Abstract
Understanding the rate of evolutionary change and the genetic architecture that facilitates rapid adaptation is a current challenge in evolutionary biology. Comparative studies show that genes with immune function are among the most rapidly evolving genes across a range of taxa. Here, we use immune defence in natural populations of Drosophila melanogaster to understand the rate of evolution in natural populations and the genetics underlying rapid change. We probed the immune system using the natural pathogens Enterococcus faecalis and Providencia rettgeri to measure post-infection survival and bacterial load of wild D. melanogaster populations collected across seasonal time along a latitudinal transect along eastern North America (Massachusetts, Pennsylvania and Virginia). There are pronounced and repeatable changes in the immune response over the approximately 10 generations between spring and autumn collections, with a significant but less distinct difference observed among geographical locations. Genes with known immune function are not enriched among alleles that cycle with seasonal time, but the immune function of a subset of seasonally cycling alleles in immune genes was tested using reconstructed outbred populations. We find that flies containing seasonal alleles in Thioester-containing protein 3 (Tep3) have different functional responses to infection and that epistatic interactions among seasonal Tep3 and Drosomycin-like 6 (Dro6) alleles underlie the immune phenotypes observed in natural populations. This rapid, cyclic response to seasonal environmental pressure broadens our understanding of the complex ecological and genetic interactions determining the evolution of immune defence in natural populations.
Keywords: rapid adaptation, innate immunity, Drosophila melanogaster, Thioester-containing protein 3, Drosomycin-like 6, epistasis
1. Introduction
The rate at which populations respond to environmental change is a fundamental parameter in the process of adaption. Evolution is historically considered to be an innately slow process that occurs over very long time scales [1], but there are now examples that evolutionary change can occur much faster [2–4]. The limits of how fast populations evolve and the genetic architecture underlying rapid evolution remain unclear [5]. The classical approach to infer adaption through the association of traits and genotypes that covary along spatial environmental gradients (e.g. latitude, longitude and altitude) [6] can be expanded across temporal environmental gradients to provide insights to the rate of adaption in the wild.
Biotic environment may shape the rate of adaptation through the immune system, which sits at the crucial interface between an organism's external and internal environment. Strong selection imposed by pathogens may result in rapid evolution of immune defence in nature, because microbiotic infection directly affects host fitness, with consequences ranging from resource reallocation to host mortality [7–13]. Comparative studies across a broad range of taxa indicate that genes with immune function are among the most rapidly evolving genes in the genome [14–19]. Drosophila melanogaster immune genes show evidence of local adaptation across large spatial gradients with high levels of population differentiation and latitudinal enrichment across multiple continents [20–23]. There is less evidence for immune differentiation at small spatial scales [24,25], although some screens of infection response in D. melanogaster indicate continental differences in defence quality [24]. Thus, immune defence in natural populations of D. melanogaster represents a tractable system to study the rate of evolution.
We predicted seasonal variation in D. melanogaster immune defence even in the absence of established clinal differences in performance. Seasonal climatic changes produce predictable environmental gradients over time that select for different phenotypes [26,27] and allele frequencies [28,29] in multivoltine organisms like D. melanogaster. Abiotic variables (e.g. temperature) that cycle across seasons can influence microbial growth, so microbial communities and pathogen diversity that vary over spatial gradients [30–36] may also change across seasonal time [37–40]. Seasonal differences in pathogen diversity and frequency may select for immune resistance or tolerance in either or both of the primary humoral immune pathways: the Toll pathway that is preferentially activated by Gram-positive bacteria or the IMD pathway that is primarily activated by Gram-negative bacteria [41].
We tested whether innate immunity evolves seasonally in mid-Atlantic D. melanogaster populations in North America (Massachusetts, Pennsylvania and Virginia). We found that immune defence changes rapidly and repeatedly from spring to autumn, and seasonally cycling alleles of immune genes determine seasonal variation in resistance to and tolerance of infection. We show that epistatic interactions among seasonally cycling SNPs produce the immune phenotypes observed in natural populations. This rapid, cyclic response to seasonal environmental pressure broadens our understanding of the complex ecological and genetic interactions determining the evolution of immune defence in natural populations.
2. Material and methods
(a). Drosophila samples
Wild D. melanogaster were collected by aspiration in early July (spring population) and late October (autumn population) repeated across 2 years at three locations spaced evenly along a 4° latitudinal gradient: George Hill Orchard in Lancaster, MA (42.500493° N, −71.563580° E), Linvilla Orchards in Media, PA (39.884179° N, −75.411227° E) and Carter Mountain Orchard in Charlottesville, VA (37.991851° N, −78.471630° E). Isofemale lines were established from wild-caught inseminated females and were maintained on standard cornmeal molasses food in standard laboratory conditions (25°C, 12 L : 12 D) on a three-week transfer cycle for six to eight generations before immune assessment.
We screened a published dataset of SNPs that oscillate in these populations across seasonal time [29] for genes with known immune function [42] to identify candidate seasonal immune SNPs. The immune function of these SNPs was assessed using recombinant outbred populations (ROPs) [43] fixed for specific seasonal allele combinations in a randomized genetic background that were constructed using lines from the Drosophila Genetics Reference Panel (DGRP) [44]. Ten gravid females from 15 lines were pooled to lay eggs for 48 h for each combination of seasonal alleles (electronic supplementary material) and the offspring mated freely for at least 10 non-overlapping generations before immune assessment. The immune function of the two SNPs in Thioester-containing protein 3 (Tep3) was tested using three genotypes that combined 2 L:7703202 and 2 L:7705370 (D. melanogaster reference genome v.5.39) spring and autumn alleles: (i) Tep3TG contained spring alleles for both 2 L:7703202 and 2 L:7705370, (ii) Tep3TT contained the spring 2 L:7703202 and the autumn 2 L:7705370 modifier allele, and (iii) Tep3CT contained autumn alleles for both SNPs. The final combination of the autumn 2 L:7703202 coding allele and the spring 2 L:7705370 modifier allele was too rare in the DGRP to create ROP. Two independent biological replicate populations were created for each of the three Tep3 genotypes. Epistatic interactions between Tep3 and either Fas-associated death domain (Fadd) or Drosomycin-like-6 (Dro6) were assessed in the same way with ROP fixed for either both spring or autumn Tep3 alleles and either Fadd or Dro6 alleles.
(b). Immune measurements
Quality of immune defence was probed using systemic bacterial infection [45] with Gram-negative Providencia rettgeri [46] and Gram-positive Enterococcus faecalis [47] strains that were originally isolated from infected wild-caught D. melanogaster. Post-infection survival was measured in males 3–5 days over two repeated blocks of five consecutive days after infection. Mortality was highest in the first 24 h and plateaued (electronic supplementary material, figure S1), so the final mortality 5 days post-infection was analysed in the model. Flies were infected with cultures started with a single colony grown to saturation in LB media at 37°C with shaking overnight and diluted to A600 nm of 1.0. Infections were delivered at a dose of 103–104 bacteria to each CO2-anaesthetized fly by inoculating the lateral thorax with a 0.15 mm minutien pin (Fine Scientific Tools) dipped into bacterial culture [45]. Two controls were used: a sterile wound by a needle disinfected in 95% ethanol and unwounded flies anaesthetized on CO2.
Systemic bacterial load of infected flies was quantified using the same infection method described above for infection survival. When evaluating the natural populations, 20 lines from each of the collection were infected during a daily infection window (09.00–12.00). All infections were repeated over two consecutive days by two infectors with infector and infection order randomized daily. Twelve males from each line were infected and maintained with food at 25°C, 12 L : 12 D for 24 h post-infection. Up to three replicate groups of three flies were homogenized in 500 ml of LB for the 2012 natural populations, and up to three single flies were homogenized in 500 ml of PBS for the 2014 natural and ROP. The samples were then plated on LB agar plates at 1 : 100 dilution for P. rettgeri, 1 : 10 for E. faecalis in natural populations and 1 : 1 for both bacteria in ROP using the Whitley Automatic Spiral Plater (Don Whitley Scientific, Shipley, UK). The plates were incubated overnight at 37°C, and the number of colony-forming units was counted using the ProtoCOL3 automated plate counter (Synbiosis, Cambridge, UK) and used to calculate the concentration of bacteria in each homogenate.
(c). Gene expression
Expression levels of Tep3, Dro6 and Fadd were determined using a published dataset of RNAseq on 185 inbred sequenced lines from the DGRP [48]. We extracted expression of Tep3, Dro6 and Fadd for each inbred line and used sequence data [44] to identify Tep3, Dro6 and Fadd haplotype.
(d). Statistical analyses
Statistical analyses were performed using R software (v. 3.2.2; R Core Team). Post-infection survival was measured daily and survival 5 days post-infection was analysed using a binomial linear regression. Survival post-infection was evaluated using the model:
| 2.5 |
Population, year and season were considered fixed effects, and replicate and line were random effects nested within season, population and year. Mean survival post-infection was standardized by survival under sterile wound control.
Concentration of bacteria in each homogenate was calculated using the number of colonies log-transformed and analysed using mixed-model ANOVAs:
Population, year and season were fixed effects, and replicate and line were random effects nested within season, population and year. Infector and infection order were initially included in model but had no significant effect and were removed.
(e). Seasonal SNPs
Seasonal immune SNPs were identified by screening a published dataset for alleles that fluctuate in frequency as a function of seasonal time [29] in 88 genes known to have immune function [49]. Seasonal SNPs were cross-referenced with a group of paired spring and autumn samples collected from 10 populations along the North American cline by the Drosophila Real Time Evolution Consortium (NCBI SRA BioProject PRJNA308584 [29,50]). Additional information collected on each SNPs included clinal q-value [29] and p-value in a genome-wide association study to identify SNPs involved with P. rettgeri pathogenic infection [51]. Enrichment for immune genes was calculated using customized python scripts that compared the proportion of seasonal and non-seasonal immune genes with control genes that were matched for size and position using χ2 with 10 000 bootstrap iterations.
Linkage disequilibrium (LD) among the candidate seasonal immune SNPs was calculated in the DGRP using allelic correlation of physical distances using the LDheatmap package [52] in R. The 205 sequenced inbred lines of the DGRP were used to examine LD among all of the candidate SNPs by chromosome [44].
(f). Seasonal genotypes
Genotypes from wild populations were determined using inbred lines originally collected in Pennsylvania in the spring and autumn of 2012. The lines were inbred by full-sib mating for 20 generations and subsequently sequenced (NCBI SRA BioProject PRJNA383555). Genotype deviation was calculated as the difference between observed frequency and a predicted frequency based on the individual alleles. Haplotype distribution of Tep3 was calculated for SNPs with a minor allele frequency greater than 0.1 using integer joining networks [53] in PopArt v. 1.7 [42].
3. Results
(a). Geographical differences in immunity
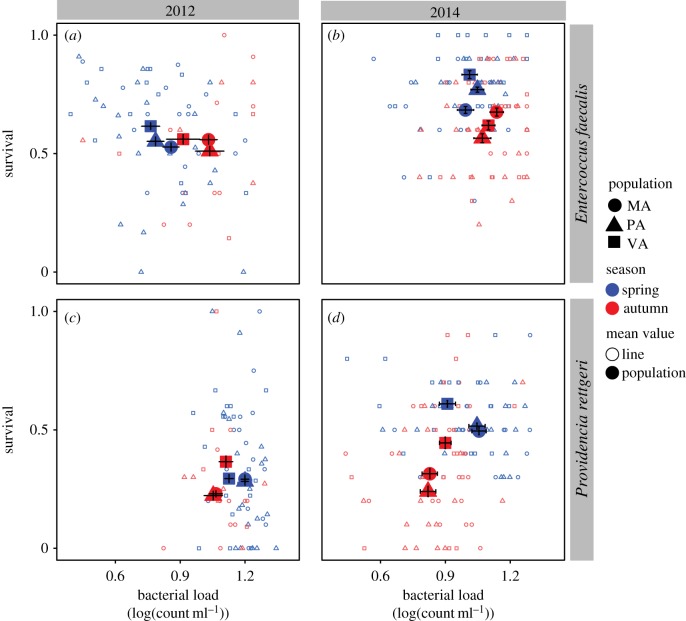
Geographical origin across the latitudinal transect determined survival post-infection, but did not predict systemic bacterial load sustained by flies infected with either pathogen. While survival after P. rettergi infection directly depended on the latitude (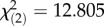 , p = 5.87 × 10−4), the interaction between geographical origin and season of collection affected survival after E. faecalis infection (
, p = 5.87 × 10−4), the interaction between geographical origin and season of collection affected survival after E. faecalis infection (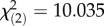 , p = 6.62 × 10−3). The lower-latitude Virginia population had higher survival after E. faecalis infection in the spring but no difference in the autumn (figure 1a,b). High-latitude Massachusetts and Pennsylvania populations had similar load and survival after P. rettgeri infection and exhibited a greater seasonal change in both survival and bacterial load compared with the lower-latitude Virginia population (figure 1c,d).
, p = 6.62 × 10−3). The lower-latitude Virginia population had higher survival after E. faecalis infection in the spring but no difference in the autumn (figure 1a,b). High-latitude Massachusetts and Pennsylvania populations had similar load and survival after P. rettgeri infection and exhibited a greater seasonal change in both survival and bacterial load compared with the lower-latitude Virginia population (figure 1c,d).
Figure 1.
Immune defence relationship between bacterial load and survival in natural spring and autumn populations. Isofemale lines (small, outline) were used to calculate population mean ± s.e. (large, filled) from natural orchard populations collected along a latitudinal gradient in Massachusetts Pennsylvania and Virginia in the spring and autumn for two replicate years: 2012 (a,c) and 2014 (b,d). Immune defence was probed with two natural pathogens: a Gram-positive bacterium Enterococcus faecalis (a,b) and a Gram-negative bacterium Providencia rettgeri (c,d). Twenty isofemale lines from each collection were measured for 5-day survival after infection and bacterial load at 24 h post-infection scaled by average load for the experiment.
(b). Immunity changes rapidly within a population over seasonal time
Immune defence changed rapidly across approximately 10 generations in the wild from spring to autumn with a pathogen-specific relationship between bacterial load and survival (figure 1). Spring populations were more resistant to E. faecalis bacterial growth (F1,219 = 87.758, p < 0.0001) and maintained low load with marginally higher survival rates (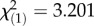 , p = 7.36 × 10−2), while the autumn populations infected with the same bacteria did not restrict bacterial growth as effectively, resulting in high load and high mortality (figure 1a,b). The converse relationship occurred when flies were infected with P. rettgeri: higher survival in spring (
, p = 7.36 × 10−2), while the autumn populations infected with the same bacteria did not restrict bacterial growth as effectively, resulting in high load and high mortality (figure 1a,b). The converse relationship occurred when flies were infected with P. rettgeri: higher survival in spring (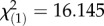 , p = 5.87 × 10−4) despite higher bacterial load (F1,215 = 7.88, p < 0.0001) and high mortality in autumn even though the bacterial growth was restricted (figure 1c,d). There is a general consistency of the patterns from spring to autumn, but the annual variation in the environment results in year and year-by-month interaction as significant factors contributing to survival after infection with E. faecalis (year:
, p = 5.87 × 10−4) despite higher bacterial load (F1,215 = 7.88, p < 0.0001) and high mortality in autumn even though the bacterial growth was restricted (figure 1c,d). There is a general consistency of the patterns from spring to autumn, but the annual variation in the environment results in year and year-by-month interaction as significant factors contributing to survival after infection with E. faecalis (year: 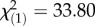 , p = 6.10 × 10−9; year × month:
, p = 6.10 × 10−9; year × month: 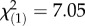 , p = 7.94 × 10−3) and P. rettgeri (year:
, p = 7.94 × 10−3) and P. rettgeri (year: 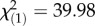 , p = 2.57 × 10−10; year × month:
, p = 2.57 × 10−10; year × month: 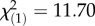 , p = 6.23 × 10−4) .
, p = 6.23 × 10−4) .
(c). SNPs in immune genes oscillate across seasonal time
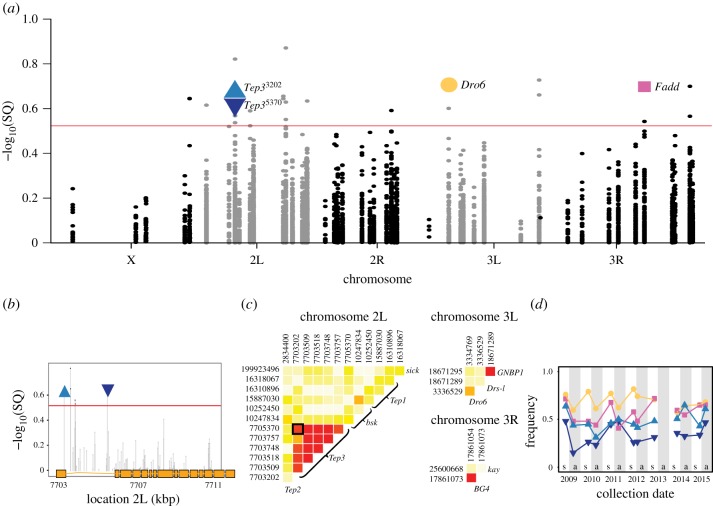
Immune genes as a functional category were not enriched among genes carrying polymorphisms that oscillate in frequency over seasonal time in these populations [29] when compared with controls matched for size and position using previously published data. Twenty-four candidate SNPs oscillated in frequency across seasonal time in these populations [29] located within or in proximity to 13 genes with known involvement in immune function [54] (electronic supplementary material, table S1; table 1 and figure 2a).
Table 1.
Seasonal immune SNPs identified using whole-genome resequencing of the Pennsylvania spring and autumn populations across three consecutive years. SNPs with a seasonal q-value (SQ)<0.3 are classified as seasonal and the SNPs investigated here are in bold. Most of seasonal SNPs do not have significant clinal q-values (CQ) and were not significant in a genome wide association study (GWAS) for response to P. rettgeri pathogenic infection [52].
| gene | position | effect | molecular function | SQ | CQ | GWAS |
|---|---|---|---|---|---|---|
| Tep2 | 2 L:2834400 | upstream modifier | effector | 0.242 | 0.956 | 0.253 |
| Tep3 | 2 L:7703202 | NS coding | effector | 0.243 | 0.159 | 0.420 |
| Tep3 | 2 L:7703509 | upstream modifier | effector | 0.151 | 0.529 | 0.084 |
| Tep3 | 2 L:7703518 | upstream modifier | effector | 0.220 | 0.643 | 0.084 |
| Tep3 | 2 L:7703748 | upstream modifier | effector | 0.271 | 0.819 | 0.114 |
| Tep3 | 2 L:7703757 | upstream modifier | effector | 0.291 | 0.956 | 0.632 |
| Tep3 | 2 L:7705370 | upstream modifier | effector | 0.219 | 0.163 | 0.385 |
| bsk | 2 L:10247834 | intron | signalling | 0.300 | 0.822 | 0.255 |
| bsk | 2 L:10252450 | intron | signalling | 0.257 | 0.749 | 0.962 |
| Tep1 | 2 L:15887030 | downstream modifier | effector | 0.227 | 0.188 | 0.089 |
| Tep1 | 2 L:15888031 | downstream modifier | effector | 0.221 | 0.520 | NA |
| cact | 2 L:16309682 | downstream modifier | signalling | 0.135 | 0.782 | 0.829 |
| cact | 2 L:16310896 | downstream modifier | signalling | 0.235 | 0.635 | 0.375 |
| cact | 2 L:16318067 | intron | signalling | 0.281 | 0.719 | 0.335 |
| sick | 2 L:19923496 | intron | signalling | 0.232 | 0.032 | 0.505 |
| IM1 | 2 R:14270817 | upstream modifier | effector | 0.256 | 0.695 | 0.423 |
| Dro6 | 3 L:3334769 | upstream modifier | effector | 0.201 | 0.427 | 0.000 |
| Drs-l | 3 L:3336529 | upstream modifier | effector | 0.251 | 0.975 | 0.028 |
| GNBP1 | 3 L:18671289 | downstream modifier | recognition | 0.187 | 0.150 | 0.729 |
| GNBP2 | 3 L:18671295 | downstream modifier | recognition | 0.218 | 0.167 | 0.666 |
| Fadd | 3 R:17861054 | UTR 3'modifier | signalling | 0.200 | 0.006 | 0.822 |
| Fadd | 3 R:17861073 | UTR 3'modifier | signalling | 0.287 | 0.425 | 0.712 |
| kay | 3 R:25600668 | intron | signalling | 0.200 | 0.588 | 0.743 |
| Tak1 | X:20388404 | intron | signalling | 0.227 | 0.326 | 0.964 |
Figure 2.
Seasonal changes in immune genes in natural populations. (a) Manhattan plot of SNPs in immune genes that change in frequency across seasonal time [29]. The red line indicates the seasonal q-value cutoff > 0.3 [29]. The SNPs on which functional analyses were performed are highlighted: Fadd, Dro6, Tep33202 and Tep35370. (b) Manhattan plot of SNPs in immune genes within Tep3 with genic structure along the x axis. Exons indicated in orange boxes. (c) Heat map showing linkage disequilibrium among SNPs in immune genes across each chromosome using DGRP. (d) Cycling of seasonal allele frequencies of candidate immune SNPs in Pennsylvania in the spring (s) and the autumn (a) from 2009 to 2015. (Online version in colour.)
(d). Seasonally oscillating Tep3 SNPs have functional differences in immunity
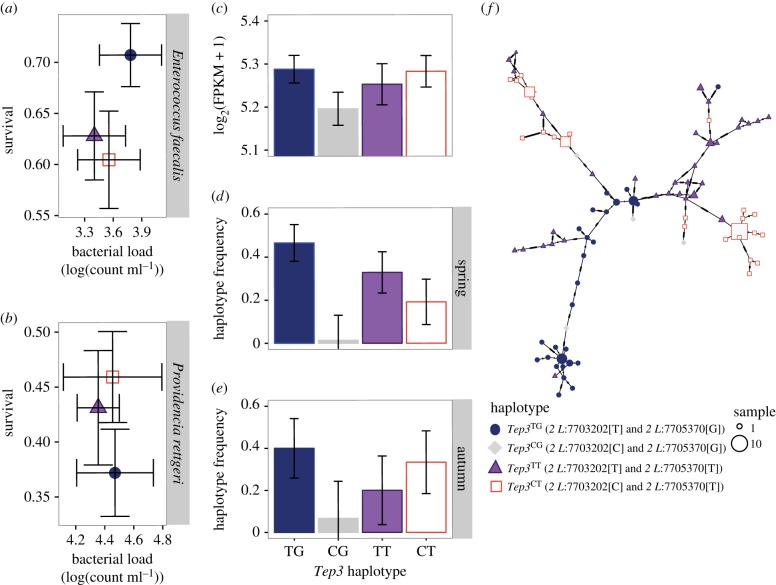
Over one-third of the seasonally variable SNPs near immune genes were near Tep family genes, with Tep homologues comprising one-fourth of all of the seasonally variable immune genes. Tep3 contained numerous seasonally oscillating loci with high LD across the 2.5 kb region in which the seasonal alleles are located in the DGRP (figure 2a,b,c). We tested the function using ROP with two loci as markers: the non-synonymous-coding change at 2 L:7703202 that is surrounded by five intronic seasonal SNPs and the intronic SNP 2 L:7705370 that is 2 kb downstream from the cluster (D. melanogaster reference genome v. 5.39). These markers are in LD in the DGRP (r2 = 0.8138) and cycle independently across seasonal time (electronic supplementary material, table S1; figure 2c), but neither allele varies along a cline (electronic supplementary material, table S1). Alleles at 2 L:7703202were non-randomly distributed with respect to karyotype: in both DGRP and Pennsylvania populations that the autumn allele (C) was strongly associated with In(2 L)t. By contrast, the spring allele (T) occurred mostly in a standard arrangement genetic background (Fisher's exact test; p < 0.0001). 2 L:7705730 had no significant association with either arrangement of In(2 L)t (Fisher's exact test; p = 0.161).
There was no difference among the Tep3 ROP in bacterial load, but there was differential survivorship after infection with both Gram-positive and Gram-negative pathogens. Flies containing the spring Tep3TG haplotype had higher survival than those containing the autumn Tep3CT or mixed Tep3CG haplotypes when infected with Gram-positive E. faecalis (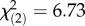 , p = 0.0346; figure 3a).The Tep3 SNPs are associated with an additive effect on survival of Gram-negative P. rettgeri infection with higher survival in flies containing the autumn haplotype than those containing the spring haplotype and intermediate survival in flies containing the mixed haplotype (
, p = 0.0346; figure 3a).The Tep3 SNPs are associated with an additive effect on survival of Gram-negative P. rettgeri infection with higher survival in flies containing the autumn haplotype than those containing the spring haplotype and intermediate survival in flies containing the mixed haplotype (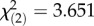 , p = 0.161; figure 3b). Flies containing the seasonal Tep3 haplotypes have no difference in Tep3 expression in the absence of infection (F3,360 = 1.419, p = 0.239, figure 3c) based on previously published RNAseq expression of the DGRP lines [48]. The Tep3TG haplotype containing spring alleles occurred at higher in the spring Pennsylvania population compared with the autumn, while the Tep3CT haplotype containing autumn alleles increased in frequency from spring to autumn (figure 3d,e). There were two primary sequence haplotypes carrying spring Tep3TG variants and two sequence haplotypes carrying the autumn Tep3CT variants in the Pennsylvania orchard (figure 3f; electronic supplementary material, table S1).
, p = 0.161; figure 3b). Flies containing the seasonal Tep3 haplotypes have no difference in Tep3 expression in the absence of infection (F3,360 = 1.419, p = 0.239, figure 3c) based on previously published RNAseq expression of the DGRP lines [48]. The Tep3TG haplotype containing spring alleles occurred at higher in the spring Pennsylvania population compared with the autumn, while the Tep3CT haplotype containing autumn alleles increased in frequency from spring to autumn (figure 3d,e). There were two primary sequence haplotypes carrying spring Tep3TG variants and two sequence haplotypes carrying the autumn Tep3CT variants in the Pennsylvania orchard (figure 3f; electronic supplementary material, table S1).
Figure 3.
Functional difference of seasonal Tep3 alleles as defined by the focal SNPs. Mean ± s.e. for bacterial load 24 h post-infection and survival 5 days post-infection for the Tep3 genotypes. (a) Higher survival for the spring genotype than the autumn or combination genotypes when infected with E. faecalis. (b) Additive effect of alleles when infected with P. rettgeri. (c) Lower constitutive Tep3 mRNA expression in the rare Tep3CG haplotype from the published dataset of DGRP flies [48]. (d,e) Frequency of Tep3 haplotypes in the Pennsylvania orchard across seasonal time. (f) Minimum spanning network illustrates that LD among the SNPs is maintained in distinct haplotypes. (Online version in colour.)
(e). Epistasis among AMP genes involved in rapid seasonal adaptation
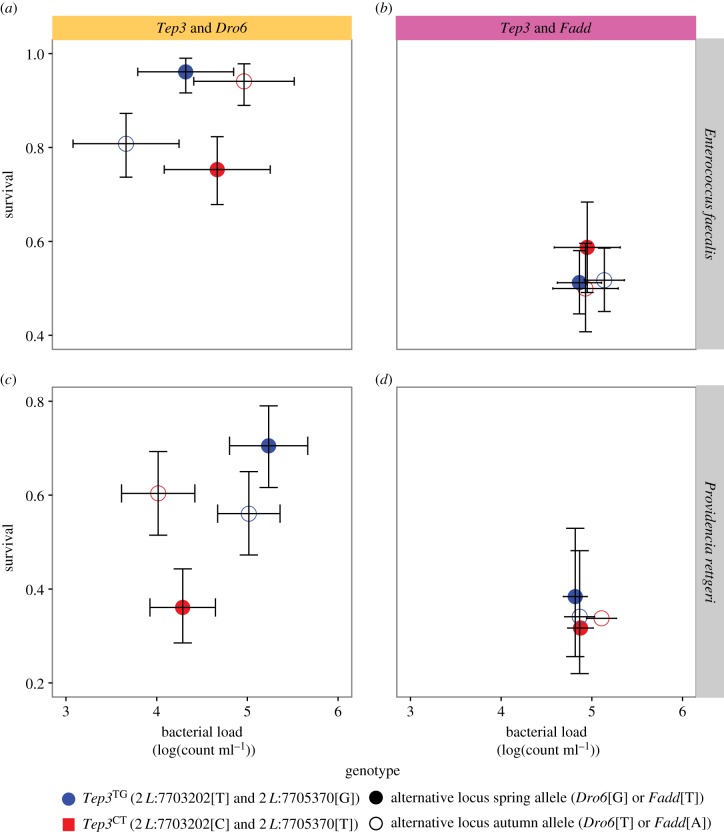
We tested whether additional seasonal SNPs in the immune pathways interact with Tep3 to facilitate rapid immune evolution across seasons: 3 L:3334769, an upstream modifier of Drosomycin-like 6 (Dro6), that was shown to significantly affect resistance to P. rettgeri in a genome-wide association study [51] and 3R:17861054, a 3′-UTR modifier in the signalling gene Fas-associated death domain orthologue (Fadd), which was the only SNP with concordant patterns between season and latitude (figure 2a and table 1). There was no difference in immune defence among ROP combinations of Tep3 and Fadd, but the non-additive interactions among ROP containing Tep3 and Dro6 alleles begin to explain the complexity of immune defence of natural populations (figure 4).
Figure 4.
Intergenic interactions among Tep3, Dro6 and Fadd. Non-additive interaction among Tep3 and Dro6 alleles. No significant interaction among Tep3 and Fadd SNPs.
4. Discussion
(a). Natural populations differ in immunity over geographical space and across seasonal time
Immune response differs among populations across space and time. Season of collection is a strong predictor of the immune response across geographical locations that span 4° latitude with a seasonal decline in resistance to E. faecalis and a seasonal decline in tolerance of P. rettgeri. The change in immunity across seasonal time occurs rapidly within each geographical location with approximately 10 generations between spring and autumn collections. Repeated seasonal change in immune defence is consistent with previous findings for other measurements of stress resistance [26,27]. Taken together, this suggests that a harsh winter selects for a suite of traits that produce a robust spring population and selection on those traits is relaxed during summer producing less stress resistant populations in autumn.
Although the strongest differentiation of immunity occurred across seasonal time, there was also a signal of geography along the sampled spatial gradient. Our results contrast with previous studies that did not detect a robust association between latitude and survival [55] or load [24,49]. The difference may be attributed to the interaction between season and latitude. It is possible that geographical differences in immune response may be even greater across a longer distance that may capture a larger difference in pathogen diversity [30–36,56]. In addition, there was secular change in post-infection survival but not in bacterial load, which may be caused by year-to-year differences in the microbial community of the environment that result in different allele frequencies in genes with immune function.
The repeatability of the general patterns of change in immune defence across space and time indicates deterministic evolutionary processes. Rearing lines for multiple generations in a common laboratory environment distinct from external sample sites removes environmental variation and ensures that differences among collections and populations can be attributed to genetic diversity among source populations. It is possible that gene flow due to migration from other latitudes contributes to the differences between spring and autumn populations. However, migration is unlikely to be the primary cause underlying seasonal immune differences, because latitudinal differentiation was weak compared to seasonal change. Furthermore, infection with different pathogens resulted in opposing clinal patterns but parallel change across seasons. Additionally, migration alone appears insufficient to explain genome-wide differences in allele frequency profiles that characterize spring and autumn populations in Pennsylvania orchard [29]; thus, migration is unlikely to explain the seasonal differences in immune response. Wild Drosophila populations live in a heterogeneous environment and evolve rapidly in response to environmental parameters that change with season [26,27], potentially including rapid turn-over in microbial and pathogen communities (electronic supplementary material, figure S2).
(b). SNPs in immune genes oscillate across seasonal time
Changes in immune defence are at least in part due to differences in genes with immune function across space and time. Genomic screens show that immune genes are enriched across latitudinal gradients [20–23], but we did not find enrichment among immune genes in SNPs that cycle in frequency with season. Seasonal differences in immunity could arise from variation in genes not classically identified as part of the immune system and were not detected from our screen. However, the D. melanogaster immune system is well characterized and changes in even a single immune gene could affect phenotypic response to infection even without enrichment for all immune genes. Alternatively, immune changes may be controlled by non-additive genetic interactions that would not be identified in enrichment analysis.
(c). Immune survival of flies containing seasonally oscillating Tep3 haplotypes
Immune responses in the ROP were consistent with the seasonal patterns in natural populations: spring populations and flies containing the spring Tep3 haplotype had a higher defence against Gram-positive E. faecalis, whereas autumn populations and flies containing the autumn Tep3 haplotype had higher defence against Gram-negative P. rettgeri. Opposite survival patterns for flies with spring and autumn Tep3 haplotypes are consistent with antagonistic pleiotropy [57] operating across distinct branches of the immune system, limiting the host such that improvements in response to one class of pathogens (e.g. Gram-negative bacteria) restrict the ability to respond to other pathogens (e.g. Gram-positive bacteria). Trade-offs within the immune system occur in several insect systems between humoral antimicrobial peptides that combat microbial infections and phenoloxidase that is deployed against eukaryotic parasites [58–60] as well as in the T-helper cells of the vertebrate immune system (reviewed in [61]). We hypothesize that genetic variation for allocation to either immune activity may be maintained if the risk of pathogenesis changes over space or time. Additivity among the loci in response to P. rettgeri, but a non-additive response to E. faecalis, suggests that the autumn allele at 2 L:7705370, or genetic variants linked to it, has a dominant effect that decreases survival to E. faecalis infection.
Our data suggest that these Tep3 loci are natural variants in immune tolerance, because flies containing the haplotypes with the same infection load had differential survivorship. The molecular function of the seasonal loci in Tep3 remains unclear. Tep proteins are α-macroglobulin protease traps that bind to pathogen surface and act as opsonins [62–64]. The polymorphism at 2 L:7703202 produces a non-synonymous Ala/Val polymorphism at residue 18, but both amino acids are hydrophobic. The intronic SNP at 2 L:7705370 is directly upstream of the exon cassette region and may regulate expression, but Tep3 is constitutively expressed and not strongly induced by E. faecalis or P. rettgeri infection [65,66]. Therefore, the SNPs we examined may most appropriately be considered as markers for a larger haplotype that contains causal variants.
Pathogen-specific higher survival associated with spring and autumn Tep3 haplotypes may increase their frequency in the wild compared with flies containing a combination of spring and autumn alleles. Inversions could theoretically maintain LD that preserves the high-fitness spring and autumn haplotypes [67,68], but this is unlikely because the In(2 L)t inversion that contains Tep3 does not cycle with season [29,50]. Additionally, Tep3 is not located near a recombination-limiting breakpoint of In(2 L)t nor is it in LD with other seasonal immune SNPs within the inversion. However, we found that in two independent populations, alleles of the intronic SNP at 2 L: 7703202 were non-randomly distributed with respect to karyotype, while 2 L:7705730 had no significant association with either arrangement of In(2 L)t. LD might be created and maintained by selection against recombinant phenotypes either due to lower immunocompetence or another pleiotropic trait or because of intraspecific genetic incompatibilities. Deleterious incompatibilities maintain distinct haplotypes in Arabidopsis thaliana NLR immune receptors [69] and may also explain the near absence of the Tep3CG combination of spring and autumn alleles in all populations examined. Flies containing the Tep3CG haplotype appear three times across the haplotype tree constructed from the seasonal Pennsylvania inbred lines, suggesting that the haplotype may form occasionally through recombination but does not proliferate in the population. Thus, it is likely that selection for the immune benefits of spring and autumn haplotypes and against combination of spring and autumn alleles maintains these distinct haplotypes in the wild. While these Tep3 haplotypes explained some of the seasonal differences in immune tolerance of natural populations, other seasonally changing genes may also contribute to the observed differences in bacterial resistance in natural populations.
(d). Epistasis among AMP genes involved in rapid seasonal adaptation
Intergenic epistatic interactions between Tep3 and Dro6 suggest that season-specific genotypes have highest fitness. In our experiment, flies having all spring or all autumn alleles had higher survival after infection, while flies that contained a combination of spring and autumn had higher mortality. This suggests that complex genetic interactions shape winter and summer fitness, with distinct haplotypes maintained by non-additive epistatic interactions [70–72].
5. Conclusion
We demonstrate that pathogen-specific innate immunity evolves rapidly in natural populations of D. melanogaster across replicate years and geographical locations. Comparative studies across species and among populations have indicated that immune genes evolve faster than other genes in the genome, but the rapid phenotypic and genetic change we observed over approximately 10 generations is a substantially faster rate than previously considered. We tested a small subset of the immune SNPs that oscillate in allele frequency over seasonal time and observed intra- and intergenic interactions consistent with changes in immune tolerance and resistance across seasons in natural populations, perhaps in response to seasonally changing bacterial communities. Epistatic interactions among seasonally oscillating immune alleles may help facilitate this rapid phenotypic change over a short seasonal time scale. This rapid, cyclic response to biotic variables broadens our understanding of the complex ecological and genetic interactions in the evolutionary dynamics of natural populations.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Robert Unckless and two anonymous reviewers for constructive feedback on the manuscript.
Data accessibility
Raw data have been deposited with Dryad (http://dx.doi.org/10.5061/dryad.qf5m8) [73].
Author contributions
E.L.B., V.M.H., B.P.L. and P.S.S. designed the project. E.L.B. and P.S.S. collected samples, and E.L.B. and V.M.H. performed infections. F.S. analysed microbial communities. A.O.B. and D.A.P. inbred and sequenced the seasonal lines. E.L.B., M.K. and P.S.S. did data analyses. E.L.B., V.M.H., M.K., F.S., A.O.B., D.A.P., B.P.L. and P.S.S. wrote the paper.
Competing interests
The authors have no competing interests to declare.
Funding
This work was supported by NSF GRF-DGE-0822 (ELB), Rosemary Grant Award from Society for the Study of Evolution (E.L.B.), Peachey Environmental Fund (E.L.B.), NSF DEB 0921307 (P.S.S.) and NIH R01GM100366 (P.S.S. and D.A.P.).
References
- 1.Darwin C. 1859. On the origin of species by means of natural selection, or, the preservation of favoured races in the struggle for life. London, UK: J Murray. [PMC free article] [PubMed] [Google Scholar]
- 2.Grant PR, Grant BR. 2002. Unpredictable evolution in a 30-year study of Darwin's finches. Science 296, 707–711. ( 10.1126/science.1070315) [DOI] [PubMed] [Google Scholar]
- 3.Thompson JN. 2013. Relentless evolution. Chicago, IL: University of Chicago Press. [Google Scholar]
- 4.Carroll SP, Hendry AP, Reznick DN, Fox CW. 2007. Evolution on ecological time-scales. Funct. Ecol. 21, 387–393. ( 10.1111/j.1365-2435.2007.01289.x) [DOI] [Google Scholar]
- 5.Messer PW, Ellner SP, Hairston NG Jr. 2016. Can population genetics adapt to rapid evolution? Trends Genet. 32, 408–418. ( 10.1016/j.tig.2016.04.005) [DOI] [PubMed] [Google Scholar]
- 6.Endler JA. 1977. Geographic variation, speciation, and clines. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 7.Sheldon BC, Verhulst S. 1996. Ecological immunology: costly parasite defenses and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321. ( 10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
- 8.Lochmiller RL, Deerenberg C. 2000. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88, 87–98. ( 10.1034/j.1600-0706.2000.880110.x) [DOI] [Google Scholar]
- 9.Schmid-Hempel P. 2003. Variation in immune defense as a question of evolutionary ecology. Proc. R. Soc. Lond. B 270, 357–366. ( 10.1098/rspb.2002.2265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moret Y, Schmid-Hempel P. 2000. Survival for immunity: the price of immune system activation for bumblebee workers. Science 290, 1166–1168. ( 10.1126/science.290.5494.1166) [DOI] [PubMed] [Google Scholar]
- 11.Ilmonen P, Taarna T, Hasselquist D. 2000. Experimentally activated immune defence in female pied flycatchers results in reduced breeding success. Proc. R. Soc. Lond. B 267, 665–670. ( 10.1098/rspb.2000.1053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svensson E, Råberg L, Koch C, Hasselquist D. 1998. Energetic stress, immunosuppression and the costs of an antibody response. Funct. Ecol. 12, 912–919. ( 10.1046/j.1365-2435.1998.00271.x) [DOI] [Google Scholar]
- 13.Zuk M, Stoehr AM. 2002. Immune defense and host life history. Am. Nat. 160, S9–S22. ( 10.1086/342131) [DOI] [PubMed] [Google Scholar]
- 14.Fumagalli M, Sironi M, Pozzoli U, Ferrer-Admettla A, Pattini L, Nielsen R. 2011. Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS Genet. 7, e1002355 ( 10.1371/journal.pgen.1002355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McTaggart SJ, Obbard DJ, Conlon C, Little TJ. 2012. Immune genes undergo more adaptive evolution than non-immune system genes in Daphnia pulex. BMC Evol. Biol. 12, 63 ( 10.1186/1471-2148-12-63) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waterhouse RM, et al. 2007. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science 316, 1738–1743. ( 10.1126/science.1139862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crawford JE, Guelbeogo WM, Sanou A, Traoré A, Vernick KD, Sagnon N, Lazzaro BP. 2010. De novo transcriptome sequencing in Anopheles funestus using Illumina RNA-Seq technology. PLoS ONE 5, e14202 ( 10.1371/journal.pone.0014202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erler S, Lhomme P, Rasmont P, Lattorff HMG. 2014. Rapid evolution of antimicrobial peptide genes in an insect host–social parasite system. Infect. Genet. Evol. 23, 129–137. ( 10.1016/j.meegid.2014.02.002) [DOI] [PubMed] [Google Scholar]
- 19.Chávez Galarza J, Henriques D, Johnston JS, Azevedo JC, Patton JC, Muñoz I, la Rúa De P, Pinto MA. 2013. Signatures of selection in the Iberian honey bee (Apis mellifera iberiensis) revealed by a genome scan analysis of single nucleotide polymorphisms. Mol. Ecol. 22, 5890–5907. ( 10.1111/mec.12537) [DOI] [PubMed] [Google Scholar]
- 20.Juneja P, Lazzaro BP. 2010. Haplotype structure and expression divergence at the Drosophila cellular immune gene eater. Mol. Biol. Evol. 27, 2284–2299. ( 10.1093/molbev/msq114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabian DK, Kapun M, Nolte V, Kofler R, Schmidt PS, Schlötterer C, Flatt T. 2012. Genome-wide patterns of latitudinal differentiation among populations of Drosophila melanogaster from North America. Mol. Ecol. 21, 4748–4769. ( 10.1111/j.1365-294X.2012.05731.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hübner S, Rashkovetsky E, Kim YB, Oh JH, Michalak K, Weiner D, Korol AB, Nevo E, Michalak P. 2013. Genome differentiation of Drosophila melanogaster from a microclimate contrast in evolution canyon, Israel. Proc. Natl Acad. Sci. USA 110, 21 059–21 064. ( 10.1073/pnas.1321533111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolaczkowski B, Kern AD, Holloway AK, Begun DJ. 2011. Genomic differentiation between temperate and tropical Australian populations of Drosophila melanogaster. Genetics 187, 245–260. ( 10.1534/genetics.110.123059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazzaro BP, Flores HA, Lorigan JG, Yourth CP. 2008. Genotype-by-environment interactions and adaptation to local temperature affect immunity and fecundity in Drosophila melanogaster. PLoS Pathog. 4, e1000025 ( 10.1371/journal.ppat.1000025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corby-Harris V, Promislow DE. 2008. Host ecology shapes geographical variation for resistance to bacterial infection in Drosophila melanogaster. J. Anim. Ecol. 77, 768–776. ( 10.1111/j.1365-2656.2008.01399.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt PS, Conde DR. 2006. Environmental heterogeneity and the maintenance of genetic variation for reproductive diapause in Drosophila melanogaster. Evolution 60, 1602–1611. ( 10.1111/j.0014-3820.2006.tb00505.x) [DOI] [PubMed] [Google Scholar]
- 27.Behrman EL, Watson SS, O'Brien KR, Heschel MS, Schmidt PS. 2015. Seasonal variation in life history traits in two Drosophila species. J. Evol. Biol. 28, 1691–1704. ( 10.1111/jeb.12690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cogni R, Kuczynski C, Koury S, Lavington E, Behrman EL, O'Brien KR, Schmidt PS, Eanes WF. 2013. The intensity of selection acting on the couch potato gene-spatial-temporal variation in a diapause cline. Evolution 68, 538–548. ( 10.1111/evo.12291) [DOI] [PubMed] [Google Scholar]
- 29.Bergland AO, Behrman EL, O'Brien KR, Schmidt PS, Petrov DA. 2014. Genomic evidence of rapid and stable adaptive oscillations over seasonal time scales in Drosophila. PLoS Genet. 10, e1004775 ( 10.1371/journal.pgen.1004775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tinsley MC, Blanford S, Jiggins FM. 2006. Genetic variation in Drosophila melanogaster pathogen susceptibility. Parasitology 132, 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Møller AP, Martín-Vivaldi M, Merino S, Soler JJ. 2006. Density-dependent and geographical variation in bird immune response. Oikos 115, 463–474. ( 10.1111/j.2006.0030-1299.15312.x) [DOI] [Google Scholar]
- 32.Møller AP, Moller AP. 1998. Evidence of larger impact of parasites on hosts in the tropics: investment in immune function within and outside the tropics. Oikos 82, 265 ( 10.2307/3546966) [DOI] [Google Scholar]
- 33.Paparazzo F, Tellier A, Stephan W, Hutter S. 2015. Survival rate and transcriptional response upon infection with the generalist parasite Beauveria bassiana in a world-wide sample of Drosophila melanogaster. PLoS ONE 10, e0132129 ( 10.1371/journal.pone.0132129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guernier V, Hochberg ME, Guégan J-F. 2004. Ecology drives the worldwide distribution of human diseases. PLoS Biol. 2, e141 ( 10.1371/journal.pbio.0020141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schemske DW, Mittelbach GG, Cornell HV, Sobel JM, Roy K. 2009. Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Evol. Syst. 40, 245–269. ( 10.1146/annurev.ecolsys.39.110707.173430) [DOI] [Google Scholar]
- 36.Nunn CL, Altizer SM, Sechrest W, Cunningham AA. 2005. Latitudinal gradients of parasite species richness in primates. Divers. Distrib. 11, 249–256. ( 10.1111/j.1366-9516.2005.00160.x) [DOI] [Google Scholar]
- 37.Gilbert JA, Field D, Swift P, Newbold L, Oliver A, Smyth T, Somerfield PJ, Huse S, Joint I. 2009. The seasonal structure of microbial communities in the western english channel. Environ. Microbiol. 11, 3132–3139. ( 10.1111/j.1462-2920.2009.02017.x) [DOI] [PubMed] [Google Scholar]
- 38.Runckel C, Flenniken ML, Engel JC, Ruby JG, Ganem D, Andino R, DeRisi JL. 2011. Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia. PLoS ONE 6, e20656 ( 10.1371/journal.pone.0020656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maurice CF, Knowles SC, Ladau J, Pollard KS, Fenton A, Pedersen AB, Turnbaugh PJ. 2015. Marked seasonal variation in the wild mouse gut microbiota. ISME J. 9, 2423–2434. ( 10.1038/ismej.2015.53) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smits SA, et al. 2017. Seasonal cycling in the gut microbiome of the Hadza hunter–gatherers of Tanzania. Science 357, 802–806. ( 10.1126/science.aan4834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann JA, Reichhart J-M. 2002. Drosophila innate immunity: an evolutionary perspective. Nat. Immunol. 3, 121–126. ( 10.1038/ni0202-121) [DOI] [PubMed] [Google Scholar]
- 42.Leigh J, Bryant D, Steel M. 2015. PopART (Population Analysis with Reticulate Trees).
- 43.Paaby AB, Bergland AO, Behrman EL, Schmidt PS. 2014. A highly pleiotropic amino acid polymorphism in the Drosophila insulin receptor contributes to life-history adaptation. Evolution 68, 3395–3409. ( 10.1111/evo.12546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackay TFC, et al. 2012. The Drosophila melanogaster genetic reference panel. Nature 482, 173–178. ( 10.1038/nature10811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khalil S, Jacobson E, Chambers MC, Lazzaro BP. 2015. Systemic bacterial infection and immune defense phenotypes in Drosophila melanogaster. J. Vis. Exp. 99, e52613 ( 10.3791/52613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juneja P, Lazzaro BP. 2009. Providencia sneebia sp. nov. and Providencia burhodogranariea sp. nov., isolated from wild Drosophila melanogaster. Int. J. Syst. Evol. Microbiol. 59, 1108–1111. ( 10.1099/ijs.0.000117-0) [DOI] [PubMed] [Google Scholar]
- 47.Lazzaro BP, Sackton TB, Clark AG. 2006. Genetic variation in Drosophila melanogaster resistance to infection: a comparison across bacteria. Genetics 174, 1539–1554. ( 10.1534/genetics.105.054593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang W, Carbone MA, Magwire MM, Peiffer JA, Lyman RF, Stone EA, Anholt RRH, Mackay TFC. 2015. Genetic basis of transcriptome diversity in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 112, E6010–E6019. ( 10.1073/pnas.1519159112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Early AM, Clark AG. 2013. Monophyly of Wolbachia pipientis genomes within Drosophila melanogaster: geographic structuring, titre variation and host effects across five populations. Mol. Ecol. 22, 5765–5778. ( 10.1111/mec.12530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kapun M, Fabian DK, Goudet J, Flatt T. 2016. Genomic evidence for adaptive inversion clines in Drosophila melanogaster. Mol. Biol. Evol. 33, 1317–1336. ( 10.1093/molbev/msw016) [DOI] [PubMed] [Google Scholar]
- 51.Unckless RL, Rottschaefer SM, Lazzaro BP. 2015. The complex contributions of genetics and nutrition to immunity in Drosophila melanogaster. PLoS Genet. 11, e1005030 ( 10.1371/journal.pgen.1005030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shin JH, Blay S, McNeney B, Graham J. 2006. LDheatmap: an R function for graphical display of pairwise linkage disequilibria between single nucleotide polymorphisms. J. Stat. Softw. 16, 1–9. ( 10.18637/jss.v016.c03) [DOI] [Google Scholar]
- 53.Sheppard SK, Meric G.2014. Campylobacter ecology and evolution. Poole, UK: Caister Academic Press.
- 54.Early AM, Arguello JR, Cardoso-Moreira M, Gottipati S, Grenier JK, Clark AG. 2016. Survey of global genetic diversity within the Drosophila immune system. Genetics 205, 353–366. ( 10.1534/genetics.116.195016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corby-Harris V, Pontaroli AC, Shimkets LJ, Bennetzen JL, Habel KE, Promislow DE. 2007. Geographical distribution and diversity of bacteria associated with natural populations of Drosophila melanogaster. Appl. Environ. Microbiol. 73, 3470–3479. ( 10.1128/AEM.02120-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dionne M, Miller KM, Dodson JJ, Caron F, Bernatchez L. 2007. Clinal variation in MHC diversity with temperature: evidence for teh role of host-pathogen interaction on local adpatation in atlantic salmon. Evolution 61, 2154–2164. ( 10.1111/j.1558-5646.2007.00178.x) [DOI] [PubMed] [Google Scholar]
- 57.Williams GC. 1957. Pleiotropy, natural-selection, and the evolution of senescence. Evolution 11, 398–411. ( 10.1111/j.1558-5646.1957.tb02911.x) [DOI] [Google Scholar]
- 58.Moret Y, Schmid-Hempel P. 2001. Entomology: immune defence in bumble-bee offspring. Nature 414, 506 ( 10.1038/35107138) [DOI] [PubMed] [Google Scholar]
- 59.Wilfert L, Gadau J, Schmid-Hempel P. 2007. The genetic architecture of immune defense and reproduction in male Bombus terrestris bumblebees. Evolution 61, 804–815. ( 10.1111/j.1558-5646.2007.00079.x) [DOI] [PubMed] [Google Scholar]
- 60.Freitak D, Wheat CW, Heckel DG, Vogel H. 2007. Immune system responses and fitness costs associated with consumption of bacteria in larvae of Trichoplusia ni. BMC Biol. 5, 56 ( 10.1186/1741-7007-5-56) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fenton A, Lamb T, Graham AL. 2008. Optimality analysis of Th1/Th2 immune responses during microparasite-macroparasite co-infection, with epidemiological feedbacks. Parasitology 135, 841–853. ( 10.1017/S0031182008000310) [DOI] [PubMed] [Google Scholar]
- 62.Blandin S. 2004. Thioester-containing proteins and insect immunity. Mol. Immunol. 40, 903–908. ( 10.1016/j.molimm.2003.10.010) [DOI] [PubMed] [Google Scholar]
- 63.Shokal U, Kopydlowski H, Eleftherianos I. 2017. The distinct function of Tep2 and Tep6 in the immune defense of Drosophila melanogaster against the pathogen Photorhabdus. Virulence 265, 1–15. ( 10.1080/21505594.2017.1330240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shokal U, Eleftherianos I. 2017. Thioester-Containing Protein-4 regulates the Drosophila immune signaling and function against the pathogen Photorhabdus. J. Innate Immun. 9, 83–93. ( 10.1159/000450610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lagueux M, Perrodou E, Levashina EA, Capovilla M, Hoffmann JA. 2000. Constitutive expression of a complement-like protein in Toll and JAK gain-of-function mutants of Drosophila. Proc. Natl Acad. Sci. USA 97, 11 427–11 432. ( 10.1073/pnas.97.21.11427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Troha K, Im JH, Revah J, Lazzaro BP, Buchon N. Submitted Comparative transcriptomics reveals CrebA as a novel regulator of infection tolerance in D. melanogaster. PLoS Pathog. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kunte K, Zhang W, Tenger-Trolander A, Palmer DH, Martin A, Reed RD, Mullen SP, Kronforst MR. 2014. Doublesex is a mimicry supergene. Nature 507, 229–232. ( 10.1038/nature13112) [DOI] [PubMed] [Google Scholar]
- 68.Nishikawa H, et al. 2015. A genetic mechanism for female-limited Batesian mimicry in Papilio butterfly. Nat. Genet. 47, 405–409. ( 10.1038/ng.3241) [DOI] [PubMed] [Google Scholar]
- 69.Chae E, et al. 2014. Species-wide genetic incompatibility analysis identifies immune genes as hot spots of deleterious epistasis. Cell 159, 1341–1351. ( 10.1016/j.cell.2014.10.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Natarajan C, Inoguchi N, Weber RE, Fago A, Moriyama H, Storz JF. 2013. Epistasis among adaptive mutations in deer mouse hemoglobin. Science 340, 1324–1327. ( 10.1126/science.1236862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tufts DM, Natarajan C, Revsbech IG, Projecto-Garcia J, Hoffmann FG, Weber RE, Fago A, Moriyama H, Storz JF. 2014. Epistasis constrains mutational pathways of hemoglobin adaptation in high-altitude pikas. Mol. Biol. Evol. 32, 287–298. ( 10.1093/molbev/msu311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanifin CT, Gilly WF. 2015. Evolutionary history of a complex adaptation: tetrodotoxin resistance in salamanders. Evolution 69, 232–244. ( 10.1111/evo.12552/pdf) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Behrman EL, Howick VM, Kapun M, Staubach F, Bergland AO, Petrov DA, Lazzaro BP, Schmidt PS. 2018. Data from: Rapid seasonal evolution in innate immunity of wild Drosophila melanogaster Dryad Digital Repository. ( 10.5061/dryad.qf5m8) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Behrman EL, Howick VM, Kapun M, Staubach F, Bergland AO, Petrov DA, Lazzaro BP, Schmidt PS. 2018. Data from: Rapid seasonal evolution in innate immunity of wild Drosophila melanogaster Dryad Digital Repository. ( 10.5061/dryad.qf5m8) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Raw data have been deposited with Dryad (http://dx.doi.org/10.5061/dryad.qf5m8) [73].