Abstract
Variation in predation risk is a major driver of ecological and evolutionary change, and, in turn, of geographical variation in behaviour. While predation risk is rarely constant in natural populations, the extent to which variation in predation risk shapes individual behaviour in wild populations remains unclear. Here, we investigated individual differences in reproductive behaviour in 16 Trinidadian guppy populations and related it to the observed variation in predator biomass each population experienced. Our results show that high heterogeneity in predator biomass is linked to individual behavioural diversification. Increased within-population heterogeneity in predator biomass is also associated with behavioural polymorphism. Some individuals adjust the frequency of consensual mating behaviour in response to differences in sex ratio context, while others display constantly at elevated frequencies. This pattern is analogous to a ‘live fast, die young’ pace-of-life syndrome. Notably, both high and low mean differences in predator biomass led to a homogenization of individual frequency of consensual mating displays. Overall, our results demonstrate that individual behavioural variation is associated with heterogeneity in predator biomass, but not necessarily with changes in mean values of predator biomass. We suggest that heterogeneity in predator biomass is an informative predictor of adaptive responses to changes in biotic conditions.
Keywords: personality, predation risk, heterogeneity, behavioural diversification, sexual behaviour
1. Introduction
Consistent behavioural differences among individuals across time and context are a ubiquitous biological feature of recognized ecological and evolutionary importance [1–3]. However, this variation in individual behaviour is expressed in an inconstant world. Ecosystems are dynamic, varying temporally in their physical and biotic conditions. Shifting community composition, particularly when it involves temporal variation in predation risk, has obvious implications for individual behaviour [4]. Yet, although individual behavioural variation is omnipresent in most natural populations, partitioning the sources of ecological conditions that fuel among-individual differences in behaviour remains unclear [5–7]. Given that the natural world is facing unprecedented change associated with the anthropocene, a clearer understanding of the link between individual behaviour and shifts in the biotic environment is needed. Here, we quantify individual behavioural variability across different mating opportunity contexts and ask the question—does individual behavioural variation across contexts increase with increasing environmental heterogeneity? To do this, we use an extensive temporal ecological dataset, and explicitly consider among- and within-individual variation in reproductive behaviour of wild populations of freshwater fish exposed to different levels of predation risk.
Fluctuating selection caused by temporal environmental heterogeneity is expected to increase the variance in fitness among individuals [8,9]. When selection favours different phenotypes at different times, investing in the diversification of individual strategies is adaptive [10–12]. As such, individual diversification in behavioural strategies is often more pronounced in temporally heterogeneous conditions than in more homogeneous ones [13]. Fluctuating selection caused by temporal environmental heterogeneity is also expected to promote among-individual differences in fitness in the trade-off between current and future fitness expectation. Such individual fitness differences are predicted to lead to the emergence of pace-of-life mating strategies within the same population [14,15], in which some individuals engage in high-risk behaviours, such as greater sexual activity, whereas others exhibit less risk-prone behaviours and prioritize future, over current, reproductive success [16,17]. It is then expected that diversification in behaviour among individuals be replicated by individuals engaging in high-risk strategies at the extremes of these distributions in the population [18].
Predation risk is a powerful force shaping individual behaviour and life-history strategies [19,20]. Further, temporal heterogeneity in predation risk modifies the fitness outcome associated with a given prey phenotype, leading to changes in individual behavioural strategies [21–23]. For example, among Trinidadian guppies (Poecilia reticulata), males are behaviourally and phenotypically more conspicuous than females, and hence potentially at greater predation risk. Accordingly, predation pressure has the potential to modify the population sex ratio, thereby influencing individual behavioural strategies [21].
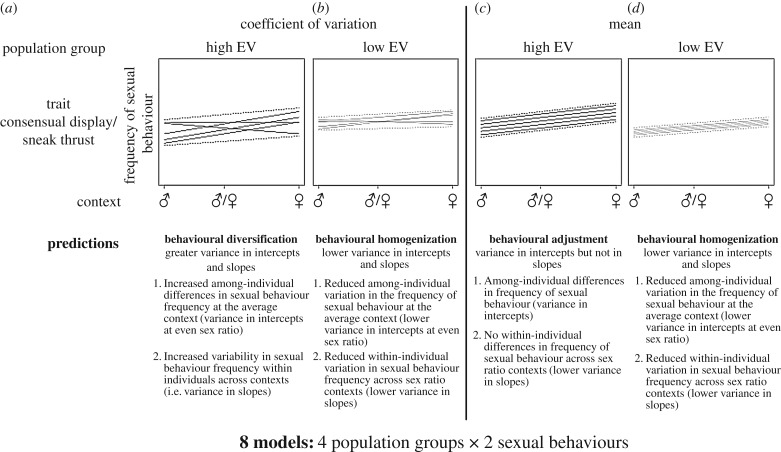
Given that consistent variation in individual behaviour, also referred to as personality, is heritable [24], exposure to constant predator pressure (i.e. always high, or always low) may favour the evolution of reduced variation (greater homogenization) in individual behavioural strategies. In contrast, individuals from populations subject to frequent changes in selection pressure will have no optimal life-history strategy, or behaviour [25]. We can thus predict greater behavioural variation among-individuals from populations exposed to greater temporal heterogeneity in predation risk [26]. To test these predictions, we quantify the frequency of male guppy sexual behaviour across contexts of contrasting operational sex ratio and relate this to temporal heterogeneity in predator biomass, a metric for perceived predation risk in the population. Individual behavioural variation in response to environmental conditions can be partitioned using reaction norms to compare three key components: (i) variation in individual intercepts, (ii) in individual slopes, and (iii) the covariance between individual intercepts and slopes [27]. Variance in the individual intercept of the reaction norm indicates that some individuals have greater mean phenotypes than others. Variance in individual slopes of the reaction norm reveals the variability in the frequency of sexual behaviours in response to differences in sex ratio contexts. Finally, covariance in intercepts and slopes of the reaction norm compares among-individual behaviour in the average context to the variation in other sex ratio contexts. By partitioning the variance into intercepts and slopes across sex ratio contexts and combining this information with environmental data, we can test the hypothesis that temporal heterogeneity in predator biomass promotes diversification in individual behavioural strategies (figure 1). Based on variance partitioning, we expect that individuals exposed to greater temporal heterogeneity in predator biomass will have greater variance in intercepts (i.e. among-individual variation in the average context) and slopes (i.e. within-individual variation across environments) of the reaction norms (figure 1a). In contrast, in more temporally homogeneous conditions the optimal phenotype is expected to remain less variable, thereby we expect comparatively less individual behavioural variation among- and within-individuals across contexts (figure 1b). Finally, higher variance in the slopes between individuals is expected when they exhibit contrasting behavioural strategies across sex ratio contexts (figure 1a).
Figure 1.
Predicted pattern of reaction norms for behavioural diversification (a), behavioural homogenization (b,d), and behavioural adjustment (c) scenarios. Each panel shows a hypothetical reaction norm plot for four predation risk population scenarios (high and low coefficient of variation (a,b), and high and low means (c,d)), across three sex ratio contexts (Male biased (♂), Even ratio (♂/♀) and Female biased (♀)). The lines represent individual reaction norms. Variance in individual intercept indicates that some individuals have greater frequency of sexual behaviour at the average sex ratio context (even sex ratio), whereas variance in slopes denotes within-individual variability in sexual behaviour across sex ratio contexts.
2. Methods
Male guppies were collected from 16 populations in Trinidad (electronic supplementary material, S1), for which we have detailed temporal information on predator biomass (see below). We quantified individual male reproductive behaviour across a range of sex ratio contexts, and then linked individual behavioural variation to the risk status (i.e. male's native population exposed to high or low temporal changes in predator biomass).
(a). Assessment of temporal heterogeneity in predator biomass
We sampled fish assemblages from 16 sites across the Northern Range of Trinidad (electronic supplementary material, S1) at three-month intervals over 5 years—each site was visited 20 times. Each site consisted of a 50 m stretch of stream, the ends of which were blocked with seine nets before each sampling session.
The primary predators of guppies in the Northern Range are the pike cichlid, Crenicichla frenata and the wolf fish, Hoplias malabaricus [28]. A survey of these predators was conducted using hand seining (64 mm mesh) followed by electrofishing [29]. On capture, all the individuals were identified, counted and weighed on a portable balance, and finally released unharmed. The combined predator biomass of the two main guppy predators was calculated for each time point at each site using all individuals heavier than two grams.
(b). Sampling and experimental set-up
Guppies were collected during the final sampling session (July–August 2015). To prevent atypical behaviour due to guppies being kept in single sex groups, we collected a sample including males, females and juveniles from each of the 16 populations (electronic supplementary material, S1). Individuals were transferred to a closed container with aeration and immediately transported to the laboratory. To mitigate stress and reduce mortality, individuals were allocated to settling tanks (90 × 30 × 40 cm) in low-density groups with a sex ratio and water temperature that matched their natural conditions. Additionally, each tank was set up with an aeration system and the bottom covered with gravel and natural plants, which provided shelter. The laboratory was kept on a 12 L-12D regime.
After settling for 48 h, each focal male was randomly allocated to a test tank containing 15 companion fish. Companion individuals all originated from the lower section of the Tacarigua River in Trinidad and were collected prior to the test individuals. We decided to use companion individuals from the same population in all tests in order to standardize variability in focal behaviour due to inherited intra-population behavioural differences. Three sex ratio contexts were used (excluding the focal male): female biased (13 females to 2 males), male biased (3 females to 12 males) or even (8 females to 7 males), with two test tanks per treatment (i.e. a total of six test tanks).
Each focal male was introduced to his test tank and kept inside a perforated transparent plastic bottle, allowing for both visual and chemical cues. As soon as the focal male appeared acclimatized (i.e. swimming normally), the bottle was removed and the focal male was allowed to interact with the companion individuals.
We quantified the type and frequency of sexual displays during a 20 min period. Male guppies perform two forms of sexual displays: the consensual sigmoid display, favoured by females and more often employed in low predation risk localities, and the sneaking thrust, a coercive form of copulation [30]. At the end of the observation period, the focal male was relocated to a new tank (60 × 25 × 30 cm) with individuals from its original population. Unique colour patterns allowed the focal male to be unambiguously recognized. After 24 h, the same focal male was re-tested as described above in a different sex ratio treatment. This process was repeated so that each focal male was tested in the three sex ratio contexts in a randomized order. The companion individuals were replaced after six observations, and were not re-used during the experiment. At the end of the three trials, the standard length of each focal male was recorded to the nearest millimetre using ImageJ [31]. A total of 20 focal males per population were tested (Ntotal focal males = 320).
(c). Statistical analysis
The main goal of the study was to test the hypothesis that populations of guppies exposed to greater heterogeneity in predator biomass display greater individual variation in behaviour. Using behavioural reaction norms [32] we explore among- and within-individual variation in sexual displays (i.e. consensual sigmoid displays and sneaking thrusts) across different sex ratios, and related the variation in reaction norms to temporal heterogeneity in predator biomass. We modelled the effect of heterogeneity (i.e. population coefficient of variation (CV) in predator biomass) in generating among-individual variation in behaviour across environments using linear mixed-effect models (LMMs). In a second phase, using LMMs we modelled the effect of mean changes (i.e. changes in mean value of predator biomass) in producing among-individual variation in behaviour. Mixed random regression models are a suitable analytical tool to quantify and test the relationship between behavioural variation among individuals and environmental variability [33]. Further, these models yield the highest power to detect variances in individual slopes and intercepts in large datasets, as in our case [34].
The 16 populations were divided into two groups according to their predator biomass CV values. Populations with a CV value greater than the overall CV mean were classified as ‘high heterogeneity’, whereas populations with CV values smaller than the overall mean CV were considered ‘low heterogeneity’. In a separate analysis, we instead split the populations according to their mean differences in predator biomass. Using the same reasoning, populations were divided into two groups of ‘high mean’ and ‘low mean’ predator biomass.
LMMs were fitted to eight separate models (figure 1). The eight models resulted from the combination of four population groups (High CV, Low CV, High mean and Low mean) for each of the two sexual behaviours (consensual mating display and sneaking thrust). These eight models shared a common form (equation (2.1))
| 2.1 |
where yij is the number of sexual behaviours of individual i of population j, αx is the intercept, α1 and α2 are fixed effects associated with the slope and curvature of the sex ratio (sr), f1 and f2 are random regression functions on natural polynomials of order n, at the individual (f1) and population (f2) groups. In both f1 and f2, n was set to 1, allowing for the estimation of random intercepts and slopes. Polynomials were applied to scaled sex ratios (male biased, even and female biased were assigned values −1, 0, and 1, respectively) to improve convergence. Finally, normally distributed heterogeneous residuals by sex ratios were estimated ɛi,j, with variance 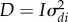 . d and p vectors with individual and population values, were assumed to follow normal distributions,
. d and p vectors with individual and population values, were assumed to follow normal distributions, 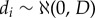 and
and 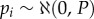 respectively, where both D(1/3) and
respectively, where both D(1/3) and 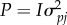 are 2 × 2 matrices, and
are 2 × 2 matrices, and 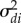 and
and 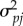 are the individual environment and the population effect of individual i and population j. Note that D[1, 1] and D[2, 2] are the variances in intercepts and slopes across sex ratio contexts, whereas D[1, 2] and D[2, 1] correspond to the covariance between the slope and the intercept of the reaction norm. Since the distributions of consensual sigmoid displays and sneak thrust counts were markedly right skewed, the models were fitted to the logarithm-transformed corresponding variables.
are the individual environment and the population effect of individual i and population j. Note that D[1, 1] and D[2, 2] are the variances in intercepts and slopes across sex ratio contexts, whereas D[1, 2] and D[2, 1] correspond to the covariance between the slope and the intercept of the reaction norm. Since the distributions of consensual sigmoid displays and sneak thrust counts were markedly right skewed, the models were fitted to the logarithm-transformed corresponding variables.
We also estimated how the variability and strength of predator biomass affected behavioural repeatability. Here, individual repeatability was calculated using (equation (2.2)). Low and high CV/mean population groups were coded as −1 and +1 with the variance arising from the slopes given by (x/−1)2
D[2, 2] in each sex ratio treatment (and zero in the even sex ratio – defined as the intercept). Therefore, the variance across sex ratio treatments arising only from slopes is (2/3)D[2, 2] + (1/3) in each sex ratio context 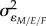 as described in equation (2.2).
as described in equation (2.2).
| 2.2 |
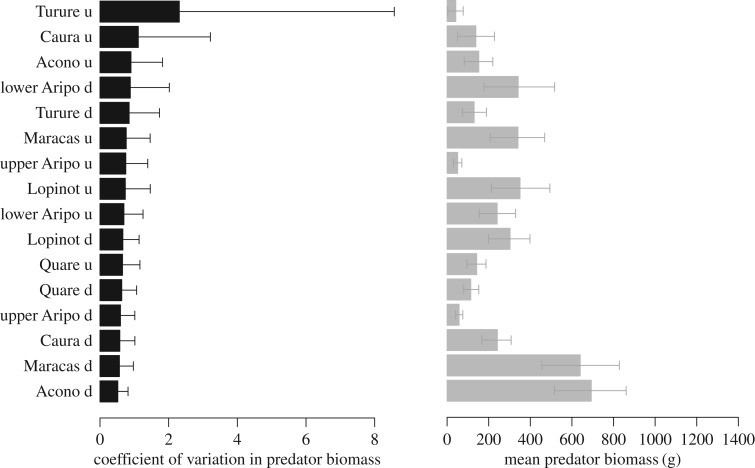
Before fitting these models, we checked how heterogeneous the original populations were. To address this, we fitted separate linear models to each population, estimating an intercept and a residual variance. The mean and variance were plotted to inform about their variability across population groups (figure 2).
Figure 2.
Differences in coefficient of variation (black) and in means (grey) in predator biomass within the 16 populations of Trinidadian guppies. Within-population differences were used to split the populations into groups of low and high CV or mean predator biomass. Error bars denote 95% credible intervals (CrI).
All models were fitted in a Bayesian framework, using MCMCglmm [35]. Convergence was checked using trace plots and posterior distribution densities. Inferences on the comparison of reaction norms between populations with low and high CV/mean predator biomass were based on 95% Highest Posterior Density (HPD) credible intervals. To make inferences about differences between population groups, for each posterior sample, we calculated the difference in the estimated parameters between the two populations, which allowed us to obtain a distribution of such differences and therefore HPD credible intervals.
3. Results
(a). Predator biomass
Our data revealed a clear differentiation between populations in terms of heterogeneity and mean values in predator biomass. Temporal differences in the coefficient of variation and in the mean value of predator biomass for the 16 populations are shown in figure 2.
(b). Consensual sigmoid display
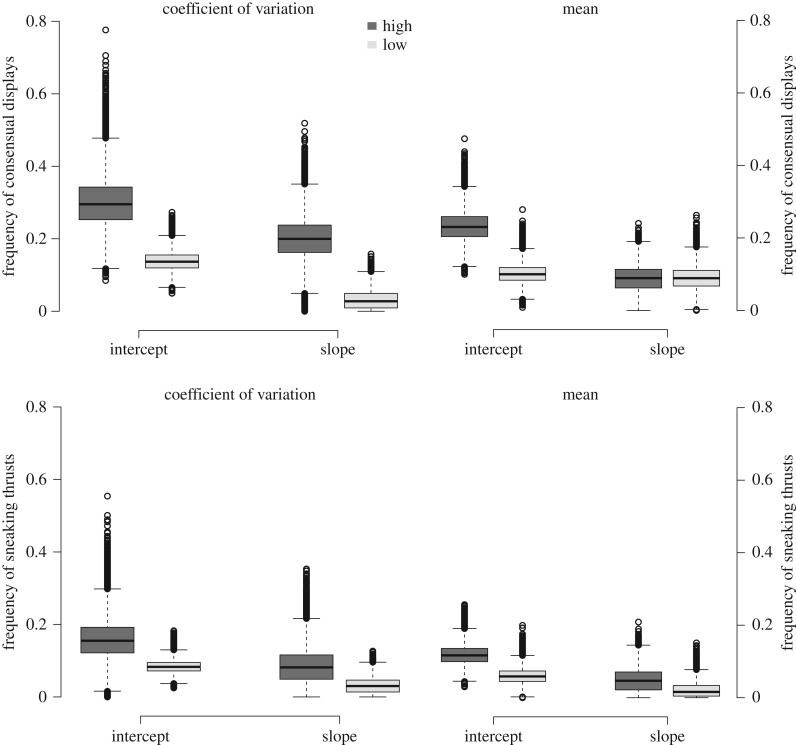
There were differences in among-individual variance in the intercepts and slopes of reaction norms between populations of low and high predator biomass CV (table 1 and figure 3; electronic supplementary material, S2 and S5). Individuals from populations with ‘high CV’ in predator biomass showed greater variance in frequency of consensual mating behaviour than individuals from ‘low CV’ populations. In contrast, mean differences in predator biomass affected the variance in intercepts, but not the variance in slopes of the reaction norms (table 1 and figure 3; electronic supplementary material, S2 and S5).
Table 1.
Variation in posterior mode at the fixed and random levels, for the predator biomass of the four population groups (low and high CV/mean). Inference about significant differences was based on 95% credible intervals (CrI) comparisons. Non-overlapping CrI are shaded in grey and denote significant differences between groups.
| population groups |
|||
|---|---|---|---|
| low coefficient of variation | high coefficient of variation | ||
| sexual behaviour | posterior mode (±CrI) | posterior mode (±CrI) | |
| consensual mating display | fixed effects | ||
| intercept | 1.772 (1.460: 2.066) | 1.598 (0.603: 2.565) | |
| slope | 0.199 (0.133: 0.272) | 0.127 (−0.381: 0.723) | |
| random effects | |||
| among individuals | 0.126 (0.087: 0.191) | 0.280 (0.170: 0.440) | |
| within individuals | 0.0004 (1.36 × 10−10: 0.080) | 0.191 (0.088: 0.321) | |
| sneaking thrusts | fixed effects | ||
| intercept | 1.754 (1.563: 1.945) | 1.315 (0.838: 1.754) | |
| slope | −0.069 (−0.168: 0.033) | −0.085 (−0.289: 0.154) | |
| random effects | |||
| among individuals | 0.079 (0.050: 0.118) | 0.155 (0.059: 0.270) | |
| within individuals | 0.0003 (1.67 × 10−10: 0.068) | 0.0004 (9.87 × 10−11: 0.172) | |
|
low mean |
high mean |
||
| posterior mode (±CrI) | posterior mode (±CrI) | ||
| consensual mating display | fixed effects | ||
| intercept | 2.053 (1.550: 2.597) | 1.506 (1.245: 1.773) | |
| slope | 0.207 (0.095: 0.342) | 0.185 (0.070: 0.278) | |
| random effects | |||
| among individuals | 0.096 (0.052: 0.156) | 0.224 (0.154: 0.314) | |
| within individuals | 0.084 (0.026: 0.151) | 0.089 (2.73 × 10−8: 0.145) | |
| sneaking thrusts | fixed effects | ||
| intercept | 1.851 (1.405: 2.314) | 1.512 (1.325: 1.701) | |
| slope | −0.126 (−0.357: 0.097) | −0.036 (−0.116: 0.046) | |
| random effects | |||
| among individuals | 0.058 (0.017: 0.103) | 0.110 (0.067: 0.173) | |
| within individuals | 0.22 × 10−3 (7.16 × 10−13: 0.062) | 0.19 × 10−3 (5.28 × 10−10: 0.103) | |
Figure 3.
Posterior samples of variation in individual intercepts and slopes for the frequency in consensual displays and sneaking thrusts between populations for the four population groups (low and high CV/mean) predator biomass. Error bars denote 95% CrI.
There was strong and positive covariance between intercepts and slopes across sex ratio context associated with individuals from populations with ‘high’ predator biomass CV values (posterior mode (95% CrI); 0.44 (−0.51: 0.68), electronic supplementary material, S2). By comparison, covariance between intercepts and slopes across sex ratios was not significantly different from zero in ‘low CV’ populations (posterior mode (95% CrI); −4.93−5 (−0.01: 0.005), electronic supplementary material, S2). In terms of mean differences in predator biomass, the values of covariance between intercepts and slopes across sex ratio treatments for both ‘low’ and ‘high’ mean populations were small and not significantly different from zero (posterior mode (95% CrI); −0.001 (−0.032: 0.151) for low; 0.0003 (−0.018: 0.024) for high, electronic supplementary material, S2).
The fixed effects structure of the model gives information about how the population, as a whole, changes behaviour across contexts. There was no effect of predator biomass in any of the population groups evaluated (table 1; electronic supplementary material, S2). Regardless of population group (i.e. low and high CV/mean) there was an increase in the frequency of consensual mating behaviour towards the female biased sex ratio context (table 1; electronic supplementary material, S2). Repeatability of frequency of consensual sigmoid display among individuals across sex ratio contexts was low for the four population groups (table 2).
Table 2.
Individual repeatability in consensual mating and sneaking thrusts across sex ratio contexts for the four population groups (low and high CV/mean). Individual repeatability was calculated as the ratio of the variance among individuals by total variation (i.e. among- and within-individual individual variation across sex ratio contexts).
| population groups |
||
|---|---|---|
| low coefficient of variation |
high coefficient of variation |
|
| sexual behaviour | posterior mode (±CrI) | posterior mode (±CrI) |
| consensual mating display | 0.349 (0.232: 0.433) | 0.478 (0.325: 0.601) |
| sneaking thrusts | 0.302 (0.192: 0.391) | 0.276 (0.118: 0.417) |
|
low mean |
high mean |
|
| posterior mode (±CrI) | posterior mode (±CrI) | |
| consensual mating display | −0.001 (−0.032: 0.015) | 0.0003 (−0.018: 0.024) |
| sneaking thrusts | 0.013 (−0.113: 0.082) | 0.0001 (−0.004: 0.014) |
(c). Sneaking thrusts
There were no differences in among-individual variation in sneaking (thrusts frequency) across sex ratio contexts between populations of ‘low’ and ‘high’ predator biomass CV, or between populations with ‘low’ and ‘high’ mean predator biomass (table 1 and figure 3; electronic supplementary material, S2 and S5).
Covariance in intercepts and slopes between individuals was close to zero in ‘low CV’ populations (posterior mode (95% CrI) −0.0006 (−0.029: 0.014), electronic supplementary material, S2), but highly negative in ‘high CV’ populations (posterior mode (95% CrI) −0.733 (−0.081: 0.086), electronic supplementary material, S2). In contrast, covariance between intercepts and slopes was not significantly different from zero across sex ratio for populations of ‘low’ and ‘high’ mean predator biomass (posterior mode (95% CrI); 0.013 (−0.113: 0.082) for low; 0.0001 (−0.004: 0.014) for high, electronic supplementary material, S2 and S5).
There was no evidence of individual adjustment in the frequency of sneaking across sex ratio contexts between populations with ‘low’ or ‘high’ CV values of predator biomass, or between populations with ‘low’ or ‘high’ mean differences in predator biomass. Intercepts and slopes of the fixed structure for all population groups (i.e. low/high CV or mean) were small and not significantly different from zero (table 1; electronic supplementary material, S2 and S5). Repeatability in the frequency of sneaking across sex ratio contexts was also low for all population groups (table 2).
4. Discussion
Our study demonstrates that temporal heterogeneity in predator biomass is key in shaping how prey individuals adjust some of their behavioural strategies in response to changes in sex ratio contexts. Individual male guppies behaved in distinct ways when faced with different sex ratio contexts, and these differences were attributable to the coefficient of variation in predator biomass associated with their original population. We detected a positive relationship between increased heterogeneity in predator biomass and diversification in individual behaviour (figure 1a; electronic supplementary material, S5). In contrast, mean differences in population predator biomass led to a homogenization in the frequency of consensual mating displays (figure 1b; electronic supplementary material, S2 and S5). Furthermore, we provide evidence that increased heterogeneity in predator biomass generates polymorphism in male prey mating behaviour. In populations with a greater coefficient of variation in predator biomass, some individuals expressed high frequencies of consensual mating behaviour across all sex ratio contexts, while others adjusted their behaviour to match the reproductive conditions. This polymorphism in mating behaviour is consistent with the assumptions of the pace-of-life syndrome hypothesis, which is predicted to emerge under heterogeneous environmental conditions [15]. Overall, our results reveal that heterogeneity in predator biomass is a vital factor in shaping the frequency of consensual mating displays and individual life strategies. In contrast, changes in mean value of predator biomass have less effect (figure 1d; electronic supplementary material, S5). The key role predators play in structuring ecological communities is widely recognized. The novelty of our study is that the key role of heterogeneity in predation risk in generating behavioural diversity within the prey population is now clear.
An individual's personality is traditionally assumed to be constant through time. It follows that individuals with stronger personality should have reduced behavioural flexibility [36]. However, temporal environmental heterogeneity can create conditions for the adaptiveness of more than one strategy within the same population [18,37]. For example, according to differences in individual fitness trade-offs, some individuals may respond to changes in conditions, whereas others may not [33]. Male guppies from populations with high predator biomass CV showed greater covariance between intercept and slope (figure 3; electronic supplementary material, S5). This indicates greater variability in personality types within these populations (i.e. temporal variation in individual personality). Our result shows that individual personality can vary over time [38] and supports the prediction that exposure to environmental heterogeneity favours variation in individual behavioural flexibility across environmental conditions (i.e. variable individual personalities) [39,40]. Taken together this result emphasizes the importance of including individual behavioural variation across contexts in personality studies.
We showed an association between the coefficient of variation in predator biomass associated with each population, and variance in individual intercepts and slopes of the reaction norms. However, when comparing populations in terms of mean differences in predator biomass, we detected an effect in individual intercepts of the reaction norm (figure 3; electronic supplementary material, S5). Male guppies from populations with ‘high mean’ values in predator biomass populations had greater variance in intercepts than individuals from low mean predator biomass populations. This indicates that while changes in heterogeneity lead to diversification in the frequency of consensual mating displays across contexts (figure 1a; electronic supplementary material, S5), differences in means affect only the diversification in the overall frequency of consensual behaviour, but not across contexts (figure 1c; electronic supplementary material, S5). This result has obvious ecological and evolutionary consequences, as it shows that our ability to fully understand the effects of biotic interactions on ecosystem structure and function may be constrained by which environmental variable is used to compare populations.
Temporal variation in predation risk shapes the link between life-history strategies and behaviour by exerting variable selection among males in the same population. Increased variation in predation risk is predicted to generate behavioural polymorphism within the prey population [41]. In populations exposed to high heterogeneity in predator biomass, some individuals always displayed consensual sigmoid behaviour at higher rates across all contexts, whereas others adjusted the frequency of behaviour to match the reproductive conditions (electronic supplementary material, S2). Such a pattern correlates with a fast-slow continuum strategy [17,42]. In wild guppy populations, males have potentially greater predation risk than females because of their conspicuous consensual mating behaviour and colouration. As a result, individuals that display constantly at high frequency can be seen as investing in a strategy of short-term fitness returns at the expense of survival.
We further note that high or low population differences in mean predator biomass did not generate behavioural polymorphism (electronic supplementary material, S2). When the probability of predation is constant over time and identical among all individuals in the population, selection favours the convergence of individual phenotypes [43]. Our study supports this by providing empirical evidence that changes in the heterogeneity in predator biomass generates polymorphism in behavioural strategies, while changes in population mean lead to homogenization of behaviours across contexts.
A common feature of personality studies is their focus on individual variation in an average context as a measure of individual personality (i.e. individual repeatability) [44–47]. We found low repeatability in the frequency of both sexual behaviours across contexts for the four population groups (electronic supplementary material, S3 and S4). This is in plain contrast to the high repeatability in male guppy behaviour reported in other studies [48,49]. While unexpected, differences between studies may be a consequence of a mathematical artefact. Repeatability is commonly measured as the proportion of total variance explained by among-individual differences in the average context [50,51]. Our study on the other hand examined both within- and among-individual variation in behaviour across contexts and related this variation to the extent of CV and mean differences in predator biomass associated with the population.
Despite the indisputable role of environmental variability as an explanatory metric of variation in individual behaviour, the effect of heterogeneity in natural populations remains largely overlooked in most studies [52]. Our study provides strong empirical, and novel, evidence for the link between diversification in behavioural strategies within and among individuals and natural temporal heterogeneity in predator biomass. We showed that changes in CV and in mean predator biomass have distinct effects on individual variation in prey behavioural strategies. The evolution of diversification in behaviour and life-history strategies is key in maintaining diversity in ecological communities. We therefore suggest that conservation practitioners should be aware of changes in both the mean and variance of predator pressure within communities before establishing plans for conservation priority of populations, as well as when removing predators of threatened populations during conservation management [53].
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to Maria Dornelas and to the Biodiversity and Behaviour Group for rewarding discussions and providing helpful comments on early drafts. We also thank Rajindra Mahabir, Kharran Deonarinesingh and Avinash Deonarinesingh for assistance with fieldwork.
Ethics
Approval was provided by the University of St Andrews Animal Welfare and Ethics Committee (2015). The review panel declared no need to obtain Animal Ethics approval.
Data accessibility
The raw data were supplied and allocated to the following repository https://doi.org/10.5061/dryad.4s4h4.
Authors' contributions
M.B., A.E.D., A.E.M. and I.R. contributed with funding; M.B., A.E.D. and A.E.M. conceived the experimental design; M.B. performed the experiments; M.B., M.J.J. and M.B.M. analysed the data; M.B., M.J.J. and M.B.M. prepared the figures and tables; M.B, A.E.D., M.J.J., M.B.M. and A.E.M. wrote the paper. All authors have read and approved the publication.
Competing interests
The authors declare there are no competing interests.
Funding
This study was funded by a Postdoctoral fellowship to M.B. (SFRH/BPD/82259/2011). M.B.M. was supported by a University Research Fellowship from the Royal Society (London). A.E.M. acknowledges the ERC (AgG BioTIME 250189, and PoC BioCHANGE 727440), and the Royal Society.
References
- 1.Bierbach D, Laskowski KL, Wolf M. 2017. Behavioural individuality in clonal fish arises despite near-identical rearing conditions. Nat. Commun. 8, 15361 ( 10.1038/ncomms15361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dingemanse NJ, Araya-Ajoy YG. 2015. Interacting personalities: behavioural ecology meets quantitative genetics. Trends Ecol. Evol. 30, 88–97. ( 10.1016/j.tree.2014.12.002) [DOI] [PubMed] [Google Scholar]
- 3.Griffin AS, Guillette LM, Healy SD. 2015. Cognition and personality: an analysis of an emerging field. Trends Ecol. Evol. 30, 207–214. ( 10.1016/j.tree.2015.01.012) [DOI] [PubMed] [Google Scholar]
- 4.Hammill E, Atwood TB, Corvalan P, Srivastava DS. 2015. Behavioural responses to predation may explain shifts in community structure. Freshw. Biol. 60, 125–135. ( 10.1111/fwb.12475) [DOI] [Google Scholar]
- 5.Biro PA, Stamps JA. 2008. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368. ( 10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 6.Alonzo SH. 2015. Integrating the how and why of within-individual and among-individual variation and plasticity in behavior. Curr. Opin. Behav. Sci. 6, 69–75. ( 10.1016/j.cobeha.2015.09.008) [DOI] [Google Scholar]
- 7.Bierbach D, Sommer-Trembo C, Hanisch J, Wolf M, Plath M. 2015. Personality affects mate choice: bolder males show stronger audience effects under high competition. Behav. Ecol. 26, 1314–1325. ( 10.1093/beheco/arv079) [DOI] [Google Scholar]
- 8.McNamara JM, Weissing FJ. 2010. Evolutionary game theory. In Social behaviour. Genes, ecology and evolution (eds Székeley T, Moore AJ, Komdeur J), pp. 109–133. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 9.Hamilton WD. 1964. The genetical evolution of social behaviour. I. J. Theor. Biol. 7, 1–16. ( 10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 10.Starrfelt J, Kokko H. 2012. Bet-hedging—a triple trade-off between means, variances and correlations. Biol. Rev. 87, 742–755. ( 10.1111/j.1469-185X.2012.00225.x) [DOI] [PubMed] [Google Scholar]
- 11.Rajon E, Desouhant E, Chevalier M, Débias F, Menu F. 2014. The evolution of bet hedging in response to local ecological conditions. Am. Nat. 184, E1–E15. ( 10.1086/676506) [DOI] [PubMed] [Google Scholar]
- 12.Barbosa M, Lopes I, Venâncio C, Janeiro MJ, Morrisey MB, Soares AMVM. 2015. Maternal response to environmental unpredictability. Ecol. Evol. 5, 4567–4577. ( 10.1002/ece3.1723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dan D, Tinbergen JM. 1997. Adaptation of Life Histories. In Behavioural ecology: an evolutionary approach, 4th edn (eds Krebs JR, Davies NB), p. 456 Hoboken, NJ: Blackwell Science Ltd. [Google Scholar]
- 14.Shuster SM, Wade MJ. 1991. Equal mating success among male reproductive strategies in a marine isopod. Nature 350, 608–610. ( 10.1038/350608a0) [DOI] [Google Scholar]
- 15.Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P-O. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B. 365, 4051–4063. ( 10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 17.Wolf M, van Doorn GS, Leimar O, Weissing FJ. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584. ( 10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 18.MacDonald K. 1995. Evolution, the five-factor model, and levels of personality. J. Pers. 63, 525–567. ( 10.1111/j.1467-6494.1995.tb00505.x) [DOI] [Google Scholar]
- 19.Sih A, Ziemba R, Harding KC. 2000. New insights on how temporal variation in predation risk shapes prey behavior. Trends Ecol. Evol. 15, 3–4. ( 10.1016/S0169-5347(99)01766-8) [DOI] [PubMed] [Google Scholar]
- 20.Lima SL, Dill LM. 1990. Behavioural decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. ( 10.1139/z90-092) [DOI] [Google Scholar]
- 21.Reznick D, Endler J. 1982. The impact of predation on life history evolution in Trinidadian guppies (Poecilia reticulata). Evolution 36, 160–177. [DOI] [PubMed] [Google Scholar]
- 22.Reznick D, Bryga H, Endler JA. 1990. Experimentally induced life-history evolution in a natural population. Nature 346, 357–359. ( 10.1038/346357a0) [DOI] [Google Scholar]
- 23.Reznick D. 1983. The structure of guppy life histories: the tradeoff between growth and reproduction. Ecology 64, 862–873. ( 10.2307/1937209) [DOI] [Google Scholar]
- 24.Dochtermann NA, Schwab T, Sih A. 2015. The contribution of additive genetic variation to personality variation: heritability of personality. Proc. R. Soc. B. 282, 20142201 ( 10.1098/rspb.2014.2201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riesch R, Martin RA, Langerhans RB. 2013. Predation's role in life-history evolution of a livebearing fish and a test of the Trexler-Deangelis model of maternal provisioning. Am. Nat. 181, 78–93. ( 10.1086/668597) [DOI] [PubMed] [Google Scholar]
- 26.Dingemanse NJ, Both C, Drent PJ, Tinbergen JM. 2004. Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. B. 271, 847–852. ( 10.1098/rspb.2004.2680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stearns SC, Koella JC. 1986. The evolution of phenotypic plasticity in life-history traits: predictions of reaction norms for age and size at maturity. Evolution 40, 893–913. ( 10.1111/j.1558-5646.1986.tb00560.x) [DOI] [PubMed] [Google Scholar]
- 28.Seghers BH. 1974. Schooling behavior in the guppy (Poecilia reticulata): an evolutionary response to predation. Evolution 28, 486–489. [DOI] [PubMed] [Google Scholar]
- 29.Deacon AE, Mahabir R, Inderlall D, Ramnarine IW, Magurran AE. 2017. Evaluating detectability of freshwater fish assemblages in tropical streams: is hand-seining sufficient? Environ. Biol. Fish. 100, 839–849. ( 10.1007/s10641-017-0610-5) [DOI] [Google Scholar]
- 30.Baerends GP, Brouwer R, Waterbolk HT. 1955. Ethological studies on Lebistes reticulatus (Peters). Behaviour 8, 249–334. ( 10.1163/156853955X00238) [DOI] [Google Scholar]
- 31.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dingemanse NJ, Kazem AJN, Réale D, Wright J. 2010. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89. ( 10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- 33.Nussey DH, Wilson AJ, Brommer JE. 2007. The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 20, 831–844. ( 10.1111/j.1420-9101.2007.01300.x) [DOI] [PubMed] [Google Scholar]
- 34.Martin JGA, Nussey DH, Wilson AJ, Réale D. 2011. Measuring individual differences in reaction norms in field and experimental studies: a power analysis of random regression models. Methods Ecol. Evol. 2, 362–374. ( 10.1111/j.2041-210X.2010.00084.x) [DOI] [Google Scholar]
- 35.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 36.Sih A, Bell AM, Chadwick J, Ziemba RE. 2004. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277. ( 10.1086/422893) [DOI] [PubMed] [Google Scholar]
- 37.Wilson DS. 1994. Adaptive genetic variation and human evolutionary psychology. Ethol. Sociobiol. 15, 219–235. ( 10.1016/0162-3095(94)90015-9) [DOI] [Google Scholar]
- 38.Harris MA, Brett CE, Johnson W, Deary IJ. 2016. Personality stability from age 14 to age 77 years. Psychol. Aging 31, 862–874. ( 10.1037/pag0000133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nettle D. 2005. An evolutionary approach to the extraversion continuum. Evol. Human Behav. 26, 363–373. ( 10.1016/j.evolhumbehav.2004.12.004) [DOI] [Google Scholar]
- 40.Nettle D. 2006. The evolution of personality variation in humans and other animals. Am. Psychol. 61, 622–631. ( 10.1037/0003-066X.61.6.622) [DOI] [PubMed] [Google Scholar]
- 41.Daan S, Tinbergen JM. 1997. Adaptation of life histories. In Behavioural Ecology: an evolutionary approach, 4th edn (eds JR Krebs, NB Davies), pp. 311–333. Oxford, UK: Blackwell Science Ltd. [Google Scholar]
- 42.Nakayama S, Rapp T, Arlinghaus R. 2017. Fast–slow life history is correlated with individual differences in movements and prey selection in an aquatic predator in the wild. J. Anim. Ecol. 86, 192–201. ( 10.1111/1365-2656.12603) [DOI] [PubMed] [Google Scholar]
- 43.Oke KB, Rolshausen G, LeBlond C, Hendry AP. 2017. How parallel is parallel evolution? A comparative analysis in fishes. Am. Nat. 190, 1–16. ( 10.1086/691989) [DOI] [PubMed] [Google Scholar]
- 44.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. ( 10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 45.Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. ( 10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 46.Stamps J, Groothuis TGG. 2010. The development of animal personality: relevance, concepts and perspectives. Biol. Rev. 85, 301–325. ( 10.1111/j.1469-185X.2009.00103.x) [DOI] [PubMed] [Google Scholar]
- 47.Wolf M, Weissing FJ. 2012. Animal personalities: consequences for ecology and evolution. Trends Ecol. Evol. 27, 452–461. ( 10.1016/j.tree.2012.05.001) [DOI] [PubMed] [Google Scholar]
- 48.Kelley JL, Phillips SC, Evans JP. 2013. Individual consistency in exploratory behaviour and mating tactics in male guppies. Naturwissenschaften 100, 965–974. ( 10.1007/s00114-013-1097-3) [DOI] [PubMed] [Google Scholar]
- 49.Harris S, Ramnarine IW, Smith HG, Pettersson LB. 2010. Picking personalities apart: estimating the influence of predation, sex and body size on boldness in the guppy Poecilia reticulata. Oikos 119, 1711–1718. ( 10.1111/j.1600-0706.2010.18028.x) [DOI] [Google Scholar]
- 50.Bell AM, Hankison SJ, Laskowski KL. 2009. The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783. ( 10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. ( 10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 52.Plard F, Gaillard J-M, Coulson T, Tuljapurkar S. 2016. Des différences, pourquoi? Transmission, maintenance and effects of phenotypic variance. J. Anim. Ecol. 85, 356–370. ( 10.1111/1365-2656.12477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith RK, Pullin AS, Stewart GB, Sutherland WJ. 2010. Effectiveness of predator removal for enhancing bird populations. Conserv. Biol. 24, 820–829. ( 10.1111/j.1523-1739.2009.01421.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data were supplied and allocated to the following repository https://doi.org/10.5061/dryad.4s4h4.