1. Introduction
In his insightful article, Speijer [1] discussed the origin of sex under multiple points of view, providing a comprehensive and balanced overview of different theories and hypotheses. However, we think that the section ‘Higher mutational loads in one gamete type and retention of uniparental mitochondrial inheritance’ (§9) needs some clarification. Speijer [1] elaborates on the correlation between gamete metabolic and physiological differences and their organellar contribution across generations. Specifically, he quotes the ‘division of labour’ hypothesis, which postulates that male gametes maximize energy production for motility by sacrificing mitochondrial DNA (mtDNA) to oxidative phosphorylation (OXPHOS) and its mutagenic by-products, while non-motile female gametes repress OXPHOS, thus being somewhat inactive [2]. Basically, we clarify two discussion points: (i) the exceptions to the strictly maternal inheritance (SMI) of mitochondria and (ii) the claim that mtDNA is highly mutated in sperm and the supposed causal relationship between such damage and OXPHOS.
2. Exceptions to the rule
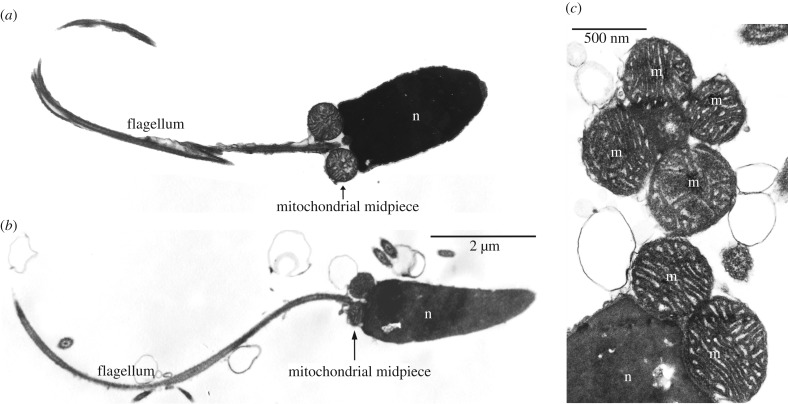
Exceptions to SMI by which bioenergetically active mitochondria are stably inherited through generations might represent a challenge for the division of labour hypothesis. Doubly uniparental inheritance (DUI) is the only known evolutionarily stable exception to the SMI typical of Metazoa. In DUI animals (approx. 100 species of gonochoric bivalve molluscs identified so far [3]), two mitochondrial lineages are inherited, one through eggs (F-type) and the other through sperm (M-type). Eggs are homoplasmic for the F-type, while spermatozoa are homoplasmic for the M-type. These ‘mother-to-daughter’ and ‘father-to-son’ mitochondrial lineages have evolved independently for millions of years (e.g. more than 200 Myr in unionids), accumulating up to 40% of DNA sequence divergence. Since eggs do not transmit the M-type, germ line mitochondria of DUI males are apportioned from the four/five mitochondria of the fertilizing spermatozoon, which carry mtDNA that must be functional and successfully inherited [4]. It is clear that the long evolutionary persistence of DUI—as inferred from the nucleotide divergence between conspecific sex-linked mtDNAs—indicates that the mtDNA transmitted through sperm can be a viable genetic template. Speijer [1] highlighted that ‘upon loss of the need for highly active sperm cells during the life cycle of an organism the strong purging of paternal mitochondria might on occasion be relaxed’ (p. 7), and—referring to DUI species of the family Mytilidae (sea mussels) and Unionidae (freshwater mussels)—claimed that the presence of paternal mtDNA inheritance in such animals can be explained by the absence of mitochondrial activity in sperm. According to Speijer [1], ‘Mussel sperm is taken along by water currents, and the energy for the final entry of the female is provided by the female incurrent siphon’ (p. 7), thus actually avoiding the proposed reactive oxygen species (ROS) generation and its consequences. In this respect, we have to clarify that spermatozoa of DUI bivalves have a well-formed flagellum, a midpiece that contains mitochondria with normal appearance (figure 1; see also [5]), and, most importantly, they actively swim. Bivalve sperm is motile—as can be easily assessed by optical microscope observation—irrespective of the type of reproduction (oviparous or larviparous), and sperm motility is needed for fertilization to take place [6,7]. Moreover, OXPHOS is required to sustain the long motility phase of Pacific oyster spermatozoa (Crassostrea gigas) [8], and spermatozoa swim to reach eggs guided by egg-produced chemoattractants and not simply carried by water currents. In the DUI marine mussel Mytilus galloprovincialis, Evans et al. [9] showed that spermatozoa exploit such chemical cues to preferentially swim towards eggs, and the presence of chemoattractants not only in bivalves, but also in several marine organisms, supports this as a shared feature of broadcast spawning animals [9–17] (table 1).
Figure 1.
Morphology of spermatozoa in bivalves that transmit sperm mitochondria to the progeny. (a) Spermatozoon of a freshwater mussel (Elliptio fumata, family Unionidae). (b) Spermatozoon of a marine clam (Ruditapes philippinarum, family Veneridae). (c) Magnification of R. philippinarum mitochondrial midpiece (transversally and longitudinally sectioned). n, nucleus; m, mitochondrion.
Table 1.
Chemotaxis and sperm swimming.
| species | phenomenon | reference |
|---|---|---|
| molluscs | ||
| Sepia officinalis | species-specific sperm chemotaxis | [10] |
| Haliotis rufescens | species-specific sperm chemotaxis | [11] |
|
Mytilus edulis Mytilus galloprovincialis |
assortative mating through gamete preference | [12] |
| M. galloprovincialis | sperm chemotaxis promoting sperm–egg encounters and facilitating species recognition; chemoattractants' unforeseen role in sexual selection by enabling sperm to ‘choose’ between the eggs of different conspecific females—mediating mate choice for genetically compatible partners |
[9] |
| M. galloprovincialis | egg chemoattraction also promotes changes in sperm behaviour and physiology; gamete ‘preferences’—sperm from individual males consistently swim towards (and fertilize) the eggs of certain females |
[13] |
| other invertebrate broadcast spawners | ||
| sperm chemotaxis—attractants and stimulators of sperm motility and respiration | [14] | |
| chemotactic signalling of sperm from marine invertebrates—sperm adjust their swimming path in a gradient of a chemical factor released by the egg | [15] | |
| species-specific sperm chemotaxis—preventing crossbreeding, especially in marine invertebrates with external fertilization | [16] | |
| Strongylocentrotus purpuratus | speract: egg peptide that regulates sperm motility and stimulates sperm mitochondrial metabolism | [17] |
3. Mitochondrial activity and mtDNA mutations
The foundation of the division of labour hypothesis is the assumption that high ATP generation in sperm leads to high ROS formation and thus to mtDNA mutations, with a consequent strong selection for the exclusion of the highly mutated paternal mtDNA from the filial generation. We point out that the correlation between OXPHOS, ROS formation and mtDNA mutations is still a matter of debate and that no clear-cut evidence unambiguously supports it. In the past decade, many studies failed to support a causal link between high OXPHOS activity and generation of hazardous amounts of ROS, so caution is advised (see [18] for a detailed discussion). The high energy demand for flagellar movements may even produce a lower amount of ROS (compared to ‘basal’ ROS production), as documented during high exercise activity [19]. In addition, it is important to mention that high levels of ROS production may inhibit sperm motility [20,21]. In DUI animals, it appears that the mtDNA is not accumulating damage faster in motile gametes, thus not being degraded on evolutionary time scales. Accordingly, there is no sign of genetic decay in the sperm-transmitted mitochondrial genome such as, for example, nonsense mutations or pseudogenization, even though multiple studies found that M-type mitochondria are clearly functional—e.g. they show a high membrane potential [18], active replication [22], transcription [18,23] and translation ([24,25] and F. Ghiselli & L. Milani 2017, unpublished results, for OXPHOS proteins)—and they do OXPHOS (S. Bettinazzi, L. Milani, E. Rodriguez, P. Blier, S. Breton 2017, unpublished results). Interestingly, Ghiselli et al. [23] used a high-throughput approach to assess the amount and type of polymorphism in the gonadal mitochondrial populations of the DUI species Ruditapes philippinarum, showing that F- and M-type mtDNAs have about the same amount of single nucleotide polymorphisms (SNPs) (actually F-type has more SNPs than M-type), and, most strikingly, M-type has significantly less SNPs with highly deleterious effects, compared to F-type. These results can be explained by two observations: (i) sperm mitochondria in DUI species are subject to selection for fundamental male functions such as spermatogenesis and fertilization performance; and (ii) selection on sperm mitochondria is more effective due to a much lower mtDNA copy number per gamete (discussed also in [18]).
Lastly, Speijer [1] also cites the ‘parent switching’, a mechanism known, among people studying DUI, as ‘role-reversal’ or ‘masculinization’ of F-type mtDNA. Role-reversal consists of F genomes that invade the male gonad, assume the role of the M genome and become sperm-transmitted. We want to point out that this phenomenon has been documented only in species of the Mytilus edulis complex, which are known to hybridize frequently—with all the expected alteration of mitochondrial heredity mechanisms (e.g. [26])—and for which DUI disruption has been reported [27] (for further details on this topic, see the discussion with Nick Lane under ‘Reviewers’ comment and response’ in [18]).
4. Conclusion
Is the DUI system undermining the division of labour hypothesis? More work is needed to assess this point, and there are at least two possibilities by which this would not be the case: (i) DUI species might use alternative energy-production pathways (e.g. [28]) and/or produce less ROS; (ii) DUI species might have evolved specific mechanisms of ROS scavenging and/or mtDNA protection. Such possibilities are under investigation. If, on the other hand, conclusive evidence about the lack of causation between energy production in sperm mitochondria and generation of hazardous amounts of ROS is eventually provided, then the division of labour as a general hypothesis to explain the evolution of anisogamy and of two sexes would be falsified. What insights can an evolutionarily derived trait as DUI provide about more general biological patterns such as the evolution of sex? Although DUI might look like a weird exception to a quite conserved biological ‘rule’, molecular and phylogenetic evidence suggests that it evolved from SMI (DUI can revert to SMI under some circumstances [3,18,29]) so, most likely, the two systems share the same basic molecular mechanism of mitochondrial inheritance. DUI has been proposed to be the result of a resolved genomic conflict triggered by a mitochondrial selfish element [30], and its apparently unusual mechanism of sperm mitochondria segregation into male germ line could be explained by their high membrane potential and other factors causing their retention, instead of the common degradation [31]. As stated by Speijer [1], the selective pressure behind the evolution of anisogamy could also be the avoidance of genomic conflicts [32] (but see [33] for additional, not mutually-exclusive explanations for the evolution of anisogamy). In SMI species, the elimination of sperm mitochondria might have been selected to avoid genomic conflicts, whereas in DUI species the same effect might have been obtained by segregating the competing mitochondrial lineages in two gamete types (see [30] for a detailed discussion on DUI origin). The DUI system seems to favour the hypothesis of genomic conflicts as the trigger for the evolution of uniparental inheritance and anisogamy over other explanations. Biology is extremely complex, and a greater effort should be made to elucidate an important and, in our opinion, overlooked topic such as the role of mitochondria in germ line evolution. Such effort should include as many taxa as possible, and, under this light, DUI organisms can be helpful in a similar way that mutants are central to understanding genetics.
Footnotes
The accompanying reply can be viewed at http://dx.doi.org/10.1098/rstb.2015.0530; http://dx.doi.org/10.1098/rstb.2017.0278.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by Italian Ministry of Education, University and Research MIUR-SIR Programme (grant no. RBSI14G0P5) funded to L.M., MIUR-FIR Programme (grant no. RBFR13T97A) funded to F.G. and FRQNT Program (grant no. 2015-NC-180242) to S.B.
References
- 1.Speijer D. 2016. What can we infer about the origin of sex in early eukaryotes? Phil. Trans. R. Soc. B 371, 20150530 ( 10.1098/rstb.2015.0530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JF. 1996. Separate sexes and the mitochondrial theory of ageing. J. Theor. Biol. 180, 135–140. ( 10.1006/jtbi.1996.0089T) [DOI] [PubMed] [Google Scholar]
- 3.Gusman A, Lecomte S, Stewart DT, Passamonti M, Breton S. 2016. Pursuing the quest for better understanding the taxonomic distribution of the system of doubly uniparental inheritance of mtDNA. PeerJ 4, e2760 ( 10.7717/peerj.2760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zouros E. 2013. Biparental inheritance through uniparental transmission: the doubly uniparental inheritance (DUI) of mitochondrial DNA. Evol. Biol. 40, 1–31. ( 10.1007/s11692-012-9195-2) [DOI] [Google Scholar]
- 5.Williams JD, Bogan AE, Garner JT. 2008. Freshwater mussels of Alabama and the mobile basin in Georgia, Mississippi and Tennessee. Tuscaloosa, AL: University of Alabama Press. [Google Scholar]
- 6.McMahon RF, Bogan AE. 2001. Mollusca: Bivalvia. In Ecology and classification of north American freshwater invertebrates, 2nd edn (eds Thorp JH, Covich AP), pp. 331–429 San Diego, CA: Academic Press. [Google Scholar]
- 7.Helm MM, Bourne N. 2004. Part 4. Hatchery operation: broodstock conditioning, spawning and fertilization. In Hatchery culture of bivalves: a practical manual (ed. Lovatelli A.). FAO Fisheries Technical Paper 471 See http://www.fao.org/docrep/007/y5720e/y5720e09.htm. [Google Scholar]
- 8.Boulais M, Soudant P, Le Goic N, Quere C, Boudry P, Suquet M. 2015. Involvement of mitochondrial activity and OXPHOS in ATP synthesis during the motility phase of spermatozoa in the Pacific oyster, Crassostrea gigas. Biol. Reprod. 93, 118 ( 10.1095/biolreprod.115.128538) [DOI] [PubMed] [Google Scholar]
- 9.Evans JP, Fracisco Garcia-Gonzalez F, Almbro M, Robinson O, Fitzpatrick JL. 2012. Assessing the potential for egg chemoattractants to mediate sexual selection in a broadcast spawning marine invertebrate. Proc. R. Soc. B 279, 2855–2861. ( 10.1098/rspb.2012.0181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zatylny C, Marvin L, Gagnon J, Henry J. 2002. Fertilization in Sepia officinalis: the first mollusk sperm-attracting peptide. Biochem. Biophys. Res. Comm. 296, 1186–1193. ( 10.1016/S0006-291X(02)02036-3) [DOI] [PubMed] [Google Scholar]
- 11.Riffell JA, Krug PJ, Zimmer RK. 2002. Fertilization in the sea: the chemical identity of an abalone sperm attractant. J. Exp. Biol. 205, 1439–1450. [DOI] [PubMed] [Google Scholar]
- 12.Bierne N, David P, Boudry P, Bonhomme F. 2002. Assortative fertilization and selection at larval stage in the mussels Mytilus edulis and M. galloprovincialis. Evolution 56, 292–298. ( 10.1111/j.0014-3820.2002.tb01339.x) [DOI] [PubMed] [Google Scholar]
- 13.Kekäläinen J, Larma I, Linden M, Evans JP. 2015. Lectin staining and flow cytometry reveals female-induced sperm acrosome reaction and surface carbohydrate reorganization. Sci. Rep. 5, 15321 ( 10.1038/srep15321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenbach M. 1999. Sperm chemotaxis. Rev. Reprod. 4, 56–66. ( 10.1530/ror.0.0040056) [DOI] [PubMed] [Google Scholar]
- 15.Kaupp UB, Hildebrand E, Weyand I. 2006. Sperm chemotaxis in marine invertebrates—molecules and mechanisms. J Cell. Physiol. 208, 487–494. ( 10.1002/jcp.20669) [DOI] [PubMed] [Google Scholar]
- 16.Yoshida M, Yoshida K. 2011. Sperm chemotaxis and regulation of flagellar movement by Ca2+. Mol. Hum. Reprod. 17, 457–465. ( 10.1093/molehr/gar041) [DOI] [PubMed] [Google Scholar]
- 17.García-Rincón J, Darszon A, Beltrán C. 2016. Speract, a sea urchin egg peptide that regulates sperm motility, also stimulates sperm mitochondrial metabolism. Biochim. Biophys. Acta 1857, 415–426. ( 10.1016/j.bbabio.2016.01.003) [DOI] [PubMed] [Google Scholar]
- 18.Milani L, Ghiselli F. 2015. Mitochondrial activity in gametes and transmission of viable mtDNA. Biol. Direct 10, 22 ( 10.1186/s13062-015-0057-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barja G. 2013. Updating the mitochondrial free radical theory of aging: an integrated view, key aspects, and confounding concepts. Antioxid. Redox Sign. 19, 1420–1445. ( 10.1089/ars.2012.5148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. 1997. Reactive oxygen species and sperm physiology. Rev. Reprod. 2, 48–54. ( 10.1530/ror.0.0020048) [DOI] [PubMed] [Google Scholar]
- 21.Baker MA, Aitken RJ. 2005. Reactive oxygen species in spermatozoa: methods for monitoring and significance for the origins of genetic disease and infertility. Reprod. Biol. Endocrinol. 3, 67 ( 10.1186/1477-7827-3-67) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerra D, Ghiselli F, Milani L, Breton S, Passamonti M. 2016. Early replication dynamics of sex-linked mitochondrial DNAs in the doubly uniparental inheritance species Ruditapes philippinarum (Bivalvia Veneridae). Heredity 116, 324–332. ( 10.1038/hdy.2015.105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghiselli F, Milani L, Guerra D, Chang PL, Breton S, Nuzhdin SV, Passamonti M. 2013. Structure, transcription and variability of metazoan mitochondrial genome: perspectives from an unusual mitochondrial inheritance system. Genome Biol. Evol. 5, 1535–1554. ( 10.1093/gbe/evt112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milani L, Ghiselli F, Maurizii MG, Nuzhdin SV, Passamonti M. 2014. Paternally transmitted mitochondria express a new gene of potential viral origin. Genome Biol. Evol. 6, 391–405. ( 10.1093/gbe/evu021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milani L, Ghiselli F, Pecci A, Maurizii MG, Passamonti M. 2015. The expression of a novel mitochondrially-encoded gene in gonadic precursors may drive paternal inheritance of mitochondria. PLoS ONE 10, e0137468 ( 10.1371/journal.pone.0137468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, Schatten G. 2000. Ubiquitinated sperm mitochondria, selective proteolysis, and the regulation of mitochondrial inheritance in mammalian embryos. Biol. Reprod. 63, 582–590. ( 10.1095/biolreprod63.2.582) [DOI] [PubMed] [Google Scholar]
- 27.Brannock PM, Roberts MA, Hilbish TJ. 2013. Ubiquitous heteroplasmy in Mytilus spp. resulting from disruption in doubly uniparental inheritance regulation. Mar. Ecol. Prog. Ser. 480, 131–143. ( 10.3354/meps10228T) [DOI] [Google Scholar]
- 28.Müller M, et al. 2012. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol. Mol. Biol. Rev. 76, 444–495. ( 10.1128/MMBR.05024-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breton S, Stewart DT, Shepardson S, Trdan RJ, Bogan AE, Chapman EG, Ruminas AJ, Piontkivska H, Hoeh WR. 2011. Novel protein genes in animal mtDNA: a new sex determination system in freshwater mussels (Bivalvia: Unionoida)? Mol. Biol. Evol. 28, 1645–1659. ( 10.1093/molbev/msq345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milani L, Ghiselli F, Passamonti M. 2016. Mitochondrial selfish elements and the evolution of biological novelties. Curr. Zool. 62, 687–697. ( 10.1093/cz/zow044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milani L. 2015. Mitochondrial membrane potential: a trait involved in organelle inheritance? Biol. Lett. 11, 20150732 ( 10.1098/rsbl.2015.0732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoekstra RF. 2011. Nucleo-cytoplasmic conflict and the evolution of gamete dimorphism. In The evolution of anisogamy: a fundamental phenomenon underlying sexual selection (eds Togashi T, Cox PA), p. 262 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 33.Togashi T, Cox PA. 2011. The evolution of anisogamy: a fundamental phenomenon underlying sexual selection. Cambridge, UK: Cambridge University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.