Abstract
The relationship between environmental stress exposure and ageing is likely to vary with stressor severity, life-history stage and the time scale over which effects are measured. Such factors could influence whether stress exposure accelerates or slows the ageing process, but their interactions have not previously been experimentally investigated. We found that experimental exposure of zebra finches to mildly challenging environmental circumstances from young to old adulthood, which increased exposure to stress hormones, reduced breeding performance during early adulthood, but had positive effects when individuals were bred in old adulthood. This difference was not due to selective mortality, because the effects were evident within individuals, and no evidence of habituation in the response to the stressor was found. The more stressful environment had no effects on survival during young or old adulthood, but substantially improved survival during middle age. Changes in the effects at different ages could be due to the duration and nature of the challenging exposure, or to variation in coping capacity or strategy with age. These results show that living under challenging environmental circumstances can influence ageing trajectories in terms of both reproductive performance and longevity. Our results provide experimental support for the emerging idea that stress exposure needs to be optimized rather than minimized to obtain the best health outcomes.
Keywords: environmental stress, glucocorticoids, reproduction, survival, hormesis
1. Introduction
Ageing, broadly defined as the decline in performance with advancing age, has been well documented among different animal taxa both in the wild and under laboratory conditions [1,2]. The pattern of ageing, that is the timing of onset and the rate at which deterioration occurs, is highly variable both among and within species. One of the major foci of ageing research is the endeavour to understand the causes of such heterogeneity [3–5]. This involves identifying selection pressures driving the evolution of species-specific patterns of ageing [1], the underlying cellular mechanisms [6], and the genetic and environmental factors that generate variation among individuals of the same species [7]. Evolutionary explanations of ageing are largely based on cost–benefit trade-offs. Two main theories currently predominate—a genetic approach centred on the antagonistically pleiotropic effects of genes that confer beneficial effects early in life but deleterious effects later in life [8,9], and a resource allocation approach, embodied in the disposable soma theory, which is concerned with the fitness effects of differential investment in self-maintenance and reproduction [3,10,11]. These two approaches are complementary, make similar predictions, and have both been applied largely in the context of variation in lifespan and reproductive performance among different species [1,3,12,13].
Variation among individuals of the same species in the pattern of ageing can also be viewed using the same framework. Allocation of resources to self-maintenance will vary due to differing capacities, constraints, priorities and resource availability. It is well recognized that intra-specific variation in the pattern of ageing is strongly influenced by environmental conditions. Shifts in ‘priority rules’ underlying optimal allocation of limiting resources between self-maintenance and reproduction are expected to become more evident when animals are exposed to challenging environments, such as when facing unpredictable, adverse environmental circumstances influencing factors such as weather, food availability, disease, parasites and predation risk [14,15]. The resultant increase in energy expenditure and stress exposure might directly damage the soma and result in faster age-related deterioration [16–19]. Alternatively, harsher environmental conditions could influence the optimal balance of resource allocation between self-maintenance and reproduction with consequences for age-related reproductive effort and survival patterns [7,20,21]. Strategic rescheduling of investment may occur, with individuals delaying reproduction if conditions are likely to improve or bringing it forwards if life expectancy is likely to be reduced, with consequences for age-specific reproductive success and the pattern of senescence [22,23].
Effects of stressful environments on ageing patterns could also vary at different life stages, for example in early life and in adulthood, or early adulthood and old age, because vulnerability to damage, and the resulting fitness consequences, may differ. An additional layer of complexity is added by the fact that the ageing process itself can alter both vulnerability and resilience to stress exposure, and stress exposure can diminish or exacerbate ageing [24]. These interactions are influenced by the severity of the stress experienced, with severe stress generally accelerating ageing, while milder stress exposure can induce resilience and extend lifespan [25]. Furthermore, the consequences of exposure to even mild stressors are likely to change with age due, at least in part, to impaired functioning of the stress–response systems with age [24]. Much attention has been devoted to the long-lasting effects of stress exposure in early life, with much less attention being given to effects in adulthood, and less still to how these effects might change across the life course [24,26]. In the majority of studies conducted to date, manipulations of environmental conditions have been conducted over a relatively short period and at a single life stage. This is, in part, due to the time investment required, the logistics of following individuals over time, and to some extent also to the largely untested assumption that what holds at one life-history stage also holds at others.
Here, we report the results of an experiment in which female zebra finches (Taeniopygia guttata) were repeatedly exposed to a relatively mild environmental stressor, to which they did not habituate, from early in their adult lives. We have previously shown that this has no effect on survival in young adulthood, but increased survival during middle age in comparison with a control group not exposed to the environmental stressor [27]. The survival advantage could have occurred due to a rescheduling of resource allocation to reproduction and/or stress-induced resilience. To examine whether this response to the mildly stressful environment involved any differences in reproductive investment over controlled age-specific breeding events, we examined reproductive performance of these birds from young adulthood into old age. We also examined whether the previously observed survival advantage of stress-exposed birds in middle age persisted into old age, or whether there was evidence that resilience then declined. Finally, we examined whether there were any changes in baseline levels of the stress hormone corticosterone with age, and whether there was any evidence of habituation to the stressor when the birds were older.
2. Material and methods
(a). Study subjects
The study was performed in female zebra finches that were produced in two replicates from parents of the same stock population at the University of Glasgow (replicate 1 birds were produced in April–June 2011; replicate 2 birds were produced in August–September 2011). To minimize potential mate familiarity [28], the stock females were paired with different mates in the two breeding events, and the resulting offspring used to form the two experimental replicates. For each replicate, the environmental manipulations started when the study females were young, fully grown, sexually mature adults (five months old on average; mean ± s.e.: 152 ± 1 days) [27]. Females were housed in treatment-specific cages (n = 7–10 per 120 × 50 × 50 cm cage) and randomly allocated to one of two experimental groups: (i) challenging environment (replicate 1: n = 45 females; replicate 2: n = 62 females) or (ii) control environment (replicate 1: n = 46 females; replicate 2: n = 61 females). When possible, females that hatched in the same nest (part of the same brood) were counterbalanced between the two treatment groups and family of origin was taken into account in all analyses. All birds were maintained throughout the experiment at a photoperiod of 14 L : 10 D cycle and the temperature was maintained in the range of 20–24°C.
(b). Environmental conditions
At five months of age, females were randomly assigned to either a challenging or control environmental condition. In the challenging environmental condition, food was made unavailable for a continuous period of 4.9 h (approx. one-third of the daylight hours), 4 days per week, on a random time schedule. For the remaining two-thirds of the day and on the remaining 3 days per week, they were provided with ad libitum food. Thus, the manipulation changed the temporal availability of food, but, when available, food was abundant. Challenged females were always kept on this food regime except when they were breeding, and received ad libitum access to food continuously from the time they were paired with a male or shortly afterwards until after they completed breeding (approx. two months for each breeding event). Females in the control group were always provided with ad libitum food and experienced exactly the same breeding regime as the challenged birds (see paragraph below). During the third breeding event only, at 1.8 years old (see also paragraph below), the birds in the challenging environment were given a single daily exposure to the glucocorticoid stress hormone corticosterone to determine whether a more protracted environmental challenge during pre-breeding/pair formation influenced reproductive investment. Specifically, two weeks prior to this breeding event, challenged birds were given oral doses of corticosterone (Sigma-Aldrich, Poole, UK) following each period of episodic food withdrawal. The hormone was administered by providing the birds with seed soaked in corticosterone suspended in peanut oil at a concentration of 0.0825 mg ml−1 (corticosterone dose was approx. 4.075 µg; 1 g of seed soaked per bird) for 10 min immediately after the end of each episodic food withdrawal. Corticosterone dosing was based on previous work in zebra finches [29]. Control birds received 1 g of seed soaked in peanut oil only for the same amount of time as the challenged females. The unpredictable food regime and corticosterone seed manipulation were continued until individual clutches were completed (mean ± s.e.: 25.8 ± 0.3 days; range: 20–33 days). A small number of females did not attempt to breed, and in these birds the oral corticosterone treatment was suspended 14 days after pairing (total duration 28 days). Following this breeding event, all experimental females were placed back on the unpredictable food regime only (i.e. no exposure to corticosterone-soaked seeds) until the next breeding event at 3.5 years of age (approx. 1.5 years later). There were no effects of the duration of corticosterone supplementation on measures of reproductive performance (clutch size or number of chicks reared) at both 1.8 years of age and 3.5 years of age (Pearson's r: −0.02 < r < 0.2, p ≥ 0.2 for all), suggesting that this short-term additional corticosterone treatment did not influence breeding investment.
We have previously shown that there is no overall significant effect of the experimental treatment on body mass up to 3 years of age [27]. Consistent with other studies, we have also found that the experimental food manipulation resulted in increases in overall exposure to glucocorticoids [30,31]. More specifically, at the end of the episodes of food withdrawal, the challenged birds showed higher baseline corticosterone (the predominant avian glucocorticoid hormone) levels than those birds living in the control environment, and this physiological response was consistent over prolonged exposure periods (up to six weeks), indicating no habituation of the birds to the unpredictable food shortages (on average 1.4-fold increase; full details in [27]). These data were collected during young adulthood (less than 1 year of age). We also measured corticosterone in a randomly chosen subset of study females (34 control and 32 challenging environment) when they were 3.5 years old. Average baseline corticosterone levels decreased by approximately 50% in both treatment groups in old adulthood compared with young adulthood, but despite this the birds in the challenging environment continued to show a similar magnitude of increase in baseline corticosterone at the end of the episodic food withdrawals also into old adulthood (on average 1.8-fold increase; electronic supplementary material, table S0; full details in electronic supplementary material). Thus, our environmental protocol mimicked the physiological effects of an environmental stressor naturally experienced by animals living under protracted exposure to unpredictable environmental conditions [32].
(c). Breeding schedule and breeding performance
Study females from both treatment groups were allowed to produce clutches of eggs four times during the study. For breeding, females were paired with a randomly assigned, relatively young male ranging in age from six months to 1.8 years; experimental females were paired with the same male partner in their first and second breeding event, whereas in the following two breeding events they were always paired with a different male. Control and challenged females were paired with males for the first time when they were on average six months old (188 ± 0.89 days of age; all females survived to this first breeding event), approximately 1 month after the start of the environmental manipulation. Each pair was housed in their own cage (60 × 50 × 50 cm) and provided with a nest-box and nest material (coconut fibre and jute; Haiths Ltd). The females were paired again at the following ages: 1.1 years (408 ± 0.82 days of age), 1.8 years (653 ± 0.78 days of age) and finally when they were 3.5 years old (1270 ± 0.92 days of age; mean ± s.e. for all). For the breeding event at 1.1 years, the pairs were not allowed to rear any chicks because the eggs were required for assays of egg composition, and were collected shortly after laying and replaced with dummy eggs. Dummy eggs were removed once individual clutches were complete and the pairs then separated. During the breeding at 1.8 years, most of the clutches (157 out of 187; logistic reasons) were cross-fostered at the end of the incubation period in order to examine egg effects on chick survival as part of a separate study to disentangle maternal from rearing environmental treatment effects; a small subset of cross-fostered clutches [43] was also subjected to brood size manipulation experiments (data to be reported in full elsewhere).
When lifetime breeding performance and survival are examined below, we only considered those birds whose clutch size was not manipulated (43 out of 214 birds excluded from these analyses; total sample size 171 birds). We quantified breeding performance of the study females by recording the following: (i) likelihood of breeding (laying a clutch), (ii) latency to lay (i.e. time from pairing to the laying of the first egg), (iii) clutch size, (iv) fledgling success (proportional data: number of chicks fledged/clutch size) and (v) the number of chicks fledged (assessed when the offspring were approx. 30 days old, including also those females that did not lay a clutch in order to assess the overall breeding performance).
(d). Survival
We monitored the survival of the birds for approximately 4 years (i.e. till 1456 days of age). Experimental birds were inspected daily, and all the birds considered here died of intrinsic causes, not of accidental injury or aggression. Where birds showed clear signs that death was imminent and their welfare was very severely compromised (the birds were not able to fly and/or feed independently, and our veterinarian confirmed that death was imminent), they were culled under the advice of our veterinarian in line with UK Home Office legislation (n = 24 out of 85 females that died—total sample size, n = 171). Generally, deaths were unpredictable, with the majority of the birds being found dead on the cage floor without having shown prior symptoms.
(e). Data analysis
Analyses were performed in R (v. 3.2.5; R Core Team, 2014). Unless otherwise specified, all final models included the effects of experimental design factors expected to influence the response variables either as parameters of interest integral to the question being investigated or for the purpose of adjustment. These relevant factors were always retained in the main models rather than tested using backwards or forwards selection to avoid overfitting. We used generalized linear mixed models (GLMMs; R packages ‘lme4’ and ‘lmerTest’ [34,35]) to examine whether the challenging treatment influenced reproductive performance, and whether any potential effect of the treatment varied across the age-specific breeding events at six months, 1.1 years, 1.8 years and 3.5 years old, as appropriate. Unless otherwise specified, final models included the following factors: treatment, age, replicate and the interaction treatment × age. In initial models, we tested the potential interaction effect of the treatment with replicate to check consistencies of treatment effects between the two replicates. Age was modelled as a categorical rather than continuous variable due to the relatively reduced number of data points per individual bird (up to two, or four as appropriate); female individual identity was always added as a random factor to control for correlations between reproductive performance traits within individuals due to the presence of repeated measurements in the data. As appropriate, we also entered family of origin and male partner identity as additional random factors to control for potential pseudo-replication due to the presence of sisters in the experiment and because some males were used more than once across the breeding events. In preliminary analyses, we also tested if previous reproductive investment decision level (investment in egg laying up until the event under consideration) influenced current reproductive investment (clutch size) at 1.1, 1.8 or 3.5 years in an interaction with the treatment. Chick mortality is low in this captive situation, and clutch size correlates well with the number of chicks reared in our population (Pearson's r = 0.6, p < 0.0001); thus, investment in egg laying is a good proxy for overall reproductive investment level at each breeding event. Across all breeding events, we found no interaction effect of the treatment with previous reproductive decisions on clutch size (p ≥ 0.5), excluding the possibility of conditionality between previous and current reproductive decisions in relation to lifetime environmental conditions. We first examined if there were any treatment differences in whether or not the females attempted to breed (i.e. laid eggs) using a GLMM with a binomial error distribution and the logit link function. The interactions treatment × age and treatment × replicate could not be assessed in the latter model due to reduced statistical power because relatively few birds did not attempt to breed during the first three breeding events. For those females that bred (i.e. laid a clutch), we then analysed the latency to lay the first egg using GLMMs with a Gaussian distribution error—data were log10-transformed to improve normality of model residuals. Clutch size (mean: 4.3, range: 1–8 eggs) was analysed using a GLMM with a Gaussian distribution error rather than with a Poisson distribution because the data were strongly under-dispersed (dispersion parameter less than 0.39) and model residuals were normally distributed. Fledging success was analysed with a GLMM using a binomial error distribution and logit link function [36], and the number of chicks fledged (range 0–6 chicks) was analysed using a GLMM with a Poisson distribution (dispersion parameters: 0.8–1.3). In the fledging success and number of chicks fledged statistics, we did not include the data at 1.8 years of age as these response variables could have been influenced by the cross-fostering experiment conducted as part of a separate study to disentangle maternal from rearing environmental treatment effects (data to be fully reported elsewhere). To assess within-female treatment effects and to exclude potential survival bias in the results caused by loss of specific phenotypes from the population (e.g. poor-quality breeders dying in early adulthood), we also performed the analyses using only those females that survived to the breeding event up to 3.5 years of age (103 out of 171 birds). We used the R package ‘lsmeans’ [37] to perform pairwise post hoc contrasts for significant outcomes in the main models (Tukey's p-value adjustment).
We have previously shown in the birds from the same study population used here that the challenging environmental conditions improved life expectancy up to 3 years of age (mixed-effects Cox models, p = 0.02; full details in [27]). We have also shown that there was no link between body mass at 1 year of age and subsequent survival up to 3 years of age [27]. Importantly, the positive effect of the challenging treatment on survival was evident prior to the start of the additional short-term corticosterone manipulation at approximately 1.8 years of age (data right-censored at 600 days of age: mixed-effects Cox models, p = 0.04) excluding the possibility that the short-term change in the severity of the stress treatment at 1.8 years of age per se was the main factor triggering the change in the survival trajectories of our study birds. Here, we further examined survival in old age (between 3 and 4 years) and tested the extent to which survival probability was dependent on individuals' lifetime reproductive effort. We excluded the females that were subjected to the brood size manipulation experiments at 1.8 years of age (43 birds) from all breeding performance and survival analyses performed here to exclude any possibility that those manipulations altered subsequent survival independently of the environmental conditions. However, the results do not differ qualitatively when these birds are included (data not shown). Data were right-censored to allow inclusions of birds still alive at the end of the survival monitoring period (49.7% out of 171 birds). We first checked if our annual measurements of body mass (i.e. 1, 2 and 3 years) predicted survival up to 4 years of life using time-dependent covariate Cox model analyses (R package ‘survival’ [38]) and found no effect of this covariate on survival (body mass, body mass × treatment, body mass × replicate, p ≥ 0.2). Therefore, body mass was dropped from the following analyses. In the following Cox model analyses (R packages: ‘survival’ and ‘coxme’ [38,39]), we entered treatment, replicate and their interaction as fixed factors, and family identity as a random factor as appropriate. Model diagnostics using Schoenfeld's residuals plotting suggested that the proportional hazards hypothesis was not met due to a nonlinear effect of the treatment with time emerging after 3 years of age, whereas it was met in our previous analysis up to 3 years. As mortality rates were clearly very low from five months to 1 year (four control and two challenged birds dead out of 171 females), and because of the change in the effect of the treatment over time after 3 years of age, we consequentially introduced in the analyses treatment time-dependent coefficients by breaking the data into three time intervals: (i) young adulthood, from the start of the experiment (five months old) up to 1 year of age; (ii) middle adulthood, from 1 to 3 years of age; and (iii) old adulthood, from 3 to 4 years of age. In the model, we also checked the potential interaction effect of treatment with replicate. The proportional hazard assumption was met in these models. To test if survival was influenced by the individual's lifetime reproductive effort, we performed separate GLMs (binomial family distribution error with logit link function) entering replicate, along with lifetime egg-laying effort (calculated as lifetime number of eggs laid divided by total number of breeding events, ranging from 1 to 4 events depending on the individual's lifespan) or chick-rearing effort (calculated as lifetime number of chicks reared by each female divided by total number of breeding events in which chicks were reared, including the event at 1.8 years of age, ranging from 1 to 3 events depending on the individual's lifespan), as continuous covariate—this standardization allowed us to overcome collinearity between longevity and lifetime number of eggs laid/chicks reared (Pearson's r = 0.1, 0.07 < p < 0.2) as females that survived longer ended up with a larger number of eggs and chicks reared over the lifespan (Pearson's r = 0.5–0.7, p < 0.0001 for both covariates). We performed the latter GLMs separately by treatment in order to simplify model interpretation and avoid issues of collinearity between the treatment and the lifetime reproductive effort. Unless otherwise specified, values are presented as mean ± s.e.
3. Results
(a). Breeding failure
Irrespective of environmental conditions, the probability of breeding failure was influenced by female age. More females failed to produce a clutch in the later breeding events at 1.8 and 3.5 years of age than the earlier events (1.8 years versus six months and 1.8 years versus 1.1 years, p ≤ 0.048; 3.5 years versus six months and 3.5 years versus 1.1 years, p ≤ 0.0007; full results in electronic supplementary material, table S1a; descriptive statistics in electronic supplementary material, table S2). There was no difference in the probability of breeding failure between the breeding events at six months and 1.1 years old (p = 1.0), or between 1.8 years and 3.5 years of age (p = 0.2; electronic supplementary material, tables S1a and S2). We found no significant effect of the treatment or replicate on the likelihood of breeding failure (electronic supplementary material, table S1a). Similar results were obtained when we carried out this analysis using only those females that survived to breed in old age at 3.5 years old (electronic supplementary material, tables S3a and S4).
(b). Latency to lay the first egg within the clutch
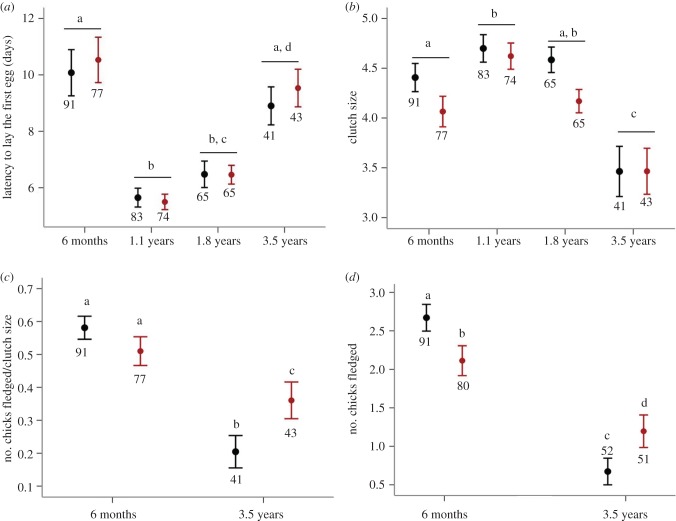
Irrespective of their environmental conditions, females at 1.1 and 1.8 years laid their first egg sooner following pairing than they did at six months of age (1.1 years versus six months, and 1.8 years versus six months, p < 0.0001 for both; full results in electronic supplementary material, table S1b; figure 1a); there were no differences in latency between 1.8 and 1.1 years (p = 0.4; figure 1a). Latency to lay increased again when the birds were old at 3.5 years to a level similar to that at six months of age (p = 0.8; electronic supplementary material, table S1b; figure 1a). Replicate 2 birds laid their first clutches slightly sooner compared with replicate 1 birds (replicate 1: 8.5 ± 0.4 days; replicate 2: 7.2 ± 0.3 days), and there were no treatment effects on latency to lay either as a main factor or in its interaction with age (electronic supplementary material, table S1b; figure 1a). Again, similar estimate parameters were obtained when carrying out the analysis only on those females that opted to breed and survived to the breeding event at 3.5 years of age (electronic supplementary material, table S3b and figure S1a).
Figure 1.
(a) Latency to lay the first egg, (b) clutch size, (c) fledging success (number of chicks fledged per clutch; proportional data) and (d) number of chicks fledged by the females exposed to the challenging environmental conditions (in red) and control environmental conditions (in black) across the age-specific breeding events. Note that eggs were allowed to hatch only during the breeding event at six months, 1.8 years and 3.5 years of age; at 1.8 years, cross-fostering was used and these data were omitted from these analyses (full details in ‘Data analysis’). Different letters indicate significant post hoc pairwise contrasts (p < 0.05 after Tukey's multiple comparison adjustment—full statistics in electronic supplementary material, table S1); numbers indicate sample sizes separately by treatment and age. (Online version in colour.)
(c). Clutch size
Irrespective of environmental conditions, clutch size (range 1–8 eggs) was influenced by female age: it increased at 1.1 years relative to six months of age (p = 0.007), did not differ between 1.1 and 1.8 years of age (p = 0.15), and then decreased in old adulthood relative to the earlier life breeding events (p ≤ 0.0001 for all contrasts; full results in electronic supplementary material, table S1c; figure 1b). We found no effect of treatment on clutch size (electronic supplementary material, table S1c; figure 1b). Replicate 2 females produced overall slightly larger clutches than replicate 1 females (replicate 1: 4.0 ± 0.1 eggs; replicate 2: 4.5 ± 0.1 eggs; electronic supplementary material, table S1c). Similar parameter estimates were obtained when carrying out the analyses only using those females that opted to breed and survived up to the breeding event at 3.5 years of age (electronic supplementary material, table S3c and figure S1b).
(d). Fledging success
We examined fledging success at the first and last breeding event (no chicks were reared at the breeding event at 1.1 and 1.8 years of age, and a separate egg cross-fostering experiment was performed, so these data have not been included). Fledging success was reduced when the birds were 3.5 years relative to six months (p < 0.0001, full results in electronic supplementary material, table S1d; figure 1c). There was no effect of replicate either as a main factor or in its interaction with the treatment (electronic supplementary material, table S1d). The effect of the treatment on fledging success was age-dependent (electronic supplementary material, table S1d; figure 1c). At six months of age, there was no detectable reduction in fledging success in the challenged females relative to controls (p = 0.2; figure 1c), while at 3.5 years, challenged females had higher fledging success than the age-matched controls (p = 0.01; figure 1c). The same results were observed when the analysis was carried out using only those females that opted to breed and survived to the breeding event at 3.5 years of age (electronic supplementary material, table S3d and figure S1c).
(e). Number of chicks fledged
As with the fledging success, we examined overall breeding performance at the first and last breeding event. As expected from the clutch size results, the number of chicks fledged (0–6) was much reduced in old adulthood compared with six months of age in both control and challenged females (p < 0.0001, full results in electronic supplementary material, table S1e; figure 1d). Replicate 2 birds reared more fledglings than replicate 1 birds (replicate 1: 1.6 ± 0.1 chicks; replicate 2: 2.1 ± 0.1 chicks, electronic supplementary material, table S1e); however, this effect was consistent between control and challenged females (electronic supplementary material, table S1e). The effect of the treatment on the number of chicks fledged was influenced by female age. Challenged females fledged fewer chicks (on average 20%) compared with controls at six months of age (p = 0.04; figure 1c), whereas at 3.5 years challenged females reared more offspring compared with age-matched controls (p = 0.008; figure 1d). Similar parameter estimates were obtained when performing analyses only using those females that survived to 3.5 years of age (p = 0.1; electronic supplementary material, table S3e and figure S1d).
(f). Survival
Mortality was very low between five months and 1 year of age, and there were no differences in survival between the two treatment groups (p = 0.5; full results in electronic supplementary material, table S5; figure 2a). Survival curves started diverging after 1 year of age (figure 2b), and from 1 to 3 years old the challenged females had, on average, a 48% reduction in relative risk of death compared with controls (p = 0.03, electronic supplementary material, table S5), as previously shown [27]. However, when we examined survival during old age, between ages 3 and 4, this effect disappeared; survival of challenged birds was no longer better than controls (p = 0.8, electronic supplementary material, table S5; figure 2c). There was no effect of replicate as a main factor or in its interaction with the treatment (electronic supplementary material, table S5). When examining survival up to 4 years of age in relation to lifetime breeding effort, we found no relationship between either laying effort or chick-rearing effort within both treatment groups (electronic supplementary material, table S6).
Figure 2.
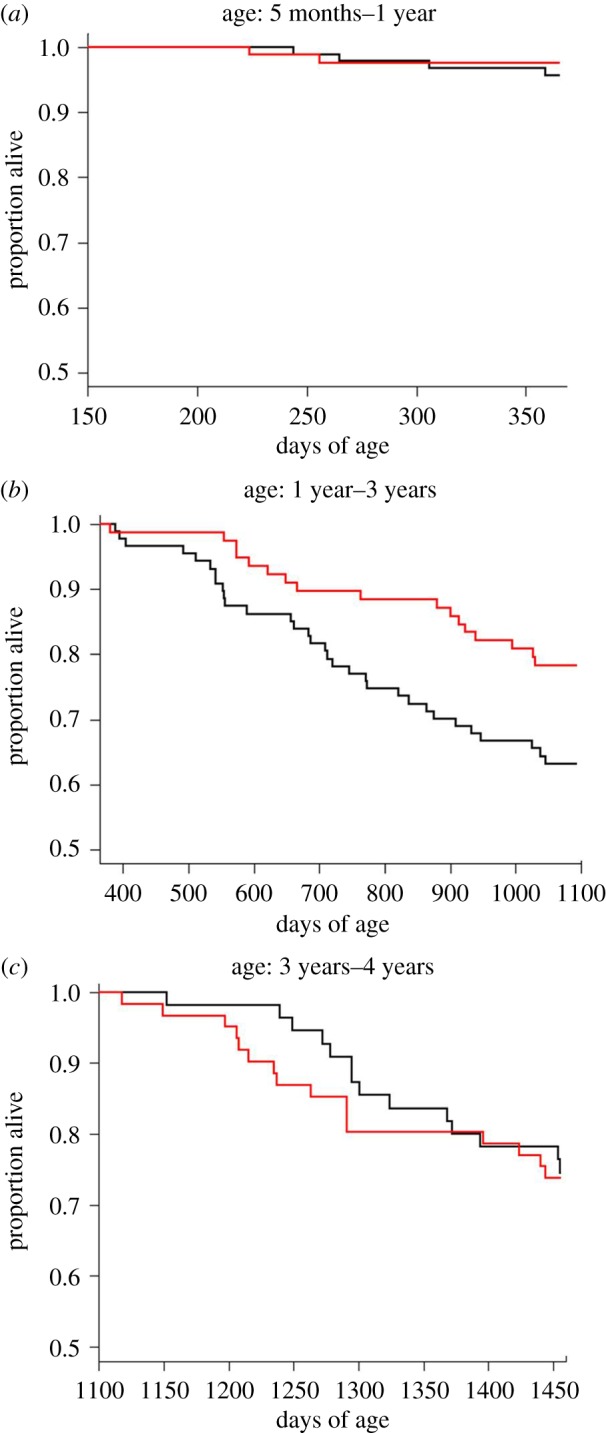
(a) Survival trajectories from the start of the experiment up to 1 year of age (i.e. 150–365 days), (b) from 1 to 3 years of age (i.e. 365–1096 days), and (c) from 3 to 4 years of age (i.e. 1096–1456 days) of zebra finch females exposed to challenging (in red) or control (in black) environmental conditions. Birds exposed to the challenging environment showed improved survival from 1 to 3 years of age (p = 0.03), whereas no treatment effects were found either from five months to 1 year of age, or from 3 to 4 years of age (full statistics in electronic supplementary material, table S5). (Online version in colour.)
4. Discussion
This is the first experimental longitudinal study in a vertebrate species to directly compare the effects of living in a challenging environment at different adult life stages, from early to old adulthood. Our key findings are as follows. (i) Regardless of environmental conditions, female reproductive performance changed across adult life (six months, 1.1 years, 1.8 years and 3.5 years) with peak performance generally occurring during middle adulthood (1.1 and 1.8 years) followed by a marked decline in old adulthood (3.5 years). Importantly, this later life decline occurred within individuals consistent with previous literature on ageing across diverse vertebrate taxa [2]. (ii) Females exposed to the challenging environmental circumstances produced relatively fewer chicks than those living in the control environmental conditions when they were young (six months of age), but, in contrast, were able to rear more chicks when they were old (3.5 years of age). Again, this effect occurred within individuals. (iii) Females living in the more challenging conditions showed no difference relative to controls in more benign conditions in the probability of survival when they were young adults (five months to 1 year of age) had a higher probability of survival in middle age (1–3 years of age), with this benefit then disappearing at older ages (from 3 to 4 years).
Our stressful environmental protocol did not influence either the likelihood of breeding or the latency to lay the first egg when the females were given the opportunity to breed across the four breeding events, from early to old adulthood. During young adulthood, the challenged females showed an overall reduction in the number of fledglings produced compared with the controls. This effect on overall breeding performance was due to additive treatment-dependent reduction in performance observed at the clutch (primarily) and fledging success level. Interestingly, in old adulthood (3.5 years of age), challenged females, despite laying similar clutch sizes to the controls, fledged proportionally more of their chicks than females living in the more predictable environment, possibly due to treatment differences in parental behaviour and/or in egg quality. Altogether, our results thus show that the mild stress exposure induced by the challenging environmental conditions resulted in females showing a relatively reduced breeding performance when they were young, but increased performance in old age. This effect occurred within individuals and thus was not due to any differential survival effects. It could be due to challenged females having either an impaired breeding capacity in young adulthood as a result of their exposure to increased levels of glucocorticoid hormones, or to their showing a strategic restraint in breeding effort during early adulthood. We have shown that our challenging environmental protocol did increase overall exposure to stress hormones without causing habituation (measured to old adulthood, 3.5 years). A reduction in reproductive performance in response to stress exposure has been reported in other studies that examined responses to stressful environments, including food shortages or increased predation pressure [32,40–42]. However, the fact that the birds in our study then showed increased breeding performance in old adulthood, despite still being exposed to higher levels of stress hormones, suggests that their breeding capacity was not impaired and supports a strategic restraint interpretation. It has been suggested that stress exposure induces shifts in energy allocation in order to promote self-maintenance strategies at the expense of reproductive behaviours and parenting [43]. It has also been suggested that environmental stressors could trigger protective and compensatory effects on reproductive physiology (see [15] for a review on the potential mechanisms). Therefore, increases in stress exposure levels experienced by the challenged birds might have activated adaptive changes that allowed individuals to better cope with the protracted exposure to the somewhat harsher environmental conditions, at the expense of earlier reproductive investment perhaps in favour of long-term maintenance processes, including survival [33]. We found no relationships between lifetime breeding effort (egg laying/chick rearing) and survival within both treatment groups. The slight treatment-dependent reduction in clutch size during the early–middle adulthood breeding events within the pool of birds that survived up to the final breeding event in old adulthood provides only very limited support to this possibility. It would be interesting in future studies to see whether similar treatment effects would be observed in animals free to reproduce. Such a design was not possible in our experiment because we were interested in determining the varying effects of the treatment with maternal age on breeding performance, while controlling for the age of the male partner. Our experimental design does not allow us to separate the effects of age and duration of the challenging exposure, because the two are interlinked, as would be the case in nature. Our comparison between exposure to repeated stress or not simulates responses of animals living in environments in which the occurrence of key stressors such as low food availability, high population density or high predation risk differs, as has been recorded in the wild in diverse species, such as black-legged kittiwakes Rissa tridactyla [44], Belding's ground squirrels Spermophilus beldingi [45] and snowshoe hares Lepus americanus (see [46] for further discussion of this). The facts that breeding performance increased at old age in the birds living in the more stressful environment and that the stress response of the birds to the random food withdrawals was not diminished with age suggest that the observed effects on reproductive performance are not due to any accumulated negative effects of stress exposure. Our data on survival show that exposure to the challenging environmental conditions had little effect on survival probability when the birds were young, as mortality was very low during this period in our study population as in previous work in captive zebra finches [17,47]. Survival of the birds in the challenging environments was better than the controls during middle age, with this effect disappearing into old adulthood. Our environmental exposure protocol only affected the temporal availability of food, which was otherwise abundant, and thus the effects on survival that we found are not likely to be attributable to caloric restriction. Indeed, body mass was not predictive of survival in our study. The challenge induced by our environmental manipulation was mild, giving rise to repeated and prolonged increases in baseline glucocorticoid secretion (this study) [27]. The effect of the treatment on survival was substantial, with the challenged birds having on average 48% decrease in the relative risk of mortality compared with control females during middle age. It is possible that the challenging environment may have induced effects that reduced the rate of ageing through hormetic processes [48,49]. This possibility fits also with our reproductive data in old adulthood as the challenged females showed less pronounced age-specific declines in reproductive performance relative to those females exposed to the more benign environmental conditions. These long-term beneficial effects of mild challenging exposure resemble those induced by various low-level/mild repeated stressors that have been shown to delay or slow the onset of senescence across a large variety of animals, including humans [49–53]. Our data are therefore compatible with the treatment exposure having induced stimulatory hormetic responses that slowed at least in part the rate of ageing. The majority of the work focusing on hormetic effects has used single or repeated exposure to mild stressors over relatively brief periods [54,55]. There is good experimental evidence that exposure to mild stressors can ‘prime’ responses such that individuals are better able to cope with challenges experienced in later life [52,56,57]. However, the survival benefits seem to be contingent on the environmental conditions to which the physiology of the animal has been conditioned being encountered again later in life [47]. In our study, the birds exposed to the challenging environment were continuously exposed to it from when they first experienced it at five months old, which may have enabled them to reap the best survival benefit from the resilience induced by the challenging exposure. We do not know the mechanism underlying the disappearance of the positive effect of the challenging environmental conditions on survival in old age. Overall, our data highlight the need for more longitudinal/long-term studies to further our understanding of interacting effects among duration of exposure to stress, stressor severity and ageing patterns—disentangling such factors would require exposing animals of different ages to different stressor duration and severity.
In conclusion, the results of this study suggest that the apparent organismal effects of living in a mildly challenging environment might vary at different life stages, something which has previously received very little consideration. We found evidence of negative effects of living under challenging environmental conditions on breeding performance across young adulthood, but positive effects in old age. Survival was not affected in young adulthood, improved in middle age, but then not affected in old age. These results, in addition to showing that exposure to challenging environments can modulate life histories with consequences for patterns of senescence, also emphasize that the duration of studies, the life-history stage at which they take place and the point at which the effects are examined can influence the interpretation. That repeated exposure to stress might slow the ageing process is an extremely interesting prospect and fits with the emerging idea that, rather than being minimized, exposure to stress levels across the life course needs to be optimized in order to obtain the best health benefits [25,48].
Supplementary Material
Acknowledgements
We thank G. Adams, G. Anderson, A. Kirk, J. Laurie, G. Law, G. Grey and A. Magierecka for excellent assistance with animal husbandry; J. C. Noguera, J. Laurie, A. Magierecka and S. Reichert for help in collecting blood samples; A. Magierecka for help with data entry; and J. C. Noguera and P. Johnson for advice with the statistical analyses. Thanks are also expressed to N. Metcalfe, D. Costantini, two reviewers and the associate editor for providing constructive comments on an earlier draft of the manuscript.
Ethics
All procedures were carried out under Home Office Project Licence (60/4109).
Data accessibility
Data are available from Dryad Digital Repository: (http://dx.doi.org/10.5061/dryad.6r273) [58].
Author contributions
V.M., W.B., B.H. and P.M. designed the study. All the authors conducted the experiment. V.M. and P.M. analysed the data and wrote the manuscript. All the authors contributed to revisions of earlier drafts of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by a European Research Council Advanced Investigator Award (268926) to P.M. V.M. was supported by a Marie Sklodowska-Curie Postdoctoral Fellowship at the time of writing (704582).
References
- 1.Monaghan P, Charmantier A, Nussey DH, Ricklefs RE. 2008. The evolutionary ecology of senescence. Funct. Ecol. 22, 371–378. ( 10.1111/j.1365-2435.2008.01418.x) [DOI] [Google Scholar]
- 2.Nussey DH, Froy H, Lemaitre JF, Gaillard JM, Austad SN. 2013. Senescence in natural populations of animals: widespread evidence and its implications for bio-gerontology. Ageing Res. Rev. 12, 214–225. ( 10.1016/j.arr.2012.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkwood TBL, Austad SN. 2000. Why do we age? Nature 408, 233–238. ( 10.1038/35041682) [DOI] [PubMed] [Google Scholar]
- 4.Jones OR, et al. 2014. Diversity of ageing across the tree of life. Nature 505, 169–173. ( 10.1038/nature12789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemaître JF, Berger V, Bonenfant C, Douhard M, Gamelon M, Plard F, Gaillard JM. 2015. Early-late life trade-offs and the evolution of ageing in the wild. Proc. R. Soc B 282, 20150209 ( 10.1098/rspb.2015.0209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging. Cell 153, 1194–1217. ( 10.1016/j.cell.2013.05.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nussey DH, Kruuk LEB, Donald A, Fowlie M, Clutton-Brock TH. 2006. The rate of senescence in maternal performance increases with early-life fecundity in red deer. Ecol. Lett. 9, 1342–1350. ( 10.1111/j.1461-0248.2006.00989.x) [DOI] [PubMed] [Google Scholar]
- 8.Medawar PB. 1952. An unsolved problem of biology. London, UK: University College London by H.K. Lewis and Co Ltd. [Google Scholar]
- 9.Williams GC. 1957. Pleiotropy, natural selection and the evolution of senescence. Evolution 11, 398–411. ( 10.1111/j.1558-5646.1957.tb02911.x) [DOI] [Google Scholar]
- 10.Kirkwood TBL. 1977. Evolution of ageing. Nature 270, 301–304. ( 10.1038/270301a0) [DOI] [PubMed] [Google Scholar]
- 11.Kirkwood TBL. 2005. Understanding the odd science of aging. Cell 120, 437–447. ( 10.1016/j.cell.2005.01.027) [DOI] [PubMed] [Google Scholar]
- 12.Ricklefs RE. 2008. The evolution of senescence from a comparative perspective. Funct. Ecol. 22, 379–392. ( 10.1111/j.1365-2435.2008.01420.x) [DOI] [Google Scholar]
- 13.van den Heuvel J, English S, Uller T. 2016. Disposable soma theory and the evolution of maternal effects on ageing. PLoS ONE 11, e0145544 ( 10.1371/journal.pone.0145544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zera AJ, Harshman LG. 2001. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 32, 95–126. ( 10.1146/annurev.ecolsys.32.081501.114006) [DOI] [Google Scholar]
- 15.Wingfield JC, Sapolsky RM. 2003. Reproduction and resistance to stress: when and how. J. Neuroendocrinol. 15, 711–724. ( 10.1046/j.1365-2826.2003.01033.x) [DOI] [PubMed] [Google Scholar]
- 16.Barnes SK, Ozanne SE. 2011. Pathways linking the early environment to long-term health and lifespan. Prog. Biophys. Mol. Biol. 106, 323–336. ( 10.1016/j.pbiomolbio.2010.12.005) [DOI] [PubMed] [Google Scholar]
- 17.Monaghan P, Heidinger BJ, D'Alba L, Evans NP, Spencer KA. 2012. For better or worse: reduced adult lifespan following early-life stress is transmitted to breeding partners. Proc. R. Soc. B 279, 709–714. ( 10.1098/rspb.2011.1291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee WS, Monaghan P, Metcalfe NB. 2016. Perturbations in growth trajectory due to early diet affect age-related deterioration in performance. Funct. Ecol. 30, 625–635. ( 10.1111/1365-2435.12538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee WS, Monaghan P, Metcalfe NB. 2013. Experimental demonstration of the growth rate-lifespan trade-off. Proc. R. Soc. B 280, 20122370 ( 10.1098/rspb.2012.2370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schultner J, Kitaysky AS, Gabrielsen GW, Hatch SA, Bech C. 2013. Differential reproductive responses to stress reveal the role of life-history strategies within a species. Proc. R. Soc. B 280, 20132090 ( 10.1098/rspb.2013.2090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid JM, Bignal EM, Bignal S, McCracken DI, Monaghan P. 2003. Environmental variability, life-history covariation and cohort effects in the red-billed chough Pyrrhocorax pyrrhocorax. J. Anim. Ecol. 72, 36–46. ( 10.1046/j.1365-2656.2003.00673.x) [DOI] [Google Scholar]
- 22.Robinson MR, Mar KU, Lummaa V. 2012. Senescence and age-specific trade-offs between reproduction and survival in female Asian elephants. Ecol. Lett. 15, 260–266. ( 10.1111/j.1461-0248.2011.01735.x) [DOI] [PubMed] [Google Scholar]
- 23.Kim SY, Metcalfe NB, Velando A. 2016. A benign juvenile environment reduces the strength of antagonistic pleiotropy and genetic variation in the rate of senescence. J. Anim. Ecol. 85, 705–714. ( 10.1111/1365-2656.12468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pardon MC. 2007. Stress and ageing interactions: a paradox in the context of shared etiological and physiopathological processes. Brain Res. Rev. 54, 251–273. ( 10.1016/j.brainresrev.2007.02.007) [DOI] [PubMed] [Google Scholar]
- 25.Sapolsky RM. 2015. Stress and the brain: individual variability and the inverted-U. Nat. Neurosci. 18, 1344–1346. ( 10.1038/nn.4109) [DOI] [PubMed] [Google Scholar]
- 26.Karatoreos IN, McEwen BC. 2013. Annual research review: the neurobiology and physiology of resilience and adaptation across the life course. J. Child Psychol. Psychiatry 54, 337–347. ( 10.1111/jcpp.12054) [DOI] [PubMed] [Google Scholar]
- 27.Marasco V, Boner W, Heidinger B, Griffiths K, Monaghan P. 2015. Repeated exposure to stressful conditions can have beneficial effects on survival. Exp. Gerontol. 69, 170–175. ( 10.1016/j.exger.2015.06.011) [DOI] [PubMed] [Google Scholar]
- 28.Zann RA. 1996. The zebra finch: a synthesis of field and laboratory studies. Oxford, UK: Oxford University Press. [Google Scholar]
- 29.Spencer KA, Evans N, Monaghan P. 2009. Postnatal stress in birds: a novel model of glucocorticoid programming of the hypothalamic-pituitary-adrenal axis. Endocrinology 150, 1931–1934. ( 10.1210/en.2008-1471) [DOI] [PubMed] [Google Scholar]
- 30.Pravosudov VV, Kitaysky AS, Wingfield JC, Clayton NS. 2001. Long-term unpredictable foraging conditions and physiological stress response in mountain chickadees (Poecile gambeli). Gen. Comp. Endocri. 123, 324–331. ( 10.1006/gcen.2001.7684) [DOI] [PubMed] [Google Scholar]
- 31.Jenni-Eiermann S, Glaus E, Gruebler M, Schwabl H, Jenni L. 2008. Glucocorticoid response to food availability in breeding barn swallows (Hirundo rustica). Gen. Comp. Endocri. 155, 558–565. ( 10.1016/j.ygcen.2007.08.011) [DOI] [PubMed] [Google Scholar]
- 32.Wingfield JC, Kitaysky AS. 2002. Endocrine responses to unpredictable environmental events: stress or anti-stress hormones? Integr. Comp. Biol. 42, 600–609. ( 10.1093/icb/42.3.600) [DOI] [PubMed] [Google Scholar]
- 33.Reed T, Kruuk L, Wanless S, Frederiksen M, Cunningham E, Harris M. 2008. Reproductive senescence in a long-lived seabird: rates of decline in late-life performance are associated with varying costs of early reproduction. Am. Nat. 171, E89–E101. ( 10.1086/524957) [DOI] [PubMed] [Google Scholar]
- 34.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 35.Kuznetsova A. 2016. Package ‘lmerTest’. See https://cran.r-project.org/web/packages/lmerTest/lmerTest.pdf.
- 36.Zuur AF, Ieno EN, Walker NJS, Saveliev AA, Smith GM. 2009. GLM and GAM for absence-presence and proportional data. In Mixed effects models and extension in ecology with R (eds Gail M, Krickeberg KS, Samet JM, Tsiatis A, Wong W), pp. 245–259. Berlin, Germany: Springer. [Google Scholar]
- 37.Lenth RV. 2016. Least-squared means: the R package lsmeans. J. Stat. Softw. 69, 1–33. ( 10.18637/jss.v069.i01) [DOI] [Google Scholar]
- 38.Therneau TM, Lumley T.2016. Package ‘survival’. See https://cran.r-project.org/web/packages/survival/survival.pdf .
- 39.Therneau TM.2015. Package ‘coxme’. See https://cran.r-project.org/web/packages/coxme/coxme.pdf .
- 40.Silverin B. 1986. Corticosterone-binding proteins and behavioral effects of high plasma levels of corticosterone during the breeding period in the pied flycatcher. Gen. Comp. Endocr. 64, 67–74. ( 10.1016/0016-6480(86)90029-8) [DOI] [PubMed] [Google Scholar]
- 41.Zanette LY, White AF, Allen MC, Clinchy M. 2011. Perceived predation risk reduces the number of offspring songbirds produce per year. Science 334, 1398 ( 10.1126/science.1210908) [DOI] [PubMed] [Google Scholar]
- 42.Thierry AM, Massemin S, Handrich Y, Raclot T. 2013. Elevated corticosterone levels and severe weather conditions decrease parental investment of incubating Adelie penguins. Horm. Behav. 63, 475–483. ( 10.1016/j.yhbeh.2012.12.011) [DOI] [PubMed] [Google Scholar]
- 43.Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. 1998. Ecological bases of hormone-behavior interactions: the ‘emergency life history stage’. Am. Zool. 38, 191–206. ( 10.1093/icb/38.1.191) [DOI] [Google Scholar]
- 44.Kitaysky AS, Wingfield JC, Piatt JF. 1999. Dynamics of food availability, body conditions and physiological stress response in breeding black-legged kittiwakes. Funct. Biol. 13, 577–584. ( 10.1046/j.1365-2435.1999.00352.x) [DOI] [Google Scholar]
- 45.Mateo JM. 2007. Ecological and hormonal correlates of antipredator behaviour in adult Belding's ground squirrels (Spermophilus beldingi). Behav. Ecol. Sociobiol. 62, 37–49. ( 10.1007/s00265-007-0436-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boonstra R, Fox C. 2013. Reality as the leading cause of stress: rethinking the impact of chronic stress in nature. Funct. Ecol. 27, 11–23. ( 10.1111/1365-2435.12008) [DOI] [Google Scholar]
- 47.Costantini D, Monaghan P, Metcalfe NB. 2014. Prior hormetic priming is costly under environmental mismatch. Biol. Lett. 10, 20131010 ( 10.1098/rsbl.2013.1010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gems D, Partridge L. 2008. Stress-response hormesis and aging: ‘That which does not kill us makes us stronger’. Cell Metab. 7, 200–203. ( 10.1016/j.cmet.2008.01.001) [DOI] [PubMed] [Google Scholar]
- 49.Masoro EJ. 1998. Influence of caloric intake on aging and on the response to stressors. J. Toxicol. Environ. Health B Crit. Rev. 1, 243–257. ( 10.1080/10937409809524554) [DOI] [PubMed] [Google Scholar]
- 50.Cypser JR, Johnson TE. 2002. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J. Gerontol. A. Biol. Sci. Med. Sci. 57, B109–B114. ( 10.1093/gerona/57.3.B109) [DOI] [PubMed] [Google Scholar]
- 51.Hercus MJ, Loeschcke V, Rattan SIS. 2003. Lifespan extension of Drosophila melanogaster through hormesis by repeated mild heat stress. Biogerontology 4, 149–156. ( 10.1023/A:1024197806855) [DOI] [PubMed] [Google Scholar]
- 52.Lopez-Martinez G, Hahn DA. 2014. Early life hormetic treatments decrease irradiation-induced oxidative damage, increase longevity, and enhance sexual performance during old age in the Caribbean fruit fly. PLoS ONE 9, e88128 ( 10.1371/journal.pone.0088128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rattan SIS, Fernandes RA, Demirovic D, Dymek B, Lima CF. 2009. Heat stress and hormetin-induced hormesis in human cells: effects on aging, wound healing, angiogenesis, and differentiation. Dose Response 7, 90–103. ( 10.2203/dose-response.08-014.Rattan) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Costantini D, Metcalfe NB, Monaghan P. 2010. Ecological processes in a hormetic framework. Ecol. Lett. 13, 1435–1447. ( 10.1111/j.1461-0248.2010.01531.x) [DOI] [PubMed] [Google Scholar]
- 55.Mattson MP, Calabrese EJ. 2010. Hormesis: a revolution in biology, toxicology and medicine. New York, NY: Springer. [Google Scholar]
- 56.Olsen A, Vantipalli MC, Lithgow GJ. 2006. Lifespan extension of Caenorhabditis elegans following repeated mild hormetic heat treatments. Biogerontology 7, 221–230. ( 10.1007/s10522-006-9018-x) [DOI] [PubMed] [Google Scholar]
- 57.Costantini D, Monaghan P, Metcalfe NB. 2012. Early life experience primes resistance to oxidative stress. J. Exp. Biol. 215, 2820–2826. ( 10.1242/jeb.072231) [DOI] [PubMed] [Google Scholar]
- 58.Marasco V, Boner W, Griffiths K, Heidinger B, Monaghan P. 2018. Data from: Environmental conditions shape the temporal pattern of investment in reproduction and survival Dryad Digital Repository. ( 10.5061/dryad.6r273) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Marasco V, Boner W, Griffiths K, Heidinger B, Monaghan P. 2018. Data from: Environmental conditions shape the temporal pattern of investment in reproduction and survival Dryad Digital Repository. ( 10.5061/dryad.6r273) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from Dryad Digital Repository: (http://dx.doi.org/10.5061/dryad.6r273) [58].