ABSTRACT
Influenza virus hemagglutinin (HA) surface glycoprotein is currently the primary target of licensed influenza vaccines. Recently, broadly reactive antibodies that target the stalk region of the HA have become a major focus of current novel vaccine development. These antibodies have been observed in humans after natural infection with influenza A virus, but the data are limited. Using samples and data from the uniquely controlled setting of an influenza A/H1N1 virus human challenge study of healthy volunteers, we performed a secondary analysis that for the first time explores the role of anti-HA stalk antibody as a human correlate of protection. An anti-HA stalk antibody enzyme-linked immunosorbent assay (ELISA) was performed on samples from 65 participants challenged with a 2009 H1N1pdm virus. Pre- and postchallenge anti-HA stalk titers were then correlated with multiple outcome measures to evaluate anti-HA stalk antibody titer as a correlate of protection. Anti-HA stalk antibody titers were present before challenge and rose in response to challenge in 64% of individuals. Those individuals with higher titers at baseline were less likely to develop shedding, but not less likely to develop symptoms. Similar to the hemagglutination inhibition (HAI) titer, the baseline anti-HA stalk antibody titer did not independently predict a decrease in the severity of influenza disease, while the antineuraminidase (neuraminidase inhibition [NAI]) titer did. As a correlate of protection, the naturally occurring anti-HA stalk antibody titer is predictive of a reduction of certain aspects of disease similar to HAI titer, but the NAI titer is the only identified correlate that is an independent predictor of a reduction of all assessed influenza clinical outcome measures.
KEYWORDS: CHIM, HA stalk, NA, antibody, human challenge, influenza, influenza A, neuraminidase, universal vaccine
IMPORTANCE
This is the first study to evaluate preexisting anti-HA stalk antibodies as a predictor of protection. We use a healthy volunteer influenza challenge trial for an examination of the role such antibodies play in protection. This study demonstrates that anti-HA stalk antibodies are naturally generated in response to an infection, but there is significant variability in response. Similar to antibodies that target the HA head, baseline anti-HA stalk antibody titer is a correlate of protection in terms of reduced shedding, but it is not a predictor of reduced clinical disease or an independent predictor of disease severity. These results, in the context of the limited data available in humans, suggest that vaccines that induce anti-HA stalk antibodies could play a role in future vaccine strategies, but alone, this target may be insufficient to induce a fully protective vaccine and overcome some of the issues identified with current vaccines.
INTRODUCTION
The influenza virus hemagglutinin (HA) surface glycoprotein is currently the primary target of all licensed vaccines for influenza and considered the dominant antigen to which individuals develop a humoral immune response. Until recently, antibodies to the antigenically and genetically variable head region of the HA have been the major focus of influenza serological studies and vaccine development, specifically antibodies that sterically inhibit hemagglutinin receptor binding, utilizing the hemagglutination inhibition (HAI) assay to assess antibody titers. Over the course of the past decade, there has been more focus on discovery of antibodies that target the more conserved stalk (or stem) region of the HA (1). These antibodies can be broadly neutralizing across one of two groups of the 18 known influenza A virus (IAV) HA subtypes, group 1, which includes seasonal human H1 and highly pathogenic avian H5, and group 2, which includes seasonal H3 and the avian H7 subtype that has caused increasing numbers of human infections during the 2016–2017 influenza season in the form of an avian H7N9 IAV throughout China (2). These broadly neutralizing anti-HA stalk antibodies have been hypothesized to have played a major role in the extinction of the previously circulating prepandemic seasonal H1N1 lineage after the 2009 H1N1pdm virus emerged (3). The possibility that these broadly neutralizing anti-stalk antibodies that bind to conserved epitopes of group 1 or group 2 HA stalk could elicit broadly protective responses has led a number of groups to investigate novel vaccines and therapeutics based on anti-stalk antibody technology (1, 4, 5). Broad protection in animal models with anti-stalk antibody-based vaccines/constructs has been shown (4), and some of these investigational products are in preparation for future clinical trial evaluation.
Anti-stalk antibodies that are broadly reactive and neutralizing in vitro and in vivo have been observed to occur naturally after infection in small studies of individuals infected with seasonal viruses, but these antibodies do not seem to be elicited significantly by seasonal vaccine (3, 6, 7). In addition, these anti-stalk antibodies have been identified in 18 patients infected with H7N9 strains in China (8), an ongoing emerging threat with pandemic potential. Insufficient serological data have been available in humans to evaluate whether anti-stalk antibodies serve as correlates of protection against influenza virus infection. Further investigation is needed to elucidate what role they play in immunity during naturally occurring infections and importantly whether novel anti-stalk-based vaccine candidates could elicit titers higher than those observed in natural infections.
The Healthy Volunteer H1N1 Influenza Challenge Model developed at the National Institutes of Health (NIH) Clinical Center (9) offers a unique opportunity to explore influenza pathophysiology and correlates of protection/disease modification in a controlled setting. Recently, it was demonstrated in the H1N1 challenge model that HAI titer and neuraminidase inhibition (NAI) titer, a measure of anti-NA antibody, predicted protection from different aspects of influenza disease (10). Using serum samples as well as clinical and virological data from this previous study, we performed a secondary analysis to explore the role of anti-HA stalk antibody in protection from and modification of various aspects of influenza disease and compared this to the previous measures of anti-HA head antibody as well as anti-NA antibody as correlates of protection and disease modification.
RESULTS
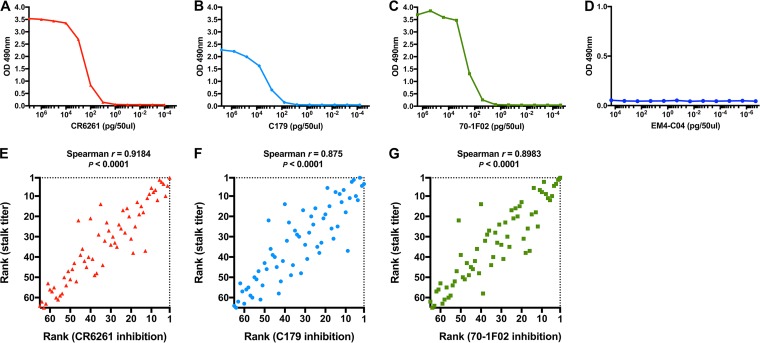
Validation of the anti-HA stalk enzyme-linked immunosorbent assay (ELISA) used in this study demonstrated that the assay was capable of measuring relevant anti-stalk antibodies that bind to important conformational epitopes on group 1 HA stalk. Antigen coating conditions for ELISA used in this study maintained important conformational epitopes of group 1 HA stalk as shown by ELISA using three well-known potent neutralizing monoclonal antibodies: CR6261, C179, and 70-1F02 (Fig. 1A to D). Inhibition ELISAs were performed that measured inhibition levels of serum samples to CR6261, C179, and 70-1F02 antibodies and resulted in strong positive correlations (P < 0.0001* [statistically significant P values are indicated with an asterisk throughout]) observed between the stalk antibody titers and the level of inhibition to these monoclonal antibodies (Fig. 1E to G). These validation results confirm that the ELISA used on the clinical samples allows assessment of the level of antibodies binding to key epitopes of the influenza HA stalk.
FIG 1 .
Evaluation of ELISA. Group 1 HA stalk antigen coated onto the wells of an ELISA plate maintained important conformational epitopes. (A to C) ELISA binding of group 1 HA stalk-binding monoclonal antibodies (in picograms per 50 μl) was measured using serial dilutions of monoclonal antibodies CR6261 (A), C179 (B), and 70-1F02 (C). (D) H1 HA globular head-binding monoclonal antibody EM4CO4 was used as a negative control. Anti-HA stalk serum titers measured from patient samples in this study showed strong positive correlation with the level of inhibition to potent group 1 HA stalk-binding monoclonal antibodies. Samples were ranked by the anti-HA stalk antibody titer and the percent inhibition to stalk-binding monoclonal antibodies for Spearman’s rank correlation analysis. (E to G) Increasing anti-HA stalk titer showed a strong and significant tendency of increasing inhibition of CR6261 (E), C179 (F), and 70-1F02 (G) binding.
The median age of clinical study volunteers was 27 years, and 48.6% were female and 51.4% were male. Of the volunteers, 43.2% were white, 48.6% were black, 6.8% were Asian, and 1.4% were American Indian. Of all subjects, 9.5% were Hispanic. No significant correlations were observed between prechallenge or postchallenge anti-HA stalk titers in relation to age, sex, or race.
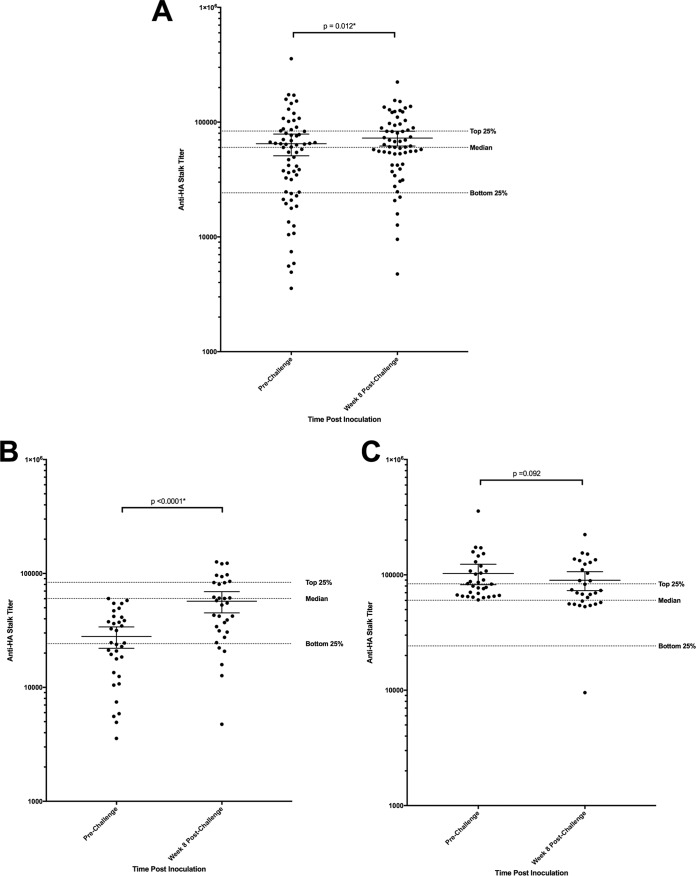
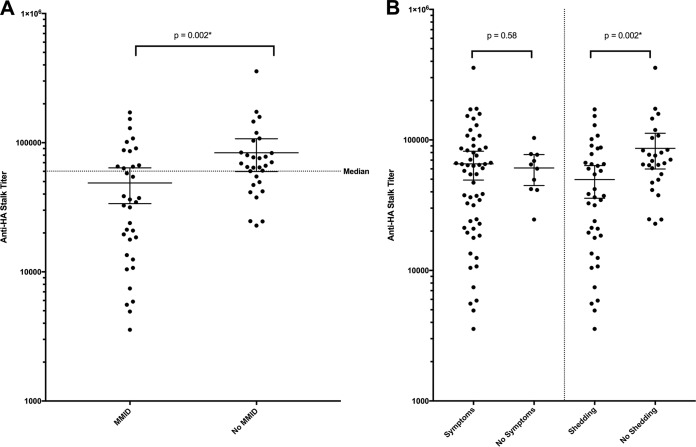
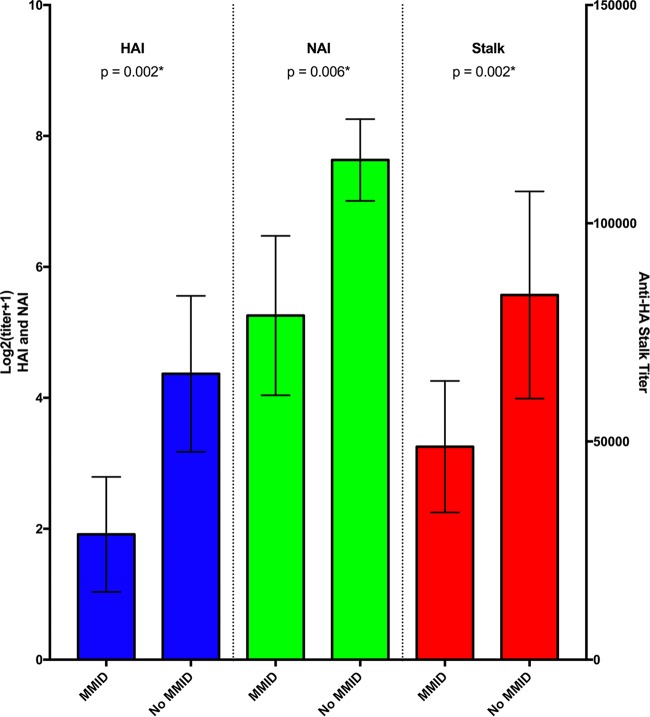
The mean anti-HA stalk antibody titer for the 65 participants prior to influenza challenge was observed to be 64,843 (median, 60,221), with a minimum titer of 3,562 and maximum titer of 356,879. The lowest quartile of participants had a titer below 24,209, and the highest quartile had a titer above 83,585 (Fig. 2). Anti-HA stalk antibody titers increased after challenge in 64% of subjects. A statistically significant increase in mean titer was observed overall to a mean titer of 72,637 by week 8 (P = 0.012*) (Fig. 2A). Individuals with a prechallenge titer below the median titer demonstrated a larger significant increase from 28,010 to 57,116 (P < 0.0001*) (Fig. 2B), while those with prechallenge titers above the median titer demonstrated no significant change in mean titer (P = 0.092) (Fig. 2C). Participants who developed mild to moderate influenza disease (MMID) (n = 35) were found to have a significantly lower mean anti-HA stalk antibody titer prechallenge than those who did not develop MMID (n = 30) (P = 0.002*) (Fig. 3A). This was primarily driven by a difference in mean prechallenge anti-HA stalk antibody between those who developed detectable shedding (n = 38) and those who did not (n = 27) (P = 0.002*) (Fig. 3B). There was no significant difference in anti-HA stalk antibody titers between those who developed influenza symptoms (n = 55) and those who did not develop influenza symptoms (n = 10) (P = 0.58) (Fig. 3B). This difference in mean prechallenge titer in those who developed MMID versus those who did not develop MMID was similar to differences observed in HAI and NAI titers as well (Fig. 4).
FIG 2 .
Anti-HA stalk antibody titers pre- and postchallenge. (A) Mean titer with 95% confidence intervals for all participants both prechallenge and 8 weeks postchallenge with 2009 H1N1pdm. This increase was significant (P = 0.012*). Each circle represents the value for an individual. (B) Mean titer with 95% confidence intervals for all participants who demonstrated a prechallenge titer less than the median prechallenge titer of 60,221. They had a larger increase postchallenge (P < 0.0001*). (C) Mean titer with 95% confidence intervals for all participants that demonstrated a prechallenge titer above the median titer (>60,221). These individuals demonstrated no significant increase in the overall titer (P = 0.092). The dotted lines represent the bottom 25%, median, and top 25% of all prechallenge anti-HA stalk antibody titers. Statistically significant P values are indicated with an asterisk.
FIG 3 .
Anti-HA stalk titers observed based on clinical outcome categories. (A) Mean prechallenge anti-HA stalk titer in those participants who experienced MMID and those who did not (P = 0.002*). The dotted line represents the median titer of all participants prechallenge. Black bars represent 95% confidence intervals. Thirty-four percent (12 of 35) of the participants who experienced MMID had titers above the median titer. (B) Mean prechallenge anti-HA stalk titer in those participants who experienced influenza symptoms versus those who did not (P = 0.58) and in those who experienced detectable viral shedding versus those who did not (P = 0.002*). Black bars represent 95% confidence intervals. Statistically significant P values are indicated with an asterisk.
FIG 4 .
Anti-HA stalk antibody titers observed based on clinical outcome categories compared to HAI and NAI titers. Mean prechallenge hemagglutinin inhibition (HAI) titer, neuraminidase inhibition (NAI) titer, and anti-HA stalk titer in those who experienced MMID (defined as experiencing symptoms and detectable shedding) versus those who did not. Black bars represent 95% confidence intervals. Statistically significant P values are indicated with an asterisk.
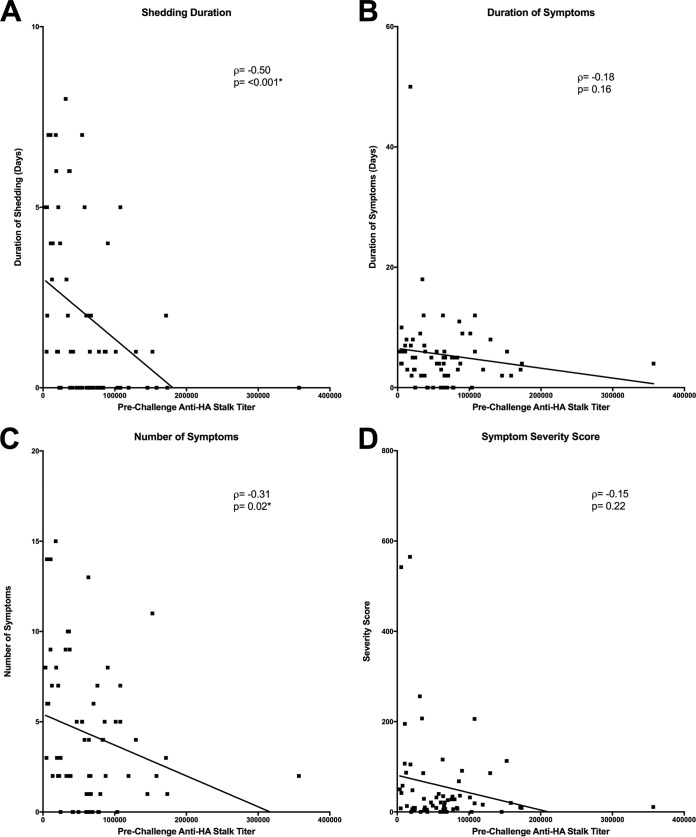
Two variable correlation analyses demonstrated that anti-HA stalk antibody titer was negatively correlated with shedding duration (P < 0.001*) (Fig. 5A), but not to duration of symptoms (P = 0.16) (Fig. 5B). Although there was a small but significant negative correlation to reduction in the number of symptoms (P = 0.02*) (Fig. 5C), there was no correlation to reduction in symptom severity (P = 0.22) (Fig. 5D). Previous multiple regression analysis to measure the independent effects of HAI and NAI on all four of these disease severity measures demonstrated that only increasing NAI titers had a statistically significant independent effect on decreasing severity of disease by all four of these clinical disease outcome measures (10). When this type of analysis was repeated in the current study with anti-HA stalk antibody titers included, a similar result was observed, demonstrating that only NAI titer was shown to be an independent predictor of a reduction in all four disease outcome measures assessed, while no statistically independent effect of either HAI or anti-HA stalk antibody titers was observed (Table 1).
FIG 5 .
Linear correlation of anti-HA stalk titers to influenza disease severity measures. (A to D) Anti-HA stalk antibody had an observed significant negative correlation to shedding duration (A), and number of symptoms (C) but was not correlated with a reduction in duration of symptoms (B) or symptom severity (D). Statistically significant P values are indicated with an asterisk.
TABLE 1 .
Multiple regression analysis of the contribution of prechallenge HAI titer, NAI titer, and anti-HA stalk titer in reduction of disease severity
| Disease severity metric | Constant or titer | Ba | Betaa | SE | P valueb | 95% CIc |
|
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Duration of shedding | Constant | 9.285 | 3.0714 | 0 | 3.144 | 15.427 | |
| HAI titer | −0.0838 | −0.1137 | 0.0818 | 0.310 | −0.2475 | −0.0799 | |
| NAI titer | −0.3484 | −0.4581 | 0.0880 | <0.001* | −0.5381 | −0.1588 | |
| Anti-HA stalk titer | −0.3080 | −0.1769 | 0.2209 | 0.168 | −0.7497 | 0.1337 | |
| Duration of symptoms | Constant | 3.969 | 10.222 | 0 | −16.472 | 24.411 | |
| HAI titer | −0.2294 | −0.1194 | 0.2726 | 0.403 | −0.7744 | 0.3155 | |
| NAI titer | −0.8259 | −0.3906 | 0.3157 | 0.011* | −1.4570 | −0.1946 | |
| Anti-HA stalk titer | 0.4987 | 0.1031 | 0.7353 | 0.500 | −0.9715 | 1.9690 | |
| No. of symptoms | Constant | 12.951 | 5.652 | 0 | 1.648 | 24.254 | |
| HAI titer | −0.0642 | −0.0536 | 0.1507 | 0.671 | −0.3655 | 0.2371 | |
| NAI titer | −0.4722 | −0.3823 | 0.1745 | 0.008* | −0.8212 | −0.1232 | |
| Anti-HA stalk titer | −0.3428 | −0.1213 | 0.4065 | 0.402 | −1.1557 | 0.4702 | |
| Symptom severity score | Constant | 251.58 | 156.4 | 0 | −61.213 | 564.379 | |
| HAI titer | 1.097 | 0.0339 | 4.171 | 0.793 | −7.2432 | 9.4365 | |
| NAI titer | −13.28 | −0.3987 | 4.830 | 0.007* | −22.9382 | −3.6218 | |
| Anti-HA stalk titer | −7.167 | −0.0940 | 11.251 | 0.526 | −29.6654 | 15.3314 | |
B represents the unstandardized regression coefficient, and Beta represents the standardized coefficient which has standard deviation as a unit of measure.
Statistically significant P values are indicated with an asterisk.
95% CI, 95% confidence interval.
In our previous report, we identified individuals with a prechallenge HAI titer of <1:40 who had no increase in HAI titer after challenge with influenza (10). These individuals had baseline prechallenge anti-HA stalk antibody titers that varied greatly and included some in the top 25%, bottom 25%, and closer to the median (Fig. 6). Compared to those with low HAI titer who did have HAI increases after challenge, there was no significant difference in mean anti-HA stalk titer prechallenge (P = 0.629) (Fig. 6). A statistically significant increase in mean anti-HA stalk titer by week 8 was noted in those individuals who had HAI responses (P < 0.001*), while no significant increase was noted in the nonresponder group (P = 0.063). In those who did not have increases in HAI antibody after challenge and had prechallenge anti-HA stalk titers below the median, many did not have an increased titer and their titer remained below the median titer or in the bottom 25% of titers (Fig. 6).
FIG 6 .
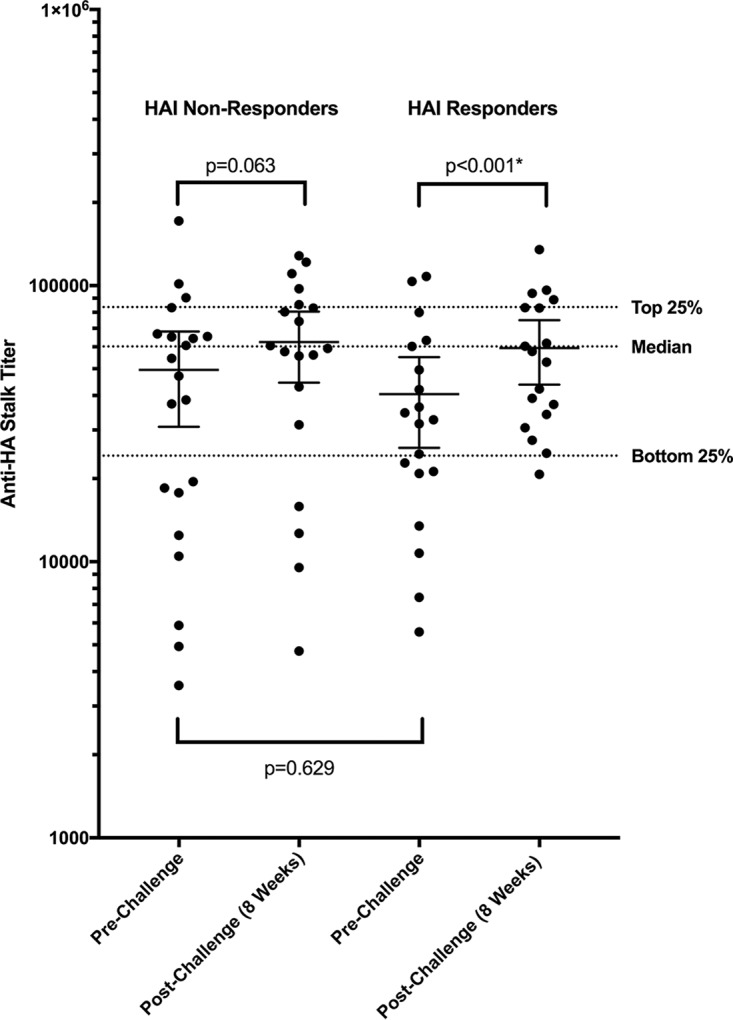
Mean anti-HA stalk titer in those individuals with a prechallenge HAI titer of <1:40 who did not develop HAI increases after challenge and those who did. Of those individuals with low prechallenge HAI titers who did or did not have increases in HAI titer after challenge, we observed variable levels of prechallenge anti-HA stalk antibody titer and no statistical difference in prechallenge anti-HA stalk antibody titer (P = 0.629). Those who responded after challenge by HAI demonstrated a significant increase in mean anti-HA stalk titer (P < 0.001*), while those who did not demonstrate a good HAI response did not (P = 0.063). The dotted lines represent the bottom 25%, median, and top 25% of all prechallenge anti-HA stalk antibody titers. Black bars represent 95% confidence intervals. Statistically significant P values are indicated with an asterisk.
DISCUSSION
A healthy volunteer influenza challenge study afforded an ideal opportunity to perform an evaluation of naturally occurring anti-HA stalk antibodies in healthy individuals in a controlled setting. The ability to measure both pre- and postexposure polyclonal anti-HA stalk antibody titer and outcomes in subjects with a wide range of prechallenge antibody titers allowed us, for the first time, to measure anti-HA stalk antibody responses after infection/exposure to IAV. This enabled us to evaluate whether preexposure levels of these antibodies could predict protection from disease or modification of illness after intranasal challenge. This type of analysis is of critical importance to better understand how new influenza vaccines that may induce anti-HA stalk antibodies as their primary mechanism of action may perform and what pitfalls and hurdles may exist with this strategy.
The data generated here clearly demonstrate that many, if not all, healthy individuals likely have detectable levels of circulating anti-HA stalk antibody in their serum and that increases in titer after intranasal challenge and infection vary from person to person, similar to titers of anti-HA head antibody as assessed by HAI (Fig. 2). After challenge with an H1N1 virus, 64% of participants developed increases in their anti-HA stalk titer from their prechallenge baseline, demonstrating that natural generation of these antibodies in response to an IAV infection/exposure occurs in the majority of individuals after infection/exposure (Fig. 2). However, we observed variability not only in baseline circulating titers of anti-HA stalk antibody but also in the quantity of the postinfection/exposure change in anti-HA stalk antibody. Those individuals with the highest levels of anti-HA stalk antibodies before exposure to influenza developed smaller increases in titer, suggesting there may be a limitation to how high a titer of anti-HA stalk antibody could be achieved naturally. This type of antibody ceiling phenomenon is consistent with what has been observed previously, as there does seem to be a limit to how high anti-HA head antibodies will increase after traditional vaccination (11, 12). In addition, variation between individuals was observed in terms of response and included a subset of individuals with a low prechallenge HAI titer and anti-HA stalk titer who did not have much increase in anti-HA stalk titer despite exposure/infection with IAV (Fig. 6). This is quite similar to the subset of individuals who demonstrated no increase in HAI titer after exposure to influenza reported previously (10).
These data suggest that antibody responses to HA stalk antigenic epitopes may be analogous to antibody responses to the HA head epitopes measured by HAI, namely, that there may be limited achievable levels and that those individuals who do not respond well by HAI to HA head antigens after infection/exposure/vaccination may also in some cases not respond well to HA stalk antigens. This would indicate that the limitations, inconsistencies, and pitfalls identified in response to current vaccines that primarily target antigenic sites on the HA head region may also still be problematic with new generations of anti-stalk vaccines. The fact that 10 to 30% of healthy individuals have poor HAI responses after vaccination, and that this response rate is even worse in the elderly (13), may mean that vaccines that solely target HA head or stalk may not be adequate to fully protect many of the individuals who are at risk of severe consequences of influenza.
As a predictor of protection from or modification of disease, naturally occurring anti-HA stalk antibody titers displayed similar predictive qualities as anti-HA head antibody titers. As with baseline HAI and NAI titers, the mean anti-HA stalk antibody titer at baseline was higher in those individuals who did not exhibit MMID than in those who did (Fig. 3A). This difference in MMID was completely driven by a reduction in shedding, with a significant difference seen in mean baseline anti-HA stalk titer between those with detectable shedding and those without detectable shedding (Fig. 3B). This observation mimics anti-HA head antibody as measured by HAI as a correlate to reduction in MMID and detection of shedding as reported previously (10). In addition, there was no significant difference in the mean anti-HA stalk titer in terms of the presence of symptoms (Fig. 3B). Some individuals with very high baseline anti-HA stalk titers demonstrated clinically symptomatic influenza, with 12 participants demonstrating MMID despite having anti-HA stalk titers above the median titer and in the same titer range as those who did not experience MMID (Fig. 3A). This again is very similar to what was observed previously when evaluating baseline HAI titers in those with or without clinically symptomatic influenza disease (10).
Baseline anti-HA stalk antibody titer was negatively correlated with shedding duration similar to baseline HAI and NAI titers (10) and also demonstrated a small but significant negative correlation with the number of symptoms (Fig. 5). No significant correlation was seen with duration of symptoms or severity of symptoms (Fig. 5). This suggests that similar to HAI titer, a higher baseline anti-HA stalk antibody titer can be used to predict that an individual will have a reduced duration of shedding of influenza virus, but not necessarily a significant reduction in duration or severity of illness, whereas NAI was previously demonstrated to predict a reduction of shedding duration, symptom duration, and a reduction in symptom severity (10). In addition, multiple regression with all three baseline antibody titers (HAI, NAI, and anti-HA stalk antibody) once again demonstrated that NAI titer was the only independent correlate that predicted a reduction in all four disease measures assessed (Table 1), suggesting that NAI may be the best currently available predictor of a reduction of all aspects of influenza A virus clinical disease.
The naturally occurring anti-HA stalk antibodies measured in this study in response to infection likely reflected a polyclonal response, and what portion of these anti-stalk antibodies may be neutralizing and/or broadly reactive against other group 1 HA subtypes is not yet known, although inhibition ELISAs showed significant correlation to binding mapped epitopes recognized by well-studied monoclonal antibodies (Fig. 1). These naturally generated anti-HA stalk responses might be somewhat different from responses from some of the proposed anti-HA stalk-based vaccine strategies currently being evaluated, especially those that might seek to induce immunity to conserved peptide motifs rather than the intact HA stalk. Therefore, it is possible that these future vaccine strategies could potentially be more broadly reactive or induce a higher level of neutralizing anti-stalk antibody titers than those produced by natural infection. However, the current data do suggest that vaccine strategies designed to generate broadly neutralizing anti-HA stalk antibodies may have some limitations, many of which seem to be similar to those seen with current seasonal vaccines that primarily target anti-HA head antibodies and must be considered.
Conclusion.
Anti-stalk-based vaccine strategies may be an improvement over current seasonal vaccines, as there are significant data to suggest that these vaccines could offer broader protection (5–8, 14–16), but the data for humans is limited, and it is yet to be determined if these vaccines will be more effective in controlling annual influenza epidemics or able to prevent the emergence of pandemics. This study is the first study of humans to evaluate naturally occurring anti-HA stalk antibody as a correlate of protection and disease modification of IAV infection. In this study, we observed that individuals with some of the highest levels of preexisting anti-HA stalk immunity may still develop significant IAV illness and that anti-HA stalk antibody was similar to anti-HA head antibody in that it predicted a reduction in shedding but was not an independent predictor of modification of human IAV disease.
It is likely that including targets that induce anti-HA stalk antibody could play an important role in future broadly protective or universal vaccine strategies, but this analysis demonstrates that antibody responses to influenza virus vary and that protection is likely to be complex and multifactorial. Our belief is that future universal vaccine strategies should ideally focus on more than one target and/or aspect of immunity. In terms of antibodies elicited by vaccines, these data suggest that targeting the generation of not only anti-HA stalk antibody but also anti-NA antibody, observed to be the only independent predictor of a reduction of all aspects of IAV disease in this study, may augment protective efficacy. Careful consideration of the complexity of influenza immune protection and evaluation of all aspects of the anti-influenza virus immune responses will ultimately be necessary in the development of a successful broadly protective or universal influenza vaccine.
MATERIALS AND METHODS
Clinical study.
A healthy volunteer challenge study was performed at the NIH Clinical Center, and healthy volunteers between the ages of 18 to 50 years were enrolled and intranasally inoculated with wild-type influenza A/H1N1pdm virus. This clinical study and the primary results were described in detail in a previous report (10). In this study, multiple clinical endpoints were measured including the presence or absence of mild to moderate influenza disease (MMID) defined as a positive molecular clinical test for influenza plus symptoms, symptom severity based on the inFLUenza patient-reported outcome (FLU-PRO) symptom assessment tool (17, 18), presence or absence of symptoms or shedding alone, duration of symptoms, and duration of shedding. This study (clinicaltrials.gov identifier NCT01971255) was approved by the NIAID Institutional Review Board and was conducted in accordance with the provisions of the Declaration of Helsinki and good clinical practice guidelines.
Immunologic assays.
Anti-hemagglutinin (HA) stalk antibody titers were determined for the 65 participants challenged with A/H1N1pdm using an enzyme-linked immunosorbent assay (ELISA) method as described below. Measurements of hemagglutination inhibition (HAI), neuraminidase inhibition (NAI), and viral shedding used for this analysis were detailed previously (10).
Production of ELISA stalk antigen.
A group 1 influenza stalk construct without the HA head was produced based on a previously described method (4) with minor modifications. The group 1 HA stalk-only construct, designated construct #4900 by Impagliazzo et al. (4) was cloned into the pFastBac1 vector (catalog no. 10360014; ThermoFisher Scientific, USA) with the Strep-Tag II (Trp-Ser-His-Pro-Gln-Phe-Glu-Lys) sequence at the 3′ end instead of the hexahistidine tag sequence. Recombinant baculovirus containing construct #4900 with Strep-Tag II (rBV_#4900_StrepII) was generated using the Bac-to-Bac baculovirus expression system (catalog no. 10359016; ThermoFisher). Sf-9 insect cells maintained in Sf-900 III medium (catalog no. 12658027; ThermoFisher) were infected with the rBV_#4900_StrepII at a multiplicity of infection (MOI) of approximately 10. Three days after infection, Sf-9 cell culture supernatant was harvested and clarified by centrifugation (3,000 × g, 20°C, 10 min). The group 1 influenza stalk construct was purified using Strep-Tactin Sepharose (IBA GmbH, Germany). Concentration and buffer exchange to phosphate-buffered saline (PBS) was done using Vivaspin 20 (10,000 [10K]-molecular-weight cutoff [MWCO]) (catalog no. VS2001; Sartorius, Germany). A bicinchoninic acid (BCA) protein assay kit (catalog no. 23225; ThermoFisher) was used to measure the concentration of the purified stalk construct.
Anti-stalk antibody ELISA.
A purified group 1 influenza stalk construct was diluted in PBS (1 µg/ml) and added to a 96-well ELISA plate (50 µl/well) (catalog no. 456537; ThermoFisher). The plates were incubated overnight at 4°C, and 100 µl of blocking buffer (1% BSA in PBS) was added to each well. After incubation with the blocking buffer (room temperature [RT], 30 min), the plates were washed three times with wash buffer (0.05% Tween 20 in PBS), followed by blotting. Serum samples from patients were diluted 1:100 with the antibody diluent (1% BSA and 0.05% Tween 20 in PBS) and added to the washed plates (100 µl/well). The dilution factor of 100 was determined by pretesting every serum sample to avoid saturation of the ELISA reaction. After incubation (37°C, 2 h), the plates were washed three times, followed by blotting. To make measurements of IgG bound to the group 1 influenza stalk construct, goat anti-human IgG antibody (catalog no. 62-8400; ThermoFisher) was labeled with horseradish peroxidase (HRP) using an HRP conjugation kit (catalog no. ab102890; Abcam, USA). The estimated concentration was 0.83 mg/ml. The HRP-conjugated anti-human IgG antibody was diluted 1:10,000 with the antibody diluent and added to the washed plates (100 µl/well). After incubation (37°C, 1 h), the plates were washed six times and blotted, and the HRP substrate solution was added (100 µl/well). The substrate solution was prepared by adding a 10-mg o-phenylenediamine dihydrochloride (OPD) tablet (catalog no. P8287; Sigma-Aldrich, USA) to 20 ml of a phosphate-citrate buffer preparation (catalog no. P4922; Sigma-Aldrich, USA). After incubation with the substrate (20°C, 30 min), 100 µl of 1 M sulfuric acid was added to each well to stop the reaction, and the optical density at 490 nm (OD490) was measured.
Calculation of anti-HA stalk antibody titers.
Antibody titers were calculated using an extrapolation method. Four serum samples with the highest reactivity to the group 1 influenza stalk construct were preselected using the ELISA described. Equal volumes of these four sera were pooled, designated standard serum, and used to generate a standard curve for each plate. To determine the titer, the standard serum was 3-fold diluted from the initial 100-fold dilution. The OD490 of each dilution was measured using the ELISA described, and the cutoff value was set as the mean OD plus 3 standard deviations (SD) of the wells containing secondary antibody only. The titer was defined as the highest dilution factor to produce an OD value above the cutoff value. The titer of this standard serum was 218,700. Serial dilutions of standard serum were added to each plate to generate a standard curve. The anti-HA stalk antibody titer of each sample was calculated from the sample OD490 using this standard curve. The anti-HA stalk antibody titers were measured in triplicate, and means of the replicates were used for further analysis.
Validation of the anti-stalk antibody ELISA.
To ensure the conformational integrity of the group 1 stalk antigen after hydrophobic binding to the ELISA plate, we performed a set of ELISAs using three well-known monoclonal antibodies binding to conformational epitopes on the stalk: CR6261 (Janssen, Netherlands), C179 (Clontech Laboratories, USA), and 70-1F02 (19). Monoclonal EM-4C04 antibody (19) binding to a globular head region of H1 HA was used as a negative control (70-1F02 and EM-4C04 were a kind gift from Rafi Ahmed at the Emory Vaccine Center). The ELISA plate was prepared as described above. Serial 10-fold dilutions of each monoclonal antibody were made with the antibody diluent and added to the washed plates (50 µl/well). After incubation (37°C, 2 h), the plates were washed three times, followed by blotting. Antibodies bound to the stalk antigen were detected using HRP-conjugated anti-human IgG secondary antibody (catalog no. A18811; ThermoFisher) for antibodies CR6261, 70-1F02, and EM-4C04. HRP-conjugated anti-mouse IgG secondary antibody (catalog no. A28177; ThermoFisher) was used for C179. Each secondary antibody was diluted 1:10,000 in antibody diluent and added to the plates (100 µl/well). After 1 h at 37°C, the plates were washed six times, followed by blotting, and the colorimetric signal from each well at OD490 was measured as described above.
To analyze a correlation between the anti-HA stalk antibody titer measured and the inhibition level to stalk-binding monoclonal antibodies, we performed a set of inhibition ELISAs. ELISA plates were prepared as described above, and the prechallenge serum samples were diluted 1:25 and added to the plates (50 µl/well) to block epitopes of the stalk antigen. Serum diluent, without serum, was added to 10 control wells (50 µl/well) in each plate to serve as a baseline of monoclonal antibody binding level without inhibition. After incubation (37°C, 2 h), the plates were washed three times, followed by blotting. CR6261, C179, and 70-1F02 monoclonal antibodies were labeled with HRP conjugation kit (ab102890; Abcam), diluted in serum diluent, and added to the plates (50 µl/well). The estimated concentrations of HRP-conjugated CR6261, C179, and 70-1F02 after dilution were 0.042, 1.042, and 0.042 µg/ml, respectively. After incubation (1 h, 37°C), the plates were washed six times and blotted, and the levels of monoclonal antibody binding were colorimetrically measured at OD490 as described above. The percent inhibition was calculated as 100 − (ODsample/ODcontrol × 100) where ODsample is the optical density of the sample and ODcontrol is the optical density of the control. For the correlation analysis, samples were separately ranked by anti-HA stalk antibody titer and the percent inhibition to each monoclonal antibody. The samples with the highest values were given a ranking of 1, and the samples with the lowest values were given a ranking of 65.
Statistical analysis.
Two variable correlations between the anti-HA stalk titer and the percent inhibition to each monoclonal antibody were performed using the nonparametric Spearman’s correlation coefficient during the validation process of the anti-HA stalk antibody assay. Changes in anti-HA stalk antibody titer in human volunteers prechallenge to postchallenge were evaluated for significance using the Wilcoxon signed-rank test. Differences in mean anti-HA stalk antibody titers based on binary outcome measures used to evaluate influenza disease were evaluated using the Wilcoxon rank sum test. Two variable correlations of all four nonbinary clinical outcome measures to anti-HA stalk titer were performed using the nonparametric Spearman’s correlation coefficient, and multiple regression analysis was performed using a linear model to examine independent effects of anti-HA stalk antibody and HAI and NAI titer on disease severity outcomes. All tests were two sided and at the 0.05 significance level. Statistical analyses were performed using R (version 3.0.1; R Development Core Team, Vienna, Austria) and GraphPad Prism software (version 7.0h; La Jolla, CA).
ACKNOWLEDGMENTS
This work was supported in part by the intramural funds of the NIH and the NIAID and also with support from the Biomedical Advanced Research and Development Authority (BARDA).
We acknowledge H. Clifford Lane, Richard T. Davey, the NIH Clinical Center Special Clinical Studies Unit staff, and the Department of Laboratory Medicine for their support of the clinical protocol.
Footnotes
Citation Park J-K, Han A, Czajkowski L, Reed S, Athota R, Bristol T, Rosas LA, Cervantes-Medina A, Taubenberger JK, Memoli MJ. 2018. Evaluation of preexisting anti-hemagglutinin stalk antibody as a correlate of protection in a healthy volunteer challenge with influenza A/H1N1pdm virus. mBio 9:e02284-17. https://doi.org/10.1128/mBio.02284-17.
REFERENCES
- 1.Neu KE, Henry Dunand CJ, Wilson PC. 2016. Heads, stalks and everything else: how can antibodies eradicate influenza as a human disease? Curr Opin Immunol 42:48–55. doi: 10.1016/j.coi.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou L, Ren R, Yang L, Bao C, Wu J, Wang D, Li C, Xiang N, Wang Y, Li D, Sui H, Shu Y, Feng Z, Li Q, Ni D. 2017. Sudden increase in human infection with avian influenza A(H7N9) virus in China, September-December 2016. Western Pac Surveill Response J 8:6–14. doi: 10.5365/WPSAR.2017.8.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, Banach D, Wrammert J, Belshe RB, García-Sastre A, Palese P. 2012. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A 109:2573–2578. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Impagliazzo A, Milder F, Kuipers H, Wagner MV, Zhu X, Hoffman RM, van Meersbergen R, Huizingh J, Wanningen P, Verspuij J, de Man M, Ding Z, Apetri A, Kükrer B, Sneekes-Vriese E, Tomkiewicz D, Laursen NS, Lee PS, Zakrzewska A, Dekking L, Tolboom J, Tettero L, van Meerten S, Yu W, Koudstaal W, Goudsmit J, Ward AB, Meijberg W, Wilson IA, Radošević K. 2015. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 349:1301–1306. doi: 10.1126/science.aac7263. [DOI] [PubMed] [Google Scholar]
- 5.Nachbagauer R, Krammer F. 2017. Universal influenza virus vaccines and therapeutic antibodies. Clin Microbiol Infect 23:222–228. doi: 10.1016/j.cmi.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, Palucka K, García-Sastre A, Palese P, Treanor JJ, Krammer F. 2013. H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J Virol 87:4728–4737. doi: 10.1128/JVI.03509-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, Denny TN, Chen X, Munshaw S, Marshall DJ, Whitesides JF, Drinker MS, Amos JD, Gurley TC, Eudailey JA, Foulger A, DeRosa KR, Parks R, Meyerhoff RR, Yu JS, Kozink DM, Barefoot BE, Ramsburg EA, Khurana S, Golding H, Vandergrift NA, Alam SM, Tomaras GD, Kepler TB, Kelsoe G, Liao HX, Haynes BF. 2011. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One 6:e25797. doi: 10.1371/journal.pone.0025797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, Nachbagauer R, Zhu L, Huang Y, Xie X, Jin S, Zhang A, Wan Y, Hirsh A, Tian D, Shi X, Dong Z, Yuan S, Hu Y, Krammer F, Zhang X, Xu J. 2017. Induction of broadly cross-reactive stalk-specific antibody responses to influenza group 1 and group 2 hemagglutinins by natural H7N9 virus infection in humans. J Infect Dis 215:518–528. doi: 10.1093/infdis/jiw608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Memoli MJ, Czajkowski L, Reed S, Athota R, Bristol T, Proudfoot K, Fargis S, Stein M, Dunfee RL, Shaw PA, Davey RT, Taubenberger JK. 2015. Validation of the wild-type influenza A human challenge model H1N1pdMIST: an A(H1N1)pdm09 dose-finding investigational new drug study. Clin Infect Dis 60:693–702. doi: 10.1093/cid/ciu924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Memoli MJ, Shaw PA, Han A, Czajkowski L, Reed S, Athota R, Bristol T, Fargis S, Risos K, Powers JH, Davey RT Jr, Taubenberger JK. 2016. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. mBio 7:e00417-16. doi: 10.1128/mBio.00417-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson RM, Grill DE, Oberg AL, Tosh PK, Ovsyannikova IG, Poland GA. 2015. Profiles of influenza A/H1N1 vaccine response using hemagglutination inhibition titers. Hum Vaccin Immunother 11:961–969. doi: 10.1080/21645515.2015.1011990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. 2011. Influenza hemagglutination inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis 204:1879–1885. doi: 10.1093/infdis/jir661. [DOI] [PubMed] [Google Scholar]
- 13.Beyer WE, Palache AM, Baljet M, Masurel N. 1989. Antibody induction by influenza vaccines in the elderly: a review of the literature. Vaccine 7:385–394. doi: 10.1016/0264-410X(89)90150-3. [DOI] [PubMed] [Google Scholar]
- 14.Kanekiyo M, Wei CJ, Yassine HM, McTamney PM, Boyington JC, Whittle JR, Rao SS, Kong WP, Wang L, Nabel GJ. 2013. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 499:102–106. doi: 10.1038/nature12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nabel GJ, Fauci AS. 2010. Induction of unnatural immunity: prospects for a broadly protective universal influenza vaccine. Nat Med 16:1389–1391. doi: 10.1038/nm1210-1389. [DOI] [PubMed] [Google Scholar]
- 16.Yassine HM, Boyington JC, McTamney PM, Wei CJ, Kanekiyo M, Kong WP, Gallagher JR, Wang L, Zhang Y, Joyce MG, Lingwood D, Moin SM, Andersen H, Okuno Y, Rao SS, Harris AK, Kwong PD, Mascola JR, Nabel GJ, Graham BS. 2015. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med 21:1065–1070. doi: 10.1038/nm.3927. [DOI] [PubMed] [Google Scholar]
- 17.Powers JH, Guerrero ML, Leidy NK, Fairchok MP, Rosenberg A, Hernández A, Stringer S, Schofield C, Rodríguez-Zulueta P, Kim K, Danaher PJ, Ortega-Gallegos H, Bacci ED, Stepp N, Galindo-Fraga A, St Clair K, Rajnik M, McDonough EA, Ridoré M, Arnold JC, Millar EV, Ruiz-Palacios GM. 2016. Development of the Flu-PRO: a patient-reported outcome (PRO) instrument to evaluate symptoms of influenza. BMC Infect Dis 16:1. doi: 10.1186/s12879-015-1330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powers JH, Bacci ED, Leidy NK, Stringer S, Kim K, Memoli MJ, Han A, Fairchok MP, Chen W, Arnold JC, Danaher PJ, Lalani T, Hansen EA, Ridore M, Burgess TH, Millar EV, Hernández A, Rodríguez-Zulueta P, Ortega-Gallegos H, Galindo-Fraga A, Ruiz-Palacios GM, Pett S, Fischer W, Gillor D, Macias LM, DuVal A, Rothman R, Dugas A, Guerrero ML. 2016. Evaluation of the performance properties of the influenza patient-reported outcomes instrument (Flu-Pro). Value Health 19:A220–A221. doi: 10.1016/j.jval.2016.03.1186. [DOI] [Google Scholar]
- 19.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng NY, Lee JH, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O’Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. 2011. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med 208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]