. Nature 350, 569–573. ( 10.1038/350569a0) [DOI] [PubMed] [Google Scholar]
- 2.De Lange T. 2004. T-loops and the origin of telomeres. Nat. Rev. Mol. Cell Biol. 5, 323–329. ( 10.1038/nrm1359) [DOI] [PubMed] [Google Scholar]
- 3.Blackburn EH, Epel ES, Lin J. 2015. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 350, 1193–1198. ( 10.1126/science.aab3389) [DOI] [PubMed] [Google Scholar]
- 4.von Zglinicki T. 2002. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344. ( 10.1016/S0968-0004(02)02110-2) [DOI] [PubMed] [Google Scholar]
- 5.Gomes NMV, Shay JW, Wright WE. 2010. Telomere biology in metazoa. FEBS Lett. 584, 3741–3751. ( 10.1016/j.febslet.2010.07.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging. Cell 153, 1194–1217. ( 10.1016/j.cell.2013.05.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simons MJ. 2015. Questioning causal involvement of telomeres in aging. Ageing Res. Rev. 24, 191–196. ( 10.1016/j.arr.2015.08.002) [DOI] [PubMed] [Google Scholar]
- 8.Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM. 2012. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 8, e1002696 ( 10.1371/journal.pgen.1002696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boonekamp JJ, Simons MJ, Hemerik L, Verhulst S. 2013. Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell 12, 330–332. ( 10.1111/acel.12050) [DOI] [PubMed] [Google Scholar]
- 10.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. 2014. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 349, g4227 ( 10.1136/bmj.g4227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. 2011. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol. Prev. Biomarkers 20, 1–13. ( 10.1158/1055-9965.EPI-11-0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willeit P, et al. 2014. Leucocyte telomere length and risk of type 2 diabetes mellitus: new prospective cohort study and literature-based meta-analysis. PLoS ONE 9, e112483 ( 10.1371/journal.pone.0112483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes NMV, et al. 2011. Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell 10, 761–768. ( 10.1111/j.1474-9726.2011.00718.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorbunova V, Seluanov A, Zhang Z, Gladyshev VN, Vijg J. 2014. Comparative genetics of longevity and cancer: insights from long-lived rodents. Nat. Rev. Genet. 15, 531–540. ( 10.1038/nrg3728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haussmann MF, Marchetto NM. 2010. Telomeres: linking stress and survival, ecology and evolution. Curr. Zool. 56, 714–727. [Google Scholar]
- 16.Monaghan P. 2010. Crossing the great divide: telomeres and ecology. Heredity 105, 574–575. ( 10.1038/hdy.2010.120) [DOI] [PubMed] [Google Scholar]
- 17.Monaghan P. 2014. Organismal stress, telomeres and life histories. J. Exp. Biol. 217, 57–66. ( 10.1242/jeb.090043) [DOI] [PubMed] [Google Scholar]
- 18.Bateson M. 2016. Cumulative stress in research animals: telomere attrition as a biomarker in a welfare context? Bioessays 38, 201–212. ( 10.1002/bies.201500127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monaghan P, Haussmann MF. 2006. Do telomere dynamics link lifestyle and lifespan? Trends Ecol. Evol. 21, 47–53. ( 10.1016/j.tree.2005.11.007) [DOI] [PubMed] [Google Scholar]
- 20.Benetos A, et al. 2013. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell 12, 615–621. ( 10.1111/acel.12086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broer L, et al. 2013. Meta-analysis of telomere length in 19 713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur. J. Hum. Genet. 21, 1163–1168. ( 10.1038/ejhg.2012.303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett ELB, Burke TA, Hammers M, Komdeur J, Richardson DS. 2013. Telomere length and dynamics predict mortality in a wild longitudinal study. Mol. Ecol. 22, 249–259. ( 10.1111/mec.12110) [DOI] [PubMed] [Google Scholar]
- 23.Haussmann MF, Winkler DW, Vleck CM. 2005. Longer telomeres associated with higher survival in birds. Biol. Lett. 1, 212–214. ( 10.1098/rsbl.2005.0301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bize P, Criscuolo F, Metcalfe NB, Nasir L, Monaghan P. 2009. Telomere dynamics rather than age predict life expectancy in the wild. Proc. R. Soc. B 276, 1679–1683. ( 10.1098/rspb.2008.1817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaulieu M, Reichert S, Le Maho Y, Ancel A, Criscuolo F. 2011. Oxidative status and telomere length in a long-lived bird facing a costly reproductive event. Funct. Ecol. 25, 577–585. ( 10.1111/j.1365-2435.2010.01825.x) [DOI] [Google Scholar]
- 27.Reichert S, Stier A, Zahn S, Arrivé M., Bize P, Massemin S, Criscuolo F. 2014. Increased brood size leads to persistent eroded telomeres. Front. Ecol. Evol. 2, 9 ( 10.3389/fevo.2014.00009) [DOI] [Google Scholar]
- 28.Sudyka J, Arct A, Drobniak S, Dubiec A, Gustafsson L, Cichon M. 2014. Experimentally increased reproductive effort alters telomere length in the blue tit (Cyanistes caeruleus). J. Evol. Biol. 27, 2258–2264. ( 10.1111/jeb.12479) [DOI] [PubMed] [Google Scholar]
- 29.Aubert G, Hills M, Lansdorp PM. 2012. Telomere length measurement: caveats and a critical assessment of the available technologies and tools. Mutat. Res. 730, 59–67. ( 10.1016/j.mrfmmm.2011.04.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nussey DH, et al. 2014. Measuring telomere length and telomere dynamics in evolutionary biology and ecology. Methods Ecol. Evol. 5, 299–310. ( 10.1111/2041-210X.12161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watson H, Bolton M, Monaghan P. 2015. Variation in early-life telomere dynamics in a long-lived bird: links to environmental conditions and survival. J. Exp. Biol. 218, 668–674. ( 10.1242/jeb.104265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrett EL. B, Richardson DS. 2011. Sex differences in telomeres and lifespan. Aging Cell 10, 913–921. ( 10.1111/j.1474-9726.2011.00741.x) [DOI] [PubMed] [Google Scholar]
- 33.Olsson M, Pauliny A, Wapstra E, Uller T, Schwartz T, Miller E, Blomqvist D. 2011. Sexual differences in telomere selection in the wild. Mol. Ecol. 20, 2085–2099. ( 10.1111/j.1365-294X.2011.05085.x) [DOI] [PubMed] [Google Scholar]
- 34.Angelier F, Vleck CM, Holberton RL, Marra PP. 2013. Telomere length, non-breeding habitat and return rate in male American redstarts. Funct. Ecol. 27, 342–350. ( 10.1111/1365-2435.12041) [DOI] [Google Scholar]
- 35.Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S. 2015. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347, 436–438. ( 10.1126/science.1261121) [DOI] [PubMed] [Google Scholar]
- 36.Bauch C, Becker PH, Verhulst S. 2014. Within the genome, long telomeres are more informative than short telomeres with respect to fitness components in a long-lived seabird. Mol. Ecol. 23, 300–310. ( 10.1111/mec.12602) [DOI] [PubMed] [Google Scholar]
- 37.Belmaker A. 2016. The role of telomere length in tree swallow behavior and life history. PhD thesis, Cornell University, NY. [Google Scholar]
- 38.Boonekamp JJ, Mulder GM, Salomons HM, Dijkstra C, Verhulst S. 2014. Nestling telomere shortening, but not telomere length, reflects developmental stress and predicts survival in wild birds. Proc. R. Soc. B 282, 20133287 ( 10.1098/rspb.2013.3287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caprioli M, Romano M, Romano A, Rubolini D, Motta R, Folini M, Saino N. 2013. Nestling telomere length does not predict longevity, but covaries with adult body size in wild barn swallows. Biol. Lett. 9, 20130340 ( 10.1098/rsbl.2013.0340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fairlie J, Holland R, Pilkington JG, Pemberton JM, Harrington L, Nussey DH. 2016. Life-long leukocyte telomere dynamics and survival in a free-living mammal. Aging Cell 15, 140–148. ( 10.1111/acel.12417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foote CG. 2009. Avian telomere dynamics. PhD thesis, University of Glasgow. [Google Scholar]
- 42.Foote CG, Daunt F, Gonzalez-Solis J, Nasir L, Phillips RA, Monaghan P. 2011. Individual state and survival prospects: age, sex, and telomere length in a long-lived seabird. Behav. Ecol. 22, 156–161. ( 10.1093/beheco/arq178) [DOI] [Google Scholar]
- 43.Reichert S, et al. 2017. Telomere length measurement by qPCR in birds is affected by storage method of blood samples. Oecologia 184, 341–350. ( 10.1007/s00442-017-3887-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geiger S, Le Vaillant M, Lebard T, Reichert S, Stier A, Le Maho Y, Criscuolo F. 2012. Catching-up but telomere loss: half-opening the black box of growth and ageing trade-off in wild king penguin chicks. Mol. Ecol. 21, 1500–1510. ( 10.1111/j.1365-294X.2011.05331.x) [DOI] [PubMed] [Google Scholar]
- 45.Ouyang JQ, Lendvai ÃZ, Moore IT, Bonier F, Haussmann MF. 2016. Do hormones, telomere lengths, and oxidative stress form an integrated phenotype? A case study in free-living tree swallows. Integr. Comp. Biol. 56, 138–145. ( 10.1093/icb/icw044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reichert S, Criscuolo FO, Zahn S, Bize P, Massemin S. 2015. Immediate and delayed effects of growth conditions on ageing parameters in nestling zebra finches. J. Exp. Biol. 218, 491–499. ( 10.1242/jeb.109942) [DOI] [PubMed] [Google Scholar]
- 47.Salomons HM, Mulder GA, van de Zande L, Haussmann MF, Linskens MHK, Verhulst S. 2009. Telomere shortening and survival in free-living corvids. Proc. R. Soc. B 276, 3157–3165. ( 10.1098/rspb.2009.0517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stier A, et al. 2014. Starting with a handicap: phenotypic differences between early- and late-born king penguin chicks and their survival correlates. Funct. Ecol. 28, 601–611. ( 10.1111/1365-2435.12204) [DOI] [Google Scholar]
- 49.Taff CC, Freeman-Gallant CR. 2017. Sexual signals reflect telomere dynamics in a wild bird. Ecol. Evol. 7, 3436–3442. ( 10.1002/ece3.2948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ujvari B, Madsen T. 2009. Short telomeres in hatchling snakes: erythrocyte telomere dynamics and longevity in tropical pythons. PLoS ONE 4, e7493 ( 10.1371/journal.pone.0007493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ujvari B, Biro PA, Charters JE, Brown G, Heasman K, Beckmann C, Madsen T. 2016. Curvilinear telomere length dynamics in a squamate reptile. Funct. Ecol. 31, 753–759. ( 10.1111/1365-2435.12764) [DOI] [Google Scholar]
- 52.Therneau T. 2014. A package for survival analysis in S. R package version 2.37–4. See http://CRAN.R-project.org/package=survival.
- 53.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. ( 10.18637/jss.v036.i03) [DOI] [Google Scholar]
- 54.Olkin I. 1995. Meta-analysis: reconciling the results of independent studies. Stat. Med. 14, 457–472. ( 10.1002/sim.4780140507) [DOI] [PubMed] [Google Scholar]
- 55.Duval S, Tweedie R. 2000. Trim and fill: a simple funnel plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463. ( 10.1111/j.0006-341X.2000.00455.x) [DOI] [PubMed] [Google Scholar]
- 56.Hatakeyama H, et al. 2016. Telomere attrition and restoration in the normal teleost Oryzias latipes are linked to growth rate and telomerase activity at each life stage. Aging 8, 62 ( 10.18632/aging.100873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anchelin M, Murcia L, Alcaraz-Perez F, Garcia-Navarro EM, Cayuela ML. 2011. Behaviour of telomere and telomerase during aging and regeneration in zebrafish. PLoS ONE 6, e16955 ( 10.1371/journal.pone.0016955) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haussmann MF, Winkler DW, Huntington CE, Nisbet ICT, Vleck CM. 2007. Telomerase activity is maintained throughout the lifespan of long-lived birds. Exp. Gerontol. 42, 610–618. ( 10.1016/j.exger.2007.03.004) [DOI] [PubMed] [Google Scholar]
- 59.McLennan D, Armstrong JD, Stewart DC, Mckelvey S, Boner W, Monaghan P, Metcalfe NB. 2017. Shorter juvenile telomere length is associated with higher survival to spawning in migratory Atlantic salmon. Funct. Ecol. 31, 2070–2079. ( 10.1111/1365-2435.12939) [DOI] [Google Scholar]
- 60.Aviv A, Hunt SC, Lin J, Cao XJ, Kimura M, Blackburn E. 2011. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 39, e134 ( 10.1093/nar/gkr634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, Martin-Ruiz C, Shiels P. 2014. Gender and telomere length: systematic review and meta-analysis. Exp. Gerontol. 51, 15–27. ( 10.1016/j.exger.2013.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cherif H, Tarry J, Ozanne S, Hales C. 2003. Ageing and telomeres: a study into organ- and gender-specific telomere shortening. Nucleic Acids Res. 31, 1576–1583. ( 10.1093/nar/gkg208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watson R, et al. 2017. Sex differences in leukocyte telomere length in a free-living mammal. Mol. Ecol. 26, 3230–3240. ( 10.1111/mec.13992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beirne C, Delahay R, Hares M, Young A. 2014. Age-related declines and disease-associated variation in immune cell telomere length in a wild mammal. PLoS ONE 9, e108964 ( 10.1371/journal.pone.0108964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schutte NS, Malouff JM. 2016. The relationship between perceived stress and telomere length: a meta-analysis. Stress Health 32, 313–319. ( 10.1002/smi.2607) [DOI] [PubMed] [Google Scholar]
- 66.Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. 2012. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc. R. Soc. B 279, 1447–1456. ( 10.1098/rspb.2011.1913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reichert S, Rojas E, Zahn S, Robin JP, Criscuolo F, Massemin S. 2015. Maternal telomere length inheritance in the king penguin. Heredity 114, 10–16. ( 10.1038/hdy.2014.60) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in the meta-analyses are published as the electronic supplementary material.