Abstract
The western honey bee (Apis mellifera) is the most frequent floral visitor of crops worldwide, but quantitative knowledge of its role as a pollinator outside of managed habitats is largely lacking. Here we use a global dataset of 80 published plant–pollinator interaction networks as well as pollinator effectiveness measures from 34 plant species to assess the importance of A. mellifera in natural habitats. Apis mellifera is the most frequent floral visitor in natural habitats worldwide, averaging 13% of floral visits across all networks (range 0–85%), with 5% of plant species recorded as being exclusively visited by A. mellifera. For 33% of the networks and 49% of plant species, however, A. mellifera visitation was never observed, illustrating that many flowering plant taxa and assemblages remain dependent on non-A. mellifera visitors for pollination. Apis mellifera visitation was higher in warmer, less variable climates and on mainland rather than island sites, but did not differ between its native and introduced ranges. With respect to single-visit pollination effectiveness, A. mellifera did not differ from the average non-A. mellifera floral visitor, though it was generally less effective than the most effective non-A. mellifera visitor. Our results argue for a deeper understanding of how A. mellifera, and potential future changes in its range and abundance, shape the ecology, evolution, and conservation of plants, pollinators, and their interactions in natural habitats.
Keywords: Apis mellifera, floral visitation, meta-analysis, plant–pollinator network, pollination, pollination effectiveness
1. Introduction
The western honey bee (Apis mellifera L.) provides highly valued pollination services for a wide variety of agricultural crops [1], and ranks as the most frequent single species of pollinator for crops worldwide [2]. A long history of domestication and intentional transport of A. mellifera by humans has resulted in its current cosmopolitan distribution that includes all continents except Antarctica and many oceanic islands. Given the advanced state of knowledge concerning this species and its role in agriculture, it seems surprising that the importance of A. mellifera as a pollinator in natural habitats remains poorly understood [3–5].
Clarifying the role of A. mellifera as a pollinator in natural habitats is important for several reasons. First, animal-mediated pollination represents a vital ecosystem service [6,7]; an estimated 87.5% of flowering plant species are pollinated by animals [8]. Quantification of the pollination services provided by the cosmopolitan, super-generalist A. mellifera [9] will thus provide insight into the functioning of many terrestrial ecosystems. Second, non-A. mellifera pollinators are declining as a result of habitat loss, habitat degradation and other factors including pesticides, pathogens, parasites and climate change [10–12]. In cases where A. mellifera populations can withstand these perturbations, the degree to which they replace pollination services formerly performed by extirpated pollinators [13–17] deserves scrutiny. Third, recent increases in the mortality of managed A. mellifera colonies in some regions of the world [11,18] may extend to populations of free-living A. mellifera [19–21]. Threats to A. mellifera populations could thus affect the reproduction and population dynamics of plants in natural areas, with potential shifts in the composition of plant assemblages [22,23], and in turn, the ecosystem services (e.g. carbon sequestration, soil retention) that these plants provide. Lastly, where introduced populations of A. mellifera attain high densities [24–26], they may compete with other pollinators [27–29] or compromise plant reproductive success [30]. These phenomena are of broad ecological, evolutionary and conservation importance, but to our knowledge, there currently exists no global quantitative synthesis of the numerical importance of A. mellifera as a pollinator in natural ecosystems in their native or introduced ranges.
Here, we address questions concerning the importance of A. mellifera by exploiting a recent trend in pollination research—the documentation of community-level, plant–pollinator interaction networks (hereafter ‘pollination networks’). Quantitative pollination network studies document the identity and frequency of each type of pollinator visiting each plant species within a locality [31]. Network data are used to address a variety of questions (e.g. [32–34]), but key for our goals here, they provide an underused opportunity to gauge the importance of A. mellifera in natural habitats, particularly because the role of A. mellifera has rarely been the focus of these studies [25,26,35]. We compiled a database of 80 quantitative pollination networks from natural habitats worldwide. To further assess the importance of A. mellifera as a pollinator, we also compiled data on per-visit pollination effectiveness of A. mellifera relative to other floral visitors from studies of 34 plant species.
Our meta-analyses address three interrelated lines of inquiry concerning the ecological importance of A. mellifera in natural habitats: (i) what proportions of floral visits are contributed by A. mellifera foragers to individual networks worldwide, and to individual plant species within networks? (ii) what environmental factors govern the relative contribution of A. mellifera to community-level floral visitation, and do levels of visitation differ between its native and introduced ranges? and (iii) given that pollination network studies often use visitation frequency as a proxy for pollinator importance (e.g. [36]), how does the per-visit pollination effectiveness of A. mellifera compare to the effectiveness of other floral visitors?
2. Material and methods
(a). Database for network synthesis
We used two approaches to compile our dataset of pollination networks. First, we performed a literature search using the ISI Web of Science database with the search terms [pollinat* network], [pollinat* web] and [pollinat* visit* community], examining all studies available as of August 2016. Second, we downloaded all pollination network data from the Interaction Web Database of the National Center for Ecological Analysis and Synthesis website (http://data.nceas.ucsb.edu/) and the Web of Life Ecological Networks Database (http://www.web-of-life.es/) available as of December 2014. We collected all studies and plant–pollinator interaction network datasets that documented visitation frequency (i.e. number of individuals observed contacting flowers or number of floral contacts per unit time) between each pair of plant and pollinator taxa. We defined a network as the sum of recorded plant–pollinator interactions in all sites from a single study that fell within a 50 km diameter circle, regardless of the number of sites that constitute the network. Sites within the same study that are separated by more than 50 km were treated as separate networks. When we encountered networks from different studies that were less than 50 km apart, we excluded those that sampled a smaller number of plant or pollinator taxa, or documented fewer interactions. We chose 50 km as a threshold to avoid over-representing studies that include many networks within a locality (e.g. [32,37]), while keeping separate those networks originating from distinct localities within the same geographical region, such as networks documented on different islands from the same archipelago (e.g. [38]). When studies included multiple years of data collection at the same sites using the same protocols, we pooled data from all study years into a single network.
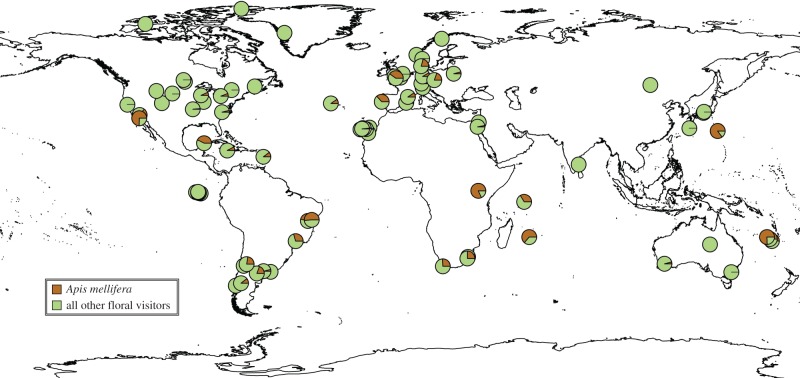
All networks retained for analyses met the following criteria. The data were collected in natural habitats, here defined as largely unmanaged assemblages of plant species where the identities and relative abundances of plant species are not purposefully manipulated (thus excluding, for example, agricultural, urban and experimental habitats; see the electronic supplementary material, table S1-1). Each network consisted of observations on five or more plant species when pooled across the sites making up an individual study. All networks documented a broad range of pollinators; studies with a narrow taxonomic scope (e.g. social bees, bird pollinators with incidental observations of A. mellifera) or those that a priori excluded A. mellifera were not included. We also excluded networks from sites that were known to be heavily influenced by A. mellifera colonies stocked for adjacent agricultural pollination. Thus, our estimates of the numerical importance of A. mellifera may be conservative with respect to mosaic landscapes where natural habitats are intermixed with agricultural fields with managed A. mellifera colonies [39]. We did not a priori exclude networks from localities outside of the presumed climatic niche of A. mellifera [40], or where A. mellifera was never introduced. In all, we obtained 80 networks (see the electronic supplementary material, table S1-1) from 60 peer-reviewed studies and three graduate theses [37,41,42]. While lacking coverage in some regions (figure 1), our dataset attains geographical coverage comparable to other recent studies that examine the importance and conservation of pollinators at a global scale [2,12,43].
Figure 1.
Proportion of all floral visits contributed by the western honey bee (Apis mellifera) in 80 plant–pollinator interaction networks in natural habitats worldwide. Apis mellifera is generally considered a native species in Europe, the Middle East, and Africa; and introduced elsewhere. (Online version in colour.)
For each network, we obtained the following data from their associated publications or from study authors when data were not available from publications: latitude, longitude and final year of data collection. When these data were not available and authors could not be reached, we used the approximate geographical centre of the study locality listed in the publication, and the year of publication as the last year of data collection. We defined the native status of A. mellifera based on [40] and [44]; although we caution that the native status of A. mellifera in the British Isles and northern Europe remains unresolved. We also extracted the following information from each study, when available: the proportion of all floral visits contributed by A. mellifera (in two networks this metric was estimated by calculating the proportion of the total visitation rate, summed across plant species, contributed by A. mellifera; see the electronic supplementary material, table S1-1), the proportion of plant species receiving at least one visit by A. mellifera, and the rank of A. mellifera with respect to both the proportion of all floral visits contributed and the proportion of plant species visited. Additionally, we used geographical information system (GIS) analysis to obtain elevation data and bioclimatic variables ([45], http://www.worldclim.org) for each network based on its global positioning system (GPS) coordinates. We also categorized each network as being on an island or a mainland; the latter category includes all continents as well as islands greater than 200 000 km2, namely Great Britain (United Kingdom), Honshu (Japan) and Greenland. For studies for which raw data were not available, we contacted the corresponding authors to request data, or, in cases where data could not be shared, requested summary statistics on plant–pollinator interactions. When raw numeric data were unavailable from the publication or from authors, we used ImageJ to extract data from figures, where possible (see the electronic supplementary material, table S1-1). Owing to the different methodologies and data reported by each study, not all of the above-mentioned variables were extracted from all networks.
(b). Frequency and patterns of Apis mellifera visitation
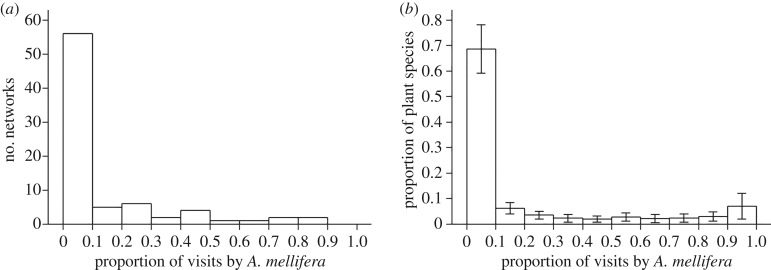
We calculated the global mean and median proportion of all floral visits contributed by A. mellifera, using each network as a data point (n = 80 networks). Calculations were repeated after excluding networks that documented no A. mellifera visits, in order to examine the role of A. mellifera specifically in localities where it occurs. Additionally, we examined plant species in 41 networks in which (i) A. mellifera was present, and (ii) data on the number of visits contributed by A. mellifera and non-A. mellifera visitors were available for each plant species. Across these networks, we calculated the mean and median proportion of plant species that were (i) not visited by A. mellifera, (ii) numerically dominated by A. mellifera (i.e. A. mellifera contributing ≥50% of all floral visits), and (iii) visited exclusively by A. mellifera. Because plant species receiving few visits overall may tend to have extreme values of proportion of visits by A. mellifera, we restricted the analysis to 834 plant taxa with ≥10 visits recorded. Additionally, to aid in visualizing the distribution of the numerical importance of A. mellifera across plant species, we also calculated for each network the proportion of plant species that fell into each of 10 bins with respect to the proportion of visits contributed by A. mellifera (range = 0–1; bin width = 0.1). We then constructed a histogram by calculating the mean and 95% confidence intervals of each bin across all 41 networks.
(c). Environmental correlates of Apis mellifera visitation frequency
We constructed multiple regression models to identify environmental factors that best explain variation in the visitation frequency of A. mellifera among networks. The response variable in these regression models was the proportion of all floral visits in each network contributed by A. mellifera. Owing to the strongly non-normal distribution of the data as well as the presence of numerous zeroes, we performed zero-inflated, multiple β regression using package gamlss [46] in R (v. 3.3.1 [47]). One network located above the Arctic Circle [48] was excluded from this analysis because bioclimatic data were unavailable (hence, n = 79). We note that the exclusion of networks with no A. mellifera visits did not qualitatively alter our results (see the electronic supplementary material, table S2-1).
To incorporate bioclimatic variables [45], we first performed principal components analysis (PCA) to avoid constructing models with highly collinear terms. We performed one PCA for the 11 variables measuring temperature, and a separate PCA for the eight bioclimatic variables measuring precipitation (see the electronic supplementary material, table S3). We then reduced bioclimatic variables to the first two principal components of the temperature and precipitation variables, which accounted for 86% and 89% of the variance, respectively. We constructed a full model containing the following explanatory variables, without interactions: latitude, longitude, altitude, land category (mainland versus island) and the first two principal components of temperature and precipitation variables. We used R package glmulti [49] to generate all possible permutations of the full model on which to perform zero-inflated, multiple β regression; and then selected the best-fit model using corrected Akaike's information criterion (AICc) scores. We also used the best-fit environmental model to address whether the proportion of visits contributed by A. mellifera, after accounting for environmental factors, was affected by (i) A. mellifera native status (native versus introduced), and (ii) year of data collection.
(d). Pollination effectiveness
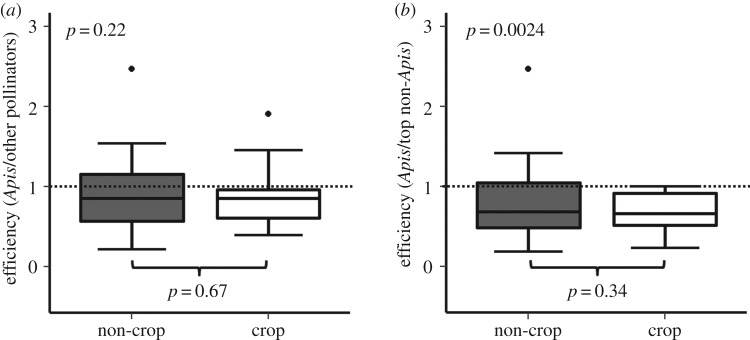
We used two approaches to compile data on pollination effectiveness. First, we performed a literature search using the ISI Web of Science database with the search term [pollinat*] in combination with one of the following terms: [efficiency], [effectiveness], [‘pollen deposition’], [‘seed set’], [‘fruit set’], or [‘pollination biology of’], examining all studies available as of August 2016. Second, we examined the literature cited sections of each of the studies found through the first approach for additional studies not captured in the initial literature search. Data points in this analysis consist of studies of focal plant species that compared A. mellifera and at least one other pollinator taxon with respect to pollen deposition, seed set, or fruit set resulting from single floral visits [50]. We used seed set data whenever available because it is most directly related to plant reproductive fitness [51], fruit set when seed counts were unavailable and pollen deposition when measures of seed and fruit set were unavailable. When raw data were unavailable, we used ImageJ to extract data from figures. In all, we obtained 32 studies reporting single-visit pollination effectiveness data for 34 plant species, spanning 22 plant families (see the electronic supplementary material, table S1-2). Of these, 18 plant species in 15 families were undomesticated, and 16 plant species in seven families were grown in agricultural settings. For each plant species considered, we divided the pollination effectiveness of A. mellifera by the mean effectiveness of all other visitors studied to obtain the relative effectiveness of A. mellifera. We also divided A. mellifera effectiveness by that of the most effective non-A. mellifera visitor. We then used one-sample t-tests to examine whether the pollination effectiveness of A. mellifera differed significantly from that of the average, or the most effective, non-A. mellifera floral visitor.
3. Results
(a). Frequency and patterns of Apis mellifera visitation
Apis mellifera was recorded in 88.89% (16 out of 18) of the pollination networks from its native range and in 61.29% (38 out of 62) of the networks from its introduced range (figure 1; see also the electronic supplementary material, table S1-1). Across all networks, the mean proportion of visits contributed by A. mellifera was 12.64% (figure 2a; median = 1.56%); among the 54 networks in which A. mellifera was recorded, this proportion increased to 18.72% (median = 8.13%). Apis mellifera was the most frequent floral visitor in 17 networks and visited the most plant species in 14 networks.
Figure 2.
The distribution of the proportion of floral visits contributed by the western honey bee (Apis mellifera) (a) across 80 plant–pollinator interaction networks in natural habitats worldwide, and (b) across plant species in 41 networks where A. mellifera was documented and where the numbers of visits to each plant species by A. mellifera and other floral visitors were available. Bars show the mean value of each bin across networks; whiskers show 95% confidence intervals.
Across 41 networks in which A. mellifera was present and the proportion of visits to each plant species by A. mellifera was recorded, we found a positively skewed distribution of the proportion of visits contributed by A. mellifera to individual plant species (figure 2b). Apis mellifera was the only documented visitor to 4.48% of plant taxa (median = 0%, range = 0%–66.67%) and contributed the majority (≥50%) of visits to 17.28% of plant taxa (median = 0%, range = 0%–100%). However, A. mellifera went unrecorded as a visitor to nearly half (49.38%) of plant taxa (median = 47.22%, range = 0%–100%). The overall patterns we report remain similar when we expand the analysis to include plant species where fewer than 10 visits were recorded (i.e. those species that might be expected to produce extreme values; see the electronic supplementary material, figure, S4-1).
(b). Environmental correlates of Apis mellifera visitation frequency
The best-fit zero-inflated, multiple beta regression model of environmental variables revealed that the proportion of visitation by A. mellifera in networks increases with the first principal component of temperature variables, with higher values corresponding to higher overall temperature, higher isothermality, lower annual temperature range and less seasonality (table 1; further statistics are reported in the electronic supplementary material, table S2-2). Apis mellifera visitation was also higher in mainland than island networks (table 1), but we found no effect of native status on the proportion of visits contributed by A. mellifera (table 1). Nevertheless, it is noteworthy that eight of the 10 networks with the highest A. mellifera visitation came from introduced range localities. In five of these networks [25,26,35,37,52], A. mellifera accounted for more than half of the total visits recorded. Lastly, we found that study year was unrelated to the proportion of A. mellifera visits in natural habitats worldwide (table 1).
Table 1.
The best-fit, zero-inflated, multiple beta regression models relating environmental variables to the proportion of visits contributed by the western honey bee (Apis mellifera) in plant–pollinator interaction networks worldwide (n = 79 networks where bioclimatic variables were available). (Temperature PC1 increases with overall temperature and isothermality, and decreases with temperature seasonality and annual range. Models examining the influence of A. mellifera native status and last year of study on proportion of visits by A. mellifera were constructed by adding these two variables to the best-fit model of environmental variables.)
| model (ΔAICc)/variable | estimate | t value | p value |
|---|---|---|---|
| best-fit environmental model (BFEM) (ΔAICc = 0) | Cox–Snell R2 = 0.19 | ||
| temperature PC1 | μ = 0.39 | 4.24 | <0.001 |
| land category (mainland = 1, island = 0) | μ = 0.81 | 2.27 | 0.026 |
| BFEM + Apis native status (ΔAICc = 1.39) | Cox–Snell R2 = 0.20 | ||
| temperature PC1 | μ = 0.41 | 4.31 | <0.001 |
| land category (mainland = 1, island = 0) | μ = 0.74 | 2.04 | 0.045 |
| Apis native status (native = 1, introduced = 0) | μ = 0.31 | 0.99 | 0.33 |
| BFEM + last study year (ΔAICc = 2.25) | Cox–Snell R2 = 0.19 | ||
| temperature PC1 | μ = 0.39 | 4.75 | <0.001 |
| land category (mainland = 1, island = 0) | μ = 0.81 | 2.26 | 0.026 |
| last study year (years CE) | μ = 0.0056 | 0.31 | 0.76 |
| BFEM + Apis native status + last study year (ΔAICc = 3.75) | Cox–Snell R2 = 0.20 | ||
| temperature PC1 | μ = 0.41 | 4.95 | <0.001 |
| Land category (mainland = 1, island = 0) | μ = 0.74 | 2.03 | 0.046 |
| Apis native status (native = 1, introduced = 0) | μ = 0.30 | 0.96 | 0.34 |
| last study year (years CE) | μ = 0.0041 | 0.23 | 0.82 |
(c). Pollination effectiveness
A literature survey of single-visit pollinator effectiveness data revealed that A. mellifera does not differ from the average non-A. mellifera floral visitor, with the effectiveness of A. mellifera averaging 90.1% that of other visitors (one-sample t-test, t33 = 1.25, p = 0.22; figure 3a). On the other hand, A. mellifera was generally less effective than the most effective non-A. mellifera visitor, with A. mellifera effectiveness averaging 75.6% that of the top non-A. mellifera visitor (one sample t-test, t33 = 3.28, p = 0.0024; figure 3b). The relative effectiveness of A. mellifera did not differ between non-agricultural (n = 18) and agricultural (n = 16) plant species, either when compared with the average non-A. mellifera visitor (figure 3a; Welch's two-sample t-test, t30.75 = 0.44, p = 0.67) or when compared with the top non-A. mellifera visitor (figure 3b; Welch's two-sample t-test, t24.46 = 0.96, p = 0.34).
Figure 3.
Average single-visit pollination effectiveness of the western honey bee (Apis mellifera) relative to (a) the mean effectiveness of all other floral visitor taxa, and (b) the effectiveness of the most effective non-A. mellifera taxon. p-values at the bottom-centre of each panel reflect two-sample t-test comparisons of A. mellifera relative effectiveness in non-crop (n = 18) versus crop (n = 16) plant species; p-values at the top-left reflect one-sample t-test comparisons of A. mellifera to the mean or most effective non-A. mellifera pollinator after combining data from non-crop and crop plant species. Boxes show central 50% of data and median; whiskers show quartiles ± 1.5× interquartile range, or most extreme values of data, whichever is closest to median. Points indicate extreme values.
4. Discussion
While A. mellifera is acknowledged to be a widely introduced [53,54], super-generalist [55,56] species that occupies a central role in many pollination networks [9,24,57], our study presents, to our knowledge, the first quantitative synthesis demonstrating the importance of A. mellifera as a floral visitor in natural habitats at a global scale. Despite considerable variance in its local abundance (figures 1 and 2a), A. mellifera appears to be the most important, single species of pollinator across the natural systems studied, owing to its wide distribution, generalist foraging behaviour and competence as a pollinator. The numerical dominance of A. mellifera is further underscored by our finding that, in a subset of 68 networks with sufficient taxonomic resolution, the average proportion of floral visits contributed by A. mellifera was more than double that contributed by all bumblebee species (Apidae: Bombus) combined (A. mellifera mean = 13.79%, Bombus mean = 6.26%, p = 0.055; see the electronic supplementary material, S5). Given that Bombus is the only other pollinator genus comparable to A. mellifera with respect to both local importance and global distribution [7,9,54], it seems unlikely that any other single pollinator species contends with A. mellifera with respect to worldwide numerical importance in natural habitats. That said, with appropriate data, it would be instructive to compare the worldwide importance of A. mellifera with that of other cosmopolitan and widely introduced pollinator taxa, such as the hover fly (Syrphidae) species Syrphus ribesii (L.) and Eristalis tenax (L.) [58], or with that of pollinator taxa that numerically dominate pollination networks in key biomes, such as stingless bees (Apidae: Meliponini) in tropical ecosystems [24,59].
We quantify for the first time, to our knowledge, that despite the global distribution and often high local abundance of A. mellifera, it is a frequent visitor to only a minority of insect-pollinated plant species (figure 2b). Even in networks where more than half of all visits are contributed by A. mellifera, approximately 16% of the plant species, on average, receive fewer than 10% of their visits from A. mellifera (see the electronic supplementary material, figure S4-2). Although individual A. mellifera colonies are known to forage extensively on only a fraction of the plant species available at any given time [60], the skewed pattern of floral visitation documented here (figure 2b) is nonetheless surprising given that A. mellifera has the greatest diet breadth of any pollinator species studied [55,56]. This result underscores the importance of maintaining robust, diverse assemblages of non-A. mellifera pollinators to provide pollination services for the majority of flowering plant species in natural habitats.
From a different perspective, A. mellifera often numerically dominated a portion of the plant species in a given network. While non-A. mellifera pollinators may find such plant taxa inherently unprofitable in some cases, they may be displaced by A. mellifera via interference or exploitative competition in other cases (e.g. [61]). In instances where A. mellifera numerically dominates plant species belonging to the ‘core’ of a pollination network (i.e. the subset of locally abundant plant species that are visited by a variety of pollinator taxa [31,62]), they may exert a strong influence on co-occurring pollinators [39]. While this phenomenon has been documented in the native range of A. mellifera [39], it may be especially consequential in its introduced range, where plant species numerically dominated by A. mellifera presumably coevolved with, and supply food for, native pollinators [63]. Our results thus suggest that A. mellifera may disrupt interactions between plants and other pollinators in many areas, including localities where A. mellifera attains only modest abundance (see the electronic supplementary material, S4-3).
Our analyses of how A. mellifera visitation correlates with environmental variables revealed significant associations with climatic and geographical predictors, but no effect of native status (table 1). Release from pathogens and parasites can contribute to the success of introduced species [64], but this mechanism may be less important for A. mellifera given that major pathogens and parasites have spread worldwide with the trafficking of managed colonies [17,18]. Nevertheless, the majority of networks with the highest proportion of A. mellifera visits come from introduced range localities. Researchers have long recognized the potential for introduced A. mellifera to impact co-occurring pollinators (e.g. [29,65]) and plants (e.g. [66]) at the local scale. Numerical dominance of introduced A. mellifera may also lead to homogenization [67] of pollinator faunas, and of pollination networks, across large spatial scales. Accordingly, further studies are needed to clarify why A. mellifera reaches high levels of abundance in some parts of its introduced range (e.g. [25,26]) and how its local abundance modifies its impacts on native plants and pollinators.
Despite recent increases in the mortality of managed A. mellifera colonies in Europe and North America [68,69], our analyses found that study year was unrelated to the proportion of A. mellifera visits in natural habitats worldwide (table 1). Agents responsible for increased mortality in managed colonies can affect wild or feral A. mellifera colonies [19–21], but ongoing research suggests that unmanaged A. mellifera populations may be better able to cope with parasites and pathogens compared to managed populations [70]. In our pollination networks, the degree to which A. mellifera foragers originated from managed versus unmanaged colonies probably varies. However, in one network numerically dominated by A. mellifera [37], genetic testing indicated that the majority of A. mellifera foragers were derived from feral, Africanized colonies [71].
Most network studies equate visitation frequency with the importance of a particular pollinator, but pollination biologists usually define pollinator importance as the per-visit effectiveness multiplied by visitation frequency [50]. Our survey of pollinator effectiveness estimates involving A. mellifera (figure 3) suggests that the average importance of A. mellifera as a pollinator is satisfactorily estimated by its visitation frequency. However, given that A. mellifera exhibits poor effectiveness at pollinating certain plant taxa [57,72], additional studies are needed to demonstrate the importance of A. mellifera as a pollinator of any particular plant species. Repeated visits by abundant pollinators, for example, can damage flowers and reduce reproductive success [73]. On plant species where A. mellifera attains high visitation rates, negative relationships between visitation frequency and plant reproductive fitness may occur [39] and are worthy of investigation [74].
As a numerically abundant, super-generalist pollinator, A. mellifera may influence the fitness [27] and behaviour [63] of competing pollinators, enhance [15] or reduce [30] plant reproduction, and facilitate the spread of non-native weeds [75] and pathogens [76]. Given the ecological importance of A. mellifera, changes in its distribution and abundance may impact the evolutionary trajectory of co-occurring animal-pollinated plants [77] and pollinators. Our study quantifies the current importance of A. mellifera in natural communities, and also highlights the vital importance of non-A. mellifera pollinators, whose key role in maintaining ecosystem function cannot be replaced by A. mellifera. Our study underscores the need for more data on how A. mellifera, and potential changes in its range and population size, shape the ecology, evolution and conservation of plants, pollinators and their interactions in natural habitats on local and global scales.
Supplementary Material
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the following individuals who provided raw data, summaries of data, and helpful discussions on the use of their data: T. Abe, R. Alarcón, J. Albrecht, I. Bartomeus, J. Bascompte, N. Blüthgen, L. Burkle, M. Campos-Navarrete, L. Carvalheiro, A. Gotlieb, M. Hagen, S. Hegland, C. Kaiser-Bunbury, M. Koski, X. Loy, H. Marrero, C. Morales, A. Nielsen, O. Norfolk, N. Rafferty, R. Ramos-Jiliberto, D. Robson, H. Taki, K. Trøjelsgaard, C. Tur, D. Vázquez, M. Vilà and Y. Yoshihara. We thank all authors who have made their raw or summarized data publicly available. J. Ollerton and R. Junker provided insightful comments in peer review.
Data accessibility
Project data are made available in the electronic supplementary material.
Authors' contributions
The study was conceived by K.-L.J.H., D.A.H. and J.R.K. Data were collected by K.-L.J.H., J.M.K., M.A and J.R.K. Data analysis was conducted by K.-L.J.H. All authors contributed to the writing of the manuscript and gave final approval for publication.
Competing interests
We have no competing interests.
Funding
Funding to K.-L.J.H. include NSF Doctoral Dissertation Improvement grant DEB-1501566; the Mildred E. Mathias Graduate Student Research grant and the Institute for the Study of Ecological and Evolutionary Climate Impacts Graduate Fellowship from the University of California Natural Reserve System; the Frontiers of Innovation Scholar Fellowship, an Academic Senate grant, and the McElroy Fellowship from the University of California, San Diego; the Sea and Sage Audubon Society Bloom-Hays Ecological Research grant; and the California Native Plants Society Educational grant.
References
- 1.Calderone NW. 2012. Insect pollinated crops, insect pollinators and US agriculture: trend analysis of aggregate data for the period 1992–2009. PLoS ONE 7, e37235 ( 10.1371/journal.pone.0037235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garibaldi LA, et al. 2013. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339, 1608–1611. ( 10.1126/science.1230200) [DOI] [PubMed] [Google Scholar]
- 3.Aebi A, Vaissière BE, vanEngelsdorp D, Delaplane KS, Roubik DW, Neumann P. 2012. Back to the future: Apis versus non-Apis pollination: a response to Ollerton et al. Trends Ecol. Evol. 27, 142–143. ( 10.1016/j.tree.2011.11.017) [DOI] [Google Scholar]
- 4.Ollerton J, et al. 2012. Overplaying the role of honey bees as pollinators: a comment on Aebi and Neumann (2011). Trends Ecol. Evol. 27, 141–142. ( 10.1016/j.tree.2011.12.001) [DOI] [PubMed] [Google Scholar]
- 5.Butz Huryn VM. 1997. Ecological impacts of introduced honey bees. Q. Rev. Biol. 72, 275–297. ( 10.1086/419860) [DOI] [Google Scholar]
- 6.Ashman T-L, et al. 2004. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85, 2408–2421. ( 10.1890/03-8024) [DOI] [Google Scholar]
- 7.Kearns CA, Inouye DW. 1997. Pollinators, flowering plants, and conservation biology. Bioscience 47, 297–307. ( 10.2307/1313191) [DOI] [Google Scholar]
- 8.Ollerton J, Winfree R, Tarrant S. 2011. How many flowering plants are pollinated by animals? Oikos 120, 321–326. ( 10.1111/j.1600-0706.2010.18644.x) [DOI] [Google Scholar]
- 9.Geslin B, et al. 2017. Massively introduced managed species and their consequences for plant–pollinator interactions. Adv. Ecol. Res. 57, 147–199. ( 10.1016/bs.aecr.2016.10.007) [DOI] [Google Scholar]
- 10.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. ( 10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 11.Goulson D, Nicholls E, Botias C, Rotheray EL. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 ( 10.1126/science.1255957) [DOI] [PubMed] [Google Scholar]
- 12.Winfree R, Aguilar R, Vázquez DP, LeBuhn G, Aizen MA. 2009. A meta-analysis of bees' responses to anthropogenic disturbance. Ecology 90, 2068–2076. ( 10.1890/08-1245.1) [DOI] [PubMed] [Google Scholar]
- 13.Xia J, Sun SG, Guo YH. 2007. Honeybees enhance reproduction without affecting the outcrossing rate in endemic Pedicularis densispica (Orobanchaceae). Plant Biol. (Stuttg) 9, 713–719. ( 10.1055/s-2007-965259) [DOI] [PubMed] [Google Scholar]
- 14.Junker RR, Bleil R, Daehler CC, Blüthgen N. 2010. Intra-floral resource partitioning between endemic and invasive flower visitors: consequences for pollinator effectiveness. Ecol. Entomol. 35, 760–767. ( 10.1111/j.1365-2311.2010.01237.x) [DOI] [Google Scholar]
- 15.Dick CW. 2001. Genetic rescue of remnant tropical trees by an alien pollinator. Proc. R. Soc. Lond. B 268, 2391–2396. ( 10.1098/rspb.2001.1781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna C, Foote D, Kremen C. 2013. Invasive species management restores a plant-pollinator mutualism in Hawaii. J. Appl. Ecol. 50, 147–155. ( 10.1111/1365-2664.12027) [DOI] [Google Scholar]
- 17.Hermansen TD, Britton DR, Ayre DJ, Minchinton TE. 2014. Identifying the real pollinators? Exotic honey bees are the dominant flower visitors and only effective pollinators of Avicennia marina in Australian temperate mangroves. Estuaries Coasts 37, 621–635. ( 10.1007/s12237-013-9711-3) [DOI] [Google Scholar]
- 18.Wilfert L, Long G, Leggett HC, Schmid-Hempel P, Butlin R, Martin SJM, Boots M. 2016. Deformed wing virus is a recent global epidemic in honey bees driven by Varroa mites. Science 351, 594–597. ( 10.1126/science.aac9976) [DOI] [PubMed] [Google Scholar]
- 19.Thompson CE, Biesmeijer JC, Allnutt TR, Pietravalle S, Budge GE. 2014. Parasite pressures on feral honey bees (Apis mellifera sp.). PLoS ONE 9, e105164 ( 10.1371/journal.pone.0105164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De la Rúa P, Jaffé R, Dall'Olio R, Muñoz I, Serrano J. 2009. Biodiversity, conservation and current threats to European honey bees. Apidologie 40, 263–284. ( 10.1051/apido/2009027) [DOI] [Google Scholar]
- 21.Kraus B, Page REJ. 1995. Effect of Varroa jacobsoni (Mesostigmata: Varroidae) on feral Apis mellifera (Hymenoptera: Apidae) in California. Environ. Entomol. 24, 1473–1480. ( 10.1093/ee/24.6.1473) [DOI] [Google Scholar]
- 22.Potts SG, et al. 2016. Safeguarding pollinators and their values to human well-being. Nature 540, 220–229. ( 10.1038/nature20588) [DOI] [PubMed] [Google Scholar]
- 23.Biesmeijer JC, et al. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354. ( 10.1126/science.1127863) [DOI] [PubMed] [Google Scholar]
- 24.Giannini TC, Garibaldi LA, Acosta AL, Silva JS, Maia KP, Saraiva AM, Guimarães PR, Kleinert AMP. 2015. Native and non-native supergeneralist bee species have different effects on plant-bee networks. PLoS ONE 10, e0137198 ( 10.1371/journal.pone.0137198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abe T, Wada K, Kato Y, Makino S, Okochi I. 2011. Alien pollinator promotes invasive mutualism in an insular pollination system. Biol. Invasions 13, 957–967. ( 10.1007/s10530-010-9882-9) [DOI] [Google Scholar]
- 26.Kato M, Kawakita A. 2004. Plant-pollinator interactions in New Caledonia influenced by introduced honey bees. Am. J. Bot. 91, 1814–1827. ( 10.3732/ajb.91.11.1814) [DOI] [PubMed] [Google Scholar]
- 27.Thomson DM. 2016. Local bumble bee decline linked to recovery of honey bees, drought effects on floral resources. Ecol. Lett. 19, 1247–1255. ( 10.1111/ele.12659) [DOI] [PubMed] [Google Scholar]
- 28.Thomson D. 2004. Competitive interactions between the invasive European honey bee and native bumble bees. Ecology 85, 458–470. ( 10.1890/02-0626) [DOI] [Google Scholar]
- 29.Roubik DW, Moreno JE, Vergara C, Wittmann D. 1986. Sporadic food competition with the African honey bee: projected impact on neotropical social bees. J. Trop. Ecol. 2, 97–111. ( 10.1017/S0266467400000699) [DOI] [Google Scholar]
- 30.Gross CL, Mackay D. 1998. Honeybees reduce fitness in the pioneer shrub Melastoma affine (Melastomataceae). Biol. Conserv. 86, 169–178. ( 10.1016/S0006-3207(98)00010-X) [DOI] [Google Scholar]
- 31.Memmott J. 1999. The structure of a plant-pollinator food web. Ecol. Lett. 2, 276–280. ( 10.1046/j.1461-0248.1999.00087.x) [DOI] [PubMed] [Google Scholar]
- 32.Albrecht M, Riesen M, Schmid B. 2010. Plant-pollinator network assembly along the chronosequence of a glacier foreland. Oikos 119, 1610–1624. ( 10.1111/j.1600-0706.2010.18376.x) [DOI] [Google Scholar]
- 33.Burkle LA, Knight TM. 2012. Shifts in pollinator composition and behavior cause slow interaction accumulation with area in plant–pollinator networks. Ecology 93, 2329–2335. ( 10.1890/12-0367.1) [DOI] [PubMed] [Google Scholar]
- 34.Rafferty NE, Ives AR. 2011. Effects of experimental shifts in flowering phenology on plant-pollinator interactions. Ecol. Lett. 14, 69–74. ( 10.1111/j.1461-0248.2010.01557.x) [DOI] [PubMed] [Google Scholar]
- 35.Olesen JM, Eskildsen LI, Venkatasamy S. 2002. Invasion of pollination networks on oceanic islands: importance of invader complexes and endemic super generalists. Divers. Distrib. 8, 181–192. ( 10.1046/j.1472-4642.2002.00148.x) [DOI] [Google Scholar]
- 36.Ballantyne G, Baldock KCR, Willmer PG. 2015. Constructing more informative plant–pollinator networks: visitation and pollen deposition networks in a heathland plant community. Proc. R. Soc. B 282, 20151130 ( 10.1098/rspb.2015.1130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hung K-LJ. 2017. Effects of habitat fragmentation and introduced species on the structure and function of plant-pollinator interactions. PhD dissertation, University of California, San Diego, La Jolla, CA, USA. [Google Scholar]
- 38.Trøjelsgaard K, Jordano P, Carstensen DW, Olesen JM. 2015. Geographical variation in mutualistic networks: similarity, turnover and partner fidelity. Proc. R. Soc. B 282, 20142925 ( 10.1098/rspb.2014.2925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magrach A, González-Varo JP, Boiffier M, Vilà M, Bartomeus I. 2017. Honeybee spillover reshuffles pollinator diets and affects plant reproductive success. Nat. Ecol. Evol. 1, 1299–1307. ( 10.1038/s41559-017-0249-9) [DOI] [PubMed] [Google Scholar]
- 40.Ruttner F. 1988. Biogeography and taxonomy of honey bees. Berlin, Germany: Springer. [Google Scholar]
- 41.Ingversen TT. 2006. Plant-pollinator interactions on Jamaica and Dominica: the centrality, asymmetry and modularity of networks. MSc thesis, University of Aarhus, Aarhus, Denmark. [Google Scholar]
- 42.Kevan PG. 1970. High arctic insect-flower visitor relations: the inter-relationships of arthropods and flowers at Lake Hazen, Ellesmere Island, Northwest Territories, Canada. PhD dissertation, University of Alberta, Edmonton, Alberta, Canada. [Google Scholar]
- 43.Rader R, et al. 2016. Non-bee insects are important contributors to global crop pollination. Proc. Natl Acad. Sci. USA 113, 146–151. ( 10.1073/pnas.1517092112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carreck NL. 2008. Are honey bees (Apis mellifera L.) native to the British Isles? J. Apicult. Res. 47, 318–322. ( 10.3896/IBRA.1.47.4.15) [DOI] [Google Scholar]
- 45.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 46.Rigby RA, Stasinopoulos DM. 2005. Generalized additive models for location, scale and shape. J. R. Stat. Soc. Ser. C Appl. Stat. 54, 507–554. ( 10.1111/j.1467-9876.2005.00510.x) [DOI] [Google Scholar]
- 47.R Development Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See: http://www.R-project.org. [Google Scholar]
- 48.Mosquin T, Martin JEH. 1967. Observations on the pollination biology of plants on Melville Island, N.W.T., Canada. Can. Field Nat. 81, 201–205. [Google Scholar]
- 49.Calcagno V, de Mazancourt C. 2010. glmulti: an R package for easy automated model selection with (generalized) linear models. J. Stat. Softw. 34, 1–29. ( 10.18637/jss.v034.i12) [DOI] [Google Scholar]
- 50.Ne'eman G, Jürgens A, Newstrom-Lloyd L, Potts SG, Dafni A. 2009. A framework for comparing pollinator performance: effectiveness and efficiency. Biol. Rev. Camb. Philos. Soc. 85, 435–451. ( 10.1111/j.1469-185X.2009.00108.x) [DOI] [PubMed] [Google Scholar]
- 51.Cane JH, Schiffhauer D. 2003. Dose-response relationships between pollination and fruiting refine pollinator comparisons for cranberry (Vaccinium macrocarpon [Ericaceae]). Am. J. Bot. 90, 1425–1432. ( 10.3732/ajb.90.10.1425) [DOI] [PubMed] [Google Scholar]
- 52.Aguiar CML. 2003. The use of floral resources by bees (Hymenoptera, Apoidea) in an area of Caatinga (Itatim, Bahia, Brazil). Revista Brasileira de Zoologia 20, 457–467. ( 10.1590/S0101-81752003000300015) [DOI] [Google Scholar]
- 53.Ollerton J, Liede S. 1997. Pollination systems in the Asclepiadaceae: a survey and preliminary analysis. Biol. J. Linn. Soc. Lond. 62, 593–610. ( 10.1111/j.1095-8312.1997.tb00324.x) [DOI] [Google Scholar]
- 54.Russo L. 2016. Positive and negative impacts of non-native bee species around the world. Insects 7, 69 ( 10.3390/insects7040069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olesen JM, et al. 2010. From Broadstone to Zackenberg: space, time and hierarchies in ecological networks. Adv. Ecol. Res. 42, 1–69. ( 10.1016/B978-0-12-381363-3.00001-0) [DOI] [Google Scholar]
- 56.Willmer P. 2011. Pollination and floral ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 57.Aslan CE, Liang CT, Galindo B, Kimberly H, Topete W. 2016. The role of honey bees as pollinators in natural areas. Nat. Area. J. 36, 478–488. ( 10.3375/043.036.0413) [DOI] [Google Scholar]
- 58.Speight MCD. 2011. Species accounts of European Syrphidae (Diptera), Glasgow 2011. Dublin, Ireland: Syrph the Net Publications. [Google Scholar]
- 59.Michener CD. 2007. The bees of the world, 2nd edn Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 60.de Vere N, Jones LE, Gilmore T, Moscrop J, Lowe A, Smith D, Hegarty MJ, Creer S, Ford CR. 2017. Using DNA metabarcoding to investigate honey bee foraging reveals limited flower use despite high floral availability. Sci. Rep. 7, 42838 ( 10.1038/srep42838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindström SAM, Herbertsson L, Rundlöf M, Bommarco R, Smith HG. 2016. Experimental evidence that honey bees depress wild insect densities in a flowering crop. Proc. R. Soc. B 283, 20161641 ( 10.1098/rspb.2016.1641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bascompte J, Jordano P, Melián CJ, Olesen JM. 2003. The nested assembly of plant-animal mutualistic networks. Proc. Natl Acad. Sci. USA 100, 9383–9387. ( 10.1073/pnas.1633576100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hansen DM, Olesen JM, Jones CG. 2002. Trees, birds and bees in Mauritius: exploitative competition between introduced honey bees and endemic nectarivorous birds? J. Biogeogr. 29, 721–734. ( 10.1046/j.1365-2699.2002.00720.x) [DOI] [Google Scholar]
- 64.Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM. 2003. Introduced species and their missing parasites. Nature 421, 628–630. ( 10.1038/nature01346) [DOI] [PubMed] [Google Scholar]
- 65.Paton DC. 1993. Honeybees in the Australian environment. BioScience 43, 95–103. ( 10.2307/1311970) [DOI] [Google Scholar]
- 66.Keys RN, Buchmann SL, Smith SE. 1995. Pollination effectiveness and pollination efficiency of insects foraging Prosopis velutina in south-eastern Arizona. J. Appl. Ecol. 32, 519–527. ( 10.2307/2404649) [DOI] [Google Scholar]
- 67.McKinney ML, La Sorte FA. 2007. Invasiveness and homogenization: synergism of wide dispersal and high local abundance. Glob. Ecol. Biogeogr. 16, 394–400. ( 10.1111/j.1466-8238.2007.000296.x) [DOI] [Google Scholar]
- 68.vanEngelsdorp D, Hayes J, Underwood RM, Pettis J. 2008. A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. PLoS ONE 3, e4071 ( 10.1371/journal.pone.0004071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moritz RFA, de Miranda J, Fries I, Le Conte Y, Neumann P, Paxton RJ. 2010. Research strategies to improve honey bee health in Europe. Apidologie 41, 227–242. ( 10.1051/apido/2010010) [DOI] [Google Scholar]
- 70.Loftus JC, Smith ML, Seeley TD. 2016. How honey bee colonies survive in the wild: testing the importance of small nests and frequent swarming. PLoS ONE 11, e0150362 ( 10.1371/journal.pone.0150362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kono Y, Kohn JR. 2015. Range and frequency of Africanized honey bees in California (USA). PLoS ONE 10, e0137407 ( 10.1371/journal.pone.0137407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Westerkamp C. 1991. Honeybees are poor pollinators—why? Plant. Syst. Evol. 177, 71–75. ( 10.1007/BF00937827) [DOI] [Google Scholar]
- 73.Sáez A, Morales CL, Ramos LY, Aizen MA. 2014. Extremely frequent bee visits increase pollen deposition but reduce drupelet set in raspberry. J. Appl. Ecol. 51, 1603–1612. ( 10.1111/1365-2664.12325) [DOI] [Google Scholar]
- 74.Aizen MA, Morales CL, Vázquez DP, Garibaldi LA, Sáez A, Harder LD. 2014. When mutualism goes bad: density-dependent impacts of introduced bees on plant reproduction. New Phytol. 204, 322–328. ( 10.1111/nph.12924) [DOI] [Google Scholar]
- 75.Barthell JF, Randall JM, Thorp RW, Wenner AM. 2001. Promotion of seed set in yellow star-thistle by honey bees: evidence of an invasive mutualism. Ecol. Appl. 11, 1870 ( 10.1890/1051-0761(2001)011%5B1870:POSSIY%5D2.0.CO;2) [DOI] [Google Scholar]
- 76.Graystock P, Blane EJ, McFrederick QS, Goulson D, Hughes WOH. 2016. Do managed bees drive parasite spread and emergence in wild bees? Int. J. Parasitol Parasites Wildl. 5, 64–75. ( 10.1016/j.ijppaw.2015.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mu J, Peng Y, Xi X, Wu X, Griffin JN, Niklas KJ, Sun S. 2014. Domesticated honey bees evolutionarily reduce flower nectar volume in a Tibetan lotus. Ecology 95, 3161–3172. ( 10.1890/13-2055.1) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Project data are made available in the electronic supplementary material.