Abstract
Controversy exists regarding whether a short-term response has an impact on the long-term survival of cervical cancer patients undergoing neoadjuvant chemotherapy (NACT). This study was designed to identify the predictive role of an early response by pooling the results of previous studies. The PubMed and Embase databases were searched through July 2016, and the associations between an early response and disease-free survival (DFS) were pooled by hazard ratio (HR) using random effects models. Six studies involving 490 cervical cancer patients, with 336 responders and 154 non-responders, were finally included in the meta-analysis. The HR for 1-year DFS between early responders and non-responders was 0.25 (95% CI 0.10–0.58, P = 0.001). The HRs for 2-, 3-, 4-, and 5-year DFS were 0.28 (95% CI 0.15–0.56), 0.27 (95% CI 0.16–0.45), 0.29 (95% CI 0.17–0.50) and 0.33 (95% CI 0.20–0.54), respectively. No obvious heterogeneity was found among the studies, with I2 = 0, and a sensitivity analysis showed that all pooled results were robust with logHR confidence limits < 0. An early response was associated with DFS, and responders achieved a significantly higher survival rate than non-responders. This finding should be validated in future prospective studies.
Introduction
Cervical cancer is a common malignant tumor disease in females worldwide. According to the latest results, more than 527,600 new cases and 265,700 deaths were estimated to be attributed to this disease in 20121. Although concurrent chemo-radiotherapy (CCRT) is the traditional treatment, the side effects in women are severe, especially for young patients2,3. Additionally, patients have become increasingly younger in recent decades. Considering the severe side effects of CCRT, great efforts have been made to develop new therapeutic drugs and devices. In recent decades, doctors and clinicians have resorted to neoadjuvant chemotherapy (NACT) as an alternative treatment for cervical cancer4.
Previous studies have shown that NACT can help to reduce tumor size and cancer cell metastasis, thus making the malignant disease operable5. Many patients choose NACT plus surgery rather than radiotherapy to avoid sacrificing their quality of life; these patients can maintain vaginal and ovarian function, as well as their pelvic organ function. These advantages are particularly important for young or pregnant women who wish to preserve their fertility6. For patients with early stage cervical cancer, NACT can result in less invasive surgery. Using NACT, minimally invasive surgery, such as cervical conization and radical trachelectomy, can be performed to spare the uterus and reproductive function7.
Although a short-term response after treatment may allow patients to choose a more effective treatment regimen in many malignant tumor diseases8, the predictive role of the short-term response to NACT on long-term survival is still unclear for cervical cancer patients9,10. This study was designed to identify the prognostic role of a short-term response on the overall survival of cervical cancer patients submitted to NACT.
Results
Literature search
A total of 583 articles were found via searches using the key words described in the methods section (Fig. 1). During the first round of screening, 428 articles were excluded after reviewing the titles and abstracts. During the second round of screening, 127 articles were excluded, as these articles were all case reports or reviews. During the third round of screening, 22 articles were excluded for reasons such as duplicated data, intractable data with neither the HR nor the survival curve reported; papers were also excluded in which the RECIST criteria were not adopted. After all three rounds of screening, 6 articles were included for further evaluation and were eventually included in the combined analysis.
Figure 1.
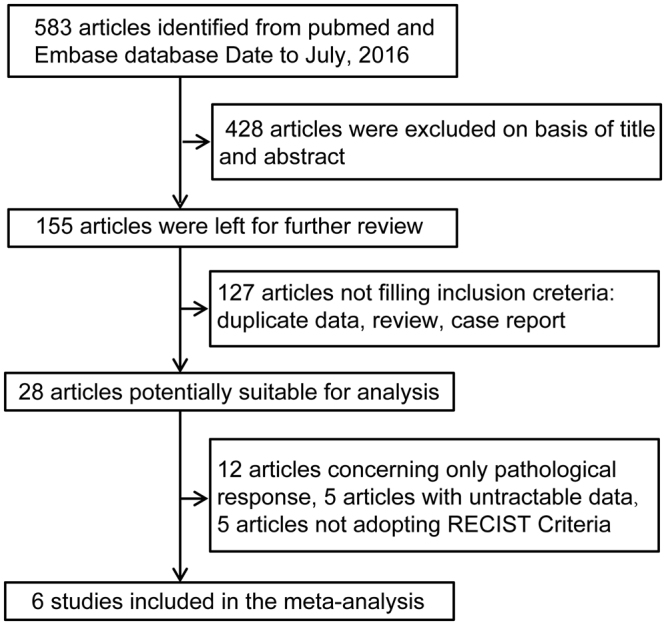
Flow chart of the meta-analysis. Studies using RECIST criteria were included; otherwise, they would be excluded. RECIST indicates Response Evaluation Criteria in Solid Tumor.
Relationship between the clinical response and disease-free survival (DFS)
Characteristics of the studies
The details of the included studies are listed in Table 1. The table reveals the association between the clinical response and DFS, either with adjustment of parameters or alone. The 6 included studies consisted of 490 patients, which included 336 clinical responders and 154 clinical non-responders. All 6 studies were conducted in East Asian areas.
Table 1.
Characteristics of studies included in the meta-analysis.
| Study | Country | Study period | No. of cases (non-responders) | No. of all patients | Adjustment | Follow-up period |
|---|---|---|---|---|---|---|
| Xie17 | China | 2003–2008 | 18 | 52 | Tumor size, the expression of ALDH1 | 3–123 months |
| Park19 | Korea | 1997–2007 | 15 | 43 | Node, the expression of ERCC1 | 6–139 months |
| Liu14 | China | 2002–2011 | 40 | 103 | None | 6–113 months |
| Yang18 | China | 2007–2012 | 33 | 115 | None | 6–75 months |
| Li12 | China | 2000–2011 | 43 | 154 | None | 6–142 months |
| Shoji10 | Japan | 2002–2011 | 5 | 23 | None | 9–90 months |
1-year HR
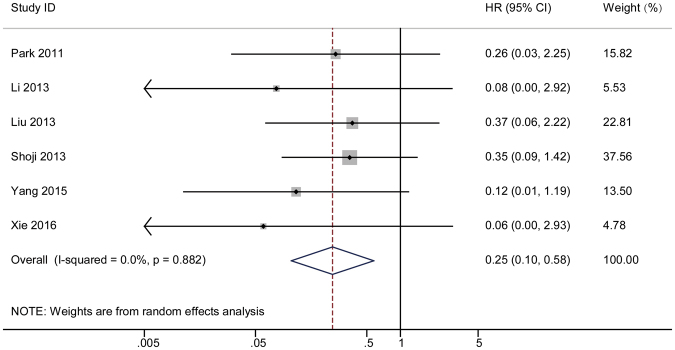
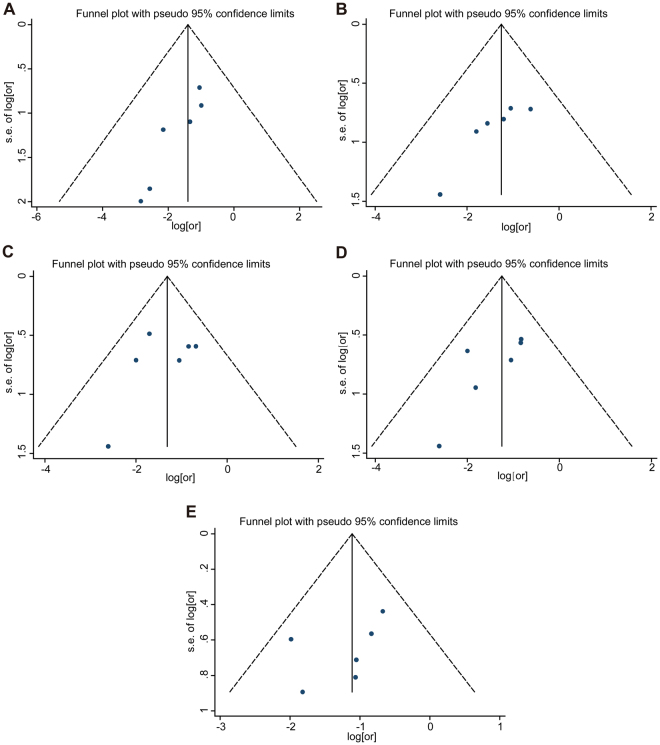
A forest plot was employed to illustrate the association between a short-term response and overall survival. The HR of each study was determined and is listed in Fig. 2. The dots in the middle of the bar indicate the HR, and the spread of the bars indicates the 95% CI of the HR. The diamond in each bar indicates the corresponding weight of the included study. The pooled result after the combination of the studies is shown at the bottom of the forest plot. The analysis showed a combined result with an HR = 0.25 and a 95% CI of 0.10–0.58. A Cochrane Q test produced a P value of 0.882 and an I2 equal to 0%. A funnel plot was constructed to visually demonstrate the probability of publication bias (Fig. 3A). Non-parametric and parametric tests were also employed to detect publication bias (Supplementary Figure S1). A sensitivity analysis was used to determine whether heterogeneity existed in the combined analysis, which is shown in Supplementary Figure S2.
Figure 2.
The pooled 1-year HR (hazard ratio) of non-responder with overall survival among cervical cancer patients undergoing neoadjuvant chemotherapy. The summary estimates were obtained by using a random-effects model. The data markers indicate the HRs comparing non-responder with responder. The size of the data markers indicates the weight of the study, which is the inverse variance of the effect estimate. The diamond data markers indicate the pooled HRs. CI indicates confidence interval.
Figure 3.
Funnel plots for detection of publication bias. The pseudo 95% confidence interval (CI) is computed as part of the analysis that produces the funnel plot, and corresponding to the expected 95% CI for a given standard error (SE). HR indicates hazard ratio. From A to E, it represents 1-year survival to 5-year survival respectively.
2-year HR
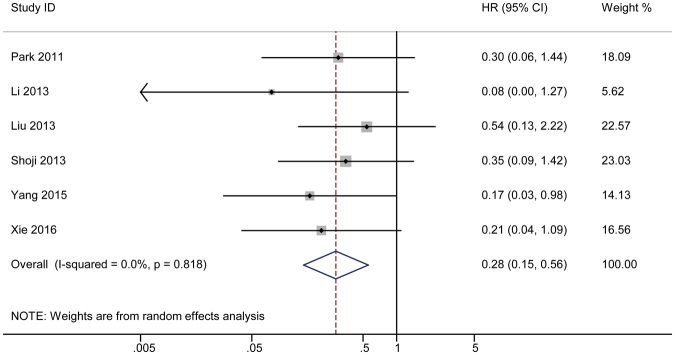
The combined results for the second year are also shown by forest plot in Fig. 4. The plot shows an HR of 0.28 with a 95% CI of 0.15–0.56. A Cochrane Q test was also performed to test the possible heterogeneity in the analysis (P = 0.818) with I2 = 0. A funnel plot was constructed to visually reveal the bias (Fig. 3B). Begg’s and Egger’s tests were also used to calculate the actual P value with non-parametric and parametric methods (Supplementary Figure S3). A sensitivity analysis was used to detect the heterogeneity in the combined analysis (Supplementary Figure S4). Each study was excluded individually, and the results of the remaining studies were pooled. Each combined HR was calculated individually.
Figure 4.
The pooled 2-year HR of non-responder with overall survival among cervical cancer patients undergoing neoadjuvant chemotherapy. The summary estimates were obtained by using a random-effects model. The data markers indicate the HRs comparing non-responder with responder. The size of the data markers indicates the weight of the study, which is the inverse variance of the effect estimate. The diamond data markers indicate the pooled HRs. CI indicates confidence interval.
3-year HR
A forest plot was created to show the combined DFS in the third year (Fig. 5). The analysis showed a final pooled HR = 0.27 for the 3-year DFS with a 95% CI of 0.16–0.45. A Cochrane Q test showed that P = 0.511; I2 test revealed a value of 0. A funnel plot (Fig. 3C) was constructed, and Begg’s and Egger’s tests (Supplementary Figure S5) were performed to observe the publication bias. A sensitivity analysis was also performed to test whether the result of the combined analysis was robust (Supplementary Figure S6).
Figure 5.
The pooled 3-year HR of non-responder with overall survival among cervical cancer patients undergoing neoadjuvant chemotherapy. The summary estimates were obtained by using a random-effects model. The data markers indicate the HRs comparing non-responder with responder. The size of the data markers indicates the weight of the study, which is the inverse variance of the effect estimate. The diamond data markers indicate the pooled HRs. CI indicates confidence interval.
4-year HR
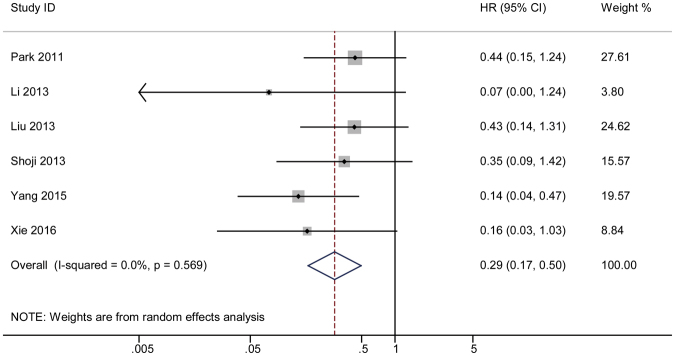
Using the same methods described above, a forest plot showed a final combined HR in the fourth year of 0.29, and the corresponding 95% CI was 0.17–0.50. A Cochrane Q test produced a P value of 0.569 and I2 test showed a value of 0 (Fig. 6). A funnel plot was also constructed (Fig. 3D). The results of the Begg’s and Egger’s tests are shown in Supplementary Figure S7. A sensitivity analysis was performed to show the distribution of the combined results by excluding each study individually (Supplementary Figure S8).
Figure 6.
The pooled 4-year HR of non-responder with overall survival among cervical cancer patients undergoing neoadjuvant chemotherapy. The summary estimates were obtained by using a random-effects model. The data markers indicate the HRs comparing non-responder with responder. The size of the data markers indicates the weight of the study, which is the inverse variance of the effect estimate. The diamond data markers indicate the pooled HRs. CI indicates confidence interval.
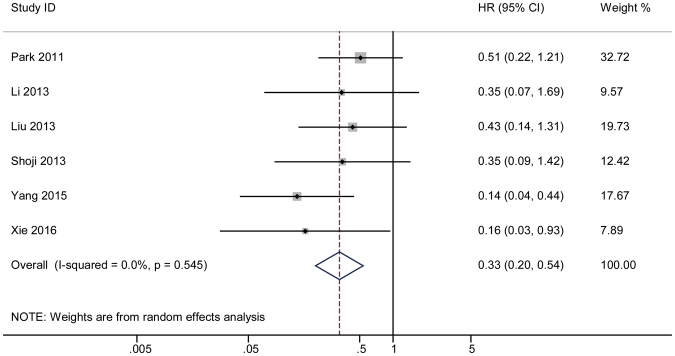
5-year HR
The combined HR for the fifth year was also determined and shown by a forest plot (HR = 0.33 and 95% CI 0.20–0.54). A Cochrane Q test revealed P = 0.545 (Fig. 7) while I2 test showed a value of 0. A funnel plot was constructed (Fig. 3E), and Begg’s and Egger’s tests were conducted to investigate the publication bias (Supplementary Figure S9). A Sensitivity analysis was also conducted to test the robustness of the combined results (Supplementary Figure S10).
Figure 7.
The pooled 5-year HR of non-responder with overall survival among cervical cancer patients undergoing neoadjuvant chemotherapy. The summary estimates were obtained by using a random-effects model. The data markers indicate the HRs comparing non-responder with responder. The size of the data markers indicates the weight of the study, which is the inverse variance of the effect estimate. The diamond data markers indicate the pooled HRs. CI indicates confidence interval.
Discussion
By combining previous study results, the present study found that a short-term response was significantly associated with the long-term survival of cervical cancer patients who underwent NACT. Additionally, overall survival may be partly predicted by the short-term response when it is evaluated by the RECIST criteria.
Our findings validated several previous studies in which the predictive role of the short-term response was also evaluated among cervical cancer patients. Chen and colleagues performed a randomized controlled trial (RCT) on 142 cervical cancer patients who underwent NACT from 1999 to 2003. They found that the response to NACT was an independent prognostic factor of long-term survival after adjustment for age, International Federation of Gynecology and Obstetrics (FIGO) stage, pathological grade, histological type, tumor size, lymph node metastasis, and parametrial infiltration. Cai and colleagues also performed a prospective RCT of 106 patients from 1999 to 2005, and they found that responders achieved a better survival rate than non-responders11. Li and colleagues performed a study on 304 patients in 2012, and they similarly found that responders achieved higher survival rates than non-responders12. Other studies that used the WHO criteria have also shown similar results indicating that a clinical response was associated with better long-term survival. In 2011, Xiong and colleagues conducted a retrospective study and demonstrated that the response to NACT was associated with long-term survival13. Our results were slightly different from those of Liu and colleagues, who found that a short-term response did not lead to a significantly higher survival rate14. We speculate that the statistical power of that study may not be sufficient to provide a definite conclusion, considering the number of subjects enrolled in the study15. Thus, we hypothesize that if the study population was larger, a significant difference would have been observed.
The predictive effect of the short-term response on long-term survival has always been a focus of research of solid tumors, as it may highlight a method for personalized treatment. The short-term response can be observed a very short time after chemotherapy, and the role of chemotherapy drugs may be quickly determined by doctors and patients. Accordingly, patients can be administered the most effective treatment regimens. A proper treatment regimen may help patients to achieve longer survival, and it may also help to decrease the cost of medical treatment.
Our study has some limitations. First, we did not pool individual data, which could have provided a more accurate result. Second, the difference in the survival rate between responders and non-responders was not investigated using WHO criteria in this study. Therefore, in future studies, we plan to collect individual data to obtain a more accurate result. We also plan to determine new methods to calculate the HR according to the WHO criteria.
In conclusion, we performed a combined analysis of the predictive role of the short-term response on long-term survival for cervical cancer patients who underwent NACT. We found that clinical responders achieved higher survival rates than non-responders. This finding may help doctors to evaluate the survival of this group of patients and may help to determine more effective treatment methods. Future RCTs should be performed to validate our results and to provide clear conclusions with less bias.
Methods
Literature search
In August 2016, a search for literature in this field was performed in the PubMed and Embase databases by two doctors independently. The key words used for the search included the following: “NACT” or “neoadjuvant chemotherapy” or “preoperative chemotherapy”, plus “cervical carcinoma” or “cervical cancer” or “uterine cervical neoplasms”, plus “responding” or “response” or “clinical response” or “responder” or “remission” or “responsiveness”. To include as many eligible articles as possible, we also reviewed the reference lists of the retrieved articles.
Study identification
Inclusion criteria. To determine their eligibility, two reviewers independently reviewed the titles and abstracts of the articles (S.Y.K. and K.C.H.). The selected articles were required to be original research articles. The articles were written in English and published in a peer-reviewed journal in a relevant discipline. All cases in the articles were cervical carcinoma patients with a definite diagnosis. Using the Newcastle-Ottawa Scale (NOS), our team performed a quality assessment of the included studies, as described in a previous study16.
Exclusion criteria. In the primary search, a total of 583 papers were retrieved. After reading the titles and abstracts, we excluded 428 articles from further analysis due to irrelevance to the present research. Then, we excluded articles that did not adopt the RECIST criteria; articles that were only concerned with the pathological response and not the clinical response were also excluded from further analysis; studies with only descriptive results but without statistical data were also excluded. These studies were carefully reviewed to exclude duplicated information. Finally, 6 articles were included in the present study, and these 6 studies were used for the final analysis10,12,14,17–19.
Statistical analyses
According to the RECIST criteria, clinical responders included individuals with a complete response (CR) or partial response (PR), while clinical non-responders included those with stable disease (SD) or progressive disease (PD). The RECIST criteria are a widely used standard for evaluating the short-term response of solid tumors20.
The hazard ratio (HR) and 95% CI were the most common statistics used across the studies to measure the association between the short-term response and survival10. When this information could not be obtained from the articles, Engauge Digitizer software was used to determine the survival curve of the included studies21 based on the calculus theory and integral theory22,23. The pooling process of the HR and its corresponding 95% CI was visually illustrated by forest plots. During pooling, a Cochrane Q test was employed to test the heterogeneity; the significance level was set at P < 0.10, according to a previous study24. The I2 statistic was also used to test the heterogeneity across the studies, and a value of I2 > 50% was considered to indicate significant heterogeneity25. A random effects model was used to calculate the combined HR according to the DerSimonian and Laird method26. The possibility of publication bias was evaluated by visual screening of a funnel plot, and both Begg’s test and Egger’s test were used to evaluate the publication bias27,28. A sensitivity analysis was conducted to evaluate the robustness of the combined results24. During our research, one study was omitted at a time to test the robustness of the combined results. Stata version 11 (Stata Corp, College Station, TX) was used for the statistical analysis. Differences with a two-sided value of P < 0.05 were considered statistically significant.
Electronic supplementary material
Acknowledgements
This research was supported by grants from the International S&T Cooperation Program of China (No. 2013DFA31400), the Program for New Century Excellent Talents in University (No. NECT-12-0646), the National Natural Science Foundation of China (No. 81370469; 81071663; 81172467; 81300460; 81402161; 81403166; 81402160; 81302267) and the National Major Science and Technology Project (No. 2009ZX09103-739).
Author Contributions
Conception, hypothesis delineation and study design: S.Y.K., K.C.H., C.Z., X.Y.M., and S.X.W.; data acquisition, analysis and interpretation: S.Y.K., K.C.H., C.Z., and X.Y.M.; writing first the draft of manuscript: S.Y.K. and X.Y.M.; revision of manuscript: all authors.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Shi-yi Kong and Kecheng Huang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19948-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiangyi Ma, Email: doctormxy@126.com.
Shixuan Wang, Email: sxwang2012@hotmail.com.
References
- 1.Torre LA, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Sardi JE, Boixadera MA, Sardi JJ. A critical overview of concurrent chemoradiotherapy in cervical cancer. Curr Oncol Rep. 2004;6:463–470. doi: 10.1007/s11912-004-0077-3. [DOI] [PubMed] [Google Scholar]
- 3.Tomao F, et al. Fertility-Sparing Options in Young Women with Cervical Cancer. Curr Treat Options Oncol. 2016;17:5. doi: 10.1007/s11864-015-0386-9. [DOI] [PubMed] [Google Scholar]
- 4.Sardi JE, Boixadera MA, Sardi JJ. Neoadjuvant chemotherapy in cervical cancer: a new trend. Curr Opin Obstet Gynecol. 2005;17:43–47. doi: 10.1097/00001703-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Hauerberg L, et al. Vaginal Radical Trachelectomy for early stage cervical cancer. Results of the Danish National Single Center Strategy. Gynecol Oncol. 2015;138:304–310. doi: 10.1016/j.ygyno.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Morice P, Uzan C, Gouy S, Verschraegen C, Haie-Meder C. Gynaecological cancers in pregnancy. Lancet. 2012;379:558–569. doi: 10.1016/S0140-6736(11)60829-5. [DOI] [PubMed] [Google Scholar]
- 7.Rob L, Skapa P, Robova H. Fertility-sparing surgery in patients with cervical cancer. Lancet Oncol. 2011;12:192–200. doi: 10.1016/S1470-2045(10)70084-X. [DOI] [PubMed] [Google Scholar]
- 8.Maas M, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 9.Chen CA, Cheng WF, Wei LH, Su YN, Hsieh CY. Radical hysterectomy alone or combined with neoadjuvant chemotherapy in the treatment of early stage bulky cervical carcinoma. J Formos Med Assoc. 2002;101:195–202. [PubMed] [Google Scholar]
- 10.Shoji T, et al. Neoadjuvant chemotherapy using platinum- and taxane-based regimens for bulky stage Ib2 to IIb non-squamous cell carcinoma of the uterine cervix. Cancer Chemother Pharmacol. 2013;71:657–662. doi: 10.1007/s00280-012-2052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai HB, Chen HZ, Yin HH. Randomized study of preoperative chemotherapy versus primary surgery for stage IB cervical cancer. J Obstet Gynaecol Res. 2006;32:315–323. doi: 10.1111/j.1447-0756.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- 12.Li R, et al. Prognostic value of responsiveness of neoadjuvant chemotherapy before surgery for patients with stage IB(2)/IIA(2) cervical cancer. Gynecol Oncol. 2013;128:524–529. doi: 10.1016/j.ygyno.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Xiong Y, Liang LZ, Cao LP, Min Z, Liu JH. Clinical effects of irinotecan hydrochloride in combination with cisplatin as neoadjuvant chemotherapy in locally advanced cervical cancer. Gynecol Oncol. 2011;123:99–104. doi: 10.1016/j.ygyno.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Liu SP, et al. Efficacy of neoadjuvant cisplatin and 5-flourouracil prior to surgery in FIGO stage IB2/IIA2 cervical cancer. Mol Clin Oncol. 2014;2:240–244. doi: 10.3892/mco.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Minckwitz G, et al. Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase III randomized GeparTrio trial. Journal of the National Cancer Institute. 2008;100:542–551. doi: 10.1093/jnci/djn085. [DOI] [PubMed] [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 17.Xie Q, et al. Aldehyde Dehydrogenase 1 Expression Predicts Chemoresistance and Poor Clinical Outcomes in Patients with Locally Advanced Cervical Cancer Treated with Neoadjuvant Chemotherapy Prior to Radical Hysterectomy. Ann Surg Oncol. 2016;23:163–170. doi: 10.1245/s10434-015-4555-7. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, et al. Clinical efficacy and safety of paclitaxel plus carboplatin as neoadjuvant chemotherapy prior to radical hysterectomy and pelvic lymphadenectomy for Stage IB2-IIB cervical cancer. Int J Clin Exp Med. 2015;8:13690–13698. [PMC free article] [PubMed] [Google Scholar]
- 19.Park JS, et al. ERCC1 (excision repair cross-complementation group 1) expression as a predictor for response of neoadjuvant chemotherapy for FIGO stage 2B uterine cervix cancer. Gynecol Oncol. 2011;120:275–279. doi: 10.1016/j.ygyno.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Wu XL, et al. Diagnostic and Prognostic Value of Circulating Tumor Cells in Head and Neck Squamous Cell Carcinoma: a systematic review and meta-analysis. Sci Rep. 2016;6:20210. doi: 10.1038/srep20210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta-analysis with time-to-event outcomes. Stat Med. 2002;21:3337–3351. doi: 10.1002/sim.1303. [DOI] [PubMed] [Google Scholar]
- 23.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan A, Wang Y, Talaei M, Hu FB. Relation of Smoking With Total Mortality and Cardiovascular Events Among Patients With Diabetes Mellitus: A Meta-Analysis and Systematic Review. Circulation. 2015;132:1795–1804. doi: 10.1161/CIRCULATIONAHA.115.017926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bi H, et al. Breakfast skipping and the risk of type 2 diabetes: a meta-analysis of observational studies. Public Health Nutr. 2015;18:3013–3019. doi: 10.1017/S1368980015000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.