Abstract
Environmental variability is ubiquitous, but its effects on populations are not fully understood or predictable. Recent attention has focused on how rapid evolution can impact ecological dynamics via adaptive trait change. However, the impact of trait change arising from plastic responses has received less attention, and is often assumed to optimize performance and unfold on a separate, faster timescale than ecological dynamics. Challenging these assumptions, we propose that gradual plasticity is important for ecological dynamics, and present a study of the plastic responses of the freshwater green algae Chlamydomonas reinhardtii as it acclimates to temperature changes. First, we show that C. reinhardtii's gradual acclimation responses can both enhance and suppress its performance after a perturbation, depending on its prior thermal history. Second, we demonstrate that where conventional approaches fail to predict the population dynamics of C. reinhardtii exposed to temperature fluctuations, a new model of gradual acclimation succeeds. Finally, using high-resolution data, we show that phytoplankton in lake ecosystems can experience thermal variation sufficient to make acclimation relevant. These results challenge prevailing assumptions about plasticity's interactions with ecological dynamics. Amidst the current emphasis on rapid evolution, it is critical that we also develop predictive methods accounting for plasticity.
Keywords: phenotypic plasticity, environmental variation, thermal performance, temperature, beneficial acclimation hypothesis, ecological forecasting
1. Introduction
Ecologists are increasingly challenged to make quantitative predictions about the behaviour and fate of ecological systems in times of significant environmental change. Producing reliable forecasts is not trivial: most ecological systems are composed of many species whose interactions are nonlinear, scale dependent and often poorly understood [1]. A promising approach for improving forecasting uses measurable properties (or traits) of organisms to understand their basic ecology, predicting how they interact with other species and react to environmental change [2–4]. Complexities at the community and ecosystem level can be simplified by focusing on the mechanistic interactions of small numbers of functional groups (taxa sharing similar traits). Dynamic trait variation within species, over time and across environments poses challenges for trait-based approaches [5]. However, preliminary successes in generating ecological predictions [6,7], and accompanying empirical and theoretical advances, highlight the field's initial contributions towards a mechanistic understanding of ecology.
Trait changes on ecological timescales, such as those driven by rapid evolution, greatly complicate predictive efforts because models can no longer treat species' traits as constants. In recent decades, researchers have dedicated substantial effort to documenting and understanding the effects of rapid evolution on ecological dynamics [8–10], discovering examples where evolutionary dynamics alter ecological predictions [11–13]. For example, the theoretically predicted quarter-period offset between predator–prey cycles were not observed in rotifer–algae systems due to rapid evolution [14]. Additionally, within three generations, the evening primrose (Oenothera biennis) evolved resistance to herbivory that influenced the abundance of its specialist seed predator moth [15]. Ecosystems can also be affected; in one case, rapid evolution in herbivore life history altered primary production [16]. While expanding literature on eco-evolutionary interactions has unarguably produced empirical and theoretical advances [17], dynamic trait variation arising from other processes can be equally important, yet seems to receive less attention.
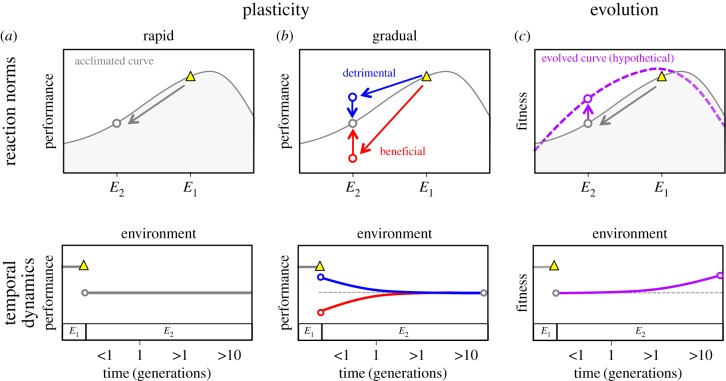
Phenotypic plasticity also drives trait change, through non-genetic mechanisms including behaviour, physiology and development. Although most plasticity research has an evolutionary focus—considering when it is adaptive and how it evolves—plastic trait changes also have important ecological consequences [18–21]. Plastic responses are sometimes incorporated into ecological models; for example, species’ reaction norms have been used to predict their occurrence, performance and growth in temporally and/or spatially variable environments [22,23] (but see [24]). These studies generally assume that plastic responses are rapid relative to environmental changes (figure 1a). However, plastic phenotypic changes may occur gradually relative to the timescales of prediction and ecological change, potentially due to time lags inherent to plasticity [25] (figure 1b). In these situations an individual's phenotype is neither fixed nor directly predicted by the current environment; instead, it is determined by complex interactions between an individual's history of environmental exposure and the mechanisms governing plasticity.
Figure 1.
Organismal performance changes in response to environmental change (say a shift from environment E1 to E2) through plastic and evolutionary processes over a range of temporal scales. Rapid plastic responses (a) allow individuals with a fixed genotype to change their phenotype quickly, leading to performance levels that match their reaction norm in environment E2. By contrast, when plastic responses are gradual (b), phenotypic adjustments occur slowly. During this period, individuals may exceed or fall short of their eventual performance once adjusted to E2 (i.e. the value given by their reaction norm). We refer to plastic responses that gradually improve performance following a perturbation as ‘beneficial’ (red); those that decrease performance are ‘detrimental’ (blue). Over longer exposures to E2, evolutionary responses (c) spanning generations may improve the fitness (often correlated with performance) of the population of individuals, changing the reaction norm. Note that plastic and evolutionary responses may occur simultaneously, depending on the rate of plasticity, the amount of heritable genetic variation present, and the rate at which new variation arises. Finally, while plasticity typically concerns phenotypic changes within the lifespan of an individual, some plastic responses can extend across generations (e.g. in populations of genetically identical, asexual microbes). (Online version in colour.)
We introduce a new term, gradual plasticity, to identify this situation and emphasize the importance of the temporal dynamics of plasticity. Specifically, we define gradual plasticity as all non-genetic phenotypic changes that are too slow to closely track the ecological or environmental changes that drive them, yet too fast to be treated as constant. This intentionally broad definition encompasses well-known processes, including mechanisms that are reversible (e.g. acclimation) or irreversible (e.g. development [26,27]), and that act within an individual's lifespan (e.g. behaviour) or potentially across generations (e.g. maternal effects [28]). Examples of gradual plasticity include leaf respiration rates (which acclimate to novel temperatures over weeks to years, [29]), and phytoplankton pigmentation (which reacts to shifts in the light spectrum over a week [30]).
Studying the temporal dynamics and effects of acclimation, a major class of plasticity, may yield insights into the broader topic of gradual plasticity. Acclimation generally consists of short term, reversible phenotypic changes stimulated by acute environmental exposure. It is commonly assumed that acclimation improves performance following exposure to a new environment (the beneficial acclimation hypothesis [31,32]; figure 1b, red). However, empirical support for this hypothesis is mixed; performance can decline with continued exposure [25,30–33], perhaps due to the costs of acclimation or investment in additional bet-hedging mechanisms [25]. We acknowledge this possibility in the gradual plasticity framework as a ‘detrimental’ response (figure 1b, blue). Organismal performance is difficult to predict when environmental variation elicits mixtures of beneficial and detrimental responses, because these responses are poorly captured by the standard reaction norm. Finally, plasticity is often heritable, it can be both adaptive and maladaptive, and ultimately it is subject to evolution. This may occur simultaneously with plastic responses [20], and act in similar or different directions [34]. Alternatively, evolutionary adaptation dependent on mutation-limited selection proceeds more slowly, ultimately increasing organismal fitness in its new environment (figure 1c). Although understanding the interaction of evolution and plasticity is an important goal, the remainder of this paper focuses primarily on plasticity, and acclimation in particular.
Here, we evaluate the hypotheses that (i) plastic changes (and acclimation in particular) can be gradual and interact with ecological variation to hinder accurate forecasts of population dynamics, and (ii) gradual acclimation responses can be both beneficial and detrimental, and differ from those produced by rapid evolution. We test these hypotheses by investigating thermal acclimation in the freshwater green alga, Chlamydomonas reinhardtii. We use laboratory experiments to test how a population's historical temperature exposure affects its growth in constant and fluctuating thermal environments, and we contrast the results with predictions that ignore gradual acclimation. We then explore whether temperature fluctuations in natural lakes might prompt meaningful acclimation responses. Our results show that (i) thermal acclimation is gradual in C. reinhardtii; (ii) gradual acclimation's beneficial and detrimental effects are critical to understanding C. reinhardtii population dynamics in the laboratory and (iii) natural thermal regimes are capable of producing similar effects in the field. Our findings show that gradual plasticity has significant ecological effects that are under-appreciated in ecology amidst the current emphasis on rapid evolution.
2. Methods
(a). Thermal acclimation effects on C. reinhardtii population growth rates
We measured the acclimated population growth rates of C. reinhardtii at five temperatures to establish its thermal performance curve [35]. Then we determined how the growth rates of populations acclimated to 14°C and 33°C responded to acute exposure to other temperatures. In all experiments, we grew populations of a single mating type of C. reinhardtii (from E. Litchman, Michigan State University) in 125 ml Erlenmeyer flasks using COMBO growth media [36]. All populations received approximately 30 µ Einsteins m−2 s−1 of light over 24 h/day within environmental control chambers (Percival I-36 & I-30VL Series Controlled Environmental Chamber; Percival Scientific, Perry, Iowa, USA) and were manually re-suspended twice daily. In all experiments, populations were inoculated at densities of approximately 10 000 cells ml−1 and maintained in exponential phase through biweekly dilutions returning densities to this level.
To measure C. reinhardtii's thermal reaction norm [35], we acclimated populations over two weeks to temperatures of 14°C, 20°C, 26°C, 30°C and 33°C, a range that includes its optimum temperature (the temperature at which growth rate is maximized). We tracked actual incubator temperatures using HOBO pendant temperature–light loggers (Onset Computer Corporation, Pocasset, MA) placed in beakers containing approximately 200 ml water (electronic supplementary material, table S1). After two weeks, we additionally established four replicates of populations acclimated to both 14°C and 33°C at all five experimental temperatures (electronic supplementary material, figure S1). We measured cell densities four times over the following 48 h, using a Spectrex Laser Particle Counter Model PC-2200 (Spectrex Corporation, Redwood City, CA). We estimated specific growth rates by taking the slope of the regression between ln(density) and time, excluding several observations where densities were too high (electronic supplementary material, appendix A). Abundances based on fluorescence and biovolume yielded similar results (not shown). After another 72 h, we repeated these measurements to monitor the decay of acclimation effects. We assessed the effects of temperature and acclimation history on growth rates using both a two-way ANOVA and generalized additive models (GAMs) to examine the shapes of acute and acclimated curves. The acclimated curve incorporates data from both measurement periods (growth rates were comparable; electronic supplementary material, figure S2 and table S2).
(b). Acclimation effects within single genotypes
The populations we studied were bottlenecked by dilutions before our initial experiments; however, they were not necessarily genetically uniform. To determine whether the responses, we observed were driven by plasticity rather than evolution, we established five isogenic lines using single cells isolated from our stock population of C. reinhardtii [37]. We measured the growth response of isogenic lines (and a mixed culture) acclimated to 14°C and 30°C when exposed acutely to 30°C (corresponding to the largest effects in our initial experiment). We analysed the effects of strain identity and acclimation history on growth rates (two-way ANOVA), and the differences between the pooled responses of the isogenic lines and the mixed culture (one-way ANOVA, unequal variances).
(c). Effects of temperature fluctuations and acclimation history on population dynamics
We next tested whether acclimation affects population dynamics in variable environments. We exposed sets of four replicate C. reinhartdii populations, acclimated to either 14°C or 30°C, to thermal regimes alternating between 14°C and 30°C every 6, 12 or 24 h for a total of 48 h (electronic supplementary material, figure S3). All populations had similar initial densities (F2,18 = 0.246, p = 0.784) and spent a total of 24 h at both temperatures. We measured the final densities of each population, and tested the effects of acclimation history and fluctuation frequency (two-way ANOVA). We also predicted final population densities using: (i) an exponential growth model that assumes growth rates acclimate instantaneously, and (ii) a new gradual acclimation model parameterized using our experimental data (details appear in electronic supplementary material, appendix B).
(d). Short-term lake temperature variation
To determine the scope of natural thermal variation to prompt acclimation responses, we analysed high spatio-temporal resolution water temperature data from two temperate lakes within the North Temperate Lakes Long-term Ecological Research Station in northern Wisconsin, USA (approx. 46°00′20′ N, approx. 89°41′44′ W) [38,39]. While abundant data exist for other lakes, we focus on this pair because they are close together, yet bathymetrically distinctive: Sparkling Lake has a surface area of 24 ha and maximum depth of 20 m, while Crystal Bog is only 0.5 ha in area and 2.5 m deep. We used hourly water temperature data over the longest overlapping interval (5 April to 2 November 2012) and from all available depths, linearly interpolating the records from Sparkling Lake to 0.25 m depth resolution (for consistency with Crystal Bog). Three gaps of 2–4 days occurred in the data from Crystal Bog; we replaced these missing values with temperatures from the same hour on the most recent day with complete records.
For each lake, we considered the thermal variation that phytoplankton might experience under three scenarios: species ‘mobile’ within the water column, species ‘recruiting’ from resting stages in the profundal benthos and species maintaining a ‘stationary’ position in the water column (electronic supplementary material, figure S4). We calculated the thermal variation members of each category might experience using a moving window approach. For ‘mobile’ species (either actively motile or subject to mixing within the water column), we calculated the maximum range of temperatures occurring within 24 h and ±1 m of each time/depth record. For ‘recruiting’ species (propagating from benthic resting stages and migrating up the water column), we calculated the temperature differential between the lake's bottom and a range of other depths at each time point. While most phytoplankton recruitment may occur in the littoral zone [40], the available data restrict our focus to profundal benthic recruitment. For ‘stationary’ species (capable of maintaining a constant depth), we calculated maximum range of temperatures occurring within 24 h at each time/depth. These categories are intentionally coarse simplifications of more detailed life-history characteristics [41,42], yet they approximate (and if anything, underestimate) the thermal experiences of different phytoplankton. For example, motile phytoplankton such Chlamydomonas are capable of 5–10 m d-1 of directed movement [43,44], including instances of crossing a 14°C temperature gradient into hypolimnetic waters [45].
3. Results
(a). Thermal acclimation in C. reinhardtii
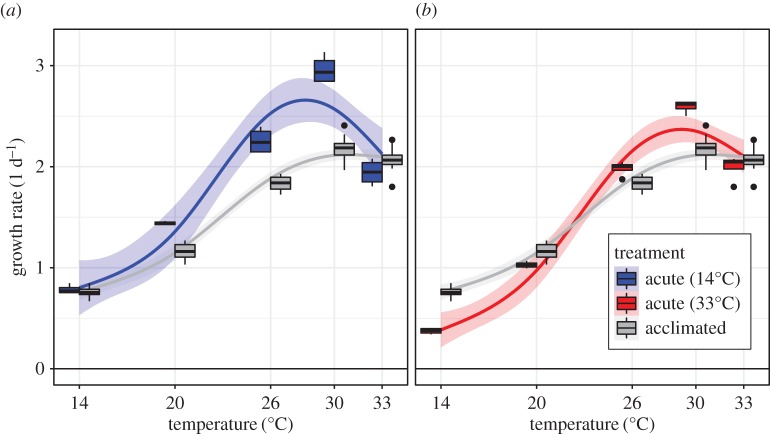
Acclimation temperature strongly affected C. reinhardtii growth rate immediately following exposure to new temperatures. Populations acclimated to 14°C and then exposed to higher temperatures achieved significantly higher acute growth rates than populations already acclimated to such temperatures (figure 2a; electronic supplementary material, tables S3–S5). These effects were most pronounced at 24°C, 30°C and 33°C, increasing growth rates by 22–35%. By contrast, populations acclimated to 33°C grew more slowly at colder temperatures than the corresponding acclimated populations (figure 2b; electronic supplementary material, tables S3–S5). At 14°C this amounted to a 50% decrease. Differences between acute and acclimated growth rates dissipated after a week of continued exposure (electronic supplementary material, figures S5 and S6 and tables S6–S8). Notably, we found examples of both beneficial acclimation (growth rates of 33°C acclimated populations at 14°C increase over time) and detrimental acclimation (growth rates of 14°C acclimated populations at 30°C decline).
Figure 2.
Depending on their prior acclimation history (a, 14°C; or b, 33°C) the acute growth rates of C. reinhardtii populations over a range of temperatures can both exceed and fall short of their long-term, acclimated growth rates (grey). Lines and shading represent GAM regression fits and 95% CIs (electronic supplementary material, table S5).
(b). Acclimation within single genotypes
The isogenic lines of C. reinhardtii exhibited the same qualitative responses as the C. reinhardtii culture we examined initially: populations acclimated to 14°C grew significantly faster at 30°C than populations acclimated to 30°C (p < 0.0001, figure 3; electronic supplementary material, table S9). The magnitude of this response varied among strains, suggesting that these lines are not identical (strain by acclimation history interaction, p < 0.0001; electronic supplementary material, table S9). The pooled response of the isogenic lines was not distinguishable from mixed culture (p = 0.46; electronic supplementary material, table S9), suggesting that these lines adequately characterize the dominant variants present in our first experiment.
Figure 3.
The growth rate of isogenic C. reinhardtii lines (left) at 30°C depend on whether they were previously acclimated to 14°C (black) or 30°C (grey). The pooled responses of strains 1–5 with a 14°C history do not differ from the original, mixed-genotype population (right). Points indicate average growth rate; error bars are 95% CIs.
(c). Phytoplankton population dynamics
If phenotypic responses to environmental change are rapid, then acclimation dynamics should have little effect on population dynamics. Consequently, C. reinhardtii population growth in variable thermal environments should be directly predictable from the acclimated thermal performance curve or reaction norm (as assumed in [22,46]). However, the final population densities we observed (figure 4) deviated dramatically from the predictions of a rapid acclimation model, which had an R2 of −0.14 (appendix B; negative R2s arise when a model's predictions are worse than the global mean across treatments). Both fluctuation frequency (F2,18 = 47.6, p < 0.001) and acclimation history (F1,18 = 162.3, p < 0.001) significantly affected populations (figure 4; electronic supplementary material, table S10). A new model accounting for gradual acclimation and acute growth rates captured these effects better (especially that of acclimation history) and offered improved predictive power (R2 = 0.497; electronic supplementary material, appendix B). Future models may yield additional improvements by considering, for example, the possibility that acclimation rate is temperature dependent.
Figure 4.
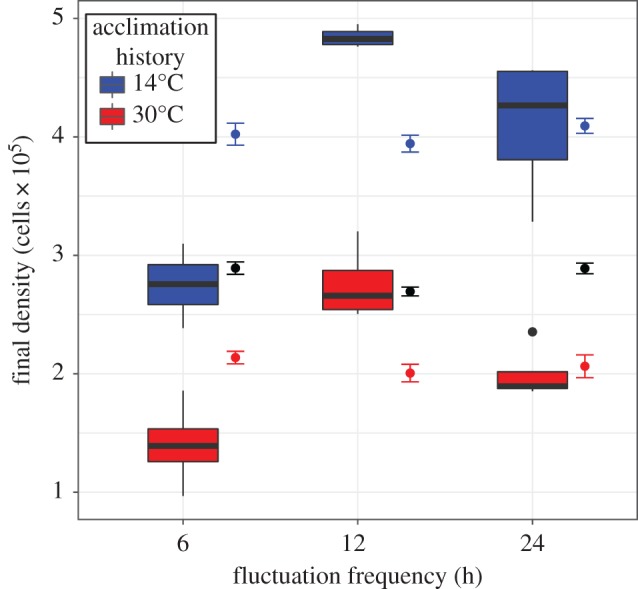
Populations of C. reinhardtii achieve different densities over 2 days of exposure to temperatures oscillating between 14°C and 30°C, depending on fluctuation frequency and their prior acclimation history (red/blue). Observations are contrasted with predicted values from: (i) a basic exponential growth model assuming rapid acclimation (black points with 95% confidence intervals). Because of this assumption, predictions are independent of acclimation history, so only a single mean prediction is provided; and (ii) an empirically parameterized model accounting for gradual acclimation (red/blue points with 95% confidence intervals).
(d). Thermal variation in natural lake ecosystems
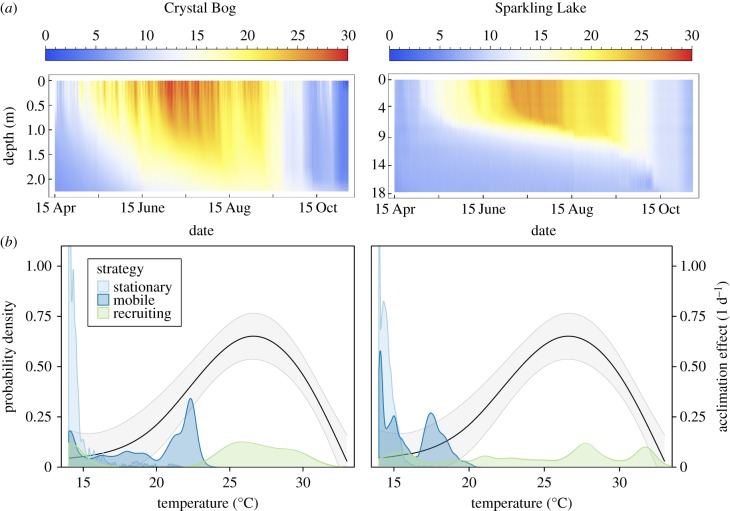
Phytoplankton experience different thermal regimes depending on their movement ability, phenology and the spatio-temporal variability of their environment (figure 5a and electronic supplementary material, figure S7). In the laboratory, temperature changes of as little as 6°C significantly affected C. reinhardtii growth rates (figure 2a). Our analyses of two stratified north temperate lakes suggest that diel changes of >6°C occur frequently enough that gradual acclimation may be ecologically important in at least some instances (figure 5 and electronic supplementary material, figure S7).
Figure 5.
Phytoplankton can experience enough thermal variation in lakes to make gradual acclimation ecologically important. (a) Depth-specific lake temperatures for Crystal Bog (left column) and Sparkling Lake (right column). (b) The difference between acute and acclimated growth rates for a 14°C acclimated population illustrates the potential effects of a range of increases in temperature (black, GAM regression line with ±1 s.e. confidence band). Shaded empirical distributions highlight the probabilities of actually experiencing these temperature increases within 24 h, depending on lake identity and movement strategy.
As they move across gradients, ‘mobile’ phytoplankton can experience high amounts of diel temperature variation (figure 5b and electronic supplementary material, figure S7b). This was common in Crystal Bog, where diel changes of greater than 6°C occurred in 25.07% of the intervals examined, but rare in Sparkling Lake (0.05%). In both lakes, ‘recruiting’ phytoplankton commonly experience temperature increases greater than 6°C (occurring with 58.67% and 71.82% frequencies in Crystal Bog and Sparkling Lake, respectively) (electronic supplementary material, figure S7c). Stationary phytoplankton only experienced changes of greater than 6°C in the shallow Crystal Bog, and then only near the surface and on 6.2% of days in the time series. Such changes did not occur in the larger and deeper Sparkling Lake (figure 5b and electronic supplementary material, figure S7d). Focusing on the times and places where lake temperatures were approximately 14°C, we found that the distributions of temperature increases within 24 h for mobile and recruiting phytoplankton are often adequate to yield significant growth rate effects, relative to our experiments (figure 5b).
4. Discussion
Thermal acclimation has broad effects on the growth and population dynamics of C. reinhardtii, especially when the timescales of acclimation and environmental change coincide. Our results additionally highlight the contrast between phenotypic changes that are plastic (which can have both beneficial and detrimental effects on performance) or driven by selection (which are usually locally adaptive). We also show that phytoplankton may experience thermal variation on scales where acclimation dynamics matter, depending on their lake environment and natural history. As such, our initial hypotheses are supported: gradual plasticity occurs (via acclimation in this case), inhibits accurate ecological predictions when ignored, and has effects that differ from rapid evolution. Below, we consider the causes and implications of these effects for C. reinhardtii, and discuss the consequences of interactions between ecology and plasticity.
(a). Mechanisms of thermal acclimation in phytoplankton
At the cellular level, a cascade of physiological changes occur as C. reinhardtii reacts to shifts in temperature by regulating its metabolism, photosynthesis, and membrane fluidity, and synthesizing new proteins [47,48]. Gradual improvements in growth rate due to acclimation (figure 2b) may occur due to delays associated with making new proteins and lipids, which can take ≥ 24 h [47,48]. Additionally, given lowered temperatures but constant light, cells still absorb the same amount of light energy, yet have less capacity to direct it into carbon fixation [49], leading to reallocation of energy [50], decreased efficiency or even cellular damage. Other mechanisms may be involved when performance declines due to acclimation (defying the beneficial acclimation hypothesis [25,31,32], figure 2a). Phytoplankton tend to have lower C : N and C : P ratios under cooler conditions [51–52], potentially due to accumulating reserves of N and P. Following a temperature increase, these reserves may fuel a temporary period of enhanced growth. However, warm-acclimated cells with depleted reserves might experience nutrient-limited growth when exposed to colder temperatures. Alternatively, detrimental acclimation could reflect an evolutionary bet-hedging strategy (sensu [53]), with moderate temperature increases prompting cells to investment in costly machinery that reduces growth, while enhancing survival at even higher temperatures. For example, high temperatures stimulate the production of heat shock proteins [54] and reduce physiological rates in ectotherms [55,56]. In C. reinhardtii, up-regulation of heat shock proteins has been reported after 2 h exposures to temperatures of ≥35°C [48]. While several mechanisms plausibly explain the mix of acclimation effects we observe, the exact mechanisms underlying acclimation in C. reinhardtii remain an important topic of study.
(b). Reconsidering the nature of acclimation responses
Other phytoplankton, and indeed many other organisms [25,32], can express a mixture of beneficial and detrimental acclimation responses depending on the environmental perturbations they experience. Collectively, this suggests that a reframing of acclimation concepts is needed: clearly, neither acclimation, nor plasticity more broadly, is universally beneficial. The existence of trade-offs may offer a general explanation for seemingly maladaptive responses like detrimental acclimation: the evolution of plasticity is driven by selection acting on organisms in the context of all the environmental variation they experience [53]. Organisms are unlikely to evolve plastic responses that improve their fitness under all possible environmental changes, due to trade-offs [30]. However, as long as situations prompting maladaptive responses are sufficiently rare or of small effect size, selection can still favour the evolution of plasticity. To understand species' responses to environmental variation, we need a more comprehensive understanding of plasticity (whether behavioural, physiological or developmental). Specifically, it is important to study plastic responses to a complex range of perturbations, rather than individual and potentially idiosyncratic treatments. Investigating the historical environments driving selection on plasticity is also critical.
(c). Towards integrating plastic and evolutionary effects
Plasticity is typically considered to occur within individuals during their lifespan, whereas evolutionary change occurs across generations. However, ‘trans-generational acclimation’ has been observed in other organisms, including fish [28,57]. It is less surprising that acclimation should occur over the course of multiple generations in fast-growing microbes such as C. reinhartdii, either due to epigenetic effects or the fact that daughter cells inherit large portions of their physical matter from their progenitors. Our results do allow us to consider the potential effects of evolution within our experiments, to a degree.
While we did detect strain-level differences in the magnitude of acclimation responses, our results strongly suggest that acclimation, not evolution, is the dominant mechanism of phenotypic change during our experiments. We base this conclusion on two factors: (i) there were very few generations for relevant de novo mutations to arise and sweep to fixation during our short-term experiments, and (ii) detrimental acclimation runs directly counter to the expected effects of natural selection. Established from single cells, our isogenic lines had no variation initially and could only reproduce asexually. A mutation accumulation study of C. reinhardtii detected only 14 mutations after approximately 350 generations, for an estimated total mutation rate of 3.23 × 10–10 mutations/site/generation [58]. By contrast, to generate the growth rate differences we observed (figures 2 and 3), mutations conveying increased growth at 30°C would have to arise and nearly fix in the 14°C acclimating populations within approximately 16 generations (assuming growth rate r = 0.8 for 14 days) and yet not appear in the 30°C acclimating populations. Furthermore, these mutations would have to be lost—despite being favoured by selection—within another 10–20 generations (assuming r ≤ 2.2 for 7 days; electronic supplementary material, figures S5 and S6). By contrast, artificial selection experiments on plankton thermal tolerance take much longer, even starting from polycultures [37,59].
Although acclimation dominates the effects we observed, there is clearly among-genotype variation in thermal acclimation capacity (consistent with prior studies showing intra-specific variation in plasticity, [21]). This implies that some change in strain frequency likely occurred in our initial experiments, and that populations exposed to different thermal regimes over longer intervals might eventually evolve different acclimation abilities. Constant thermal environments might lead to a loss of acclimation ability, while variability might foster increased acclimation capacity, or alter which temperature combinations elicit detrimental or beneficial responses.
(d). When are overlapping eco-physiological timescales important?
Existing patterns of environmental variation both dictate selection on plastic responses and determine whether they are sufficiently gradual to impact ecological dynamics (e.g. figure 5). In the stratified north temperate lakes, we considered the most consistent source of thermal variation existed across depths. Phytoplankton recruiting from the profundal benthos to near-surface waters will typically experience a 10–15°C temperature change during spring and summer months. This places the 16°C temperature differential used in our laboratory fluctuation experiment (figure 4) near the upper end of thermal variation phytoplankton might experience. However, acclimation still produced significant effects on growth rate for smaller temperature perturbations (figure 2) such that smaller amplitude fluctuations (6°C) are still likely to affect realized population dynamics (figure 5b). The importance of benthic recruitment of phytoplankton for maintaining pelagic populations is variable [60], but can be substantial in certain systems [61,62]. Additionally, these life stages also correspond to points in time where populations are not likely to experience density dependence.
Our results provide context for understanding when and where gradual acclimation is likely to affect phytoplankton ecology, due to the temporal overlap of plastic and environmental changes. Acclimation will have its largest effects when phytoplankton experience temperature perturbations of ≥6°C on timescales of 1–2 days. This is most likely to occur in smaller lakes, during spring and summer, and for species that recruit from the profundal benthos, migrate through the water column or reside in surface waters. Estimates of mean daily surface temperature fluctuations in lakes less than 3 km2 area are 4–7°C [63]. This is significant considering that such small systems represent the overwhelming majority of all lakes [64]. Importantly, patterns of thermal variability also change through time. In temperate systems, the largest temperature swings appear either early in the growing season when temperatures are most temporally dynamic [63] or spatially after stratification establishes strong thermal gradients [65]. Temperature variation reaches its lowest from late fall through early spring, where temperate lake temperatures only span 0–4°C [65]. Accordingly, when lake temperatures are stable through time and across depths, and change slowly, acclimation effects will be minor, as cellular physiology equilibrates faster than environments change. From an applied perspective, ongoing difficulties in predicting important dynamical phenomena in phytoplankton, such as the onset of harmful algal blooms, may be directly related to a lack of understanding of acclimation effects in variable aquatic habitats.
Beyond phytoplankton and acclimation, there are many reasons to believe that the timescales of plastic and ecological changes will overlap in a wide range of ecosystems and organisms. Other small taxa with short generation times (e.g. protists, bacteria and rotifers) will experience analogous environmental fluctuations and may depend similarly on acclimation history over timescales from days to weeks. Acclimation is typically assessed in longer-lived organisms by measuring physiological variables over brief intervals. It remains unclear how the effects of such short-term responses accumulate over the lifespan of an organism. Other forms of plastic responses, including developmental plasticity and maternal effects may interact with ecology over longer timescales by irreversibly fixing the traits of an organism based on conditions prevailing early in its life, or that of its parents [26]. In general, measuring the plastic responses of organisms to environmental change, including both the direction and rate of plastic changes, will be important in determining when gradual plasticity may complicate ecological dynamics.
5. Conclusion
The future of ecology as a predictive science depends on being able to accurately connect environmental conditions and organismal traits with particular ecological outcomes. Currently, process-based models rarely improve forecasting horizons relative to simple statistical models [1]. Additionally, studies of population dynamics in fluctuating environments generally reveal mismatches between measurements in static conditions and observed values in fluctuating environments over short timescales [66]. Our study of C. reinhardtii shows that accounting for gradual acclimation can reduce such mismatches and provide enhanced predictions. These results provide a microcosm against which we can better understand the effects of gradual plasticity on ecology in general. We argue that alongside the current integration of rapid evolution and ecology, it is critical to appreciate that gradual plasticity can also have large effects on ecological processes. While recent efforts to increase the accuracy of ecological forecasts have embraced incorporating the capacity for adaptive evolution, acclimation and plasticity more broadly remain ignored [22]. Given that patterns of environmental variation are changing now and will continue to do so over the coming century [67], and that predictions are often most needed in variable and perturbed systems, it is critical that we develop predictive methods that integrate the joint effects of plasticity, evolution and ecology.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank K. L. Cottingham and the Dartmouth College Department of Biological Sciences for equipment, supply and facility access; M. K. Thomas and K. L. Cottingham and for helpful comments; E. Litchman and P. Woodruff for supplying C. reinhardtii; and C. Layne and B. Labbadia for laboratory assistance.
Data accessibility
Datasets supporting this article are included with the electronic supplementary material.
Authors' contributions
C.T.K., S.B.F. and D.A.V. conceived of the study; S.B.F. and A.A.A. performed the experiments; C.T.K. analysed the results; S.B.F. analysed lake temperature data; all authors contributed to writing and approved the final manuscript.
Competing interests
We have no competing interests.
Funding
Support came from an NSF PRFB no. 1402074 to C.T.K., a James S. McDonnell Foundation Postdoctoral Fellowship to S.B.F., a James O. Friedman Presidential Scholarship and Porter Family Fund for Sustainability Science Award to A.A.A., Yale University, and NSF grant OCE-1638958 to Elena Litchman.
References
- 1.Petchey OL, et al. 2015. The ecological forecast horizon, and examples of its uses and determinants. Ecol. Lett. 18, 597–611. ( 10.1111/ele.12443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGill BJ, Enquist BJ, Weiher E, Westoby M. 2006. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185. ( 10.1016/j.tree.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 3.Litchman E, Klausmeier CA. 2008. Trait-based community ecology of phytoplankton. Annu. Rev. Ecol. Evol. Syst. 39, 615–639. ( 10.1146/annurev.ecolsys.39.110707.173549) [DOI] [Google Scholar]
- 4.Westoby M, Wright IJ. 2006. Land-plant ecology on the basis of functional traits. Trends Ecol. Evol. 21, 261–268. ( 10.1016/j.tree.2006.02.004) [DOI] [PubMed] [Google Scholar]
- 5.Kremer CT, Williams AK, Finiguerra M, Fong AA, Kellerman A, Paver SF, Tolar BB, Toscano BJ. 2017. Realizing the potential of trait-based aquatic ecology: new tools and collaborative approaches. Limnol. Oceanogr. 62, 253–271. ( 10.1002/lno.10392) [DOI] [Google Scholar]
- 6.Edwards KF, Litchman E, Klausmeier CA. 2013. Functional traits explain phytoplankton responses to environmental gradients across lakes of the United States. Ecology 94, 1626–1635. ( 10.1890/12-1459.1) [DOI] [PubMed] [Google Scholar]
- 7.Webb CT, Hoeting JA, Ames GM, Pyne MI, LeRoy Poff N. 2010. A structured and dynamic framework to advance traits-based theory and prediction in ecology. Ecol. Lett. 13, 267–283. ( 10.1111/j.1461-0248.2010.01444.x) [DOI] [PubMed] [Google Scholar]
- 8.Post DM, Palkovacs EP. 2009. Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play. Phil. Trans. R. Soc. B 364, 1629–1640. ( 10.1098/rstb.2009.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson JN. 1998. Rapid evolution as an ecological process. Trends Ecol. Evol. 13, 329–332. ( 10.1016/S0169-5347(98)01378-0) [DOI] [PubMed] [Google Scholar]
- 10.Hairston NG, Ellner SP, Geber MA, Yoshida T, Fox JA. 2005. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 8, 1114–1127. ( 10.1111/j.1461-0248.2005.00812.x) [DOI] [Google Scholar]
- 11.Kremer CT, Klausmeier CA. 2013. Coexistence in a variable environment: eco-evolutionary perspectives. J. Theor. Biol. 339, 14–25. ( 10.1016/j.jtbi.2013.05.005) [DOI] [PubMed] [Google Scholar]
- 12.Barabás G, D'Andrea R. 2016. The effect of intraspecific variation and heritability on community pattern and robustness. Ecol. Lett. 19, 977–986. ( 10.1111/ele.12636) [DOI] [PubMed] [Google Scholar]
- 13.Shoresh N, Hegreness M, Kishony R. 2008. Evolution exacerbates the paradox of the plankton. Proc. Natl Acad. Sci. USA 105, 12 365–12 369. ( 10.1073/pnas.0803032105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida T, Jones LE, Ellner SP, Fussmann GF, Hairston NG. 2003. Rapid evolution drives ecological dynamics in a predator–prey system. Nature 424, 303–306. ( 10.1038/nature01767) [DOI] [PubMed] [Google Scholar]
- 15.Agrawal AA, Johnson MTJ, Hastings AP, Maron JL. 2013. A field experiment demonstrating plant life-history evolution and its eco-evolutionary feedback to seed predator populations. Am. Nat. 181, S35–S45. ( 10.1086/666727) [DOI] [PubMed] [Google Scholar]
- 16.Walsh MR, DeLong JP, Hanley TC, Post DM. 2012. A cascade of evolutionary change alters consumer–resource dynamics and ecosystem function. Proc. R. Soc. B 279, 3184–3192. ( 10.1098/rspb.2012.0496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoener TW. 2011. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331, 426–429. ( 10.1126/science.1193954) [DOI] [PubMed] [Google Scholar]
- 18.Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA. 2005. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 20, 685–692. ( 10.1016/j.tree.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 19.Yamamichi M, Yoshida T, Sasaki A. 2011. Comparing the effects of rapid evolution and phenotypic plasticity on predator–prey dynamics. Am. Nat. 178, 287–304. ( 10.1086/661241) [DOI] [PubMed] [Google Scholar]
- 20.Kasada M, Yamamichi M, Yoshida T. 2014. Form of an evolutionary tradeoff affects eco-evolutionary dynamics in a predator–prey system. Proc. Natl Acad. Sci. USA 111, 16 035–16 040. ( 10.1073/pnas.1406357111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer BB, Kwiatkowski M, Ackermann M, Krismer J, Roffler S, Suter MJF, Eggen RIL, Matthews B. 2014. Phenotypic plasticity influences the eco-evolutionary dynamics of a predator–prey system. Ecology 95, 3080–3092. ( 10.1890/14-0116.1) [DOI] [Google Scholar]
- 22.Thomas MK, Kremer CT, Klausmeier CA, Litchman E. 2012. A global pattern of thermal adaptation in marine phytoplankton. Science 338, 1085–1088. ( 10.1126/science.1224836) [DOI] [PubMed] [Google Scholar]
- 23.Vasseur DA, DeLong JP, Gilbert B, Greig HS, Harley CDG, McCann KS, Savage V, Tunney TD, O'Connor MI. 2014. Increased temperature variation poses a greater risk to species than climate warming. Proc. R. Soc. B 281, 20132612 ( 10.1098/rspb.2013.2612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kingsolver JG, Buckley LB. 2017. Quantifying thermal extremes and biological variation to predict evolutionary responses to changing climate. Phil. Trans. R. Soc. B 372, 20160147 ( 10.1098/rstb.2016.0147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press. [Google Scholar]
- 26.Kaplan RH, Phillips PC. 2006. Ecological and developmental context of natural selection: maternal effects and thermally induced plasticity in the frog Bombina Orientalis. Evolution 60, 142–156. ( 10.1111/j.0014-3820.2006.tb01089.x) [DOI] [PubMed] [Google Scholar]
- 27.Brakefield PM, Pijpe J, Zwaan BJ. 2007. Developmental plasticity and acclimation both contribute to adaptive responses to alternating seasons of plenty and of stress in Bicyclus butterflies. J. Biosci. 32, 465–475. ( 10.1007/s12038-007-0046-8) [DOI] [PubMed] [Google Scholar]
- 28.Donelson JM, Munday PL, McCormick MI, Pitcher CR. 2011. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat. Clim. Change 2, 30–32. ( 10.1038/nclimate1323) [DOI] [Google Scholar]
- 29.Reich PB, Sendall KM, Stefanski A, Wei X, Rich RL, Montgomery RA. 2016. Boreal and temperate trees show strong acclimation of respiration to warming. Nature 531, 633–636. ( 10.1038/nature17142) [DOI] [PubMed] [Google Scholar]
- 30.Stomp M, van Dijk MA, van Overzee HMJ, Wortel MT, Sigon CAM, Egas M, Hoogveld H, Gons HJ, Huisman J. 2008. The timescale of phenotypic plasticity and its impact on competition in fluctuating environments. Am. Nat. 172, E169–E185. ( 10.1086/591680) [DOI] [PubMed] [Google Scholar]
- 31.Leroi AM, Bennett AF, Lenski RE. 1994. Temperature acclimation and competitive fitness: an experimental test of the beneficial acclimation assumption. Proc. Natl Acad. Sci. USA 91, 1917–1921. ( 10.1073/pnas.91.5.1917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett AF, Lenski RE. 1997. Evolutionary adaptation to temperature. VI. Phenotypic acclimation and its evolution in Escherichia coli. Evolution 51, 36 ( 10.2307/2410958) [DOI] [PubMed] [Google Scholar]
- 33.Kingsolver JG, Woods HA. 2016. Beyond thermal performance curves: modeling time-dependent effects of thermal stress on ectotherm growth rates. Am. Nat. 187, 283–294. ( 10.1086/684786) [DOI] [PubMed] [Google Scholar]
- 34.Ghalambor CK, Hoke KL, Ruell EW, Fischer EK, Reznick DN, Hughes KA. 2015. Non-adaptive plasticity potentiates rapid adaptive evolution of gene expression in nature. Nature 525, 372–375. ( 10.1038/nature15256) [DOI] [PubMed] [Google Scholar]
- 35.Boyd PW, et al. 2013. Marine phytoplankton temperature versus growth responses from polar to tropical waters — outcome of a scientific community-wide study. PLoS ONE 8, e63091 ( 10.1371/journal.pone.0063091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kilham SS, Kreeger DA, Lynn SG, Goulden CE, Herrera L. 1998. COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377, 147–159. ( 10.1023/A:1003231628456) [DOI] [Google Scholar]
- 37.Huertas IE, Rouco M, López-Rodas V, Costas E. 2011. Warming will affect phytoplankton differently: evidence through a mechanistic approach. Proc. R. Soc. B 278, 3534 ( 10.1098/rspb.2011.0160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NTL LTER. 1991. North Temperate Lakes LTER: High Frequency Water Temperature Data — Sparkling Lake Raft 1989 — current. Environmental Data Initiative. See 10.6073/pasta/42bae8ac8d59cfa3fb550dfd9aff479d (accessed 25 May 2017). [DOI]
- 39.NTL LTER. 2012. North Temperate Lakes LTER: High Frequency Water Temperature Data — Crystal Bog Buoy 2005 — current. Environmental Data Initiative. See 10.6073/pasta/b80c4f285ae88d230d9180a072312dc8 (accessed 25 May 2017). [DOI]
- 40.Rengefors K, Gustafsson S, SthlDelbanco A. 2004. Factors regulating the recruitment of cyanobacterial and eukaryotic phytoplankton from littoral and profundal sediments. Aquat. Microb. Ecol. 36, 213–226. ( 10.3354/ame036213) [DOI] [Google Scholar]
- 41.Reynolds CS, Huszar V, Kruk C, Naselli-Flores L, Melo S. 2002. Towards a functional classification of the freshwater phytoplankton. J. Plankton Res. 24, 417–428. ( 10.1093/plankt/24.5.417) [DOI] [Google Scholar]
- 42.Kruk C, Huszar VLM, Peeters ETHM, Bonilla S, Costa L, Lürling M, Reynolds CS, Scheffer M. 2010. A morphological classification capturing functional variation in phytoplankton. Freshw. Biol. 55, 614–627. ( 10.1111/j.1365-2427.2009.02298.x) [DOI] [Google Scholar]
- 43.Ojakian GK, Katz DF. 1973. A simple technique for the measurement of swimming speed of Chlamydomonas. Exp. Cell Res. 81, 487–491. ( 10.1016/0014-4827(73)90540-5) [DOI] [PubMed] [Google Scholar]
- 44.Byrne TE, Wells MR, Johnson CH. 1992. Circadian rhythms of chemotaxis to ammonium and of methylammonium uptake in Chlamydomonas. Plant Physiol. 98, 879–886. ( 10.1104/pp.98.3.879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones RI. 1988. Vertical distribution and diel migration of flagellated phytoplankton in a small humic lake. Hydrobiologia 161, 75–87. ( 10.1007/BF00044102) [DOI] [Google Scholar]
- 46.Thomas MK, Aranguren-Gassis M, Kremer CT, Gould MR, Anderson K, Klausmeier CA, Litchman E. 2017. Temperature–nutrient interactions exacerbate sensitivity to warming in phytoplankton. Glob. Change Biol. 23, 3269–3280. ( 10.1111/gcb.13641) [DOI] [PubMed] [Google Scholar]
- 47.Schroda M, Hemme D, Mühlhaus T. 2015. The Chlamydomonas heat stress response. Plant J. Cell Mol. Biol. 82, 466–480. ( 10.1111/tpj.12816) [DOI] [PubMed] [Google Scholar]
- 48.Tanaka Y, Nishiyama Y, Murata N. 2000. Acclimation of the photosynthetic machinery to high temperature in Chlamydomonas reinhardtii requires synthesis de novo of proteins encoded by the nuclear and chloroplast genomes. Plant Physiol. 124, 441–450. ( 10.1104/pp.124.1.441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anning T, Harris G, Geider R. 2001. Thermal acclimation in the marine diatom Chaetoceros calcitrans (Bacillariophyceae). Eur. J. Phycol. 36, 233–241. ( 10.1080/09670260110001735388) [DOI] [Google Scholar]
- 50.Fanesi A, Wagner H, Becker A, Wilhelm C. 2016. Temperature affects the partitioning of absorbed light energy in freshwater phytoplankton. Freshw. Biol. 61, 1365–1378. ( 10.1111/fwb.12777) [DOI] [Google Scholar]
- 51.Toseland A, et al. 2013. The impact of temperature on marine phytoplankton resource allocation and metabolism. Nat. Clim. Change 3, 979–984. ( 10.1038/nclimate1989) [DOI] [Google Scholar]
- 52.Yvon-Durocher G, Dossena M, Trimmer M, Woodward G, Allen AP. 2015. Temperature and the biogeography of algal stoichiometry: temperature dependence of algal stoichiometry. Glob. Ecol. Biogeogr. 24, 562–570. ( 10.1111/geb.12280) [DOI] [Google Scholar]
- 53.Botero CA, Weissing FJ, Wright J, Rubenstein DR. 2015. Evolutionary tipping points in the capacity to adapt to environmental change. Proc. Natl Acad. Sci. USA 112, 184–189. ( 10.1073/pnas.1408589111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saidi Y, Finka A, Muriset M, Bromberg Z, Weiss YG, Maathuis FJM, Goloubinoff P. 2009. The heat shock response in moss plants is regulated by specific calcium-permeable channels in the plasma membrane. Plant Cell 21, 2829–2843. ( 10.1105/tpc.108.065318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Potter K, Davidowitz G, Woods HA. 2009. Insect eggs protected from high temperatures by limited homeothermy of plant leaves. J. Exp. Biol. 212, 3448–3454. ( 10.1242/jeb.033365) [DOI] [PubMed] [Google Scholar]
- 56.Kingsolver JG, Diamond SE, Buckley LB. 2013. Heat stress and the fitness consequences of climate change for terrestrial ectotherms. Funct. Ecol. 27, 1415–1423. ( 10.1111/1365-2435.12145) [DOI] [Google Scholar]
- 57.Munday PL. 2014. Transgenerational acclimation of fishes to climate change and ocean acidification. F1000 Prime Rep. 6, 99 ( 10.12703/P6-99) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ness RW, Morgan AD, Colegrave N, Keightley PD. 2012. Estimate of the spontaneous mutation rate in Chlamydomonas reinhardtii. Genetics 192, 1447–1454. ( 10.1534/genetics.112.145078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Padfield D, Yvon-Durocher G, Buckling A, Jennings S, Yvon-Durocher G. 2016. Rapid evolution of metabolic traits explains thermal adaptation in phytoplankton. Ecol. Lett. 19, 133–142. ( 10.1111/ele.12545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carey CC, Weathers KC, Ewing HA, Greer ML, Cottingham KL. 2014. Spatial and temporal variability in recruitment of the cyanobacterium Gloeotrichia echinulata in an oligotrophic lake. Freshw. Sci. 33, 577–592. ( 10.1086/675734) [DOI] [Google Scholar]
- 61.Perakis SS, Welch EB, Jacoby JM. 1996. Sediment-to-water blue-green algal recruitment in response to alum and environmental factors. Hydrobiologia 318, 165–177. ( 10.1007/BF00016678) [DOI] [Google Scholar]
- 62.Ståhl-Delbanco A, Hansson L-A, Gyllström M. 2003. Recruitment of resting stages may induce blooms of microcystis at low N:P ratios. J. Plankton Res. 25, 1099–1106. ( 10.1093/plankt/25.9.1099) [DOI] [Google Scholar]
- 63.Woolway RI, et al. 2016. Diel surface temperature range scales with lake size. PLoS ONE 11, e0152466 ( 10.1371/journal.pone.0152466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cael BB, Seekell DA. 2016. The size-distribution of Earth's lakes. Sci. Rep. 6, 29633 ( 10.1038/srep29633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Read JS, Winslow LA, Hansen GJA, Van Den Hoek J, Hanson PC, Bruce LC, Markfort CD. 2014. Simulating 2368 temperate lakes reveals weak coherence in stratification phenology. Ecol. Model. 291, 142–150. ( 10.1016/j.ecolmodel.2014.07.029) [DOI] [Google Scholar]
- 66.Jiang L, Morin PJ. 2004. Temperature-dependent interactions explain unexpected responses to environmental warming in communities of competitors. J. Anim. Ecol. 73, 569–576. ( 10.1111/j.0021-8790.2004.00830.x) [DOI] [Google Scholar]
- 67.Wang G, Dillon ME. 2014. Recent geographic convergence in diurnal and annual temperature cycling flattens global thermal profiles. Nat. Clim Change 4, 988–992. ( 10.1038/nclimate2378) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets supporting this article are included with the electronic supplementary material.