Abstract
A series of 2-aryl-9-methyl-β-carbolinium bromides (B) were synthesized and explored for anti-acetylcholinesterase (AChE) activities in vitro, action mechanism and structure-activity relationship. All the compounds B along with their respective 3,4-dihydro intermediates (A) presented anti-AChE activity at 10 μM. Thirteen compounds B showed the excellent activity with IC50 values of 0.11–0.76 μM and high selectivity toward AChE relative to butyrylcholinesterase (BChE), superior to galantamine (IC50 = 0.79 μM), a selective AChE inhibitor drug. Kinetic analysis showed that the action mechanisms of both compounds B and A are a competitive inhibition model. Structure-activity relationship analyses showed that the C = N+ moiety is a determinant for the activity. Substituents at 6, 7 or 4′ site, the indole-N-alkyl and the aromatization of the C-ring can significantly improve the activity. Molecular docking studies showed that the compounds could combine with the active site of AChE by the π-π or cation-π action between the carboline ring and the phenyl rings of the residues, and the β-carboline moiety is embedded in a cavity surrounded by four aromatic residues of Trp86, Tyr337, Trp439 and Tyr449. The present results strongly suggest that the para-position of the D-ring should be a preferred modification site for further structural optimization design. Thus, 2-aryl-9-methyl-β-carboliniums emerged as novel and promising tool compounds for the development of new AChE inhibitor agents.
Introduction
As the most common form of adult onset dementia, Alzheimer’s disease (AD) is an age-related irreversible neurodegenerative disorder characterized by a progressive memory loss, a decline in language skills and other cognitive impairments1. It was estimated that 47 million people suffered from dementia worldwide in 2016, and this number is predicted to increase to more than 131 million by 2050. The total estimated worldwide cost of dementia is US$818 billion, and it will become a trillion dollar disease by 20302. Therefore, given the aging of the population, AD is becoming one of the main public health issues we have to face.
AD is a complicated disease involving different molecular events. Its pathological features include tau-protein aggregation in nerve cells, β-amyloid protein aggregation in the spaces between nerve cells, oxidative stress and lowered levels of acetylcholine in the brain3. Among them, cholinergic hypothesis is the most popular explanation of mechanism of AD development4. Neuropathological evidence has proved that the memory impairment and behavioral abnormalities in patients with AD results from low level of acetylcholine (ACh) in different areas of the central nervous system, mainly the cerebral cortex and the hippocampus. Therefore, AChE inhibitors has been the leading strategy for the development of AD drugs, which can elevate the level of acetylcholine in the synapses between cholinergic neurons and enhance cholinergic function5. In addition, AChE inhibitors are also used for the treatment of senile dementia, ataxia, myasthenia gravis and Parkinson’s disease6, or as insecticides in modern agricultural procedures7.
Acetylcholinesterase and butyrylcholinesterase (BuChE) are two different types of cholinesterases present in the human brain, which catalyze the hydrolysis of choline-based esters to terminate cholinergic signal transmission. The two enzymes are distinguished on the basis of substrate specificities, tissue distribution and sensitivity to inhibitors8,9. AChE mainly exist in the synapses of the central and peripheral nervous systems and in the membranes of erythrocytes, while BuChE is primarily present in the blood and glial cells or neurons10,11. AChE hydrolyzes acetylcholine (ACh) more quickly whereas BuChE hydrolyzes butyrylcholine (BuCh) more quickly. Under normal conditions, acetylcholine (ACh) is dominantly decomposed by AChE instead of BuChE although both AChE and BuChE can hydrolyze ACh12,13. Therefore, AChE has been considered the main targeting cholinesterase in the AD scenario. Current clinical therapeutic strategy for mild-to-moderate AD is mainly to improve cholinergic neurotransmission by using AChE inhibitors14. However, it is worth mentioning that BuChE was also found to have a critical role for ACh hydrolysis in AD, especially in the late stage of AD15,16. In progressed AD, level of AChE in brain declines while BuChE increases, leading to ratio of BuChE/AChE shifting from 0.6 to 1.117,18. At this point, BuChE can compensate for AChE loss by hydrolyzing ACh in cholinergic transmission. Therefore, to avoid the adverse effects caused by suppression of AChE, exploits of BuChE inhibitors for AD treatment have also aroused a worldwide popularity. A lot of effective and selective BuChE inhibitors have been discovered19. Furthermore, it was found that the two enzymes are also related with the formation of amyloid protein plaques, which is encouraging the development of dual- or multi-functional ChE inhibitors20.
At present, there are four FDA-approved AChE inhibitor-type drugs (tacrine, donepezil, galantamine and rivastigmine) for treatment of cognitive dysfunction and memory loss associated with mild-to-moderate AD21. Among them, donepezil and galantamine are reversible and selective AChE inhibitors that compete with acetylcholine for AChE binding22. Recent researches showed that AChE inhibitors not only alleviate the cognitive defect of AD patients by elevating acetylcholine (ACh) levels, but also act as disease modifying agents by preventing the early step of AD, the assembly of β-amyloid peptide (Aβ) into amyloid plaque23,24. Nevertheless, some obvious adverse effects including nausea and vomiting, decreased appetite, weight loss, hepatotoxicity or problems associated with bioavailability were also reported for these drugs25–28. Additionally, these drugs are only focused on the symptomatic aspects but cannot prevent, halt or reverse the progression of the disease14. Therefore, the search of central selective AChEIs devoid of adverse effects is still a challenging research topic. It is very necessary to find better AChE inhibitor agents and effective therapeutics for AD desease29.
To date, a lot of highly active AChE and/or BuChE inhibitors had been found, including synthetic, semi-synthetic and natural compounds30. It is worth noting that most of the compounds are nitrogen-containing compounds or alkaloids. Among them, β-carboline (pyrido[3,4-b]indole) compounds are one important type. β-Carboline alkaloids, also referred to as harman alkaloids, were widely distributed in organisms including plants, animals, halobios and human being31. In addition, β-carbolines occur in foods and cigarette smoke, suggesting human uptake and exposure to these compounds32. In the past decades, β-carbolines have attracted lots of attention from researchers due to their diverse biological activities such as antimicrobial33, antitumor34, antiviral35 and antiparasitic36, anti-inflammatory37, vasorelaxant38, antioxidant39, neuroactive or neurotoxic actions40. Especially interesting for us is that some natural or synthetic quaternary 2-methyl-β-carboline salts were also found to have strong AChE inhibitory activity41,42. The results suggest that β-carboline is a promising molecular framework for development of new anti-AChE agents.
With the aim of finding more potent β-carboline-type AChE inhibitors for treatment of AD, herein, a series of new β-carboline derivatives (Fig. 1) were designed and explored for AChE inhibition activity, action mechanism as well as structure-activity relationship (SAR). These compounds are characterized by a quaternary 2-phenyl-9-methyl-β-carbolinium skeleton with various substituents on the 2-aryl ring.
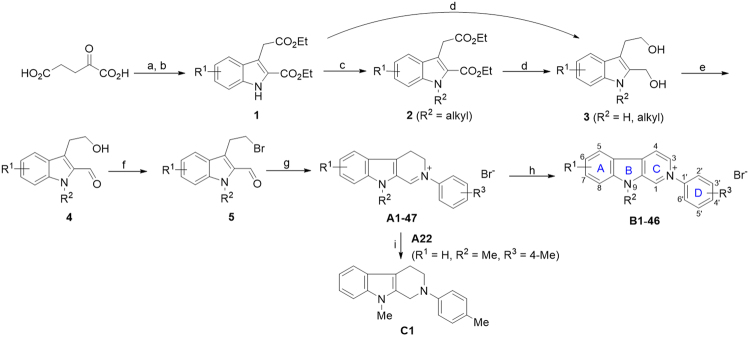
Figure 1.
Synthetic route of target compounds B1-B34 and C1. Reagents and conditions: (a) phenylhydrazine-HCl, H2O, 12 h at r.t.; (b) EtOH, con. H2SO4, reflux for 12 h; (c) R2I or R2Br, NaH, dry DMF, 0 °C; (d) LiAlH4, dry THF, 0 °C to r.t.; (e) active MnO2, CHCl3, r.t.; (f) MsCl, Et3N, LiBr, dry THF, 0 °C to r.t.; (g) R-PhNH2, TsOH·H2O, EtOH, r.t.; (h) Pd/C, acetonitrile, reflux; (i) NaBH4, EtOH, r.t.
Results and Discussion
Chemistry
Based on the consideration of SAR and structural diversity, we designed a series of 2-aryl-9-mehtyl-β-carboline salts (B1-B33) with various substituents on the D-ring (Fig. 1) in order to get an insight into the effect of the substitution pattern of the D ring on anti-AChE activity. The substituents include both electron-withdrawing groups like halogen atom, CN, CF3, NO2, and electron-donating groups like CH3, OCH3 and OH. The substitution position involves the 2′, 3′ or 4′ site of the D-ring. In addition, we also designed one the indole-N-H instead of N-Me derivative (B34) and one 1,2,3,4-tetrahydro derivative (C1) of compounds B in order to explore the effect of the indole-N-substituents and the C-ring aromatization on the activity. The substitution patterns of target compounds are depicted in Table 1.
Table 1.
Structures and inhibitory activity of compounds A and intermediates B against AChE. aThe test concentration of the compound is 10 μM. The differences between data with the different lowercases within a column are significant (p < 0.05).
| Compound | Inhibition rate (%)a | Compound | Inhibition rate (%)a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | R1 | R2 | R3 | No. | R1 | R2 | R3 | ||
| B1 | H | Me | H | 71.5 ± 0.3 l | A1 | H | Me | H | 40.9 ± 2.1 ij |
| B2 | H | Me | 2′-F | 59.8 ± 3.2 n | A2 | H | Me | 2′-F | 15.1 ± 2.8 mnop |
| B3 | H | Me | 3′-F | 70.5 ± 2.2 l | A3 | H | Me | 3′-F | 22.4 ± 1.1 l |
| B4 | H | Me | 4′-F | 84.3 ± 1.5 hi | A4 | H | Me | 4′-F | 49.3 ± 2.2 fgh |
| B5 | H | Me | 2′-Cl | 38.5 ± 1.9 st | A5 | H | Me | 2′-Cl | 2.4 ± 8.0 stu |
| B6 | H | Me | 3′-Cl | 56.4 ± 2.5 no | A6 | H | Me | 3′-Cl | 19.3 ± 4.0 lm |
| B7 | H | Me | 4′-Cl | 91.2 ± 1.5 cde | A7 | H | Me | 4′-Cl | 60.1 ± 2.9 d |
| B8 | H | Me | 2′-Br | 32.1 ± 0.9 u | A8 | H | Me | 2′-Br | 5.9 ± 1.0 qrs |
| B9 | H | Me | 3′-Br | 48.8 ± 6.6 q | A9 | H | Me | 3′-Br | 16.2 ± 3.3 mno |
| B10 | H | Me | 4′-Br | 95.4 ± 0.8 ab | A10 | H | Me | 4′-Br | 60.3 ± 1.6 d |
| B11 | H | Me | 2′-I | 43.3 ± 1.8 r | A11 | H | Me | 2′-I | −10.6 ± 3.0 w |
| B12 | H | Me | 3′-I | 56.4 ± 5.9 no | A12 | H | Me | 3′-I | 10.1 ± 2.8 pqr |
| B13 | H | Me | 4′-I | 93.8 ± 0.4 abcd | A13 | H | Me | 4′-I | 54.2 ± 3.2 ef |
| B14 | H | Me | 2′-OH | 23.9 ± 4.3 v | A14 | H | Me | 2′-OH | 23.2 ± 2.5 kl |
| B15 | H | Me | 3′-OH | 72.1 ± 1.8 l | A15 | H | Me | 3′-OH | 51.8 ± 9.1 fg |
| B16 | H | Me | 4′-OH | 82.1 ± 2.5 ij | A16 | H | Me | 4′-OH | 64.3 ± 0.3 cd |
| B17 | H | Me | 2′-OMe | 41.0 ± 2.9 rs | A17 | H | Me | 2′-OMe | 5.8 ± 2.4 qrs |
| B18 | H | Me | 3′-OMe | 53.5 ± 0.4 op | A18 | H | Me | 3′-OMe | 4.7 ± 1.5 rst |
| B19 | H | Me | 4′-OMe | 90.7 ± 0.8 def | A19 | H | Me | 4′-OMe | 66.1 ± 0.5 c |
| B20 | H | Me | 2′-Me | 59.6 ± 3.2 n | A20 | H | Me | 2′-Me | 44.3 ± 1.7 j |
| B21 | H | Me | 3′-Me | 72.4 ± 3.9 l | A21 | H | Me | 3′-Me | 28.6 ± 1.7 k |
| B22 | H | Me | 4′-Me | 95.2 ± 0.5 abc | A22 | H | Me | 4′-Me | 77.6 ± 1.5 a |
| B23 | H | Me | 2′-CN | 68.7 ± 0.7 lm | A23 | H | Me | 2′-CN | 10.1 ± 1.6 pqr |
| B24 | H | Me | 3′-CN | 50.7 ± 2.2 pq | A24 | H | Me | 3′-CN | 18.9 ± 0.3 lmn |
| B25 | H | Me | 4′-CN | 36.8 ± 0.8 t | A25 | H | Me | 4′-CN | 15.6 ± 1.8 mnop |
| B26 | H | Me | 3′-CF3 | 41.0 ± 1.9 rs | A26 | H | Me | 3′-CF3 | 12.8 ± 2.1 kln |
| B27 | H | Me | 4′-CF3 | 91.4 ± 0.3 bcde | A27 | H | Me | 4′-CF3 | 28.4 ± 1.0 k |
| B28 | H | Me | 3′-NO2 | 34.9 ± 1.8 tu | A28 | H | Me | 3′-NO2 | 14.5 ± 3.9 mnop |
| B29 | H | Me | 2′,6′-diF | 48.5 ± 3.1 pq | A29 | H | Me | 2′,6′-diF | −3.3 ± 2.0 uv |
| B30 | H | Me | 2′,4′-diCl | 90.6 ± 1.0 def | A30 | H | Me | 2′,4′-diCl | −0.5 ± 2.4 tu |
| B31 | H | Me | 3′,5′-diCl | 18.9 ± 2.5 v | A31 | H | Me | 3′,5′-diCl | 0.7 ± 0.5 stu |
| B32 | H | Me | 2′-F-4′-Br | 92.8 ± 1.0 abc | A32 | H | Me | 2′-F-4′-Br | 11.1 ± 2.8 opq |
| B33 | H | Me | 2′,4′-diBr | 96.7 ± 0.05 a | A33 | H | Me | 2′,4′-diBr | −7.0 ± 8.3 vw |
| B34 | 6-OMe | Me | H | 85.7 ± 2.5 ghi | A34 | 6-OMe | Me | H | 68.4 ± 2.8 c |
| B35 | 6-Me | Me | H | 88.6 ± 2.5 efg | A35 | 6-Me | Me | H | 70.3 ± 2.2 bc |
| B36 | 7-F | Me | H | 76.2 ± 1.1 k | A36 | 7-F | Me | H | 59.3 ± 2.1 de |
| B37 | 7-Cl | Me | H | 89.9 ± 0.7 def | A37 | 7-Cl | Me | H | 66.6 ± 4.7 c |
| B38 | H | Et | H | 87.8 ± 1.1 efgh | A38 | H | Et | H | 76.6 ± 3.7 a |
| B39 | H | Pr | H | 78.8 ± 0.9 jk | A39 | H | Pr | H | 67.1 ± 2.4 c |
| B40 | H | iPr | H | 86.9 ± 0.8 fgh | A40 | H | iPr | H | 68.9 ± 5.1 bc |
| B41 | H | allyl | H | 78.2 ± 1.6 k | A41 | H | allyl | H | 74.4 ± 1.1 ab |
| B42 | H | Bu | H | 65.7 ± 0.6 m | A42 | H | Bu | H | 53.9 ± 1.5 ef |
| B43 | H | iBu | H | 84.1 ± 0.6 hi | A43 | H | iBu | H | 46.5 ± 1.9 ghi |
| B44 | H | Bn | H | 47.8 ± 2.0 q | A44 | H | Bn | H | 31.2 ± 4.4 ef |
| B45 | H | CH2CO2Et | H | 42.0 ± 0.7 rs | A45 | H | CH2CO2Et | H | 38.8 ± 4.6 j |
| B46 | H | H | H | 43.0 ± 1.8 r | A46 | H | H | H | 41.3 ± 1.4 ij |
| C1 | H | Me | 4′-Me | −2.3 ± 3.2 w | A47 | H | H | 4′-Br | 16.0 ± 1.7 mnop |
| Galantamine | 91.2 ± 1.1 cde | ||||||||
The synthetic route of target compounds is shown in Fig. 1, in which phenylhydrazine–HCl and α-ketoglutaric acid were used as the starting materials. According to our reported method33, key intermediates A1-A35 were synthesized via 6–7 steps. In sequence, the intermediates A1-A34 were dehydrogenated by Pd/C in acetonitrile or toluene to provide the final compounds B1-B34. Compound A1 was reduced with NaBH4 to obtain its 1,2,3,4-tetrahydro derivative C1.
Compounds B include 32 new compounds (B2-B33) and 2 known compounds (B1, B34). The known compounds B1, B34 and intermediates A1-A35 were confirmed by comparison of NMR and MS data and those reported in literature33. New compounds B2-B33 were elucidated by 1H NMR, 13C NMR and HRMS analyses. All compounds B showed some similar spectroscopic characteristics because of the structural similarity. Each compound showed a characteristic ion peak at m/z [M-Br]+ in positive ESI-HRMS spectra. The presence of bromide anion was confirmed by ion peaks at m/z 79 and 81 in negative ESI-MS spectra. In 1H and 13C NMR spectra, each compound B revealed one signal of H-1 at δH ca. 10.0 (1 H, s or d, J = ca 1.2 Hz) and one signal of C-1 in the range of δC 152–163, one doublet signal of H-4 at δH ca. 9.02 (1 H, d, J = ca 6.5 Hz), one doublet or double doublet signal of H-3 at δH ca. 9.00 (1 H, d, J = ca. 6.5 Hz, or dd, J = ca. 6.5, ca. 1.2 Hz), one signal of C-4 at δC ca. 130 and one signal of C-3 at δC ca.133. The NMR spectra of C1 were similar to that of B22 except for three additional CH2 signals at δH 4.38 (2 H, s, H-1)/δC 46.6 (C-1), 3.60 (2 H, t, J = 5.6 Hz, H-3)/δC 48.7 (C-3), 2.93 (2 H, t, J = 5.6 Hz, H-4)/δC 21.8 (C-4) and fewer signals of one HC=N+ unit and one AX system [C(3)H=C(4)H].
AChE inhibition activity
According to Ellman’s coupled enzyme assay43, compounds B1-B34, C1 along with intermediates A1-A35 were screened for inhibition activity on AChE at 10 μM. Galantamine, a selective and competitive AChE inhibitor drug for treatment of AD, was used as a reference control. The results are shown in Table 1.
The data in Table 1 showed that all compounds B and the majority of intermediates A presented some anti-AChE activity at 10 μM. However, compared with compounds A, except for B14, all compounds B showed the much higher activity. Among compounds B, thirty-two compounds displayed inhibition rates of >50%, and nine compounds (B7, B10, B13, B19, B22, B27, B30, B32 and B33) showed the inhibition rates of 90.6% to 96.7%, higher or equal to galantamine (91.2%). B33 (R = 2′,4′-diBr, R′ = Me) showed the highest activity. In contrast to compounds B, only the minority of compounds A (A1, A4, A7, A10, A13, A15, A16, A19, A22, A34-A43 and A46) showed the moderate inhibition rates of 40% to 78%.
In order to explore anti-AChE potential more detail, the compounds with the higher initial activity in Table 1 were further determined for median inhibition concentrations (IC50) on AChE. Galantamine was used as a reference standard. The results are listed in Table 2. Gratifyingly, thirteen compounds B (B7, B10, B13, B19, B22, B27, B30, B32, B33, B35, B37, B38 and B40) showed the excellent activity with IC50 values of ≤0.76 μM, higher or approximately equal to that of galantamine (IC50 = 0.79 μM), agreement with that showed in Table 1. Among them, B33 (IC50 = 0.11 μM) was most active, superior to rivastigmine (IC50 = 9.94 μM)44 and tacrine (IC50 = 0.25 μM)45 but inferior to donepezil (IC50 = 0.03 μM)46. In contrast to compounds B, all the compounds A in Table 2 showed the low to the moderate activity (IC50 values > 1.4 μM).
Table 2.
Median inhibition concentrations of part of compounds B and intermediates A against AChE and BuChE. aSI: selectivity index, the ratio value of IC50 (BuChE)/IC50 (AChE); bEstimated values based on the results in Table 1; cND: no determination. dNA: no inhibition activity at 10 μM; eThe data are cited from ref.44; fThe data are cited from ref.45. gThe data are cited from ref.46.
| Compound | IC50 ± S.D (μM) | SIa | Compound | IC50 ± S.D (μM) | SIa | ||
|---|---|---|---|---|---|---|---|
| AChE | BuChE | AChE | BuChE | ||||
| B1 | 3.18 ± 0.12 | 124.9 ± 7.8 | 39.3 | A1 | 18.6 ± 2.93 | 10.1 ± 1.66 | 0.54 |
| B2 | 6.65 ± 1.07 | 23.0 ± 0.53 | 3.49 | A2 | >10b | ND | |
| B3 | 4.73 ± 0.33 | 72.4 ± 4.25 | 15.3 | A3 | >10b | ND | |
| B4 | 1.46 ± 0.12 | 51.6 ± 2.43 | 35.3 | A4 | 14.1 ± 1.41 | 59.5 ± 2.72 | 4.22 |
| B5 | >10b | NDc | A5 | ≫10b | ND | ||
| B6 | 9.65 ± 0.78 | 33.1 ± 1.07 | 3.40 | A6 | >10b | ND | |
| B7 | 0.56 ± 0.07 | 60.0 ± 1.52 | 107.1 | A7 | 7.58 ± 1.29 | 42.2 ± 0.86 | 5.57 |
| B8 | >10b | ND | A8 | ≫10b | ND | ||
| B9 | ≈10b | ND | A9 | >10b | ND | ||
| B10 | 0.43 ± 0.04 | 55.4 ± 1.73 | 128.8 | A10 | 6.79 ± 2.29 | 76.2 ± 11.0 | 11.2 |
| B11 | >10b | ND | A11 | NA | ND | ||
| B12 | 11.1 ± 1.34 | 21.1 ± 0.46 | 1.90 | A12 | >10b | ND | |
| B13 | 0.24 ± 0.03 | 40.4 ± 1.83 | 168.3 | A13 | 5.50 ± 0.37 | 53.8 ± 2.51 | 9.80 |
| B14 | >10b | ND | A14 | >10b | ND | ||
| B15 | 3.92 ± 0.30 | 29.6 ± 2.48 | 7.60 | A15 | 6.12 ± 0.61 | 10.0 ± 0.59 | 1.63 |
| B16 | 1.50 ± 0.19 | 67.9 ± 11.5 | 45.3 | A16 | 2.81 ± 0.22 | 28.0 ± 2.88 | 9.96 |
| B17 | >10b | ND | A17 | ≫10b | ND | ||
| B18 | 9.69 ± 0.80 | 62.2 ± 3.62 | 6.42 | A18 | ≫10b | ND | |
| B19 | 0.47 ± 0.04 | 34.0 ± 2.00 | 72.3 | A19 | 2.92 ± 0.28 | 137.0 ± 0.79 | 46.9 |
| B20 | 9.71 ± 0.55 | 154.0 ± 9.1 | 15.9 | A20 | >10b | ND | |
| B21 | 5.05 ± 0.26 | 68.6 ± 8.06 | 13.6 | A21 | >10b | ND | |
| B22 | 0.29 ± 0.03 | 32.2 ± 1.65 | 111.6 | A22 | 1.45 ± 0.21 | 80.1 ± 5.27 | 55.2 |
| B23 | 4.41 ± 0.29 | 195.6 ± 13.4 | 44.4 | A23 | >10b | ND | |
| B24 | 10.3 ± 0.60 | 212.2 ± 17.4 | 20.6 | A24 | >10b | ND | |
| B25 | >10b | ND | A25 | >10b | ND | ||
| B26 | >10b | ND | A26 | >10b | ND | ||
| B27 | 0.70 ± 0.11 | 77.9 ± 4.97 | 111.3 | A27 | >10b | ND | |
| B28 | >10b | ND | A28 | >10b | ND | ||
| B29 | ≈10b | ND | A29 | NA | ND | ||
| B30 | 0.55 ± 0.07 | 132.6 ± 26.3 | 241.1 | A30 | NA | ND | |
| B31 | >10b | ND | A31 | NA | ND | ||
| B32 | 0.30 ± 0.08 | 115.5 ± 18.2 | 385.0 | A32 | >10b | ND | |
| B33 | 0.11 ± 0.07 | 30.6 ± 2.31 | 278.2 | A33 | NA | ND | |
| B34 | 0.94 ± 0.23 | 33.3 ± 2.34 | 35.4 | A34 | 2.04 ± 0.33 | 37.6 ± 2.84 | 18.4 |
| B35 | 0.42 ± 0.15 | 17.2 ± 1.86 | 41.0 | A35 | 2.66 ± 0.37 | 10.6 ± 0.58 | 3.98 |
| B36 | 2.61 ± 0.51 | 27.0 ± 1.14 | 10.3 | A36 | 4.74 ± 0.90 | 14.2 ± 0.44 | 3.00 |
| B37 | 0.57 ± 0.08 | 14.4 ± 0.84 | 25.3 | A37 | 4.02 ± 0.95 | 11.7 ± 0.50 | 2.91 |
| B38 | 0.76 ± 0.21 | 17.1 ± 0.78 | 22.5 | A38 | 3.69 ± 0.40 | 10.4 ± 0.40 | 2.82 |
| B39 | 3.08 ± 0.66 | 37.0 ± 5.40 | 12.0 | A39 | 5.72 ± 0.66 | 13.0 ± 0.62 | 2.27 |
| B40 | 0.54 ± 0.16 | 16.5 ± 2.37 | 30.6 | A40 | 3.73 ± 0.34 | 16.3 ± 0.56 | 4.37 |
| B41 | 1.18 ± 0.30 | 16.2 ± 1.80 | 13.7 | A41 | 3.91 ± 0.36 | 6.83 ± 0.20 | 1.75 |
| B42 | 2.91 ± 0.33 | 22.8 ± 3.18 | 7.84 | A42 | 9.79 ± 3.55 | 10.0 ± 0.68 | 1.02 |
| B43 | 0.98 ± 0.03 | 6.53 ± 0.61 | 6.66 | A43 | >10b | ND | |
| B44 | 11.2 ± 2.54 | 24.1 ± 2.23 | 2.15 | A44 | >10b | ND | |
| B45 | >10b | ND | A45 | >10b | ND | ||
| B46 | 18.0 ± 2.42 | 18.7 ± 2.80 | 1.04 | A46 | 18.2 ± 1.97 | 3.90 ± 0.53 | 0.21 |
| C1 | NAd | ND | A47 | >10b | ND | ||
| Galantamine | 0.79 ± 0.05 | 13.7 ± 0.71 | 18.0 | Tacrine | 0.25 ± 0.01e | 0.05 ± 0.00e | 0.22 e |
| Rivastigmine | 9.94 ± 0.83f | 2.86 ± 0.22f | 0.29f | Donepezil | 0.03 ± 0.01g | 5.40 ± 0.27g | 180g |
There is increasing recognition that BuChE may play an important role in cholinergic transmission15,16. While the level of AChE decreases dramatically in the advanced stage of AD, BuChE increases in brain regions involved in cognitive functions17,18. At this point, BuChE is able to compensate for AChE loss by hydrolyzing ACh. These observations lead one to believe that inhibition of both AChE and BuChE may contribute to improvement of current symptomatic treatments of AD. Therefore, the compounds in Table 2 were also evaluated for BuChE inhibition activity by means of Ellman’s method43. Galantamine was used as a reference standard. The results in Table 2 showed that the compounds also exhibited some inhibition activity on BuChE with IC50 values of 3.90 to 212.2 μM, but compared with the anti-AChE activity of each the compound, its anti-BuChE activity is obviously lower except for A1 and A34. The results above showed that both the compounds B and A had the higher selectivity for AChE than BuChE. Compared with galantamine, thirteen compounds B (B7, B10, B13, B19, B22, B27, B30, B32, B33, B34, B35, B38 and B40) also showed the higher selectivity for AChE in addition to the higher anti-AChE activity. Among them, the selectivity indexes (SI) of compounds B30, B32 and B33 reached up to >240, comparable with that of donepezil (SI = 180)46, a selective AChE inhibitor drug. Relative to compounds B, compounds A in Table 2 showed the lower selectivity to AChE. Unexpectedly, compounds A1 and A46 (SI < 1.0) showed the higher selectivity to BuChE but not AChE.
As the representative compounds, compound B33 with the highest anti-AChE activity among compounds B and compound A22 with the highest anti-AChE activity among compounds A were further evaluated for cytotoxic activities on both mouse neuroblastoma N2a cells and primary cultured porcine fetal kidney cells. Meanwhile, in order to compare the cytotoxicity of compounds A and B, compound B22 was subjected to the assay. The results in Table 3 showed that both B33 and B22 showed the much lower cytotoxicity with IC50 values of 10.6 and 19.2 μM on mouse neuroblastoma N2a cells compared with their respective anti-AChE activity (IC50 = 0.11, 0.29 μM). Similarly, both B33 and B22 also showed the much lower cytotoxicity on primary cultured porcine fetal kidney cells (IC50 = >20, 18.1 μM). Compared with B22, compound A22 showed the higher cytotoxicity with IC50 values of 2.13 and 5.18 μM on the two strains of cells although its cytotoxicities are also lower than its anti-AChE activity (IC50 = 1.45 μM).
Table 3.
Cytotoxicity of the compounds on mouse neuroblastoma N2a cells and primary cultured porcine fetal kidney cells (48 h). a95% CI: the confidence interval of IC50 at 95% probability.
| Compound | Mouse neuroblastoma N2a cells | Primary cultured porcine fetal kidney cells | ||
|---|---|---|---|---|
| IC50 (μM) | 95% CI (μM) | IC50 (μM) | 95% CI (μM) | |
| B33 | 10.6 | 8.26–14.4 | >20 | |
| B22 | 19.2 | 13.0–33.2 | 18.1 | 13.5–27.2 |
| A22 | 2.13 | 1.88–2.41 | 5.18 | 4.72–5.68 |
Mechanism of AChE inhibition
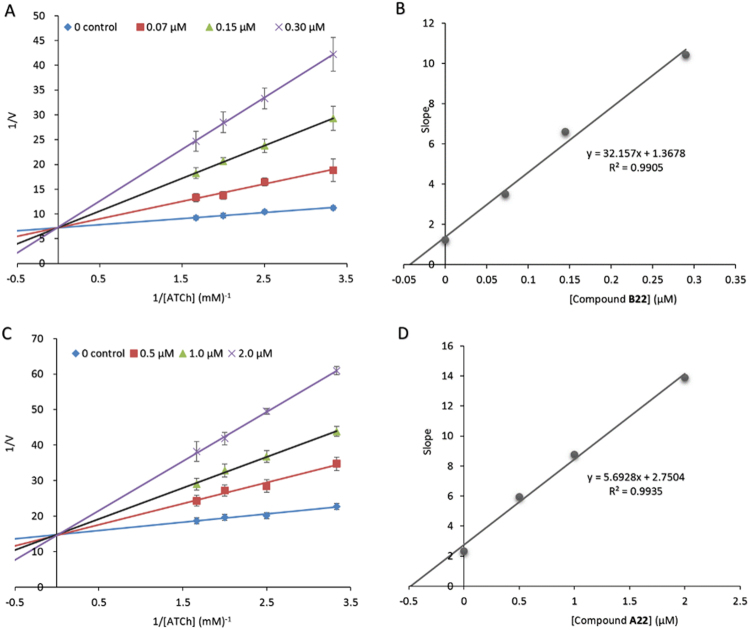
In order to know the mechanism of AChE inhibition, compounds B22 and A22 as two representative compounds were conducted for kinetic analysis of AChE inhibition. The graphical analysis of the inhibition data for B22 and A22 in comparison with galantamine is shown in Fig. 2. The results in Fig. 2A and C clearly showed that both B22 and A22 inhibited AChE in a competitive manner with the substrate acetylthiocholine iodide (ATCh). The estimates of the inhibition constants Ki of B22 and A22 for AChE were 4.25 × 10−8 M and 4.83 × 10−7 M, respectively, while the Km value of acetylthiocholine iodide (ATCh) for AChE was 1.63 × 10−4 M. The results showed that the binding capacity of B22 with AChE is approximately 11-fold that of A22 and 3835-fold that of the substrate ATCh.
Figure 2.
The Mechanism of AChE inhibition by compound B22 (A) and intermediate A22 (C) respective to ATCh, and their Ki determination (B,D). (A and B) The reciprocals of the initial reaction rates and substrate concentrations are plotted. (C and D) The slope values of the lines from graph A or C are plotted versus the inhibitor concentration, affording an equation of linear regression. When y is 0, the equations give Ki values of 4.25 × 10−8 M for compound B22 and 4.83 × 10−7 M for compound A22.
Structure-activity relationships
By comparison of the activity and structures of the compounds in Tables 1 and 2, we can find some clear and regular structure-activity relationships for compounds B and A (Tables 4, 5 and 6).
Table 4.
Effect of substitution patterns of the D-ring on the activity of compounds B and A. aArrows outside of parentheses represent compounds B; arrows inside parentheses represent compounds A. b↑significantly increasing the activity relative to R = H; ↓significantly decreasing the activity; ±, slight change of the activity. c“nd” means no determination.
| Substituent (R) | Anti-AChE | Selectivity toward AChE | Anti-BChE | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2′-R | 3′-R | 4′-R | 2′-R | 3′-R | 4′-R | 2′-R | 3′-R | 4′-R | |
| F | ↓ (↓) | ± (↓) | ↑ (↑) | ↓ (nd) | ↓ (nd) | ↓ (↑) | ↑ (nd) | ↑ (nd) | ↑ (↓) |
| Cl | ↓ (↓) | ↓ (↓) | ↑ (↑) | nd | ↓ (nd) | ↑ (↑) | nd | ↑ (nd) | ↑ (↓) |
| Br | ↓ (↓) | ↓ (↓) | ↑ (↑) | nd | nd | ↑ (↑) | nd | nd | ↑ (↓) |
| I | ↓ (↓) | ↓ (↓) | ↑ (↑) | nd | ↓ (nd) | ↑ (↑) | nd | ↑ (nd) | ↑ (↓) |
| OH | ↓ (↓) | ± (↑) | ↑ (↑) | nd | ↓ (↑) | ↑ (↑) | nd | ↑ (±) | ↑ (↓) |
| OMe | ↓ (↓) | ↓ (↓) | ↑ (↑) | nd | ↓ (nd) | ↑ (↑) | nd | ↑ (nd) | ↑ (↓) |
| Me | ↓ (±) | ± (↓) | ↑ (↑) | ↓(nd) | ↓ (nd) | ↑ (↑) | ↓ (nd) | ↑ (nd) | ↑ (↓) |
| CN | ± (↓) | ↓ (↓) | ↓ (↓) | ↑ (nd) | ↓ (nd) | nd | ↓ (nd) | ↓ (nd) | nd |
| CF3 | nd | ↓ (↓) | ↑ (↓) | Nd | nd | ↑(nd) | nd | nd | ↑ (nd) |
| NO2 | nd | ↓ (↓) | nd | Nd | nd | nd | nd | nd | nd |
Table 5.
Effect of substitution patterns of the D-ring on the activity of compounds B and A. a↑significantly increasing the activity relative to R = H; ↓significantly decreasing the activity; ±, slight change of the activity. b“nd” means no determination.
| Dihalogenation | Anti-AChE | Selectivity toward AChE | Anti-BChE | |||
|---|---|---|---|---|---|---|
| Compound B | Compound A | Compound B | Compound A | Compound B | Compound A | |
| 2′,4′-diCl | ↑ | ↓ | ↑ | nd | ↓ | nd |
| 2′,4′-diBr | ↑ | ↓ | ↑ | nd | ↑ | nd |
| 2′-F-4′-Br | ↑ | ↓ | ↑ | nd | ↑ | nd |
| 2′,6′-diF | ↓ | ↓ | nd | nd | nd | nd |
| 3′,5′-diCl | ↓ | ↓ | nd | nd | nd | nd |
Table 6.
Effect of the indole-N-alkyl and substitution patterns of the A-ring on the activity of compounds B and A. a↑, significantly increasing the activity relative to R = H; ↓, significantly decreasing the activity; ±, slight change of the activity. b“nd” means no determination.
| Substituent | Anti-AChE | Selectivity toward AChE | Anti-BChE | |||
|---|---|---|---|---|---|---|
| Compound B | Compound A | Compound B | Compound A | Compound B | Compound A | |
| 6-OMe | ↑ | ↓ | ↓ | ↑ | ↑ | ↓ |
| 6-Me | ↑ | ↓ | ± | ↑ | ↑ | ± |
| 7-F | ↑ | ↓ | ↓ | ↑ | ↑ | ↓ |
| 7-Cl | ↑ | ↓ | ↓ | ↑ | ↑ | ± |
| (N)Me | ↑ | ± | ↑ | ↑ | ↓ | ↓ |
| (N)Et | ↑ | ↑ | ↑ | ↑ | ± | ↓ |
| (N)Pr | ↑ | ↑ | ↑ | ↑ | ↓ | ↓ |
| (N)iPr | ↑ | ↑ | ↑ | ↑ | ± | ↓ |
| (N)Allyl | ↑ | ↑ | ↑ | ↑ | ± | ↓ |
| (N)Bu | ↑ | ↑ | ↑ | ↑ | ↓ | ↓ |
| (N)iBu | ↑ | ± | ↑ | nd | ↑ | nd |
| (N)Bn | ↑ | ↓ | ↑ | nd | ↓ | nd |
| (N)CH2CO2Et | ± | ↓ | nd | nd | nd | |
Firstly, the substitution pattern of the D-ring can dramatically influence the activity. For compounds B, the p-substitution of all the substituents except cyano group (B25, A25) can significantly increase the anti-AChE activity (B4, B7, B10, B13, B16, B19, B22, B27 vs B1) whereas the o- or m-substitution generally leads to decrease or slight change of the activity. A similar trend was also found in compounds A (A4, A7, A10, A13, A16, A19, A22 vs A1). The substituents above not only include electron-donating groups like OH, OMe or Me and electron-withdrawing groups like halogen atoms or CF3 but also involve hydrogen-bond acceptors (OMe) and hydrogen-bond donors (OH). Therefore, we speculated that the effect of the p-substituents on the activity should be mainly steric effect but not electron effect or hydrogen-bond effect. For p-mono-substituted compounds B, the order of the anti-AChE activity is the iodide (B13) ≈ the Me-substituted compound (B22) > the bromide (B10) ≈ the MeO-substituted compound (B19) > the chloride (B7) > the CF3-substituted compound (B27) > the fluoride (B4) ≈ the OH-substituted compound (B16). The results above strongly suggest that the 4′ site of the D-ring should be one important modifiable position for further structure optimization.
For BuChE, the effect of substituents on the D-ring on the activity strongly depends on the structure of the C-ring. For compounds B with the aromatic C-ring, the introduction of substituents to the D-ring causes increase of the anti-BuChE activity in most cases. However, the opposite was observed for compounds A with a nonaromatic C-ring. Nevertheless, 4′-substituents (Cl, Br, I, Me, OMe, OH, CF3) can significantly increase the selectivity of both compounds B and A to AChE at the same time.
For the dihalides (B29‒33), the presence of 2′,4′-dihalogen atoms (B30, 32, 33) can significantly improve both the anti-AChE activity and selectivity, whereas the presence of 2′,6′-difluoro or 3′,5′-dichloro leads to dramatic decrease of the activity (B29, B31 vs B1). Compared the corresponding 4′-mono-halogenated compounds, 2′,4′-dihalogenated compounds showed the slight improvement of the anti-AChE activity although they exhibited the much higher selectivity for AChE (B30 vs B7; B32 or B33 vs B10). The results above show that for the 2′,4′-halogenated compounds, 2′-halogen atom can significantly increase the selectivity to AChE but not greatly influences the anti-AChE activity (B30 vs B7; B32 or B33 vs B10). In other words, there may be a synergistic effect on the anti-AChE selectivity between 2′-Cl and 4′-Cl, 2′-F and 4′-Br or 2′-Br and 4′-Br. The selectivity indexes for AChE of the 2′,4′-dibromo compound (B33), the 2′-fluoro-4′-bromo compound (B32), 2′,4′-dichloro compound (B30) reach up to 278, 385 and 241, respectively.
As strong electron-withdrawing substituents, cyano, nitro or trifluoromethyl group on the D-ring generally causes decrease of the activity of compounds B (B24-26, B28 vs B1). The exception is that 4′-CF3 is able to dramatically improve the activity (B27 vs B1). Unlike compounds B, the above substituents or double halogen atoms on the D-ring always leads to decrease of the activity of compounds A (A22-33 vs A1).
Secondly, the C=N+ moiety of the compounds should be a determinant for the activity because transformation of the 3,4-dihydro-β-cabolinium (A22) or aromatic β-cabolinium (B22) to its 1,2,3,4-tetrahydro-derivative C1 leads to complete loss of the activity. A similar case was also observed in some tertiary aromatic β-carbolines and their 1,2,3,4-tetrahydro-β-carbolines, where only quaternary aromatic 2-methyl-β-carboliniums showed the higher activity, whereas its corresponding tertiary aromatic β-carboline derivatives without 2-methyl, 2-methyl-1,2,3,4-tetrahydro-β-carbolines or quaternary 2,2-dimethyl-1,2,3,4-tetrahydro-β-carboline salts only exhibited very weak or no activity41,42.
Thirdly, compared with B46 (the indole-N-H), the indole-N-alkylation can significantly improve the anti-AChE activity of compounds B. This effect varies with the type and steric hindrance of the alkyl groups. A similar case was also observed for compounds A. Among all the substituents, iso-propyl (B40) and ethyl (B38) can give the best improvement of the anti-AChE activity followed by iso-butyl (B43) and allyl (B41) while benzyl has the weakest effect on the activity. Methyl, propyl or butyl cause the moderate effect. The only exception is that —CH2CO2Et has almost no effect on the activity (B45 vs B46, Table 1). On the contrary, for anti-BuChE activity, whether for compounds B or A, the indole-N-alkylation always causes decrease of the activity compared with B46 or A46 (the indole-N-H). The above SARs are obviously different from that of 2-methyl-β-carbolinium salts, where the indole-N-methylation is not able to significantly increase the anti-AChE activity41, even decrease the activity42. The results above suggest that the effect of the indole-N-alkylation on the activity of the aromatic 2-substituted-β-carboliniums is also related with the type of the pyridine-N-substituents.
It is worth noting that for compounds A, the effect of the indole-N-alkylation on the activity varies with the substituents on the D-ring. When the D-ring is phenyl (R=H), the indole-N-methylation hardly impact the activity (A1 vs A46). However, when the D-ring is 4′-bromrophenyl, the indole-N-methylation causes the significant increase of the activity (A10 vs A47, Table 1). We assume that a similar case might also exist in compounds B.
Fourthly, comparison of B34-B37 and B1 or A34-A37 and A1 suggests that substitution patterns of the A-ring can also dramatically influence the activity. The introduction of 6-OMe, 6-Me, 7-F or 7-Cl to the A-ring leads to obvious increase of the anti-AChE activity of both compounds B and A. However, different cases exist in aspect of anti-BuChE activity. The substituents on the A-ring can also improve the anti-BuChE activity of compounds B (B37-B37 vs B1) but decrease that of compounds A (A37-A37 vs A1). Since the substituents above involve different electron effect, we speculated that the effect of substituents on the A-ring might mainly be steric effect but not electron effect.
Finally, besides the substituents on the A- and D-ring and the indole-N-substituents, the aromatization of the C-ring can also intensely influence the anti-AChE activity. Among these factors, there is an interaction effect on the activity. The aromatization of the C-ring dramatically enhances the activity of the indole-N-alkyl compounds (B1‒B45 vs A1‒A45), but hardly influences the activity of the indole-N-H compounds (A45 vs B45), indicating that the influence of the aromatization of the C-ring on the activity is related with the indole-N-substituents. Compound A33 was no activity at 10 μM whereas B33 showed the highest activity; compound A7 was more active than compound A4, but B7 was less active than B4. This result shows that the influence of the aromatization of the C-ring on the activity also closely varies with the substituents on the D-ring. Furthermore, the presence of 4′-Br can improve the activity of the indole-N-Me compounds (A10 vs A1, B10 vs B1) but decrease the activity of the indole-N-H compounds (A47 vs A46, Table 1), suggesting that an interaction effect on the activity also exists between the substituents on the D-ring and the indole-N-substituents.
For BuChE, a similar interaction effect on the activity also exists among the four factors. For example, for the N9-Me compounds, the C-ring aromatization improves that activity of the 4′-F, 4′-Br, 4′-I, 4′-OMe or 4′-Me compounds (B4 vs A4, B10 vs A10, B13 vs A13, B19 vs A19, B22 vs A22) but decreases the activity of the 4′-Cl, 3′-OH or 4′-OH compounds (B7 vs A7, B15 vs A15, B16 vs A16). For the indole-N-H compound, the C-ring aromatization also causes decrease of the activity (B46 vs A46). Interestingly, whether for the indole-N-alkyl compounds or for the indole-N-H compounds, the C-ring aromatization always leads to increase of the selectivity toward AChE (B1 vs A1; B4 vs A4; B7 vs A7; B10 vs A10; B15 vs A15; B16 vs A16; B19 vs A19; B22 vs A22; B34-45 vs A34-45; B46 vs A46) (Table 2).
Molecular docking of compounds with AChE and BuChE
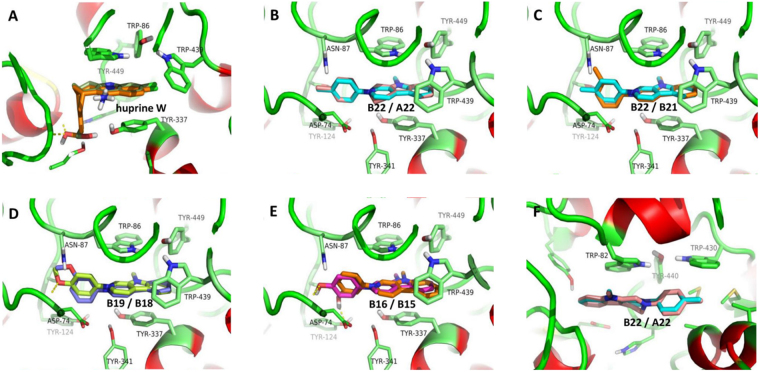
To gain insight into binding interactions of the compounds in the hydrolytic active site of AChE and BuChE, molecular docking studies were performed for the representative compounds B15, B16, B18, B19, B21, B22 and A22. These studies were performed into an AChE structure (PDB 4BDT) and BuChE (PDB 5K5E). The results are shown in Figure 3 and Table 7. Figure 3A revealed that the binding modes of huprine W and 6QS obtained by AutodDock are almost identical to the crystallographic structure, which proves the feasibility of the docking protocol. The data in Table 5 show that the binding free energies (<−9.3 kcal/mol) of compounds A or B with the catalytic site of AChE are larger than that (−7.2 to −8.3 kcal/mol) with BuChE, indicating that compounds A or B have more inhibition potential to AChE than BuChE, agreement with the measured inhibition activities. The results of molecular docking showed that the compounds have very similar binding modes (Fig. 1B–E and F). Figure 3B–E showed that the compounds could be stabilized in the active site of AChE by interactions similar to those described for huprine W. The β-carboline moiety is embedded in a remarkable group of aromatic rings including Trp86, Tyr337, Trp439 and Tyr449. The B-ring of the β-carboline is facing Tyr337 while the C-ring is facing Trp86 (Fig. 3B). Obviously, the full aromatic β-carboline moiety should be more beneficial than its 3,4-dihydro structure for enhancing the binding affinity of the ligands for AChE due to the π-π action between the carboline ring and the indole ring of the residue Trp86. The speculation was further supported by the compounds B having larger binding free energy with the catalytic site of AChE than compounds A (Table 7, B13 vs A13, B15 vs A15, B19 vs A19, B22 vs A22).
Figure 3.
The estimated binding modes of the compounds into the active site of AChE and BuChE. The protein structures are show in Ribbon style with coil in green, helix in red and strand in yellow. The binding site residues are displayed using a stick model with carbon in green. The potential hydrogen contacts between compounds and protein were highlighted by yellow dashed lines. (A) Superposition of huprine W in the X-ray crystallographic structure (PDB code 4BDT; orange) and in docking resultant complex structure of the AChE (dark green). (B–E) The binding modes of the compounds (in stick model with carbon) into the active site of AChE: (B) B22 (cyan) and A22 (pink); (C) B22 (cyan) and B21 (goldenrod); (D) B19 (chartreuse) and B18 (medium blue); (E) B16 (purple) and B15 (orange red). (F) The binding modes of compounds B22 (cyan) and A22 (pink) into the active site of BuChE. Other atoms were colored as follows: nitrogen, blue; oxygen, red; hydrogen, gray.
Table 7.
The binding free energies of compounds with AChE and BuChE. aThe inhibitor co-crystals with AChE in the crystal structure of the protein complex (PDB code: 4BDT). bThe inhibitor co-crystals with BuChE in the crystal structure of the protein complex (PDB code: 5K5E).
| Compound | IC50 (μM) | FBE (kcal/mol) | ||
|---|---|---|---|---|
| AChE | BuChE | AChE | BuChE | |
| A13 | 5.5 | 53.8 | −10.38 | −7.51 |
| A15 | 6.12 | 10 | −9.38 | −7.7 |
| A16 | 2.81 | 28 | −9.48 | −7.69 |
| A19 | 2.92 | 137 | −10.04 | −7.25 |
| A22 | 1.45 | 80.1 | −9.46 | −7.52 |
| B12 | 11.1 | 21.1 | −9.78 | −8.26 |
| B13 | 0.24 | 40.4 | −10.46 | −7.46 |
| B15 | 3.92 | 29.6 | −9.57 | −7.8 |
| B16 | 1.5 | 67.9 | −9.43 | −7.76 |
| B18 | 9.69 | 62.2 | −9.46 | −7.65 |
| B19 | 0.47 | 34 | −10.11 | −7.65 |
| B21 | 5.05 | 68.6 | −9.37 | −7.82 |
| B22 | 0.29 | 32.2 | −9.86 | −7.38 |
| B33 | 0.11 | 30.6 | −10.28 | −7.97 |
| huprine W a | 0.0011 | −10.23 | / | |
| 6QS b | 0.443 | / | −11.49 | |
The binding models of B22/21 (Fig. 3C) or B19/18 (Fig. 3D) showed that the 3′-substituents may bring some inappropriate clash penetration by the ligand into the enzyme, whereas 4′-substituents generally increase the binding free energy of AChE-the compound. Similar situations arise in other compounds, such as B16/B15 (Fig. 3E), B13/B12, A16/A15, et al. Interestingly, Fig. 3E showed that the hydrogen atom of 3′-OH group and the phenolic oxygen atom of Tyr124 could form an H-bond (Fig. 3E), which compensates the inappropriate clash penetration, resulting in the comparable binding free energy for A16 vs A15 or B16 vs B15. These results support our presumption above that the effect of the p-substituents on the activity should be mainly steric effect but not electron effect or hydrogen-bond effect.
All of the current marketed AChEIs are nitrogen-contained compounds, which are partly protonated at physiological pH. In the acid-base equilibrium, the cations formed by protonation can enhance the overall affinity of AChE for the ligands by additional cation–π interactions with aromatic residues at the active site of AChE, whereas their neutral can cross the blood‒brain barrier (BBB) due to their good lipophilicity47. In the present study, ionic compounds A22 and B22 showed the higher activity against AChE, but their neutral derivative C1 lost the activity (Table 2). Obviously, the positive charge of compounds A and B should be a crucial factor for the activity. Molecular docking showed that the β-carboline moiety locates in the cavity consisting of four aromatic residues of Trp-86, Trp-439, Tyr-337 and Tyr-449 at the active site of AChE (Fig. 3B–E). All the aromatic rings of these residues are rich in π-electrons. Therefore, the positively charged β-carboline moiety with the π-electron poor property can form stronger π-π action with the residues to improve the overall affinity of AChE for the compounds or the activity of the compounds.
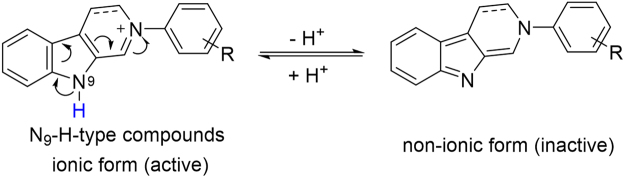
Similarly, the low activity of the indole-N-H compounds may also be explained by loss of the positive charge. Theoretically, the indole-N-H has stronger acidity because of the presence of the C=N+ moiety adjacent to the N9 site. Therefore, the indole-N-H compounds such as B34 and A35 can partly release their protons in aqueous solution to form inactive or low active neutral forms (Fig. 4). In other word, the indole-N-H compounds can co-exist in two forms of ion and non-ion in aqueous solution, but only their ionic forms have the good activity. Unlike the indole-N-H compounds, the indole-N-Me compounds always exist in ionic forms. This may be why the indole-N-methylation can increase the activity of compounds B (B1 vs B34, Table 2) or A (A10 vs A35, Table 1). Additionally, the similar case was not observed for 2-methyl-β-carbolinium salts41,42, where their indole-N-substiutents did not significantly influent the activity against AChE. The reason is that the non-ionic form formed by release of the indole-N-H of 2-methyl-β-carbolinium salts is unstable because of the lack of conjugation action of the D-aryl ring.
Figure 4.
Possible existing forms of N9-H-type compounds in an aqueous solution.
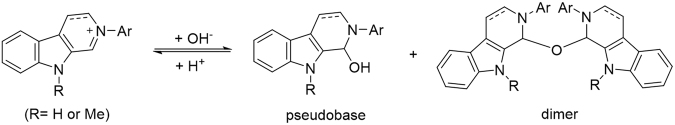
Sanguinarine and chelerythrine are two quaternary benzo[c]phenanthridine alkaloids, which contain a C=N+ moiety with high chemical activity. Studies showed that the two can react with OH− or H2O to yield the corresponding pseudobase or dimer by its C=N+ moiety, and mainly exist in its dimer in pH 7.0 aqueous solution48,49. Therefore, both sanguinarine and chelerythrine can easily cross the membrane of cells to quickly enter living cells although they are ionic compounds50. Additionally, we also found the similar chemical transformation for 2-aryl-3,4-dihydroisoquinoliniums containing a C=N+ moiety (unpublished). Based on the results above and the structural similarity, we speculated that compounds A or B could also co-exist in two forms of ion and their corresponding pseudobases or dimers in human blood (pH 7.35–7.45) due to the presence of a C=N+ moiety with high chemical activity (Fig. 5). The resulting pseudobase or dimer forms can allow compounds A or B to cross the blood brain barrier (BBB) due to its good lipophilicity. At present, the investigation on BBB permeability of the compounds is ongoing in our lab.
Figure 5.
Plausible existing forms of compounds A or B in an aqueous solution.
Besides higher anti-AChE activity and higher selectivity to AChE, compound B22 also showed the lower cytotoxicity than A22, which should be attributed to the aromatization of the C-ring. A similar case was observed for the antifungal activity of compounds A, where the aromatization of the C-ring causes reduction of the activity33. This similarity might be related with both cytotoxicity and antifungal activity involving inhibition of cell proliferation. Obviously, for potent and high selective β-carboline-type AChE inhibitors for treatment of AD, the full aromatic 9-alkyl-β-carboline skeleton is crucial whereas for anticancer or antimicrobial, the 9-alkyl-3,4-dihydro-β-carboline skeleton is favorable.
In conclusion, a series of new 2-aryl-9-methyl-β-carboline bromides (B) were synthesized in the present study. All compounds B along with most of their 3,4-dihydro intermediates (A) showed anti-AChE activities in vitro at 10 μM. Among them, nine compounds B showed higher anti-AChE activity and selectivity relative to BChE than galantamine, a selective AChE inhibitor drug, which showed great potential to be developed as new selective AChE inhibitor agents. Kinetic analysis proved that both compounds B and A are able to inhibit AChE in a competitive manner, and the binding capacity of B22 with AChE reaches up to 3835-fold that of substrate acetylthiocholine iodide (ATCh). In aspect of SAR, the C=N+ moiety was proved to be a determinant for the activity, and 4′-substituents of the D-ring, the indole-N-methyl and C-ring aromatization can dramatically increase the anti-AChE activity and selectivity by independent or synergistic effect. These findings will be of great importance for further structural optimization design. Thus, we succeeded in discovering a class of new and promising tool compounds for development of new AChE inhibitor agents.
Methods
Chemicals
Acetylthiocholine iodide (ATCh), butyrylthiocholine iodide (BuTCh), 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), and galantamine were purchased from Sigma Chemicals Co., Ltd. (Shanghai, China). Phenylhydrazine-HCl and α-ketoglutaric acid were purchased from Aladdin Industrial Inc. (Shanghai, China). Other reagents and solvents were obtained locally and of analytical grade or purified according to standard methods before use. The water used was redistilled and ion-free.
Enzymes
AChE (E.C. 3.1.1.7) (BR, 200 u/mg) from fly’s head and BuChE (E.C. 3.1.1.8) (BR, 20 u/mg) from horse serum were purchased from Shanghai Yuanye biological technology Co. Ltd (Shanghai, China).
Instruments
Melting points were determined on a digital melting point apparatus. Nuclear magnetic resonance spectra (NMR) were performed on an Avance III 500 MHz instrument (Bruker, Karlsruhe, Germany). Chemical shifts (δ values) and coupling constants (J values) are given in parts per million and hertz, respectively. High-resolution mass spectra (HR-MS) were carried out with a microTOFQ II instrument (Bruker). Low-resolution mass spectra were carried out with a LCQ Fleet instrument (Thermo Fisher, Shanghai, China).
Synthesis
General procedure for the synthesis of compounds A1-A47
According to our previous method33, intermediates A1-A47 were prepared via 5 or 6 steps starting from phenylhydrazine-HCl and α-ketoglutaric acid. The compounds were confirmed by 1H NMR, 13C NMR and MS analyses, and their spectral data were agreement with those in literature23.
General procedure for the synthesis of compounds B1-B46
To a solution of compounds A (0.2 mmol) in acetonitrile (40 mL) was added 5% Pd/C (wetted with ca. 55% water) (0.17 mmol, 80 mg). The mixture was refluxed at 80 °C for about 3 days to complete the reaction. The Pd/C powders in the reaction solution was filtered off through a sand core funnel and completely washed with methanol. The combined solution was evaporated under vacuum to yield the desired compounds.
9-Methyl-2-phenyl-β-carboline bromide (B1): Yield 78%; yellow powders; mp 271.2–272.8 °C; The 1H NMR, 13C NMR and MS data are consistent with those in the literature33.
9-Methyl-2-(2-fluorophenyl)-β-carboline bromide (B2): Yield, 29%; golden yellow powders; mp 247.6–249.0 °C; 1H NMR (500 MHz, DMSO-d6) δ: 9.99 (1 H, s, H-1), 9.07 (1 H, d, J = 6.5 Hz, H-4), 9.00 (1 H, d-like, J = 6.5 Hz, H-3), 8.67 (1 H, d, J = 8.0 Hz, H-5), 8.04 (1 H, 2 × t, J = 7.9, 1.7 Hz, H-6′), 8.01–7.95 (2 H, m, H-7, H-8), 7.84 (1 H, m, H-4′), 7.75 (1 H, t-like, J = 9.4, 8.5, 1.3 Hz, H-3′), 7.63 (1 H, t, J = 7.7 Hz, H-5′), 7.58 (1 H, 2 × t, J = 7.9, 1.5 Hz, H-6), 4.17 (3 H, s, NCH3); 13C NMR (125 MHz, DMSO-d6) δ 155.4 (d, J = 252.1 Hz, C-2′), 146.0 (C-1), 136.4 (C-8a), 134.5 (C-8a), 133.7 (d, J = 7.9 Hz, C-4′), 133.5 (C-3), 133.1 (C-9a), 131.5 (d, J = 11.7 Hz, C-1′), 131.0 (C-4b), 128.9 (C-6′), 126.3 (d, J = 3.8 Hz, C-5′), 124.7 (C-7), 122.7 (C-5), 119.4 (C-6), 118.1 (C-8), 117.7 (d, J = 18.8 Hz, C-3′), 112.0 (C-4a), 31.2 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H14FN2+, 277.1136, found 277.1170.
9-Methyl-2-(3-fluorophenyl)-β-carboline bromide (B3): Yield, 50%; orangered powders; mp 143.0–144.0 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.97 (1 H, d, J = 1.1 Hz, H-1), 9.04 (2 H, s, H-3, H-4), 8.67 (1 H, d, J = 8.0 Hz, H-5), 8.06 (1 H, 2 × t, J = 9.5, 2.3 Hz, H-2′), 8.01–7.93 (2 H, m, H-7, H-8), 7.92–7.82 (2 H, m, H-5′, H-6′), 7.66 (1 H, t-like, J = 8.4 Hz, H-4′), 7.57 (1 H, 2 × t, J = 8.0, 1.4 Hz, H-6), 4.20 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 162.5 (d, J = 247.1 Hz, H-3′), 145.9 (C-1), 145.0 (d, J = 10.3 Hz, H-1′), 136.4 (C-8a), 133.32 (C-3), 133.26 (C-4b), 132.9 (C-9a), 132.5 (d, J = 8.8 Hz, C-5′), 129.8 (C-4), 124.7 (C-7), 122.7 (C-5), 122.1 (d, J = 3.2 Hz, C-6′), 119.4 (C-6), 118.23 (d, J = 21.1 Hz, C-2′), 118.19 (C-8), 113.8 (d, J = 26.3 Hz, C-4′), 112.0 (C-4a), 31.0 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H14FN2+, 277.1136, found 277.1170.
9-Methyl-2-(4-fluorophenyl)-β-carboline bromide (B4): Yield, 76%; yellow powders; mp 296.8–297.8 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.95 (1 H, s, H-1), 9.03 (1 H, d, J = 6.5 Hz, H-3), 8.99 (1 H, dd, J = 6.5, 1.3 Hz, H-2), 8.67 (1 H, d, J = 8.0 Hz, H-5), 8.08 (2 H, dd, J = 8.8, 4.5 Hz, H-2′, H-6′), 8.01–7.93 (2 H, m, H-7, H-8), 7.67 (2 H, t, J = 8.7 Hz, H-3′, H-5′), 7.57 (1 H, 2 × t, J = 8.0, 1.3 Hz, H-6), 4.19 (3 H, s, NCH3). 13C NMR (126 MHz, DMSO-d6) δ 162.9 (d, J = 249.0 Hz, C-4′), 145.2 (C-1), 140.0 (d, J = 2.9 Hz, C-1′), 136.0 (C-8a), 132.9 (C-3), 132.6 (C-9a), 132.1 (C-4b), 129.4 (C-4), 127.7 (d, J = 9.4 Hz, C-2′, C-6′), 124.1 (C-7), 122.1 (C-5), 118.9 (C-6), 117.6 (C-8), 116.9 (d, J = 23.6 Hz, C-3′, C-5′), 111.4 (C-4a), 30.5 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H14FN2+, 277.1136, found 277.1118.
9-Methyl-2-(2-chlorophenyl)-β-carboline bromide (B5): Yield, 36%; golden yellow powders; mp 206.9–207.7 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.99 (1 H, d, J = 1.1 Hz, H-1), 9.10 (1 H, d, J = 6.5 Hz, H-4), 8.96 (1 H, dd-like, J = 6.5, 1.4 Hz, H-4), 8.69 (1 H, d, J = 8.0 Hz, H-5), 8.04 (dd, J = 7.8, 1.4 Hz, H-6′), 8.02–7.96 (2 H, m, H-7, H-8), 7.94 (1 H, d, J = 8.0, 1.4 Hz, H-3′), 7.82 (1 H, 2 × t, J = 7.8, 1.6 Hz, H-4′), 7.77 (1 H, 2 × t, J = 7.7, 1.5 Hz, H-5′), 7.59 (1 H, dt-like, J = 8.0, 1.5 Hz, H-6), 4.17 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 145.4 (C-1), 140.6 (C-1′), 135.8 (C-8a), 134.0 (C-2′), 133.0 (C-3), 132.8 (C-9a), 132.6 (C-4b), 130.6 (C-4), 130.4 (C-4′), 128.9 (C-3′), 128.8 (C-5′), 128.6 (C-6′), 124.3 (C-7), 122.2 (C-5), 119.0 (C-6), 117.7 (C-8), 111.6 (C-4a), 30.7 (NCH3). HR-ESI-MS [M-Br]+: Calcd for C18H14ClN2+, 293.0840, found 293.0848.
9-Methyl-2-(3-chlorophenyl)-β-carboline bromide (B6): Yield, 60%; orangered powders; mp 206.7–207.5 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.97 (1 H, d, J = 1.1 Hz, H-1), 9.04 (2 H, broad s, H-3, H-4), 8.67 (1 H, d, J = 8.0 Hz, H-5), 8.23 (1 H, t, J = 2.1 Hz, H-2′), 8.01–7.94 (3 H, m, H-7, H-8, H-6′), 7.86 (1 H, 2 × t, J = 8.2, 1.6 Hz, H-4′), 7.83 (1 H, t, J = 7.9 Hz, H-5′), 7.57 (1 H, t, J = 6.6 Hz, H-6), 4.19 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 145.3 (C-1), 144.4 (C-1′), 140.1 (C-3′), 135.9 (C-8a), 134.1 (C-5′), 132.8 (C-3), 132.3 (C-9a), 131.6 (C-4b), 130.6 (C-2′), 129.2 (C-4), 125.5 (C-4′), 124.2 (C-7), 124.1 (C-6′), 122.1 (C-5), 118.8 (C-6), 117.6 (C-8), 111.4 (C-4a), 30.5 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H14ClN2+, 293.0840, found 293.0847.
9-Methyl-2-(4-chlorophenyl)-β-carboline bromide (B7): Yield, 89%; golden yellow powders; mp 265.5–266.5 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.94 (1 H, d, J = 1.2 Hz, H-1), 9.03 (1 H, d, J = 6.5 Hz, H-4), 9.00 (1 H, dd, J = 6.5, 1.2 Hz, H-3), 8.66 (1 H, d, J = 8.0 Hz, H-5), 8.05 (2 H, d, J = 8.7 Hz, H-3′, H-5′H-2′, H-6′), 8.01–7.92 (2 H, m, H-7, H-8), 7.90 (2 H, d, J = 8.7 Hz, H-2′, H-6′), 7.56 (1 H, t, J = 7.3 Hz, H-6), 4.19 (3 H, s, NCH3). 13C NMR (126 MHz, DMSO-d6) δ 145.2 (C-1), 142.3 (C-1′), 136.0 (C-8a), 135.5 (C-4′), 132.8 (C-3), 132.7 (C-3′, C-5′), 132.2 (C-9a), 130.0 (C-4b), 129.2 (C-4), 127.2 (C-2′, C-6′), 124.1 (C-7), 122.1 (C-5), 118.9 (C-6), 117.7 (C-8), 111.5 (C-4a), 30.6 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H14ClN2+, 293.0840, found 293.0933; Negative ESI-MS m/z: 78.68 [79Br−], 80.70 [81Br−].
9-Methyl-2-(2-bromophenyl)-β-carboline bromide (B8): Yield, 32%; golden yellow powders; mp 212.6–213.5 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.00 (1 H, d, J = 1.3 Hz, H-1), 9.12 (1 H, d, J = 6.4 Hz, H-4), 8.93 (1 H, dd, J = 6.4, 1.4 Hz, H-3), 8.70 (1 H, d, J = 8.1 Hz, H-5), 8.07 (1 H, dd, J = 8.1, 1.4 Hz, H-6′), 8.03 (1 H, dd, J = 8.0, 1.6 Hz, H-2′), 8.02–7.96 (2 H, m, H-7, H-8), 7.80 (1 H, 2 × t, J = 7.8, 1.4 Hz, H-4′), 7.74 (1 H, 2 × t, J = 7.8, 1.6 H, H-5′), 7.59 (1 H, 2 × t, J = 8.0, 1.4 Hz, H-6), 4.17(3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 145.9 (C-1), 142.7 (C-1′), 136.3 (C-8a), 134.6 (C-4′), 134.2 (C-3′), 133.5 (C-3), 133.4 (C-5′), 133.2 (C-9a), 130.8 (C-4b), 129.9 (C-4), 129.2 (C-6′), 124.8 (C-7), 122.8 (C-5), 119.4 (C-2′), 119.3 (C-6), 118.1 (C-8), 112.1 (C-4a), 31.1 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H14BrN2+, 337.0335 (79Br), 339.0314 (81Br), found 337.0340, 339.0329.
9-Methyl-2-(3-bromophenyl)-β-carboline bromide (B9): Yield, 53%; yellow powders; mp 219.2–220.2 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.96 (1 H, s, H-1), 9.03 (2 H, s, H-3, H-4), 8.66 (1 H, d, J = 8.0 Hz, H-5), 8.33 (1 H, d, J = 2.1 Hz, H-2′), 8.02 (1 H, dd, J = 8.2, 2.1 Hz, H-4′), 8.00–7.94 (3 H, m, H-7, H-8, H-6′), 7.75 (1 H, t, J = 8.1 Hz, H-5), 7.57 (1 H, t, J = 7.3 Hz, H-6), 4.19 (3 H, s, NCH3); 13C NMR (125 MHz, DMSO-d6) δ 145.8 (C-1), 145.0 (C-1′), 136.4 (C-8a), 134.1 (C-6′), 133.32 (C-5′), 133.30 (C-3), 132.8 (C-9a), 132.4 (C-4b), 129.8 (C-4), 128.7 (C-4′), 125.1 (C-3′), 124.6 (C-7), 122.8 (C-2′), 122.7 (C-5), 119.4 (C-6), 118.1 (C-8), 112.0 (C-4a), 31.0 (NCH3). HR-ESI-MS [M-Br]+: Calcd for C18H14BrN2+, 337.0335 (79Br), 339.0314 (81Br), found 337.0342, 339.0323.
9-Methyl-2-(4-bromophenyl)-β-carboline bromide (B10): Yield, 80%; faint yellow powders; mp 260.0–261.1 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.93 (1 H, d, J = 1.4 Hz, H-1), 9.03 (1 H, d, J = 6.5 Hz, H-3), 9.00 (1 H, dd, J = 6.5, 1.4 Hz, H-4), 8.66 (1 H, d, J = 8.0 Hz, H-5), 8.03 (2 H, d, J = 8.8 Hz, H-2′, H-6′), 7.97 (2 H, d, J = 8.8 Hz, H-3′, H-5′), 7.99–7.93 (2 H, m, H-7, H-8), 7.57 (1 H, 2 × t, J = 8.0, 1.4 Hz, H-6), 4.18 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 145.3 (C-1), 142.7 (C-1′), 136.0 (C-8a), 133.0 (C-3), 132.8 (C-3′), 132.7 (C-5′), 132.2 (C-9a), 131.3 (C-4b), 129.2 (C-4), 127.8 (C-3′), 127.4 (C-5′), 124.2 (C-2′), 124.1 (C-6′), 122.2 (C-5), 118.9 (C-6), 117.7 (C-8), 111.5 (C-4a), 30.6 (NCH3). HR-ESI-MS [M-Br]+: Calcd for C18H14BrN2+, 337.0335 (79Br), 339.0314 (81Br), found 337.0337, 339.0316.
9-Methyl-2-(2-iodohenyl)-β-carboline bromide (B11): Yield, 34%; golden yellow powders; mp 253.6–254.4 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.92 (1 H, d, J = 1.2 Hz, H-1), 9.09 (1 H, d, J = 6.4 Hz, H-4), 8.87 (1 H, dd, J = 6.4, 1.4 Hz, H-3), 8.68 (1 H, d, J = 8.1 Hz, H-5), 8.23 (1 H, dd, J = 7.9, 1.4 Hz, H-6′), 8.02–7.96 (2 H, m, H-7, H-8), 7.93 (1 H, dd, J = 7.9, 1.5 Hz, H-2′), 7.78 (1 H, td, J = 7.7, 1.4 Hz, H-4′), 7.60 (1 H, td, J = 8.0, 2.0 Hz, H-6), 7.53 (1 H, td, J = 7.8, 1.5 Hz, H-5′), 4.17 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 146.4 (C-1′), 145.9 (C-1), 140.2 (C-3′), 136.3 (C-8a), 134.6 (C-3), 133.5 (C-9a), 133.15 (C-4′), 133.12 (C-4b), 130.6 (C-5′), 130.4 (C-4), 128.2 (C-6′), 124.7 (C-7), 122.8 (C-5), 119.4 (C-6), 118.2 (C-8), 112.1 (C-4a), 96.7 (C-2′), 31.1 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H14IN2+, 385.0196, found 385.0198.
9-Methyl-2-(3-iodohenyl)-β-carboline bromide (B12): Yield, 60%; brown red powders; mp 243.7–244.0 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.93 (1 H, d, J = 1.1 Hz, H-1), 9.01(1 H, d, J = 6.5 Hz, H-4), 9.00 (1 H, dd, J = 6.5, 1.1 Hz, H-3), 8.66 (1 H, d, J = 8.0 Hz, H-5), 8.42 (1 H, t, J = 1.9 Hz, H-2′), 8.13 (1 H, d, J = 8.2 Hz, H-6′), 8.01 (1 H, dd, J = 8.1, 2.2 Hz, H-4′), 8.00–7.93 (2 H, m, H-7, H-8), 7.60–7.54 (2 H, m, H-6, H-5′), 4.19 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 145.8 (C-1′), 144.8 (C-1), 139.9 (C-4′), 136.4 (C-8a), 134.0 (C-2′), 133.3 (C-5′), 133.2 (C-9a), 132.7 (C-3), 132.2 (C-4b), 129.7 (C-4), 125.3 (C-6′), 124.6 (C-7), 122.6 (C-5), 119.4 (C-6), 118.1 (C-8), 112.0 (C-4a), 96.0 (C-3′), 31.0 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H14IN2+, 385.0196, found 385.0188.
9-Methyl-2-(4-iodohenyl)-β-carboline bromide (B13): Yield, 92%; orangered powders; mp 281.6–282.8 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.92 (1 H, d, J = 1.3 Hz, H-1), 9.02 (1 H, d, J = 6.5 Hz, H-4), 8.98 (1 H, dd, J = 6.5, 1.4 Hz, H-3), 8.65 (1 H, dd, J = 8.0, 1.0 Hz, H-5), 8.18 (2 H, d, J = 8.7 Hz, H-3′, H-5′), 7.99–7.93 (2 H, m, H-7, H-8), 7.80 (2 H, d, J = 8.7 Hz, H-2′, H-6′), 7.56 (1 H, 2 × t, J = 8.0, 1.4 Hz, H-6), 4.18 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 145.2 (C-1), 143.2 (C-1′), 138.8 (C-3′, H-5′), 136.0 (C-8a), 132.7 (C-3), 132.5 (C-9a), 132.1 (C-4b), 129.0 (C-4), 127.2, 124.1 (C-7), 122.1 (C-5), 118.9 (C-6), 117.7 (C-8), 111.4 (C-4a), 97.7 (C-4′), 30.5 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H14IN2+, 385.0196, found 385.0150; Negative ESI-MS m/z: 78.69 [79Br−], 80.68 [81Br−].
9-Methyl-2-(2-hydroxyhenyl)-β-carboline bromide (B14): Yield, 33%; red brown powders; mp 189.7–191.2 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.72 (1 H, s, H-1), 8.82 (1 H, d, J = 6.4 Hz, H-4), 8.76 (1 H, d, J = 6.5 Hz, H-3), 8.59 (1 H, d, J = 8.0 Hz, H-5), 7.94 (1 H, d, J = 8.4 Hz, H-8), 7.89 (1 H, 2 × t, J = 8.4, 2.1 Hz, H-7), 7.56–7.48 (2 H, m, H-4′), 7.33 (1 H, dd, J = 7.6, 2.0 Hz, H-6′), 7.15 (1 H, d, J = 7.9 Hz, H-3′), 7.07 (1 H, 2 × t, J = 8.0, 1.8 Hz, H-5′), 6.58 (1 H, 2 × broad s, OH), 4.14 (s, 3 H, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 145.1 (C-2′), 136.1 (C-1), 134.1 (C-8a), 131.9 (C-3), 131.4 (C-9a), 130.8 (C-4b), 129.6 (C-4′), 128.4 (C-3), 125.9 (C-6′), 125.5 (C-1′), 124.6 (C-7), 124.0 (C-5′), 121.9 (C-5), 119.6 (C-6), 117.0 (C-8), 112.4 (C-3′), 111.5 (C-4a), 30.6 (NCH3). HR-ESI-MS [M-Br]+: Calcd for C18H15N2O+, 275.1179, found 275.1178.
9-Methyl-2-(3-hydroxyhenyl)-β-carboline bromide (B15): Yield, 69%; red brown powders; mp 233.8–235.1 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.42 (1 H, s, OH), 9.89 (1 H, s, H-1), 8.99 (1 H, d, J = 6.5 Hz, H-4), 8.96 (1 H, dd, J = 6.5, 1.3 Hz, H-3), 8.65 (1 H, d, J = 8.1 Hz, H-5), 8.04–7.89 (2 H, m, H-7, H-8), 7.58 (1 H, t, J = 8.0 Hz, H-5′), 7.54 (1 H, 2 × t, J = 8.0, 1.4 Hz, H-6), 7.38 (1 H, dd, J = 7.8, 2.1 Hz, H-6′), 7.35 (1 H, t, J = 2.3 Hz, H-2′), 7.17 (1 H, dd, J = 8.2, 2.2 Hz, H-4′), 4.18 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 158.5 (C-3′), 145.1 (C-1), 144.5 (C-1′), 136.0 (C-8a), 132.52 (C-9a), 132.48 (C-3), 132.0 (C-4b), 130.9 (C-5′), 128.8 (C-4), 124.0 (C-7), 121.9 (C-5), 118.8 (C-6), 117.6 (C-8), 117.5 (C-6′), 115.3 (C-4′), 112.1 (C-4a), 111.3 (C-2′), 30.4 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H15N2O+, 275.1179, found 275.1190.
9-Methyl-2-(4-hydroxyhenyl)-β-carboline bromide (B16): Yield, 93%; red brown powders; mp 267.9–268.5 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.72 (1 H, s, H-1), 8.88 (1 H, d, J = 6.6 Hz, H-4), 8.86 (1 H, d, J = 6.6 Hz, H-3), 8.60 (1 H, d, J = 8.0 Hz, H-5), 7.95–7.88 (2 H, m, H-7, H-8), 7.56 (2 H, d, J = 8.1 Hz, H-2′, H-6′), 7.53 (1 H, t, J = 7.3 Hz, H-6), 6.71 (2 H, d, J = 8.1 Hz, H-3′, H-5′), 4.18 (1 H, b s, OH), 4.18 (3 H, s, NCH3). 13C NMR (126 MHz, DMSO-d6) δ 144.7 (C-1), 136.3 (C-4′), 132.1 (C-1′), 132.0 (C-8a), 131.8 (C-3), 130.4 (C-9a), 127.5 (C-4), 125.4 (C-4b), 125.33 (C-2′), 125.31 (C-6′), 123.7 (C-7), 121.7 (C-5), 119.0 (C-6), 118.0 (C-3′), 117.9 (C-5′), 117.6 (C-8), 111.1 (C-4a), 30.4 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H15N2O+, 275.1179, found 275.1185.
9-Methyl-2-(2-methoxylphenyl)-β-carboline bromide (B17): Yield, 46%; orange powders; mp 181.7–182.4 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.85 (1 H, d, J = 1.3 Hz, H-1), 8.99 (1 H, d, J = 6.4 Hz, H-4), 8.84 (1 H, dd, J = 6.5, 1.3 Hz, H-3), 8.64 (1 H, d, J = 8.1 Hz, H-5), 8.00–7.94 (2 H, m, H-7, H-8), 7.81 (1 H, dd, J = 7.8, 1.6 Hz, H-6′), 7.74 (1 H, 2 × t, J = 8.0, 1.7 Hz, H-4′), 7.57 (1 H, 2 × t, J = 7.2, 1.7 Hz, H-6), 7.49 (1 H, dd, J = 8.5, 1.2 Hz, H-5′), 7.31 (1 H, 2 × t, J = 7.7, 1.2 Hz, H-3′), 4.15 (3 H, s, OCH3), 3.86 (3 H, s, NCH3); 13C NMR (125 MHz, DMSO-d6) δ 152.9 (C-2′), 145.7 (C-1), 136.4 (C-8a), 135.0 (C-4′), 133.2 (C-3), 133.0 (C-9a), 132.7 (C-4b), 131.1 (C-4), 127.9 (C-6′), 124.6 (C-7, C-1′), 122.5 (C-5), 121.6 (C-5′), 119.4 (C-6), 117.7 (C-8), 113.9 (C-3′), 111.9 (C-4a), 57.0 (OCH3), 31.0 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C19H17N2O+, 289.1335, found 289.1367.
9-Methyl-2-(3-methoxylphenyl)-β-carboline bromide (B18): Yield, 70%; yellow powders; mp 204.0–205.1 °C; 11H NMR (500 MHz, DMSO-d6) δ 9.93 (1 H, s, H-1), 9.01 (2 H, s, H-3, H-4), 8.66 (1 H, d, J = 8.0 Hz, H-5), 8.03–7.92 (2 H, m, H-7, H-8), 7.70 (1 H, t, J = 8.2 Hz, H-5′), 7.63 (1 H, t, J = 2.2 Hz, H-2′), 7.61–7.52 (2 H, m, H-6, H-6′), 7.33 (1 H, dd, J = 8.4, 2.4 Hz, H-4′), 4.19 (3 H, s, OCH3), 3.93 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 160.6 (C-3′), 145.7 (C-1), 145.1 (C-1′), 136.5 (C-8a), 133.3 (C-5′), 133.2 (C-9a), 132.7 (C-3), 131.5 (C-4b), 129.6 (C-4), 124.6 (C-7), 122.6 (C-5), 119.4 (C-6′), 118.2 (C-6), 117.6 (C-8), 116.9 (C-2′), 111.9 (C-4′), 111.7(C-4a), 56.5 (OCH3), 31.1 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C19H17N2O+, 289.1335, found 289.1340.
9-Methyl-2-(4-methoxylphenyl)-β-carboline bromide (B19): Yield, 93%; faint yellow powders; mp 225.5–226.8 °C; 11H NMR (500 MHz, DMSO-d6) δ 9.87 (1 H, d, J = 1.3 Hz, H-1), 8.99 (1 H, d, J = 6.5 Hz, H-4), 8.95 (1 H, dd, J = 6.5, 1.4 Hz, H-3), 8.65 (1 H, d, J = 8.0 Hz, H-5), 7.99–7.92 (2 H, m, H-7, H-8), 7.93 (2 H, d, J = 9.0 Hz, H-2′, H-6′), 7.55 (1 H, 2 × t, J = 8.0, 1.2 Hz, H-6), 7.32 (2 H, d, J = 9.0 Hz, H-3′, H-5′), 4.18 (3 H, s, OCH3), 3.92 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 160.7 (C-4′), 145.1 (C-1), 136.8 (C-8a), 136.1 (C-1′), 132.8 (C-9a), 132.4 (C-3), 131.7 (C-4b), 129.0 (C-4), 126.4 (C-2′, C-6′), 124.0 (C-7), 122.0 (C-5), 118.9 (C-6), 117.7 (C-8), 115.1 (C-3′, C-5′), 111.3 (C-4a), 55.9 (OCH3), 30.5 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C19H17N2O+, 289.1335, found 289.1342; Negative ESI-MS m/z: 78.68 [79Br−], 80.67 [81Br−].
9-Methyl-2-(2-methylphenyl)-β-carboline bromide (B20): Yield, 43%; yellow powders; mp 122.6–130.5 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.83 (1 H, s, H-1), 9.03 (1 H, d, J = 6.3 Hz, H-4), 8.85 (1 H, d, J = 6.3 Hz, H-3), 8.68 (1 H, d, J = 8.0 Hz, H-5), 8.02–7.93 (2 H, m, H-7, H-8), 7.74 (1 H, d, J = 7.8 Hz, H-6′), 7.67 (1 H, t, J = 8.0 Hz, H-4′), 7.64 (1 H, t, J = 8.0 Hz, H-5′), 7.58 (2 H, m, H-6, H-3′), 4.16 (3 H, s, NCH3), 2.17 (3 H, s, CH3); 13C NMR (126 MHz, DMSO-d6) δ 145.2 (C-1), 143.0 (C-1′), 136.0 (C-8a), 133.6 (C-9a), 133.0 (C-3), 132.6 (C-2′), 132.2 (C-4b), 131.5 (C-4), 130.9 (C-3′), 130.0 (C-4′), 127.4 (C-5′), 126.5 (C-6′), 124.1 (C-7), 122.0 (C-5), 119.0 (C-6), 117.6 (C-8), 111.4 (C-4a), 30.5 (NCH3), 16.6 (CH3); HR-ESI-MS [M-Br]+: Calcd for C19H17N2+, 273.1386, found 273.1392.
9-Methyl-2-(3-methylphenyl)-β-carboline bromide (B21): Yield, 68%; luminous yellow powders; mp 227.9–229.3 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.96 (1 H, s, H-1), 9.05 (1 H, d, J = 6.5 Hz, H-4), 9.03 (1 H, d, J = 6.7 Hz, H-3), 8.69 (1 H, d, J = 8.0 Hz, H-5), 8.06–7.94 (2 H, m, H-7, H-8), 7.87 (1 H, s, H-2′), 7.82 (1 H, d, J = 8.0 Hz, H-6′), 7.70 (1 H, t, J = 7.8 Hz, H-5′), 7.63–7.56 (2 H, m, H-6, H-4′), 4.23 (3 H, s, NCH3), 2.54 (3 H, s, CH3); 13C NMR (126 MHz, DMSO-d6) δ 145.7 (C-1), 144.1 (C-1′), 140.6 (C-3′), 136.6 (C-8a), 133.2 (C-3), 133.1 (C-9a), 132.6 (C-4b), 131.7 (C-2′), 130.4 (C-4′), 129.5 (C-4), 126.0 (C-5′), 124.6 (C-7), 122.7 (C-6′), 122.6 (C-5), 119.4 (C-6), 118.2 (C-8), 111.9 (C-4a), 31.1 (NCH3), 21.4 (CH3); HR-ESI-MS [M-Br]+: Calcd for C19H17N2+, 273.1386, found 273.1416.
9-Methyl-2-(4-methylphenyl)-β-carboline bromide (B22): Yield, 82%; orange powders; mp 290.2–290.9 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.89 (1 H, s, H-1), 9.00 (1 H, d, J = 6.5 Hz, H-4), 8.97 (1 H, dd, J = 6.5, 1.4 Hz, H-3), 8.65 (1 H, d, J = 8.0 Hz, H-5), 8.00–7.92 (2 H, m, H-7, H-8), 7.88 (2 H, d, J = 8.3 Hz, H-2′, H-6′), 7.59 (2 H, d, J = 8.3 Hz, H-3′, H-5′), 7.56 (1 H, t, J = 8.1 Hz, H-6), 4.18 (3 H, s, NCH3), 2.49 (3 H, s, CH3); 13C NMR (126 MHz, DMSO-d6) δ 145.1 (C-1), 141.3 (C-4′), 140.7 (C-1′), 136.1 (C-8a), 132.7 (C-9a), 132.5 (C-3), 131.9 (C-4b), 130.4 (C-3′, C-5′), 128.9 (C-4), 124.8 (C-2′, C-6′), 124.0 (C-7), 122.0 (C-5), 118.9 (C-6), 117.7 (C-8), 111.4 (C-4a), 30.5 (NCH3), 20.6 (CH3); HR-ESI-MS [M-Br]+: Calcd for C19H17N2+, 273.1386, found 273.1390; Negative ESI-MS m/z: 78.72 [79Br−], 80.72 [81Br−].
9-Methyl-2-(2-cyanophenyl)-β-carboline bromide (B23): Yield, 57%; brown yellow powders; mp 179.5–179.8 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.08 (1 H, s, H-1), 9.14 (1 H, d, J = 6.5 Hz, H-4), 9.12 (1 H, d, J = 6.5 Hz, H-3), 8.68 (1 H, d, J = 8.0 Hz, H-5), 8.34 (1 H, d, J = 7.8 Hz, H-6′), 8.21–8.12 (2 H, m, H-3′, H-4′), 8.06–7.96 (3 H, m, H-7, H-8, H-5′), 7.61 (1 H, 2 × t-like, J = 8.0, 1.6 Hz, H-6), 4.18 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 145.6 (C-1), 144.3 (C-1′), 135.7 (C-8a), 135.0 (C-5′), 134.2 (C-3′), 133.5 (C-4′), 133.2 (C-9a), 132.9 (C-3), 131.8 (C-4b), 130.2 (C-3), 128.1 (C-6′), 124.3 (C-7), 122.4 (C-5), 118.9 (C-6), 117.6 (C-8), 114.8 (C≡N), 111.6 (C-4a), 109.4 (C-2′), 30.6 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C19H14N3+, 284.1182, found 284.1193.
9-Methyl-2-(3-cyanophenyl)-β-carboline bromide (B24): Yield, 79%; orange-yellow powders; mp 271.0–272.8 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.0 (1 H, s, H-1), 9.08 (1 H, s, H-4), 8.67 (1 H, d, J = 8.0 Hz, H-5), 8.63 (1 H, br s, H-3), 8.37 (1 H, dd, J = 8.2, 2.3 Hz, H-6′), 8.27 (1 H, d, J = 7.9 Hz, H-4′), 8.03 (1 H, d, J = 7.9 Hz, H-4′), 8.01–7.95 (2 H, m, H-7, H-8), 7.99 (1 H, s, H-3′), 7.58 (1 H, 2 × t, J = 8.0, 1.4 Hz, H-6), 4.20 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 147.0 (C-1′), 145.3 (C-1), 143.6 (C-4′), 135. 9 (C-8a), 134.3 (C-5′), 132.9 (C-9a), 132.7 (C-3), 132.4 (C-4b), 131.4 (C-6′), 130.3 (C-4), 129.3 (C-2′), 124.2 (C-7), 122.2 (C-5), 118.8 (c-6), 117.7 (C-8), 117.5 (C≡N), 112.7 (C-3′), 111.5 (C-4a), 30.5 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C19H14N3+, 284.1182, found 284.1187.
9-Methyl-2-(4-cyanophenyl)-β-carboline bromide (B25): Yield, 74%; orange-yellow powders; mp 188.6–189.9 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.0 (1 H, d, J = 1.2 Hz, H-1), 9.07 (1 H, d-like, J = 6.5 Hz, H-4), 9.04 (1 H, dd-like, J = 6.5, 1.2 Hz, H-3), 8.67 (1 H, d, J = 8.0 Hz, H-5), 8.34 (2 H, d, J = 8.7 Hz, H-2′, H-6′), 8.24 (2 H, d, J = 8.7 Hz, H-3′, H-5′), 8.03–7.94 (2 H, m, H-7, H-8), 7.58 (1 H, 2 × t, J = 8.0, 1.4 Hz, H-6), 4.20 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 146.4 (C-1′), 145.4 (C-1), 135.9 (C-8a), 134.2 (C-3′, C-5′), 132.9 (C-3), 132.6 (C-9a), 132.5 (C-4b), 129.2 (C-4), 126.6 (C-2′, C-6′), 124.2 (C-7), 122.2 (C-5), 118.9 (C-6), 117.74 (C-8), 117.71 (C-4′), 113.5 (C≡N), 111.5 (C-4a), 30.6 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C19H14N3+, 284.1182, found 284.1180.
9-Methyl-2-(3-trifluoromethylphenyl)-β-carboline bromide (B26): Yield, 82%; orange-yellow powders; mp 125.4–126.1 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.0 (1 H, s, H-1), 9.08 (1 H, dd, J = 6.5, 1.3 Hz, H-3), 9.06 (1 H, d, J = 6.5 Hz, H-4), 8.68 (1 H, d, J = 8.0 Hz, H-5), 8.48 (1 H, s, H-2′), 8.33 (1 H, dd, J = 8.0, 2.2 Hz, H-6′), 8.16 (1 H, d, J = 7.9 Hz, H-4′), 8.05 (1 H, t, J = 8.0 Hz, H-5′), 8.02–7.94 (2 H, m, H-7, H-8), 7.58 (1 H, 2 × t, J = 8.0, 1.3 Hz, H-6), 4.20 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 145.3 (C-1), 143.9 (C-1′), 135.9 (C-8a), 133.0 (C-3), 132.8 (C-9a), 132.3 (C-4b), 131.4 (C-4), 130.4 (q, J = 33.0 Hz, C-3′), 129.7 (C-5′), 129.5 (C-6′), 127.5 (q, J = 3.7 Hz, C-4′), 124.2 (C-7), 123.4 (q, J = 273.3 Hz, CF3), 122.7 (q, J = 4.0 Hz, C-2′), 122.2 (C-5), 118.9 (C-6), 117.6 (C-8), 111.5 (C-4a), 30.6 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C19H14F3N2+, 327.1104, found 327.1081.
9-Methyl-2-(4-trifluoromethylphenyl)-β-carboline bromide (B27): Yield, 86%; yellow powders; mp 152.7–153.8 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.0 (1 H, s, H-1), 9.07 (2 H, s, H-3, H-4), 8.67 (1 H, d, J = 8.0 Hz, H-5), 8.26 (2 H, d, J = 8.6 Hz, H-2′, H-6′), 8.22 (2 H, d, J = 8.6 Hz, H-3′, H-5′), 8.03–7.94 (2 H, m, H-7, H-8), 7.58 (1 H, 2 × t, J = 8.0, 1.4 Hz, H-6), 4.20 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 146.3 (d-like, J = 4.5 Hz, H-1′), 145.3 (C-1), 135.9 (C-8a), 132.9 (C-3), 132.7 (C-9a), 132.4 (C-4b), 130.8 (q, J = 32.3 Hz, C-4′), 129.3 (C-4), 127.3 (q, J = 3.8 Hz, C-3′, C-5′), 126.6 (C-2′, C-6′), 123.6 (q, J = 273.5 Hz, CF3), 124.2 (C-7), 122.2 (C-5), 118.9 (C-6), 117.7 (C-8), 111.5 (C-4a), 30.6 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C19H14F3N2+, 327.1104, found 327.1130.
9-Methyl-2-(3-nitrophenyl)-β-carboline bromide (B28): Yield, 79%; orange-yellow powders; mp 182.2–183.5 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.1 (1 H, d, J = 1.3 Hz, H-1), 9.09 (1 H, dd, J = 6.5, 1.4 Hz, H-3), 9.07 (1 H, d, J = 6.5 Hz, H-4), 8.96 (1 H, t, J = 2.3 Hz, H-2′), 8.68 (1 H, d, J = 8.1 Hz, H-5), 8.61 (1 H, dd, J = 8.2, 2.1 Hz, H-6′), 8.48 (1 H, dd, J = 7.8, 2.2 Hz, H-4′), 8.10 (1 H, t, J = 8.2 Hz, H-5′), 8.04–7.95 (2 H, m, H-7, H-8), 7.58 (1 H, dt-like, J = 8.0, 1.3 Hz, H-6), 4.21 (3 H, s, NCH3); 3C NMR (126 MHz, DMSO-d6) δ 148.2 (C-3′), 145.4 (C-1), 143.8 (C-1′), 135.9 (C-8a), 132.92 (C-3), 132.89 (C-9a), 132.5 (C-4b), 132.0 (C-5′), 131.6 (C-6′), 129.6 (C-4), 125.4 (C-4′), 124.2 (C-7), 122.2 (C-5), 121.0 (C-2′), 118.9 (C-6), 117.7 (C-8), 111.5 (C-4a), 30.6 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H14N3O2+, 304.1081, found 304.1089.
9-Methyl-2-(2,6-difluorophenyl)-β-carboline bromide (B29): Yield, 68%; yellow powders; mp 259.5–261.0 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.10 (1 H, s, H-1), 9.15 (1 H, d, J = 6.5 Hz, H-4), 9.06 (1 H, d, J = 6.4 Hz, H-3), 8.67 (1 H, d, J = 8.1 Hz, H-5), 8.03–7.98 (2 H, m, H-7, H-8), 7.92 (1 H, nonet, J = 8.7, 6.3 Hz, H-4′), 7.66 (2 H, t, J = 8.8 Hz, H-3′, H-5′), 7.60 (1 H, dt-like, J = 8.0, 2.2 Hz, H-6), 4.14 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 156.1 (d, J = 254.2 Hz, C-2′, C-6′), 145.8 (C-1), 136.0 (C-8a), 134.4 (C-3), 133.8 (C-9a), 133.5 (C-4b), 133.2 (C-4), 131.2 (C-4′), 124.4 (C-7), 122.4 (C-5), 119.0 (C-6), 118.0 (C-8), 113.6 (d, J = 12.3 Hz, C-1′), 113.2 (dd, J = 18.8, 3.2 Hz, C-3′, C-5′), 111.7 (C-4a), 30.6 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H13F2N2+, 295.1041, found 295.1049.
9-Methyl-2-(2,4-dichlorophenyl)-β-carboline bromide (B30): Yield, 63%; brown powders; mp 215.6–216.8 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.97 (1 H, d, J = 1.3 Hz, H-1), 9.10 (1 H, d, J = 6.4 Hz, H-4), 8.94 (1 H, dd, J = 6.4, 1.3 Hz, H-3), 8.67 (1 H, d, J = 8.0 Hz, H-5), 8.21 (1 H, d, J = 2.3 Hz, H-3′), 8.07 (1 H, d, J = 8.5 Hz, H-6′), 8.05–7.95 (2 H, m, H-7, H-8), 7.91 (1 H, dd, J = 8.5, 2.3 Hz, H-5′), 7.59 (1 H, dt-like, J = 8.0, 2.2 Hz, H-6), 4.15 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 145.5 (C-1), 139.5 (C-1′), 136.8 (C-4′), 136.6 (C-2′), 135.8 (C-8a), 133.9 (C-3), 133.1 (C-9a), 132.8 (C-4b), 130.4 (C-3′), 130.2 (C-5′), 130.1 (C-4), 128.9 (C-6′), 124.2 (C-7), 122.3 (C-5), 118.9 (C-6), 117.6 (C-8), 111.6 (C-4a), 30.6 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H13Cl2N2+, 327.0450 (35Cl), 329.0421 (37Cl) found 327.0458, 329.0428.
9-Methyl-2-(3,5-dichlorophenyl)-β-carboline bromide (B31): Yield, 73%; yellow powders; mp 272.6–273.8 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.01 (1 H, s, H-1), 9.08 (2 H, t-like, J = 6.0 Hz, H-3, H-4), 8.69 (1 H, d, J = 8.0 Hz, H-5), 8.27 (2 H, d, J = 1.8 Hz, H-2′, H-6′), 8.13 (1 H, t, J = 1.8 Hz, H-4′), 8.08–7.94 (2 H, m, H-7, H-8), 7.61 (1 H, dt-like, J = 8.0, 1.6 Hz, H-6), 4.22 (3 H, s, NCH3). 13C NMR (126 MHz, DMSO-d6) δ 145.4 (C-1), 144.8 (C-1′), 135.8 (C-8a), 135.0 (C-3′, C-5′), 133.0 (C-3), 132.8 (C-9a), 132.5 (C-4b), 130.3 (C-4′), 129.3 (C-4), 124.8 (C-7), 124.2 (C-2′, C-6′), 122.2 (C-5), 118.8 (C-6), 117.6 (C-8), 111.5 (C-4a), 30.6 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H13Cl2N2+, 327.0450 (35Cl), 329.0421 (37Cl) found 327.0429, 329.0399.
9-Methyl-2-(2-fluoro-4-bromophenyl)-β-carboline bromide (B32): Yield, 56%; orange-yellow powders; mp 211.2–212.9 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.97 (1 H, s, H-1), 9.07 (1 H, d, J = 6.5 Hz, H-4), 8.98 (1 H, dt, J = 6.4, 1.5 Hz, H-3), 8.66 (1 H, d, J = 8.0 Hz, H-5), 8.18 (1 H, dd, J = 9.9, 2.1 Hz, H-6′), 8.04–7.96 (3 H, m, H-7, H-8, H-5′), 7.89 (1 H, dt, J = 8.5, 1.5 Hz, H-5′), 7.59 (1 H, dt-like, J = 8.0, 1.7 Hz, H-6), 4.16 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 145.5 (C-1), 139.5 (C-1′), 136.8 (C-4′), 136.6 (C-2′), 135.8 (C-8a), 133.9 (C-3), 133.1 (C-9a), 132.8 (C-4b), 130.4 (C-3′), 130.2 (C-5′), 130.1 (C-4), 128.9 (C-6′), 124.2, 122.3, 118.9, 117.6 (C-8), 111.6 (C-4a), 30.6 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H13BrFN2+, 355.0241 (79Br), 357.0220 (81Br) found 355.0244, 357.0223.
9-Methyl-2-(2,4-dibromophenyl)-β-carboline bromide (B33): Yield, 55%; yellow powders; mp 258.4–258.7 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.97 (1 H, d, J = 1.3 Hz, H-1), 9.10 (1 H, d, J = 6.4 Hz, H-4), 8.92 (1 H, dd, J = 6.4, 1.3 Hz, H-4), 8.68 (1 H, d, J = 8.1 Hz, H-5), 8.41 (1 H, d, J = 2.0 Hz, H-3′), 8.06 (1 H, dd, J = 8.5, 2.2 Hz, H-5′), 8.03–7.94 (3 H, m, H-7, H-8, H-6′), 7.59 (1 H, dt-like, J = 8.0, 1.7 Hz, H-6), 4.16 (3 H, s, NCH3); 13C NMR (126 MHz, DMSO-d6) δ 145.5 (C-1), 141.5 (C-1′), 135.7 (C-3′), 135.6 (C-8a), 133.9 (C-3), 133.1 (C-9a), 132.7 (C-4b), 132.3 (C-5′), 130.2 (C-6′), 130.1 (C-4), 125.1 (C-2′), 124.2 (C-7), 122.3 (C-5), 120.4 (C-4′), 118.9 (C-6), 117.6 (C-8), 111.6 (C-4a), 30.6 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H13Br2N2+, 414.9440 (2 × 79Br), 416.9420 (79Br + 81Br), 418.9399 (2 × 81Br) found 414.9439, 416.9418, 418.9398.
6-Methoxy-9-methyl-2-phenyl-β-carboline bromide (B34): Yield, 88%; orange-yellow powders; 1H NMR (500 MHz, MeOD) δ 9.64 (1 H, s, H-1), 8.82 (1 H, d, J = 6.5 Hz, H-4), 8.73 (1 H, d, J = 6.5 Hz, H-3), 8.00 (1 H, d, J = 2.6 Hz, H-5), 7.91 (2 H, d-like, J = 6.6 Hz), 7.82 (1 H, d, J = 9.2 Hz), 7.78–7.74 (3 H, m), 7.58 (1 H, dd, J = 9.2, 2.7 Hz), 4.17 (NCH3), 3.99 (OCH3); 13C NMR (126 MHz, CD3OD) δ 157.5 (C-6), 145.6 (C-1), 142.8 (C-1′), 138.0, 133.8, 132.9, 132.1, 131.6 (C-3′, C-5′), 129.5, 126.1 (C-2′, C-6′), 125.8, 121.2, 118.7, 113.3, 104.6, 56.6 (OCH3), 30.9 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C21H21N2+, 289.1335 found 289.1342.
6,9-Dimethyl-2-phenyl-β-carboline bromide (B35): Yield, 90%; orange-yellow powders; 1H NMR (500 MHz, MeOD) δ 9.66 (1 H, s, H-1), 8.81(1 H, d, J = 6.5 Hz, H-4), 8.79 (1 H, d, J = 6.5 Hz, H-3), 8.34 (1 H, s, H-5), 7.94 (2 H, d, J = 6.9 Hz, H-2′, H-6′), 7.79–7.82 (5 H, m), 4.19 (3 H, NCH3), 2.63 (3 H, CH3); 13C NMR (126 MHz, MeOD) δ 145.9 (C-1), 145.6 (C-1′), 138.1, 136.1, 134.2, 133.9, 133.5, 132.1, 131.6 (C-3′, C-5′), 129.3, 126.1 (C-2′, C-6′), 124.0, 120.9, 118.6, 111.9, 30.9 (NCH3), 21.4 (CH3); HR-ESI-MS [M-Br]+: Calcd for C19H17N2+, 273.1386 found 273.1393.
7-Fluoro-9-methyl-2-phenyl-β-carboline bromide (B36): Yield, 93%; orange-yellow powders; 1H NMR (500 MHz, CD3Cl) δ 9.94 (1 H, s, H-1), 9.06 (1 H, dd, J = 6.5, 1.2 Hz, H-3), 9.02 (1 H, d, J = 6.5 Hz, H-4), 8.75 (1 H, dd, J = 8.5, 5.5 Hz, H-5), 8.01 (2 H, d-like, J = 8.0 Hz, H-8), 7.94 (1 H, dd, J = 10.0, 2.0 Hz, H-8), 7.84–7.78 (3 H, m), 7.50–7.47 (1 H, m), 4.19 (3 H, s, CH3); 13C NMR (126 MHz, DMSO-d6) δ 165.2 (d, J = 249.2 Hz, C-7), 146.6 (d, J = 13.7 Hz, C-1), 143.6 (C-1′), 136.9 (d, J = 1.2 Hz, C-8a), 133.5 (C-3), 132.1 (C-4′), 130.8 (C-9a), 130.2 (C-3′, C-5′), 129.1, 128.9, 126.6 (d, J = 11.4 Hz, C-5), 125.5 (C-4), 125.2 (C-2′, C-6′), 117.6 (C-4b), 115.8 (C-4a), 111.3 (d, J = 25.7 Hz, C-8), 98.2 (d, J = 27.5 Hz, C-6), 30.9 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H14FN2+, 277.1136 found 277.1145.
7-Chloro-9-methyl-2-phenyl-β-carboline bromide (B37): Yield, 93%; orange-yellow powders; 1H NMR (500 MHz, CD3Cl) δ 9.98 (1 H, s, H-1), 9.08–9.04 (2 H, m), 8.80 (1 H, d, J = 6.0 Hz, H-4), 8.70 (1 H, d, J = 8.5 Hz, H-5), 8.20 (1 H, d, J = 1.6 Hz, H-8), 8.03–8.01 (2 H, m), 7.84–7.78 (3 H, m), 7.63 (1 H, dd, J = 8.5, 1.7 Hz, H-6), 7.48 (1 H,d, J = 8.0 Hz, H-4′), 7.1.2 (1 H, d, J = 7.4 Hz, H-6), 4.20 (3 H, s, CH3); 13C NMR (126 MHz, DMSO-d6) δ 145.7 (C-1), 143.6 (C-1′), 136.7 (C-8a), 133.5, 130.8, 130.2 (C-3′, C-5′), 129.6, 128.0, 125.8, 125.5, 125.2 (C-2′, C-6′), 122.7, 118.0, 118.2 (C-8), 111.7 (C-4a), 30.8 (NCH3); HR-ESI-MS [M-Br]+: Calcd for C18H14ClN2+, 293.0840 found 293.0851.
9-Ethyl-2-phenyl-β-carboline bromide (B38): Yield, 90%; orange-yellow powders; 1H NMR (500 MHz, CD3Cl) δ 10.1 (1 H, s, H-1), 8.80 (1 H, d, J = 6.0 Hz, H-4), 8.71 (1 H, d, J = 6.0 Hz, H-3), 8.31 (1 H, d, J = 8.0 Hz, H-5), 8.06 (2 H, d, J = 7.5 Hz, H-2′, H-6′), 7.78 (1 H, t, J = 7.5 Hz), 7.61 (1 H, d, J = 8.4 Hz), 7.56 (2 H, t, J = 7.5 Hz, H-3′, H-5′), 7.48 (1 H, t, J = 7.5 Hz), 7.40 (1 H, t, J = 7.4 Hz, H-4′), 4.96 (2 H, q, J = 7.0 Hz, H-1″), 1.49 (3 H, t, J = 7.0 Hz, H-2″); 13C NMR (126 MHz, CD3Cl) δ 144.7 (C-1), 143.1 (C-1′), 135.5 (C-8a), 133.0 (C-3), 132.9 (C-9a), 131.9 (C-4′), 130.7 (C-3′, C-5′), 130.5 (C-4b), 127.8 (C-4), 124.8 (C-7), 124.0 (C-2′, C-6′), 122.4 (C-5), 119.5 (C-6), 118.3 (C-8), 110.8 (C-4a), 40.2 (C-1″), 14.5 (C-2″); HR-ESI-MS [M-Br]+: Calcd for C19H17N2+, 273.1386 found 273.1385.
9-Propyl-2-phenyl-β-carboline bromide (B39): Yield, 86%; orange-yellow powders; 1H NMR (500 MHz, CD3Cl) δ 10.1 (1 H, s, H-1), 8.81 (1 H, d, J = 6.0 Hz, H-4), 8.72 (1 H, d, J = 6.0 Hz, H-3), 8.30 (1 H, d, J = 7.8 Hz, H-5), 8.05 (2 H, d, J = 7.5 Hz, H-2′, H-6′), 7.77 (1 H, t, J = 7.4 Hz), 7.59 (1 H, d, J = 8.3 Hz), 7.54 (2 H, t, J = 7.4 Hz, H-3′, H-5′), 7.46 (1 H, t, J = 6.9 Hz), 7.38 (1 H, t, J = 7.4 Hz, H-4′), 4.86 (2 H, t, J = 6.7 Hz, H-1″), 1.97–1.87 (2 H, m, H-2″), 0.94 (3 H, t, J = 7.0 Hz, H-3″); 13C NMR (126 MHz, CD3Cl) δ 145.2 (C-1), 143.1 (C-1′), 136.1, 132.92, 132.86, 132.0, 130.7, 130.4 (C-3′, C-5′), 127.9, 124.9 (C-2′, C-6′), 124.0, 122.4, 119.3, 118.4, 111.0, 46.5 (C-1″), 22.7 (C-2″), 11.5 (C-3″); HR-ESI-MS [M-Br]+: Calcd for C20H19N2+, 287.1543 found 287.1538.
9-Iso-propyl-2-phenyl-β-carboline bromide (B40): Yield, 85%; orange-yellow powders; 1H NMR (500 MHz, CDCl3) δ 10.1 (1 H, s, H-1), 8.84 (1 H, d, J = 6.4 Hz, H-4), 8.76 (1 H, d, J = 6.4 Hz, H-3), 8.34 (1 H, d, J = 8.0 Hz, H-5), 8.06–8.04 (2 H, m), 7.82 (1 H, d, J = 8.5 Hz), 7.76 (1 H, t, J = 7.7 Hz), 7.55 (2 H, t, J = 7.7 Hz), 7.48 (1 H, t, J = 7.7 Hz), 7.40 (1 H, t, J = 7.5 Hz), 5.84 (1 H, p, J = 7.1 Hz, NCHMe2), 1.77 (6 H, d, J = 6.9 Hz, 2 × CH3); 13C NMR (126 MHz, CDCl3) δ 143.9 (C-1), 143.3 (C-1′), 135.5 (C-8a), 133.0 (C-3), 132.6, 132.0, 130.8, 130.5 (C-3′, C-5′), 128.1, 124.9 (C-2′, C-6′), 124.2, 122.2, 120.3, 118.3 (C-8), 113.2 (C-4a), 49.8 (NCHMe2), 21.3 (2 × CH3); HR-ESI-MS [M-Br]+: Calcd for C20H19N2+, 287.1543 found 287.1539.
9-Allyl-2-phenyl-β-carboline bromide (B41): Yield, 74%; orange-yellow powders; 1H NMR (500 MHz, CD3Cl) δ 10.1 (1 H, s, H-1), 8.82 (1 H, d, J = 6.0 Hz, H-4), 8.80 (1 H, d, J = 6.0 Hz, H-3), 8.32 (1 H, d, J = 8.0 Hz, H-5), 8.13 (2 H, d, J = 7.6 Hz, H-2′, H-6′), 7.79 (1 H, t, J = 7.6 Hz), 7.61–7.55 (3 H, m), 7.50 (1 H, t, J = 7.2 Hz), 7.43 (1 H, t, J = 7.5 Hz), 6.09–6.03 (1 H, m, H-2″), 5.70 (2 H, br s, H-1″), 5.17 (1 H, d, J = 10.3 Hz, H-3″a), 5.06 (1 H, d, J = 17.1 Hz, H-3″b); 13C NMR (126 MHz, CD3Cl) δ 145.2 (C-1), 143.1 (C-1′), 135.8, 133.0, 132.04, 133.01, 131.3, 130.8, 130.5 (C-3′, C-5′), 128.4, 124.8 (C-2′, C-6′), 123.9, 122.6, 119.4, 118.4, 117.9 (C-3″), 111.2, 47.3 (C-1″); HR-ESI-MS [M-Br]+: Calcd for C20H17N2+, 285.1386 found 285.1383.
9-Butyl-2-phenyl-β-carboline bromide (B42): Yield, 83%; orange-yellow powders; 1H NMR (500 MHz, CD3Cl) δ 10.1 (1 H, s, H-1), 8.81 (1 H, d, J = 5.9 Hz, H-4), 8.72 (1 H, d, J = 5.9 Hz, H-3), 8.30 (1 H, d, J = 7.8 Hz, H-5), 8.05 (2 H, d, J = 7.3 Hz, H-2′, H-6′), 7.77 (1 H, t, J = 7.3 Hz), 7.59–7.53 (3 H, m), 7.46 (1 H, t, J = 7.1 Hz), 7.38 (1 H, t, J = 7.1 Hz), 4.88 (2 H, t, J = 6.5 Hz, H-1″), 1.88–1.80 (2 H, m, H-2″), 1.34–1.31 (2 H, m, H-3″), 0.85 (3 H, t, J = 6.9 Hz, H-3″); 13C NMR (126 MHz, CD3Cl) δ 145.1 (C-1), 143.1 (C-1′), 135.92, 132.87, 132.0, 130.7, 130.5 (C-3′, C-5′), 127.8, 124.8 (C-2′, C-6′), 124.0, 122.4, 119.3, 118.4, 111.0, 57.9 (C-1″), 31.4 (C-2″), 20.3 (C-3″), 13.8 (C-4″); HR-ESI-MS [M-Br]+: Calcd for C21H21N2+, 301.1699 found 301.1696.
9-Iso-butyl-2-phenyl-β-carboline bromide (B43): Yield, 80%; orange-yellow powders; 1H NMR (500 MHz, CD3Cl) δ 10.1 (1 H, s, H-1), 8.84 (1 H, d, J = 5.7 Hz, H-4), 8.76 (1 H, d, J = 5.7 Hz, H-3), 8.30 (1 H, d, J = 7.8 Hz, H-5), 8.13 (2 H, d, J = 7.3 Hz, H-2′, H-6′), 7.75 (1 H, t, J = 7.3 Hz), 7.57 (1 H, d, J = 8.3 Hz), 7.52 (2 H, J = 7.1 Hz, H-3′, H-5′), 7.43 (1 H, t, J = 7.1 Hz), 7.37 (1 H, t, J = 7.1 Hz), 4.74 (2 H, d, J = 7.1 Hz, H-1″), 2.28–2.36 (1 H, m, H-2″), 0.93 (6 H, d, J = 6.0 Hz, 2 × CH3); 13C NMR (126 MHz, CD3Cl) δ 145.5 (C-1), 143.1 (C-1′), 136.3, 132.9, 132.8, 132.2, 130.7, 130.4 (C-3′, C-5′), 128.1, 124.9 (C-2′, C-6′), 123.9, 122.4, 119.2, 118.5, 111.3, 51.9 (C-1″), 29.2 (C-2″), 20.2 (2 × CH3); HR-ESI-MS [M-Br]+: Calcd for C21H21N2+, 301.1699 found 301.1697.
9-Benzyl-2-phenyl-β-carboline bromide (B44): Yield, 76%; orange-yellow powders; 1H NMR (500 MHz, CD3Cl) δ 10.2 (1 H, s, H-1), 8.69 (1 H, d, J = 6.3 Hz, H-3), 8.55 (1 H, d, J = 6.3 Hz, H-4), 8.37 (1 H, dd, J = 8.1 Hz), 8.16 (1 H, d, J = 7.7 Hz), 7.87 (1 H, d, J = 7.8 Hz), 7.74 (1 H, J = 8.6 Hz), 7.66 (2 H, t, J = 7.6 Hz), 7.60 (1 H, t, J = 7.4 Hz), 7.53 (1 H, t, J = 7.7 Hz), 7.34 (3 H, d-like, J = 7.1 Hz), 7.28 (3 H, d-like, J = 7.2 Hz), 6.43 (2 H, s, NCH2); 13C NMR (126 MHz, CD3Cl) δ 145.7 (C-1), 143.3 (C-1′), 136.0, 135.6, 133.3, 133.1, 131.4, 131.0, 130.6, 129.5, 129.0, 128.1, 127.0, 124.8, 123.7, 122.8, 119.4, 117.7, 111.4, 48.3 (NCH2); HR-ESI-MS [M-Br]+: Calcd for C24H19N2+, 335.1544 found 335.1543.
9-(Ethoxy-2-oxyethyl)-2-phenyl-β-carboline bromide (B45): Yield, 75%; orange-yellow powders; 1H NMR (500 MHz, CD3Cl) δ 10.5 (1 H, s, H-1), 8.59 (1 H, d, J = 6.1 Hz, H-3), 8.48 (1 H, d, J = 6.3 Hz, H-4), 8.31 (1 H, d, J = 7.8 Hz), 8.16 (2 H, d, J = 7.4 Hz), 7.86 (1 H, t, J = 7.6 Hz), 7.66 (2 H, t-like, J = 7.3 Hz), 7.59 (2 H, t-like, J = 8.8 Hz), 7.50 (1 H, t, J = 7.6 Hz), 6.17 (2 H, s, N-CH2), 4.28 (2 H, q, J = 7.1 Hz, CH2CH3), 1.36 (3 H, t, J = 7.1 Hz, CH3); 13C NMR (126 MHz, CDCl3) δ 168.2 (C = O), 145.2 (C-1), 143.2 (C-1′), 136.7, 133.22, 133.17, 131.2, 131.0, 130.6, 130.5, 124.7, 123.6, 122.9, 119.6, 117.5, 110.7, 62.4 (OCH2), 47.0 (NCH2), 14.1 (CH3); HR-ESI-MS [M-Br]+: Calcd for C21H19N2O2+, 331.1450 found 331.1441.
2-Phenyl-β-carboline bromide (B46): Yield 72%; yellow powders; mp 228.1–229.8 °C; The 1H NMR, 13C NMR and MS data are consistent with those in the literature33.
Synthesis of 9-methyl-2-p-toly-1,2,3,4-tetrahydro-β-carboline (C1)
To the solution of compound A22 (6.1 mmol) in 50 mL ethanol was slowly added NaBH4 (0.347 g, 9.2 mmol) under stirring. After completion of the reaction by TLC, the solution was concentrated under reduced pressure. 100 mL water was added to the residue, extracted with EtOAc (2 × 60 mL), washed with brine and dried over anhydrous Na2SO4. After filtration, the solvent was removed under reduced pressure to yield compound C1 as white powders in 98% yield. Mp 118.4–118.7 °C; 1H NMR (125 MHz, D3COD) δ 7.52 (1 H, d, J = 7.8 Hz, H-8), 7.30 (1 H, d, J = 8.2 Hz, H-5), 7.20 (1 H, 2 × t, J = 8.1, 1.0 Hz, H-6), 7.11 (2 H, d, J = 8.5 Hz, H-3′, H-5′), 7.10 (1 H, 2 × t, J = 7.8, 0.9 Hz, H-7), 7.00 (2 H, d, J = 8.5 Hz, H-2′, H-6′), 4.38 (2 H, s, H-1), 3.69 (3 H, s, NCH3), 3.60 (2 H, t, J = 5.6 Hz, H-3), 2.93 (2 H, t, J = 5.6 Hz, H-4), 2.30 (3 H, s, CH3); 13C NMR (500 MHz, D3COD) δ 149.1 (C-1′), 137.3 (C-8a), 133.2 (C-9a), 129.9 (C-4′), 129.3 (C-3′, C-5′), 126.8 (C-4b), 121.1 (C-7), 119.1 (C-6), 118.2 (C-5), 116.9 (C-8), 108.8 (C-2′, C-4′), 108.1 (C-4a), 48.7 (C-3), 46.6 (C-1), 29.4 (NCH3), 21.8 (C-4), 20.6 (CH3); HR-ESI-MS [M−H]+ calcd for C19H19N2, 275.1543, found 275.1536.
Assay of AChE and BuChE
The inhibitory activities of the compounds against AChE were evaluated according to Ellman’s coupled enzyme assay47 with the following modifications. 90 μL potassium phosphate buffer (PBS, 0.1 M, pH 7.4), 10 μL the solution of 2 units/mL AChE in 0.1 M PBS (pH 7.4) and 10 μL the solution of 200 μM test compounds in the mixed solution of methanol-PBS (pH 7.4) (1:9, V/V) were added to each well of a 96-well plate. The methanol-PBS solution was used as a blank control. After incubation at room temperature for 10 min, 60 μL the solution of 10 mM DTNB in PBS was added into each the well. The plate was placed on crushed ices and cooled for 20 min. To each the well was added 30 μL the solution of 7.5 mM ATCh in PBS (pH 7.4). After incubation at 37 °C for 40 min, the initial rate of the enzyme was analyzed by measuring absorbance at wavelength of 412 nm with a microplate reader (Molecular Devices Co., Ltd.). Each test was performed in triplicate. Inhibition rates of the enzyme were calculated relative to a control sample. The inhibitory activities against BuChE were measured as described above for AChE, using 0.7 unit/mL BuChE and 4 mM BuTCh instead of AChE and ATCh as the enzyme and substrate, respectively.
The compounds with higher initial activities were further assayed for IC50 values according to the method described above. Based on the screening results, a series of test concentrations of the compound were set and tested for inhibition rate against AChE and BChE. The probit value of the average inhibition rate for each test concentration and the corresponding lg[concentration] were used to establish enzyme inhibition regression equation by the linear least-square fitting method. IC50 values were calculated from the equations.
Duncan multiple comparison test was used to evaluate significant difference between the average inhibition rates of various compounds at the same concentration by using PRISM software ver. 5.0 (GraphPad Software Inc., San Diego, CA, USA).
Data
Data will be made available upon request to the corresponding authors.
Electronic supplementary material
Acknowledgements
This project was financially supported by the National Natural Science Foundation of China (Nos 31572038, 21602172). This study was also supported by the Agricultural Science and Technology Innovation Project of Shaanxi Province (2016NY-171).
Author Contributions
L.Z. and H.G. conceived the research. L.Z. and B.Z. wrote the manuscript. L.Z., B.Z., B.-H.Z., B.-Y. Z., X.L., H.L. and Z.C. performed all experiments. D.L. conducted molecular docking. All authors designed experiments, analyzed data, edited and approved the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Bohang Zhou and Bingyu Zhang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19999-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Huiling Geng, Email: genghuiling5@163.com.
Le Zhou, Email: zhoulechem@nwsuaf.edu.cn.
References
- 1.Thies W, Bleiler L. Alzheimer’s association Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Prince, M., Comas-Herrera, A., Knapp, M., Guerchet, M. & Karagiannidou, M. World Alzheimer report 2016: Impoving healthcare for people living with dementia, coverage, quality and costs now and in the future. Alzheimer’s Disease International, 2016.
- 3.Singh A. Alzheimer’s disease inhibitors: current status and future prospects. Int. J. Pharm. Life Sci. 2014;5:3734–3740. [Google Scholar]
- 4.Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp. Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- 5.Cummings JL. Cholinesterase inhibitors: expanding applications. Lancet. 2000;356:2024–2025. doi: 10.1016/S0140-6736(00)03393-6. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Seal CJ, Okello EJ. Kinetics of acetylcholinesterase inhibition by an aqueous extract of Withania somnifera roots. Int. J. Pharm. Sci. Res. 2011;2:1188–1192. [Google Scholar]
- 7.Finkelstein BL, et al. Tricyclic cyanoguanidines: Synthesis, site of action and insecticidal activity of a novel class of reversible acetylcholinesterase inhibitors. Bioorg. Med. Chem. 2002;10:599–613. doi: 10.1016/S0968-0896(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 8.Lane RM, Potkin SG, Enz A. Targetting acetylcholinesterase and butyrylcholinesterase in dementia. Int. J. Neuropsychopharmacol. 2006;9:101–124. doi: 10.1017/S1461145705005833. [DOI] [PubMed] [Google Scholar]
- 9.Zimmerman G, Soreq H. Termination and beyond: acetylcholinesterase as a modulator of synaptic transmission. Cell Tissue Res. 2006;326:655–669. doi: 10.1007/s00441-006-0239-8. [DOI] [PubMed] [Google Scholar]
- 10.Wright CI, Geula C, Mesulam MM. Neurological cholinesterases in the normal brain and in Alzheimer’s disease: relationship to plaques, tangles, and patterns of selective vulnerability. Ann. Neurol. 1993;34:373–384. doi: 10.1002/ana.410340312. [DOI] [PubMed] [Google Scholar]
- 11.Mesulam M, Geula C. Butyrylcholinesterase reactivity differentiates the amyloid plaques of aging from those of dementia. Ann. Neurol. 1994;36:722–727. doi: 10.1002/ana.410360506. [DOI] [PubMed] [Google Scholar]
- 12.Chatonnet A, Lockridge O. Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem. J. 1989;260:625–634. doi: 10.1042/bj2600625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mack A, Robitzki A. The key role of butyrylcholinesterase during neurogenesis and neural disorders: an antisense-5′-butyrylcholinesterase-DNA study. Prog. Neurobiol. 2000;60:607–628. doi: 10.1016/S0301-0082(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 14.Arai T, et al. Design, synthesis, and evaluation of Trolox-conjugated amyloid-β C-terminal peptides for therapeutic intervention in an in vitro model of Alzheimer’s disease. Bioorg. Med. Chem. 2016;24:4138–4143. doi: 10.1016/j.bmc.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 15.Greig NH, et al. A new therapeutic target in Alzheimer’s disease treatment: attention to butyrylcholinesterase. Curr. Med. Res. Opin. 2001;17:159–165. doi: 10.1185/03007990152673800. [DOI] [PubMed] [Google Scholar]
- 16.Mushtaq GN, Greig H, Khan JA, Kamal MA. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets. 2014;13:1432–1439. doi: 10.2174/1871527313666141023141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arendt T, Bruckner MK, Lange M, Bigl V. Changes in acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease resemble embryonic developmentea study of molecular forms. Neurochem. Int. 1992;21:381–396. doi: 10.1016/0197-0186(92)90189-X. [DOI] [PubMed] [Google Scholar]
- 18.Arendt T, Bigl V, Walther F, Sonntag M. Decreased ratio of CSF acetylcholinesterase to butyrylcholinesterase activity in Alzheimer’s disease. Lancet. 1984;1:173. doi: 10.1016/S0140-6736(84)90116-8. [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Yang H, Chen Y, Sun H. Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer’s disease. Eur. J. Med. Chem. 2017;132:294–309. doi: 10.1016/j.ejmech.2017.03.062. [DOI] [PubMed] [Google Scholar]
- 20.Yu L, et al. Synthesis and binding ability of 1,2,3-triazole-based triterpenoid receptors for recognition of Hg(2+) ion. Bioorg. Med. Chem. Lett. 2010;20:3254–3258. doi: 10.1016/j.bmcl.2010.04.059. [DOI] [PubMed] [Google Scholar]
- 21.Cacabelos R, Torrellas C, Teijido O, Carril JC. Pharmacogenetic considerations in the treatment of Alzheimer’s disease. Pharmacogenomics. 2016;17:1041–1074. doi: 10.2217/pgs-2016-0031. [DOI] [PubMed] [Google Scholar]
- 22.Munoz-Torrero D. Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimer’s disease. Curr. Med. Chem. 2008;15:2433–2455. doi: 10.2174/092986708785909067. [DOI] [PubMed] [Google Scholar]
- 23.Pang YP, Quiram P, Jelacic T, Hong F, Brimijoin S. Highly potent, selective, and low cost bis-tetrahydroaminacrine inhibitors of acetylcholinesterase. Steps toward novel drugs for treating Alzheimer’s disease. J. Biol. Chem. 1996;271:23646–23649. doi: 10.1074/jbc.271.39.23646. [DOI] [PubMed] [Google Scholar]
- 24.Munoz-Torrero D, Camps P. Dimeric and hybrid anti-Alzheimer drug candidates. Curr. Med. Chem. 2006;13:399–422. doi: 10.2174/092986706775527974. [DOI] [PubMed] [Google Scholar]
- 25.Khorshid MA, Fatma Hassan AM, Mona Abd El-Gawad AM, Enab AK. Effect of some plants and pesticides on acetylcholinesterase. Am. J. Food Technol. 2015;10:93–99. doi: 10.3923/ajft.2015.93.99. [DOI] [Google Scholar]
- 26.Thomsen T, Zendeh B, Fischer JP, Kewitz H. In vitro effects of various cholinesterase inhibitors on acetyl- and butyrylcholinesterase of healthy volunteers. Biochem. Pharmacol. 1991;41:139–141. doi: 10.1016/0006-2952(91)90022-W. [DOI] [PubMed] [Google Scholar]
- 27.Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int. J. Clin. Pract. Suppl. 2002;127:45–63. [PubMed] [Google Scholar]
- 28.Noetzli M, Eap CB. Pharmacodynamic, pharmacokinetic and pharmacogenetic aspects of drugs used in the treatment of Alzheimer’s disease. Clin. Pharmacokinet. 2013;52:225–41. doi: 10.1007/s40262-013-0038-9. [DOI] [PubMed] [Google Scholar]
- 29.Leon R, García AG, Marco-Contelles J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med. Res. Rev. 2013;33:139–189. doi: 10.1002/med.20248. [DOI] [PubMed] [Google Scholar]
- 30.Singh M, et al. Acetylcholinesterase inhibitors as Alzheimer therapy: From nerve toxins to neuroprotection. Eur. J. Med. Chem. 2013;70:165–188. doi: 10.1016/j.ejmech.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 31.Herraiz T. N-methyltetrahydropyridines and pyridinium cations as toxins and comparison with naturally-occurring alkaloids. Food Chem. Toxicol. 2016;97:23–39. doi: 10.1016/j.fct.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Herraiz, T. β-Carboline Alkaloids (eds Gilbert, J. et al.), Ch. 8, 199–223 (Blackwell Publishing Ltd., Oxford, UK, 2008).
- 33.Hou Z, et al. Design, synthesis, and structure-activity relationship of new 2-aryl-3,4-dihydro-β-carbolin-2-ium salts as antifungal agents. J. Agric. Food Chem. 2016;64:2847–2854. doi: 10.1021/acs.jafc.6b00505. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M-M, Sun D-Q. Recent advances of natural and synthetic β-carbolines as anticancer agents. Anti-Cancer Agents Med. Chem. 2015;15:537–547. doi: 10.2174/1871520614666141128121812. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, et al. Skeletal modifications of β-carboline alkaloids and their antiviral activity profile. Mol. Divers. 2016;20:829–835. doi: 10.1007/s11030-016-9669-8. [DOI] [PubMed] [Google Scholar]
- 36.Lunagariya NA, et al. Design, synthesis and biological evaluation of 1,3,6-trisubstituted β-carboline derivatives for cytotoxic and anti-leishmanial potential. Bioorg. Med. Chem. Lett. 2016;26:789–794. doi: 10.1016/j.bmcl.2015.12.095. [DOI] [PubMed] [Google Scholar]
- 37.Cao RH, Peng WL, Wang ZH, Xu AL. β-Carboline alkaloids: biochemical and pharmacological functions. Curr. Med. Chem. 2007;14:479–500. doi: 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]
- 38.Shi CC, Chen SY, Wang GJ, Liao JF, Chen CF. Vasorelaxant effect of Harman. Eur. J. Pharmacol. 2003;390:319–325. doi: 10.1016/S0014-2999(99)00928-0. [DOI] [PubMed] [Google Scholar]
- 39.Herraiz T, Galisteo J. Hydroxyl radical reactions and the radical scavenging activity of β-carboline alkaloids. Food Chem. 2015;172:640–649. doi: 10.1016/j.foodchem.2014.09.091. [DOI] [PubMed] [Google Scholar]
- 40.Herraiz, T. Current Topics in Neurotoxicity, Vol. 1 (eds Antkiewicz-Michaluk, L. et al.), Ch. 5, 77‒103 (Springer, US, 2012).
- 41.Schott Y, Michael D, Rommelspacher H, Lehmann J. 6-Hydroxy- and 6-methoxy-β-carbolines as acetyl- and butyrylcholinesterase inhibitors. Bioorg. Med. Chem. Lett. 2006;16:5840–5843. doi: 10.1016/j.bmcl.2006.08.067. [DOI] [PubMed] [Google Scholar]
- 42.Ghosal S, Bhattacharya SK, Mehta R. Naturally occurring and synthetic β-carbolines as cholinesterase inhibitors. J. Pharm. Sci. 1972;61:808–810. doi: 10.1002/jps.2600610535. [DOI] [PubMed] [Google Scholar]
- 43.Cho K-M, Yoo I-D, Kim W-G. 8-Hydroxydihydrochelerythrine and 8-hydroxydihydrosanguinarine with a potent acetylcholinesterase inhibitory activity from Chelidonium majus L. Biol. Pharm. Bull. 2006;29:2317–2332. doi: 10.1248/bpb.29.2317. [DOI] [PubMed] [Google Scholar]
- 44.Xiao G, et al. Design, synthesis and biological evaluation of 4′-aminochalconerivastigmine hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2017;25:1030–1041. doi: 10.1016/j.bmc.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 45.Valenti P, et al. Acetylcholinesterase inhibition by tacrine analogs. Bioorg. Med. Chem. Lett. 1997;7:2599–2602. doi: 10.1016/S0960-894X(97)10025-7. [DOI] [Google Scholar]
- 46.Sultana N, et al. Synthesis, crystal structure determination, biological screening and docking studies of N1-substituted derivatives of 2,3-dihydroquinazolin-4(1H)-one as inhibitors of cholinesterases. Bioorg. Chem. 2017;72:256–267. doi: 10.1016/j.bioorg.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Peauger L, et al. Donepezil-based central acetylcholinesterase inhibitors by means of a “bio-oxidizable” prodrug strategy: design, synthesis, and in vitro biological evaluation. J. Med. Chem. 2017;60:5909–5926. doi: 10.1021/acs.jmedchem.7b00702. [DOI] [PubMed] [Google Scholar]
- 48.Jones RR, Harkrader RJ, Southard GL. The effect of pH on sanguinarine iminium ion form. J. Nat. Prod. 1986;49:1109–1111. doi: 10.1021/np50048a025. [DOI] [Google Scholar]
- 49.Dostal J, Taborska E, Slavik J, Potacek M, de Hoffmann E. Structure of chelerythrine base. J. Nat. Prod. 1995;58:723–729. doi: 10.1021/np50119a010. [DOI] [Google Scholar]
- 50.Slaninova I, Slanina J, Taborska E. Quaternary benzo[c]phenanthridine alkaloids-Novel cell permeant and red fluorescing DNA probes. Cytometry, Part A. 2007;71A:700–708. doi: 10.1002/cyto.a.20423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.