Abstract
Recent research has demonstrated that tumor microenvironments play pivotal roles in tumor development and metastasis through various physical, chemical, and biological factors, including extracellular matrix (ECM) composition, matrix remodeling, oxygen tension, pH, cytokines, and matrix stiffness. An emerging trend in cancer research involves the creation of engineered three-dimensional tumor models using bioinspired hydrogels that accurately recapitulate the native tumor microenvironment. With recent advances in materials engineering, many researchers are developing engineered tumor models, which are promising platforms for the study of cancer biology and for screening of therapeutic agents for better clinical outcomes. In this review, we discuss the development and use of polymeric hydrogel materials to engineer native tumor ECMs for cancer research, focusing on emerging technologies in cancer engineering that aim to accelerate clinical outcomes.
Keywords: polymeric hydrogels, engineered tumor models, tumor microenvironments, cancer research
1. INTRODUCTION
In developing new cancer therapeutics, there is a great need for in vitro platforms that will allow better screening of novel treatments. The US National Institutes of Health (NIH) (1) have estimated that in 2016, 1.68 million people will be diagnosed with cancer and 595,690 people will die of it. The average cost of a new therapy is around US$2.6 billion (2). Two reasons for these high costs are research and development expenses and the extensive animal testing necessary to comply with US Food and Drug Administration (FDA) guidelines. Moreover, these therapies also have a high failure rate because current in vitro and even in vivo models are unable to predict their efficacy and effects in humans. At present, the likelihood that a drug will complete all stages of clinical trials is 12%; 67% of drugs fail Phase 2 clinical trials (3). Therefore, we need to better understand the mechanisms of action underlying potential targets of therapeutics, and to better predict the clinical outcomes of such potential therapeutics. To this end, researchers must gain a better understanding of the cancer microenvironment in order to enable the development of research tools that more accurately portray the pathophysiological aspects of the tumor and its surroundings. Such research and development tools could be used for diagnostic applications. The average cost of a cancer therapy is $8,500 per patient per month, and global spending on cancer therapy is estimated to be $91 billion (4). In addition, many patients have to go through multiple rounds of treatment to eradicate their cancer. Thus, the technologies aiming to better mimic the tumor microenvironment in order to study and screen potential therapeutics could be adapted for diagnostic and preventive applications.
The tumor microenvironment is an intricate and dynamic milieu containing supporting cells, blood vessels, and extracellular matrix (ECM). It is also characterized by a complex combination of chemical, physical, and biological cues that regulate cancer progression, such as stiffness of the ECM, pressure of the growing tumor mass, secreted and sequestrated cytokines and growth factors, oxygen (O2), pH, and glucose concentration. All of these cues help determine whether cancer cells grow or migrate, contributing to cancer development and metastasis. These environmental cues are present in a three-dimensional (3D) environment, which is drastically different from the conventional two-dimensional (2D) culture regimes used to study mechanisms that regulate cancer growth. These in vitro 2D culture regimes have minimal physiological relevance, as they do not accurately portray the complexity of the tumor environment. As a result, current studies using 2D culture regimes have shown limited translational impact, as well as a low screening ability for successful therapies.
A potential approach is to develop polymeric hydrogel materials that recapitulate the 3D cancer microenvironment. Hydrogels are cross-linked polymeric networks that are extensively swollen with water (5). These polymer networks can be fabricated from a wide variety of materials, including natural, synthetic, and semisynthetic polymers (6). Hydrogel properties such as viscoelasticity, swelling, and degradation, as well as cell-adhesion domains, can be engineered to tune a hydrogel for use with a targeted cell type (7). Such platforms can be used for the study of mechanisms underlying cancer development, growth, and metastasis, as well as to screen cancer therapeutics. A better understanding of the tumor microenvironment must be established in order to improve the design of these novel hydrogel materials.
In this review, we discuss the development and use of polymeric hydrogel materials to engineer native tumor microenvironments for cancer research, focusing on emerging technologies in cancer engineering to accelerate clinical outcomes.
2. PHYSICOCHEMICAL PROPERTIES OF TUMOR MICROENVIRONMENTS
The native tumor microenvironment is composed of a complex structure of ECM with varying chemical and physical gradients that lead to the migration and proliferation of cancer cells. The extracellular environment provides many cues that result in cancer development and metastasis. In the following subsections, we focus on key aspects of the tumor microenvironment that regulate cancer cell fates, including ECM composition, matrix remodeling, O2, pH, cytokines, and matrix stiffness.
2.1. Extracellular Matrix
The ECM is the acellular component of a tissue made of a meshwork of highly cross-linked biomacromolecules (8). The ECM is composed of a wide variety of proteins and polysaccharides, such as fibronectin, collagen, laminin, glycoprotein, hyaluronic acid (HA), chondroitin sulfate, heparan sulfate, and so forth (8). Collagen is the most abundant protein in the ECM; it constitutes up to 30% of the total protein in the body and 90% of the ECM (9, 10). Collagen is a substantial structural feature of the ECM that regulates tensile strength and cell adhesion (11). Collagen molecules are made of three α-helical structures that together form a fibril structure. These fibers form larger supramolecular structures that create the backbone of collagen bundles (10). These fibrous bundles are modified through the hydroxylation of proline and lysine resides, allowing for the strengthening and cross-linking of collagen fibers (12). Another major ECM component is fibronectin. Fibronectin is made from a dimer that is joined by C-terminal disulfide bonds. These bonds can bind to other fibronectin dimers, to collagen, and to cell integrin receptors (13). Laminin is another crucial ECM protein. This molecule is important for cell attachment to the matrix (14), and it is known to increase migration in breast carcinomas (15, 16). All of these proteins in conjunction create a fibrillar network. This fibrillar network morphology can be imaged using second harmonic generation (SHG) microscopy (17, 18).
2.2. Matrix Remodeling
Within the ECM are cancer-associated fibroblasts (CAFs), which modify the ECM, leading to fibrillar ECM (19). Specifically, CAFs contribute to the synthesis of type I, type III, and type IV collagen, fibronectin, and laminin (20–22). An increased number of fibroblasts leads to an increase in type I collagen as well as to remodeling of the matrix, both factors that contribute to tumorigenesis (23).
Both CAFs and cancer cells secrete a wide variety of proteases and ECM modifiers. Two of the most-studied enzymes are matrix metalloproteinase (MMP) and thrombospondin motifs (ADAMTSs), which specialize in degrading the ECM (23). There are approximately 23 members of the MMP family (24). MMPs target a large range of extracellular proteins, including MMP-1, which targets collagen; MMP-2, which targets gelatin (denatured collagen); and MMP-7, which targets fibronectin (25, 26). These MMPs are of two types: soluble (secreted-type) and membrane-bound MMPs (27, 28). Soluble MMPs are secreted from the cell and are made up of pro-MMPs (29). In the prodomain of the soluble MMPs resides a cysteine residue, which ligates catalytic zinc (29). This cysteine–zinc interaction is disrupted in a two-step reaction. The reaction starts by cleaving the propeptide region to remove the N-terminal polypeptide. The second step occurs through an autoproteolytic reaction, which generates an active enzyme (30). Membrane-type MMPs are anchored to the membrane and are activated by proprotein convertases (27). ADAMTSs share a similar structure to MMPs in that both have prodomains that need to be activated, degrading the surrounding matrix. The difference between MMPs and ADAMTSs is that ADAMTSs have a thrombospondin domain instead of a cytosine-rich domain (31).
Cancer cells also modify the ECM to facilitate migration. Because of the prevalence of interstitial collagen in the tumor microenvironment, the ECM is subject to numerous posttranslational modifications (32). Collagen cross-linking is mediated primarily by lysyl oxidase (LOX) and LOX-like enzymes. LOX cross-linking increases both cancer cell migration and ECM stiffness (33, 34). Baker et al. (35) have shown that this increase in stiffness (due to LOX cross-linking) leads to an increase in colorectal cancer migration. In breast cancer, LOX cross-linking induces β1-integrin clustering, PI3K (phosphatidylinositol 3-kinase) signaling, and focal adhesions (36). Another key collagen modifier is procollagen-lysine 2-oxoglutarate 5-dioxygenase (PLOD2), which initiates lysine hydroxylation of collagen (37–39). These cross-links are crucial for the formation of mature collagen fibers, are regulated by hypoxia, and cause an increase in tumorigenesis (40, 41).
2.3. O2 and pH
O2 and pH are two important factors that regulate cell growth. Changes in these factors have been suggested to lead to changes in cancer cell growth and migration (42, 43). The relative distance to a blood vessel determines O2 tension and pH in the tumor microenvironment. Datta et al. (44) have shown that changes in the O2 and pH gradients around the tumor can be observed depending on the state of the vasculature. As the distance from the blood vessel to the tumor increases, O2 concentration decreases to levels below 2%, a state known as hypoxia (45, 46). Hypoxia causes an increase in angiogenesis (47), metastatic potential (48, 49), and DNA replication (48, 50) and reduces protein synthesis (51).
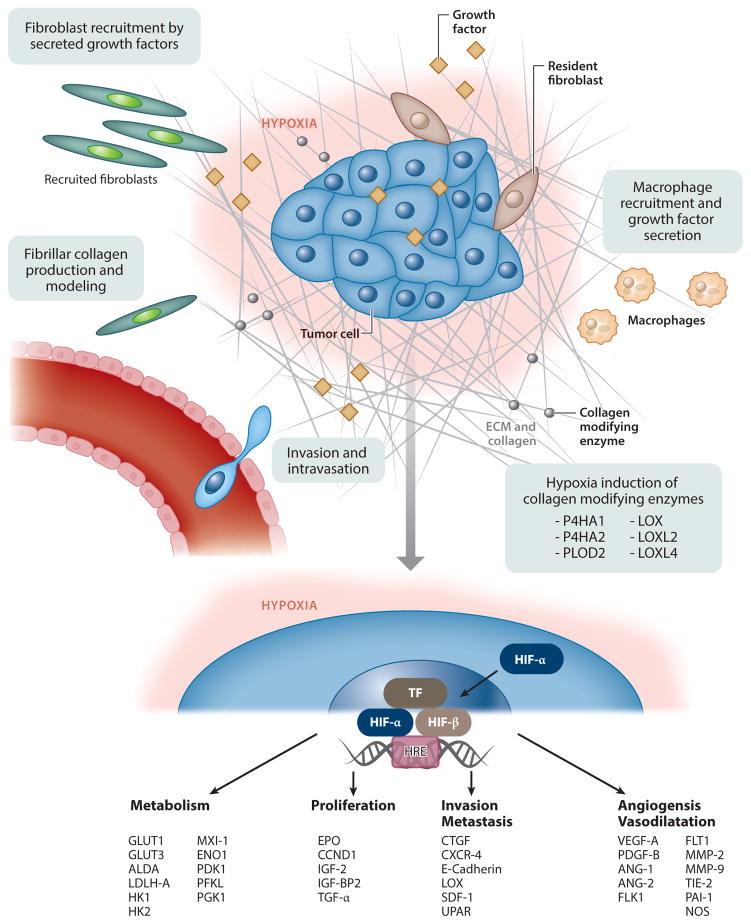
The O2-sensing mechanism occurs through transcription factors termed hypoxia-inducible factors (HIFs). HIFs are composed of α and β subunits (52). HIF1α is accumulated in severe hypoxic conditions, typically below 1.5% O2 (53), and is rapidly degraded in a nonhypoxic environment. Less is known about HIF2α and HIF3α; whereas HIF1α is constantly expressed ubiquitously in most tissues, HIF2α and HIF3α are found in only a subset of tissues (54–56). Hypoxia in general causes upregulation of a variety of factors (Figure 1), from proangiogenic to ECM modifiers (57). Moreover, cells in a hypoxic environment are usually far away from blood vessels and therefore less exposed to drugs, and they have lower proliferation rates and thus less sensitivity to radiotherapy and p53-mediated apoptosis (58). Recently, our lab developed an in vitro platform to better screen potential cancer therapeutics in a hypoxic cancer tumor microenvironment (42).
Figure 1.
Cancerous pathways affected by accumulation of hypoxia-inducible factor (HIF). Abbreviations: ALDA, aldolase A; ANG-1, angiopoietin 1; ANG-2, angiopoietin 2; BMDSC, bone marrow–derived stem cell; CCND1, cyclin D1; CTGF, connective tissue growth factor; CXCR-4, C-X-C chemokine receptor type 4; ECM, extracellular matrix; ENO1, enolase 1; EPO, erythropoietin; FLK1, VEGF receptor 2; FLT-1, VEGF receptor 1; GLUT, glucose transporter; HK, hexokinase; IGF-2, insulin growth factor 2; IGF-BP2, insulin-like growth factor–binding protein 2; LDHA, lactate dehydrogenase A; LOX, lysyl oxidase; miRNA, microRNA; LOXL, lysyl oxidase homolog 1; MMP, matrix metalloproteinase; MXI-1, max interactor 1; P4HA, prolyl 4-hydroxylase subunit α1; PAI-1, plasminogen activator inhibitor 1; PDGF-B, platelet-derived growth factor B; PDK1, pyruvate dehydrogenase kinase 1; PFKL, phosphofructokinase L; PGK1, phosphoglycerate kinase 1; PLOD, procollagen-lysine 2-oxoglutarate 5-dioxygenase; SDF-1, stromal cell–derived factor 1; TF, transcription factor; TGF-α, transforming growth factor α; TIE-2, angiopoietin receptor 2; UPAR, urokinase plasminogen activator receptor; VEGF, vascular endothelial growth factor. Modified from Reference 46.
Hypoxia also affects cell metabolism. The normal metabolism and generation of adenosine triphosphate (ATP) are highly sensitive to O2 concentration. Hypoxia causes a substantial decrease in ATP production (59). Normally, cancer cells have to change their metabolism to rapidly produce ATP (60). Under hypoxia, cancer cells switch from their normal oxidative phosphorylation to glycolysis. In order to maintain high levels of ATP, these cells drastically increase their glucose uptake. By using glycolysis, cancer cells can generate ATP even faster than conventional oxidative phosphorylation. However, this type of metabolism causes the pH in the extracellular environment to fall from a physiological value of 7.2 to an acidic value of between 5.6 and 6.8 (46).
2.4. Stiffness
Stiffness of the ECM is a crucial characteristic of the tumor microenvironment, with the majority of the ECM composed of collagen type I. As described in detail above, the density of ECM in the tumor microenvironment increases as a result of new ECM secretion and upregulation of cross-linkers by CAFs and cancer cells. In fact, an increase in collagen cross-linkers such as LOX and PLOD2 facilitates tumor progression (61). Cancer cells respond to an increase in LOX secretion by clustering nonreceptor tyrosine kinases, specifically the focal adhesion kinase (FAK). Paszek et al. (34) have shown that 3D matrices with a Young’s modulus of 400 Pa enable a significant increase in the size of colonies compared with colonies cultured in 3D matrices with a Young’s modulus of 160–170 Pa. They linked the increase in size to clustering of α5β1-integrin, which caused stimulation of FAK phosphorylation, activated RhoA, and resulted in cytoskeletal contractility.
Another important factor of the tumor microenvironment is the porosity (specifically, pore size) of the surrounding matrix. Pathak & Kumar (62) have shown that ECM matrix stiffness and confinement play a role in cancer cell migration, demonstrating that cells in stiffer and narrower channels migrate faster. Wong et al. (63) demonstrated on polyacrylamide pillars that cells will spread more quickly on stiffer substrates. Haeger et al. (64) explored the concepts of confinement and cell–cell junctions in collagen gels. They concluded that, depending on the type and density of the ECM, the mode of migration changes; cells in denser matrixes show “follow-the-leader”-type migration, whereas less porous matrices demonstrate single-cell migration.
ECM stiffness also increases the differentiation of epithelial cancer cells to a more mesenchymal phenotype. Wei et al. (65) recently reported that nuclear translocation of TWIST1 (twist family basic helix–loop–helix transcription factor 1) leads to an increase in mesenchymal transition via this mechanotransduction pathway.
3. TUMOR ANGIOGENESIS
Angiogenesis is the growth of new capillaries from existing vasculature. Tumors have a high demand for nutrients due to an increase in the generation of ATP. This high demand for O2 and glucose via the vascular network results in upregulation of proangiogenic signals that stimulate blood vessel growth and increased blood supply to the tumor.
3.1. Endothelial Recruitment
In order to meet the demand for nutrients, tumors have developed a variety of strategies for releasing cytokines and growth factors to activate the quiescent endothelial cells (ECs) around them to generate new blood vessels. Unique features of the tumor’s environment such as O2 and glucose deprivation affect blood vessels, leading to migration of the ECs (66). Comprehensive reviews by Carmeliet and Jain explore the complex mechanism of tumor angiogenesis (67–69).
A key mechanism underlying tumor angiogenesis is the response of ECs to vascular endothelial growth factor (VEGF), which induces their sprouting and proliferation (70). A secondary mechanism involves platelet-derived growth factor (PDGF), which activates perivascular cells to enable migration of ECs. Perivascular cells usually provide stability and vessel maturation. However, because of the constant release of cytokines in the tumor environment, vessels can never become truly mature. Angiogenic factors are also upregulated because of the hypoxic environment caused by the irregular blood supply (67).
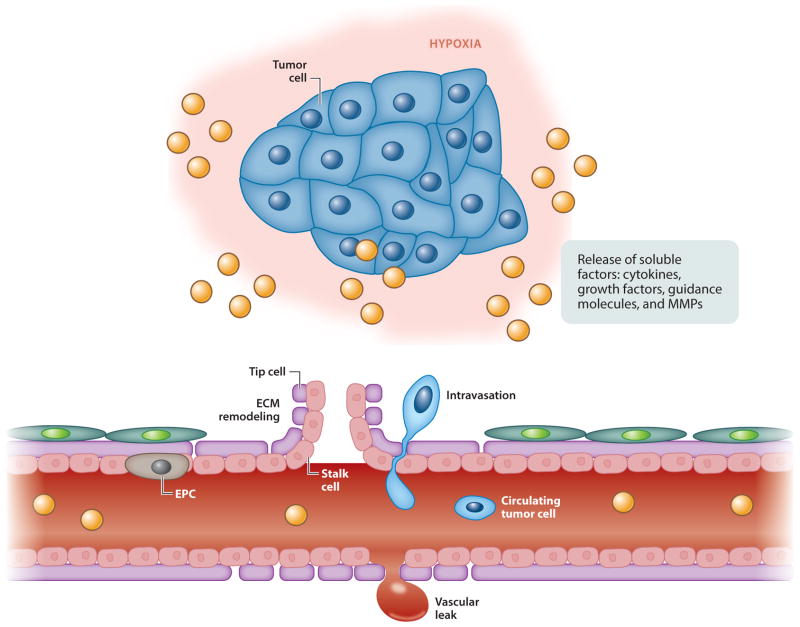
CAFs further help the migration of blood vessels by secreting MMPs that degrade the surrounding matrix. Throughout this process, inflammatory cells infiltrate the site, which can positively or negatively increase the migration speed of ECs. This constant inflammatory response has given rise to an analogy between tumors and wounds—a tumor is “the wound that never heals” (Figure 2) (71).
Figure 2.
Tumor angiogenesis, in which certain growth and environmental factors lead to the recruitment of endothelial cells (70). Abbreviations: ECM, extracellular matrix; EPC, endothelial progenitor cell; MMP, matrix metalloproteinase.
3.2. Vascular Structure
In healthy tissue, there is an orderly and efficient network of blood vessels that provides nutrients to tissue. These vessels are kept in balance due to a cocktail of pro- and antiangiogenic factors and a systematic network of lymphatic vessels to drain fluid and metabolic waste. In tumors, the exact opposite is observed. Due to the high levels of VEGF and other proangiogenic factors, tumor vasculature is immature, tortuous, and hyperpermeable (72). These vessels have an uneven diameter and blind ends that can lead to turbulent blood flow. Moreover, lymphatic vessels are highly disorganized and dilated (72). Due to these abnormalities, the efficiency of the vasculature in delivering nutrients and removing waste is greatly reduced.
The ECs within the tumor’s vessels are also deformed. Tumor ECs have long, ruffled margins and fragile cytoplasmic projections (73). The tips of the tumor ECs can branch into the lumen, creating intercellular gaps in the vessel wall. These gaps lead to leaky blood vessels, a condition known as the enhanced permeability and retention (EPR) effect. The gaps between the ECs are ~1.5 μm wide (73), causing blood leakage and reduced blood flow to the tumor site.
3.3. Vascular–Extracellular Matrix Microenvironment
The tumor vasculature contains key integrins that interact with the primary ECM proteins within the tumor (collagen and laminin). ECs express numerous integrins, including α1β1, α2β1 αvβ3, αvβ5, αvβ8, and α5β1 (74). These integrins, especially αvβ3 and αvβ5, which are specific for type I collagen, have been the targets of some current therapies.
Another key characteristic of the tumor ECM is its relatively organized, aligned fibrous structure. Aligned fibrous collagen increases the migratory potential of ECs (75). Levels of VEGF and VEGF receptor (VEGFR) are increased through the interaction between ECs and basement membrane proteins such as laminin and collagen IV (76, 77). These, in turn, stimulate the angiogenesis of vessels, thereby causing the secretion of MMPs, such as MMP-8 and MMP-9 (78), to enable the intravasation of cancer cells.
To study the formation of tumor vasculature, researchers have been using Matrigel, a mixture containing mouse sarcoma ECM as the substrate to culture and form EC networks. An important drawback of Matrigel is that, because it is generated from mouse sarcoma, it is completely undefined and has significant batch-to-batch variation (79). The main components of Matrigel are laminin, type IV collagen, and enactin. Although it mimics the basal lamina, this composition does not accurately mimic the complex tumor ECM. To address this shortcoming, investigators have developed biomaterials to study cancerous ECM–EC interactions.
3.4. Trends in Targeting of Tumor Angiogenesis
The role of tumor vasculature in cancer growth, development, and metastasis has prompted the development of a wide variety of therapeutics. One of the first was an anti-VEGF inhibitor called bevacizumab (80). This drug is a humanized neutralizing antibody that blocks VEGF-A or inhibits the activation of VEGFR2. Another strategy has been to target tyrosine kinase, which participates in many proangiogenic pathways. Drugs such as sunitinib, sorafenib, pazopanib, and axitinib have been approved for cancer treatment. These drugs simultaneously target multiple cell types, such as cancer cells, pericytes, and ECs, through the inhibition of PDGF and VEGF signaling pathways. However, these drugs show minimal efficacy in the clinic. Therefore, combination therapies of these antiangiogenic inhibitors and standard chemotherapeutic agents are being considered. For example, Heist et al. (81) have shown an increase in survival of patients with non-small-cell lung cancer when they are treated with the chemotherapeutics carboplatin and paclitaxel following anti-VEGF therapy. The main reason for this increase in efficacy has been hypothesized to be due to the restoration of normal vasculature (69). This blood vessel restoration prevents the EPR effect and enables better drug delivery and an increase in O2 supply. This increase in turn improves the efficacy of conventional drugs. Drugs such as doxorubicin have significantly decreased efficacy in a hypoxic environment (82), and their use in combination with an anti-VEGF therapy could dramatically increase their efficacy.
4. POLYMERIC HYDROGELS AS ENGINEERED THREE-DIMENSIONAL MICROENVIRONMENTS
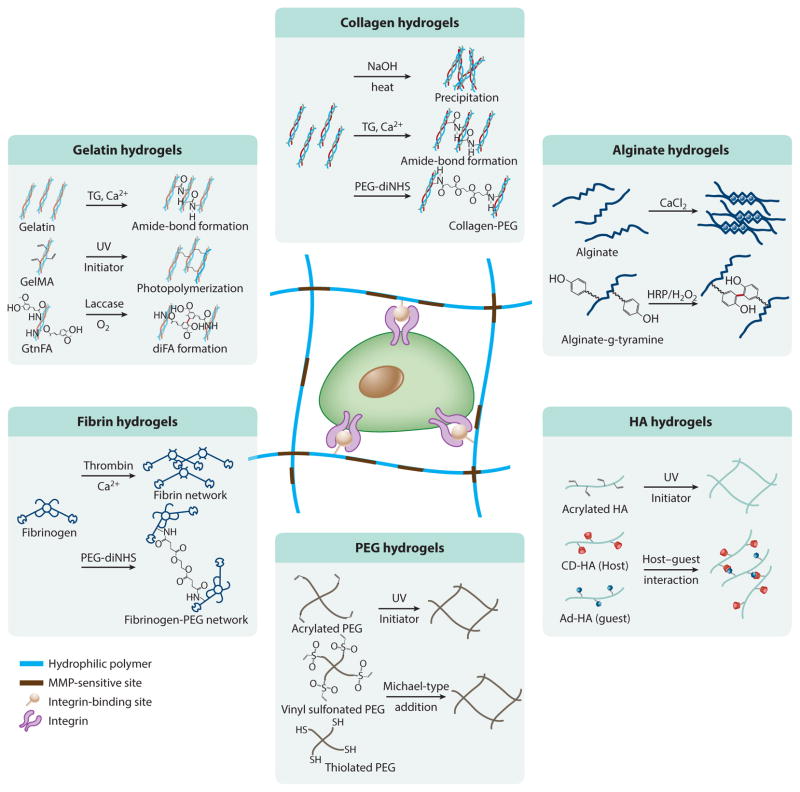
Polymeric hydrogels, which are composed of a 3D hydrophilic network, are a promising material for regenerative medicine and pharmacological applications due to their multitunable properties and structural similarity to native ECM (83–85). Their physicochemical and biological properties, such as mechanical strength, degradation behavior, nutrient transport, and spatiotemporal topography, can be regulated by use of polymers with different compositions, by their cross-linking density, and by tailoring with various bioactive molecules (such as cell-adhesion ligands or proteolytic degradation sites). In addition, these matrices are utilized as artificial cellular microenvironments for recapitulating ECM in soft tissues due to their high swelling properties and mechanical resemblance. In particular, in situ cross-linkable hydrogels, which undergo a phase transition from solution to gel through various cross-linking chemistries, have attracted substantial attention as engineered cellular microenvironments and drug-delivery carriers because they are minimally invasive and amenable to encapsulation of therapeutic agents during hydrogel formation under mild conditions (86, 87). Advances in materials engineering have prompted the development of various in situ–forming hydrogels and cross-linking strategies to create engineered microenvironments for supporting cell growth and directing cell fate (Figure 3) (88). In this section, we discuss the various types of polymeric hydrogels, and their cross-linking chemistries, that are used to create 3D hydrogel matrices for a broad range of biomedical applications.
Figure 3.
In situ cross-linkable hydrogels as an artificial cellular microenvironment. Cross-linking strategies are detailed for each hydrogel system. Abbreviations: CD-HA, β-cyclodextrin-modified hyaluronic acid; FA, ferulic acid; GelMA, methacrylated gelatin; GtnFA, gelatin grafted with ferulic acid; HA, hyaluronic acid; MMP, matrix metalloproteinase; PEG, poly(ethylene glycol); PEG-diNHS, poly(ethylene glycol) conjugated to di(succinic acid N-hydroxysuccinimide ester).
4.1. Natural Hydrogel Matrices
Collagen type I is a well-known ECM protein that provides the structural frame required to support cell growth and adhesion through cell-to-cell and cell-to-matrix interaction through its various binding sites that interact with cell surface receptors and other ECM components (e.g., fibronectin) (89, 90). Collagen type I also includes proteolytic degradable sites for ECM remodeling within cellular microenvironments, which is critical for regulating cell behavior in these microenvironments (90). Generally, collagen hydrogels are prepared via physical cross-linking by pH and temperature sensitivity, which can provide 3D fibrillar matrices recapitulating the ECM in vivo. Increasing the pH and temperature of the acidic collagen precursor solution induces the formation of fibrillar hydrogel matrices (91). Because of its unique properties, collagen has been widely used as a scaffold for tissue engineering and regenerative medicine, as well as in other biomedical applications. However, collagen has critical disadvantages, namely weak mechanical properties and batch-to-batch variation. In an effort to overcome these limitations, investigators have developed many chemical cross-linking methods, including chemical glycation (92) and the enzyme-mediated cross-linking reaction, to generate stiffer and more stable collagen hydrogels through chemical cross-linking. For example, Vorp and colleagues (93) fabricated chemically cross-linked collagen hydrogels by using transglutaminase that catalyzes amide cross-linking from specific functional groups (e.g.,γ-carboxamide and primary amine in collagen chains). Using this approach, these authors created more collagen hydrogels that were more stable than physically cross-linked collagen hydrogels. Li and colleagues (94) utilized a different cross-linking condition by using genipin, an excellent natural cross-linker for proteins with low cytotoxicity (e.g., collagen and gelatin). The mechanical properties of the collagen hydrogels can be controlled by varying genipin concentrations, ranging from 2 to 50 kPa of compressive strength. However, a disadvantage of this system is its slow chemical reaction rate (more than 24 h to fully cross-link the networks). More recently, Kong and colleagues (95) developed a novel strategy to create more stable and multitunable collagen hydrogels by using poly(ethylene glycol) conjugated to di(succinic acid N-hydroxysuccinimide ester) (PEG-diNHS); these hydrogels induce interconnection between collagen fibers through cross-linking of primary amines in polymer backbones. Using the engineered collagen hydrogels, these authors investigated the effect of matrix stiffness on the behavior of cancer spheroids of hepatocarcinoma cells, demonstrating that carcinoma cells embedded in soft hydrogels form malignant cancer spheroids, whereas cells encapsulated in stiff hydrogels form compact hepatoids with suppressed malignancy.
Although many approaches have been developed to enhance the stability of collagen matrices, advanced engineering technologies are still required to improve these materials’ cytocompatibility and multitunable properties. Fibrin hydrogels have been used in a wide range of biomedical applications due to their biocompatibility, biodegradability, and ease of fabrication (96). Fibrin hydrogels are fabricated by the cross-linking of fibrinogens through thrombin-mediated ionic interactions (97, 98). In this reaction, thrombin cleaves fibrogen’s N-terminal end, which can induce polymerization of fibrin into fibrous hydrogel matrices (97, 98). These fibrin matrices contain various cellular responsible domains, including integrin, heparin, and fibronectin binding sites, that are critical in regulating cellular activities in wound healing and tissue regeneration (99). Because of their unique properties, fibrin gels have been utilized as therapeutic implants and as vehicles for regenerative medicine and drug delivery, as well as for clinical treatments. Compared with collagen hydrogel, fibrin gels have a relatively higher and broader range of matrix mechanical properties (approximately 1 to 30 kPa) because of variations in their fibrinogen and thrombin concentrations. However, fibrin gels degrade quickly because of the tissue factors and other enzymes secreted by cells, limiting their ability to support cell growth. Therefore, many studies have focused on improving fibrin gel stability toward the goal of long-term cell culture, which will lead to a better understanding of the effect of 3D matrices on cellular activities. The main goal of these studies is to incorporate functionalized PEG as a cross-linker, defined as a PEGylation. Del Bufalo et al. (100) utilized PEG–fibrin hydrogels to provide artificial tumor microenvironments for cancer research. They prepared PEGylated fibrin hydrogels by simply mixing fibrinogen and functionalized PEG in order to induce cross-linking between the polymer backbones through chemical reactions. Using the stable matrices, these authors investigated the effect of 3D microenvironments on the growth of cancer cells (e.g., lung adenocarcinoma cell lines, A549, and H1299), demonstrating that the 3D matrices supported tumor growth similar to that in in vivo models.
4.2. Synthetic Hydrogel Matrices
Synthetic polymers have several advantages over natural polymers, such as more controllable physicochemical properties. PEG is the representative biomaterial for medical applications. PEG is a bioinert and hydrophilic polymer used as both a surface-grafting material, to create an antifouling surface on biomaterials, and as a coating material, to enhance the lubricity of medical catheters. PEG hydrogels are used commercially as clinical tissue sealants, and PEG has been utilized as a hydrogel matrix for tissue regeneration and drug-delivery systems due to its biocompatibility and multitunable properties. However, PEG should be modified with bioactive molecules (e.g., cell-adhesion sites and MMP-sensitive moieties) to support cell growth within 3D microenvironments. The most common strategy is to fabricate PEG hydrogels through photopolymerization of diacrylated PEG (PEGDA) chains (101). During hydrogel formation, the bioactive molecules can be incorporated into the matrices. Anseth and colleagues (102) utilized photocurable PEG hydrogels as a template to study the effect of mechanical memory on stem cell fate, demonstrating that stem cells have mechanical memory that stores information from past physicochemical conditions and regulates their fate.
There are many other ways to create PEG hydrogels, including Michael-type addition reactions, thiolene reactions, and enzyme-mediated cross-linking reactions. Lutolf & Hubbell (103) developed a PEG hydrogel incorporating cysteine oligopeptides via Michael-type additions. The conjugative reaction occurs under mild conditions, which is critical for in vitro and in vivo applications. Segura and colleagues (104) developed similar PEG hydrogels through a thiol-maleimide Michael-type addition by using zinc, thereby demonstrating the multitunable properties of the hydrogel matrices, such as kinetics of hydrogel formation and matrix stiffness. Using this matrix, these authors investigated the impact of matrix stiffness on cellular activities of human dermal fibroblasts.
4.3. Semisynthetic Hydrogel Matrices
Although natural and synthetic hydrogels have been widely used as 3D matrices in tissue engineering and regenerative medicine, they have some limitations, such as matrix stability, batch-to-batch variation, and the challenge of accurately recapitulating native ECM. To overcome these limitations, investigators have developed chemically modified natural hydrogels (defined as semisynthetic hydrogels). There are various approaches to creating engineered polymeric hydrogels for tissue engineering and regenerative medicine.
Alginate is a naturally derived anionic polymer that is composed of repeating units of (1–4)-linked β-D-mannurinate (M units) and α-L-gluronate (G units) (105). Alginate hydrogels form in the presence of divalent cations (e.g., Ca2+ and Mg2+) through ionic interactions between the G units in the polymer backbones. Due to its biocompatibility and easy fabrication process, alginate hydrogels have been used in various clinical applications, including wound dressing, drug delivery, and tissue regeneration, as well as in treatments of myocardial infraction (106, 107). Although alginate-based hydrogels are useful in various biomedical applications, improving their mechanical properties and bioactivity remains a challenge. To address these limitations, researchers have endeavored to chemically modify alginate chains. Dhoot et al. (108) have conjugated cell-adhesive sites [L-arginine, glycine, L-aspartic acid (RGD)] into alginate chains to enhance cellular activities. Zhu and colleagues (109) have developed an enzymatically cross-linked alginate hydrogel, which is composed of tyramine-conjugated alginate that can form chemically cross-linked hydro-gels through a horseradish peroxidase–mediated oxidative reaction. More recently, Mooney and colleagues (110) developed a novel strategy to create highly stretchable and tough alginate hydrogels through the formation of interpenetrating networks. To improve mechanical stiffness and hydrogel stability, these authors fabricated the hydrogels by hybridization of ionically cross-linked alginate networks using Ca2+ and photo-cross-linkable polyacrylamide gels, resulting in highly elastomeric alginate hydrogels. With advances in engineering approaches, alginate hydrogels with biological activities have the potential to provide 3D matrices for tissue regeneration and other clinical applications.
Gelatin, which is denatured collagen type I, has been widely used in tissue engineering and regenerative medicine because of its biocompatibility, biodegradability, ease of fabrication, and cost-effectiveness [reviewed by Klotz et al. (111)]. It also contains cellular responsive sites, such as integrin binding sites and proteolytically degradable domains, which play a critical role in regulating cellular activities within 3D microenvironments (112). In order for gelatin to be used as a biomaterial, the polymer backbone must be chemically cross-linked to create stable gelatin matrices at body temperature. There are various ways to form covalently cross-linked gelatin networks, including enzyme-mediated cross-linking and photo-cross-linking reactions. Using transglutaminase and formaldehyde, one can prepare gelatin hydrogels with unmodified gelatin. However, the process must overcome certain critical limitations, such as slow phase transition and cytotoxicity. Toward that end, many approaches have been developed to create gelatin hydrogels using chemically modified gelatin. Khademhosseini and colleagues (113) created methacrylated gelatin (GelMA), which can form hydrogels through photopolymerization. These authors used the bioactive hydrogels to create various cell-laden matrices for regenerative medicine, as well as engineered tissue models (114, 115). Recently, they also developed grapheme oxide (GO)-incorporated GelMA hydrogels to enhance mechanical properties through additional physical interaction between GO and polymer chains (39). More recently, we developed a novel gelatin hydrogel composed of gelatin grafted with ferulic acid (FA) that formed hydrogel matrices with O2 consumed in the laccase-mediated chemical cross-linking reaction (87). In this reaction, laccase catalyzes the cross-linking of FA with O2 consumption, resulting in 3D hydrogel networks created through di-FA formations. We demonstrated that O2-controllable hydrogels provide the artificial hypoxic microenvironment necessary to support enhanced vascular differentiation and tube formation of endothelial progenitor cells (EPCs). Using advanced hydrogel matrices, we are applying the engineered vasculatures to the treatment of ischemic tissues and are using engineered tumor models to study tumor development.
HA, an anionic linear glycosaminoglycan consisting of D-glucuronic acid and N-acetyl-D-glucosamine, is commonly used in clinical applications as a soft tissue filler and an antiadhesion adjuvant, as well as a scaffold for tissue engineering and regenerative medicine (116, 117). Although HA generates very viscous solutions, it needs chemical cross-linking in order to create a stable hydrogel for in vitro and in vivo applications. HA hydrogels have been widely utilized as 3D cellular microenvironments to support vascular morphogenesis and cancer cell growth, as HA has several binding sites (e.g., CD44 and CD168) that play a critical role in regulating cellular signaling (118). However, HA–cell bonds are relatively weak, and there is no key cell-adhesion site in the HA backbone; therefore, cell-adhesion moiety should be incorporated into the hydrogel matrices to enhance cell binding affinity. Moreover, HA does not have proteolytic degradable sites (e.g., MMP-sensitive sites), which are crucial for matrix remodeling within cellular microenvironments. Thus, investigators should consider employing both cell-adhesion and MMP-sensitive sites when designing 3D cellular microenvironments using HA hydrogels.
There are many ways to engineer microenvironments using HA hydrogels. Burdick and colleagues (119) developed physically cross-linked HA hydrogels through host–guest interactions. They synthesized two kinds of precursor polymers: adamantine-modified HA (guest macromer) and β-cyclodextrin-modified HA (host macromer). The HA hydrogels are rapidly formed by simple mixing of the solutions, resulting in physically cross-linked HA networks through guest–host bonds. The same research group also developed photocurable HA hydrogels consisting of RGD-modified methacrylated HA (120). The functionalized HA hydrogels were formed by photopolymerization, and their mechanical properties and degradation behaviors could be controlled by varying relevant parameters (e.g., cross-linking density, polymer concentrations, degree of substitution of methacrylate, MMP-sensitive sites). Using multitunable HA hydrogel matrices, these authors investigated the effect of matrix stiffness and degradation on stem cell fate. They demonstrated that the differentiation of human mesenchymal stem cells (hMSCs) was regulated by matrix degradation–mediated cellular traction, independently of cell morphology or matrix stiffness. Our group has used acrylated HA (AHA) hydrogels as 3D microenvironments to support vascular assembly of human pluripotent stem cell–derived early vascular cells (EVCs) to create stable engineered vasculatures (121). We encapsulated the EVCs within RGD-modified AHA hydrogels, as the EVCs can mature into ECs and pericytes to create multicellular vasculatures within 3D matrices. We also demonstrated that the engineered vasculatures connect to host vasculatures with blood perfusion following transplantation.
With advances in biomaterials engineering, various polymeric hydrogels have been used as 3D matrices for a wide range of medical applications. Although these engineered matrices are promising materials, well-defined 3D cellular microenvironments are still required in order to accurately recapitulate the native cellular environments, which present myriad physical, chemical, and biological challenges, as well as spatiotemporal complexity.
5. ENGINEERING TUMOR MICROENVIRONMENTS
Tumor microenvironments, with their complexity, diversity, and dynamic nature, play a critical role in cancer development and progression (122). Thus, many researchers have endeavored to create artificial tumor microenvironments recapitulating native tumors by using various engineered biomaterials (123). Advances in materials engineering have enabled the use of various polymeric hydrogels as engineered 3D matrices that recapitulate the pathological tumor ECM to allow studies of basic cancer biology and screening of the efficacy of anticancer agents (124, 125). In this section, we discuss the most recently engineered tumor microenvironments aiming for a better understanding of cancer biology and emerging anticancer therapeutics. In particular, we focus on models of the tumor ECM used to study angiogenesis, invasion, and metastasis occurring in native tumor microenvironments, as well as screening of anticancer drugs.
Breast cancer is the most common and deadly tumor in women worldwide (126). Various polymeric hydrogels have been used to study the effect of numerous factors on breast cancer progression, including invasion, metastasis, and tumor-associated angiogenesis (127). These engineered platforms offer the ability to evaluate drug efficacy and screen newly developed anticancer drugs in order to obtain a better understanding of breast cancer biology and improved treatment outcomes.
Charoen et al. (128) utilized biomimetic 3D breast cancer models comprising collagen hydrogels encapsulating multicellular spheroids (MDA-MB-231) to evaluate the anticancer therapeutic effect of paclitaxel-loaded polymeric nanoparticles. They fabricated the multicellular spheroids by culturing the cells on the top of agarose hydrogels, to which the cells could not attach as they did not contain cell-adhesive motives. The authors controlled the size of the tumor spheroids (diameters ranging from 100 to 400 μm) by varying the cell-seeding density. The cancer spheroids were encapsulated within collagen hydrogels by simple mixing of cell suspension and polymer solutions. Using the tumor models, these authors investigated the therapeutic efficacy of nanoparticles encapsulating paclitaxel versus delivery of the drug without carriers. To this end, they loaded the drug into pH-sensitive polymeric nanoparticles and treated the tumor models. Interestingly, the drug combination with nanoparticles had a greater therapeutic effect than the drug alone, suggesting that the engineered tumor models are a promising platform to evaluate drug candidates and delivery systems for new cancer therapies.
Shoichet and colleagues (129) utilized HA hydrogels as a platform for studying variations in matrix stiffness, MMP sensitivity, and the concentration of cell-adhesion sites in breast cancer cell invasion. They fabricated HA hydrogels with independently varying cross-linking (mechanical) and ligand (chemical) densities to study the effects of each factor on breast cancer invasion. They observed that soft hydrogels tailored with an MMP-sensitive moiety exhibited extensive cancer invasion compared with stiff matrices without MMP-sensitive sites. This finding demonstrates the importance of matrix stiffness and cell-mediated matrix degradation in breast cancer invasion. Interestingly, these authors also showed that cell-adhesive ligand density affects cancer cell proliferation, rather than migration.
Sarcomas are heterogeneous mesenchymal tumors diagnosed in more than 2,000 people worldwide each year (130). Anseth and colleagues (131) utilized PEG-based hydrogels in an investigation of the effect of biochemical and biophysical matrix properties on fibrosarcoma (HT1080 cell) migration. They prepared peptide-functionalized PEG hydrogels through thiolene photopolymerization; the hydrogel matrices could be spatially and temporally patterned using standard lithography. The authors used this technique to fabricate hydrogel matrices with mechanical gradients ranging from 60 to 260 Pa, and they examined the effect of matrix adhesion at different moduli on HT1080 cell migration. They demonstrated that HT1080 cells on soft matrices migrate faster and over longer distances than HT1080 cells on stiff matrices. The authors also found that cells tended to migrate from stiff to soft areas. These results suggest that the engineered tumor models are innovative tools for investigating several unresolved questions in tumor development, including the effect of tissue interfaces on tumor motility and invasion.
Our lab has studied sarcoma cell migration in an O2-controllable hydrogel model (132). We generated a gelatin-based hydrogel cross-linked with O2 consumption in a laccase-mediated reaction. This reaction created a hypoxia-inducible gel with O2 tension ranging from 1% to 5%, providing a biomimetic environment of a 3D hypoxic O2 gradient. Using this model, we showed that sarcoma cells migrate along increased O2 tension via PLOD2 modification of secreted collagen. Cells in the hypoxic gradient hydrogels have a faster migration rate and migrate for longer distances, and they upregulate the expression of PLOD2, LOX, and type I collagen. This migration was halted when the cells were treated with a PLOD2 inhibitor, minoxidil. This finding demonstrates that O2 plays a critical role as a 3D physicotactic agent in sarcoma metastasis through remodeling of collagen matrices.
Glioblastoma multiforme (GBM) is a common and deadly form of brain tumor that is aggressive and malignant (133). Various engineering approaches have been developed to recapitulate and model GBM tumor microenvironments in vitro in order to obtain a better understanding of GBM molecular and systems biology (134). Using PEG-based hydrogels, Wang et al. (135) bioengineered 3D brain tumor models to investigate the effects of matrix stiffness on GBM cells. The hydrogels were prepared through photopolymerization and incorporated RGD- and MMP-sensitive sites to support 3D cell growth and matrix remodeling. HA was also included in order to mimic its concentration in the brain ECM. To examine the effect of matric moduli on GBM cell fate in three dimensions, Wang et al. encapsulated U87 cells (a model GBM cell line) in soft (1-kPa) or stiff (26-kPa) hydrogels, mimicking the matrix stiffness of normal brain or GBM tissue. Interestingly, they found that increasing stiffness led to slower cell proliferation but that the cells formed denser tumor spheroids as well as upregulated matrix degradation (through HA synthase 1 and MMP-1). These results demonstrate the importance of ECM decomposition and remodeling during tumor invasion and metastasis. This bioengineered 3D hydrogel platform may provide an innovative brain tumor model for investigating the mechanisms underlying GBM progression, as well as for evaluating the efficacy of potential drug candidates for the treatment of GBM.
Prostate cancer is one of the most commonly diagnosed cancers in men worldwide (136). Many researchers have utilized advanced engineering platforms to determine the molecular mechanism underlying growth and metastasis (137). Fong et al. (138) utilized HA hydrogel–based 3D models using patient-derived prostate tumors for drug screening. As is well known, patient-derived xenograft (PDX) cells show poor cell viability in standard culture conditions. To overcome this limitation, these authors encapsulated the PDX cells in the HA hydrogel matrices and evaluated both cell viability and drug efficacy. They observed that most cells were viable within the matrices until day 14. Fong et al. also investigated the drug sensitivity of 3D tumor models from different patients. Interestingly, the 3D PDX cells were much more resistant to docetaxel compared with the cell lines. This result suggests that the primary culture system offers potential for direct culture of patients’ tumor tissues for rapid drug screening, and thus may represent an innovative platform for personalized cancer therapeutics.
Whereas prostate cancer is the most commonly diagnosed cancer in men, representing 29% of all incident cases, ovarian cancer accounts for only 3% of all incident cancer cases in women each year (139). It is challenging to model ovarian cancer because of its genetic complexity, diverse pathology, and the unique mechanisms underlying its metastasis. Thus, many studies have attempted to recreate sophisticated ovarian cancer microenvironments to study growing cancer spheroids and metastasis of ovarian cancer cells (137). Kaemmerer et al. (140) developed 3D ovarian cancer models by using GelMA hydrogels encapsulating a human epithelial ovarian cancer cell line (OV-MZ-6) derived from a patient. They investigated the effect of matrix stiffness, matrix degradation, and incorporation of ECM components (laminin-411 and HA) on cancer spheroid formation. The hydrogels’ matrix stiffness was modulated by varying the polymer concentrations from 0.5 to 9.0 kPa. Hydrogels with medium stiffness (3.4 kPa) enabled the formation of tumor spheroids and led to higher proliferation compared with more or less stiff matrices. In addition, the researchers noticed that inhibition of proteolytic degradation of matrices reduced the formation of spheroids and that incorporating laminin-411 and HA further facilitated spheroid growth within the gelatin-based hydrogels. These results demonstrate that semisynthetic gelatin hydrogels may provide alternative, bioengineered 3D ovarian cancer culture models in vitro.
6. EMERGING TECHNIQUES TO CREATE ENGINEERED TUMOR/TUMOR ANGIOGENESIS MODELS
Recently, advanced tools have been developed to accurately mimic natural tumor microenvironments composed of ECM, vasculature, and supporting stromal cells (141, 142). These innovative approaches include 3D coculture systems, micropatterning, microfluidics, and 3D bioprinting, which provide unique opportunities to recapitulate complex and sophisticated tumor microenvironments, such as multicellular microenvironments and spatiotemporal gradients of various physicochemical and biological cues. In this section, we discuss the most recent tools used to recreate tumor microenvironments using these advanced fabrication methods.
Our lab has evaluated the effect of hydrogel stiffness and O2 tension on cancer cell fate and vascular cell invasion. We fabricated HA-based hydrogels tailored with RGD- and MMP-sensitive sequences through a Michael-type addition reaction to support the growth of 3D cancer cells (HT1080 cells) (143). The stiffness of the hydrogel matrices was modulated by varying the amount of cross-linkers (e.g., cysteine-containing peptides; GCRDGPQGWGQDRCG) ranging from 80 to 600 Pa. We observed different cell morphologies depending on the stiffness. While fibrosarcoma cells spread on relatively stiff hydrogels (300 Pa), they formed aggregates at lower stiffnesses, demonstrating that matrix stiffness influences sarcoma morphology. We also utilized coculture systems of HT1080 cells and vascular EPCs [e.g., endothelial colony-forming cells (ECFCs)] to study the effect of matrix stiffness and O2 tension on vascular cell invasion of 3D tumor microenvironments (Figure 4a). Interestingly, we found that ECFCs cultured on top of the 3D tumor models under hypoxic conditions exhibited extensive vascular invasion of 3D tumor models at all levels of matrix stiffness, through MMP-mediated matrix degradation and upregulation of proangiogenic factors [e.g., VEGFs and angiopoietin-1 (ANG-1)]. This result clearly demonstrated that O2 tension affects vascular invasion into the tumor microenvironment, suggesting that coculture models may provide a promising platform for basic studies of tumor biology, including the vascular invasion that selectively impedes cancerous processes.
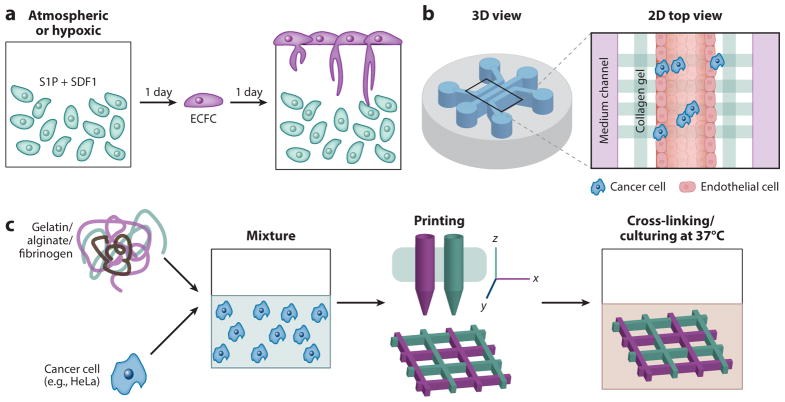
Figure 4.
Emerging techniques to create three-dimensional (3D) tumor microenvironments. (a) Strategy for studying vascular cell invasion into tumor microenvironments. (b) A microfluidic system consisting of three independently addressable media channels separated from extracellular matrix (ECM)-mimicking hydrogel matrices. (c) The printing process used to create 3D HeLa–hydrogel matrices. Abbreviations: ECFC, endothelial colony-forming cell; SDF-1, stromal cell–derived factor 1; S1P, sphingosine-1-phosphate; 2D, two-dimensional. Panel a modified from Reference 143. Panel b modified from Reference 146. Panel c modified from Reference 148.
Microfluidic 3D tumor models recreate the complex and heterogeneous aspects of the native tumor environment (144, 145). A critical advantage is the ability of the 3D microscale system to provide multifunctionality (e.g., spatial/temporal controls), including perfusable fluid systems that can accurately recapitulate the native tumor microenvironment (144). Jeon et al. (146) developed an in vitro model of tumor cell extravasation by using the microfluidic technique and collagen hydrogels. They created a microfluidic system consisting of three independently addressable media channels separated by ECM-mimicking chambers (Figure 4b). The inner channels are composed of an EC monolayer surrounding a collagen hydrogel, which allowed cancer cell migration (e.g., breast cancer cells, MDA-MB-231) from the vessel-mimicking regions to the hydrogel matrices (defined as tumor extravasation). These authors evaluated the permeability of the endothelial monolayer, which showed excellent media permeability, then examined cancer extravasation into the collagen hydrogel matrices through the EC monolayers. The microscaled perfusable tumor models allowed the study of breast cancer extravasation, resulting in extravasation of 38.8% of the tumor cells within 1 day of their introduction. This result suggests that advanced tumor models present the opportunity to develop therapeutic strategies for the treatment of metastatic tumors, as well as personalized medicine applications.
3D bioprinting techniques have been widely used to create highly precise and complex 3D architectures with living cells. The key advantage of 3D printing is its ability to create complex architectures with high efficiency and customizability on a desktop printing scale. For example, multicellular 3D tissue constructs can be printed by use of a multichannel cartilage filled with bioinks encapsulating the targeted cells. Many researchers have used this innovation to fabricate sophisticated artificial tissues as well as tumor microenvironments (147). Sun and colleagues (148) fabricated in vitro cervical tumor models by using gelatin-based hydrogels (Figure 4c). They used these models to compare the cellular behavior in a 3D cervical tumor model with that in traditional 2D culture models, including cancer cell proliferation, MMP protein expression, and chemoresistance. Interestingly, they found that the HeLa cells in the 3D construct had a higher proliferation rate, resulting in tumor spheroid formation. They also found that the cells embedded into the 3D matrices showed higher MMP protein expression and chemoresistance than those in the 2D cultures. These findings suggest that this novel 3D printing technique may ultimately lead cancer studies toward better clinical outcomes.
7. FUTURE DIRECTIONS AND CONCLUSION
Significant recent advances in synthesis of hydrogel biomaterials and other 3D miniature devices have been explored for tissue regenerative applications, but they have not been fully taken advantage of in the field of cancer research. For example, following recent advances in organon-a-chip technologies, the next logical step would be to make a tumor on a chip or to create more physiologically relevant diseases on a chip. For example, Huh et al. (149) have developed a lung-on-a-chip model that can mimic the physiological environment of the lung. The next step for this technology should be to integrate cancer phenotypes in specific locations into the chip in order to mimic tumor metastasis. Many examples of angiogenic devices on a chip are beginning to be used in intravasation or extravasation of tumor cells into and out of existing vasculature (42). However, most of these devices use collagen gel as the 3D hydrogel portion of the device. The next generation of these approaches should incorporate advanced materials to augment these natural ECMs, thereby enabling more accurate mimicking of physiologically relevant chemical and cytokine gradients.
These technologies will also have to incorporate multiple cell types. As discussed above, the tumor microenvironment is a complex combination of ECM and multicellular cues. CAFs have been studied for their importance in tumor migration and ECM modification. Preliminary studies have incorporated stromal cells into systems to study changes in cancer migration. Moreover, CAFs change the barrier and structure of ECs and vasculature. In order to culture multiple cell types that require different culture conditions and ECM components, investigators must develop more complex patterning of hydrogel networks and matrices. Our lab has shown that cancer and EC patterning can be controlled solely via ECM proteins (150). Technologies developed by Burdick and colleagues (151) take advantage of different properties of “self-healing” and shear-thinning gels to print patterns that could be adapted for multiple cell types and ECM coculture platforms.
The ultimate goal of most of these complex systems is to discover new mechanisms in cancer development, growth, and metastasis. However, due to the complexity of these devices, they have limited use outside bioengineering laboratories. Classical biological researchers do not have the instruments (e.g., 3D printers, syringe pumps, chemical equipment for biopolymer synthesis) to perform these assays. Efforts should be made to simplify the mode of operation for ease of use. Moreover, these devices can be employed for potential therapeutic evaluation and possible replacement of in vivo models. Such trials, though complex, are still less expensive and more controllable than current in vivo studies. Simplifying current technologies into one- or two-component devices would greatly enhance their translatability and promote their widespread use. If this task is accomplished, the transition from drug discovery to personalized medicine could be achieved.
Personalized medicine is a new and exciting field in cancer research; several technologies in this area have made the transition from benchtop to clinic. Recently, Jonas et al. (152) demonstrated the use of a complex system that enables clinical testing of up to 16 different therapeutics in microdoses. The researchers created Delrin acetal resin blocks that can hold 16 different individual therapies by controlling the drug-release kinetics in the tumor microenvironment. This device is implanted via a biopsy needle into mice bearing human tumors, then extracted via a corning needle and evaluated histologically. More technologies like this one need to be adapted for use in a clinical setting and, more importantly, over a wide variety of cancer cell types. Other challenges include the inherent heterogeneity of tumors, with a wide variety of genomic changes, and the ability to keep tumor samples viable outside the body. Finally, these advanced tissue culture technologies have to be mass produced for broader application and ease of use. Mass production will enable researchers across disciplines to employ the latest technologies. Furthermore, mass production requires a more scalable synthetic design for wide-scale use. This may fulfill the need for controllable and reproducible systems that can be used for cancer diagnosis and treatment.
Acknowledgments
The writing of this review was supported by a fellowship from the National Cancer Institute (T-32), a Nanotechnology Cancer Research training grant (2T32CA153952-06 to D.M.L.), a National Research Foundation of Korea grant funded by the Korean government (NRF-2015R1C1A1A01054498 to K.M.P.), and a President’s Frontier Award from Johns Hopkins University (to S.G.).
Footnotes
All authors contributed equally to the writing of this review.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.NIH (Nat. Inst. Health) SEER Stat Fact Sheets: Cancer of Any Site. Washington, DC: NIH; 2016. http://seer.cancer.gov/statfacts/html/all.html. [Google Scholar]
- 2.Mullard A. New drugs cost US$2.6 billion to develop. Nat Rev Drug Discov. 2014;13:877. [Google Scholar]
- 3.Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat Biotechnol. 2014;32:40–51. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Fojo T, Mathisen M, Zwelling LA. Cancer drugs in the United States: justum pretium—the just price. J Clin Oncol. 2013;31:3600–4. doi: 10.1200/JCO.2013.49.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res. 2015;6:105–21. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices. 2011;8:607–26. doi: 10.1586/erd.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanjaya-Putra D, Bose V, Shen Y-I, Yee J, Khetan S, et al. Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood. 2011;118:804–15. doi: 10.1182/blood-2010-12-327338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteom. 2011;11:014647. doi: 10.1074/mcp.M111.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Rest M, Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814–23. [PubMed] [Google Scholar]
- 10.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–40. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robins S. Biochemistry and functional significance of collagen cross-linking. Biochem Soc Trans. 2007;35:849–52. doi: 10.1042/BST0350849. [DOI] [PubMed] [Google Scholar]
- 13.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–63. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 14.Ioachim E, Charchanti A, Briasoulis E, Karavasilis V, Tsanou H, et al. Immunohistochemical expression of extracellular matrix components tenascin, fibronectin, collagen type IV and laminin in breast cancer: their prognostic value and role in tumour invasion and progression. Eur J Cancer. 2002;38:2362–70. doi: 10.1016/s0959-8049(02)00210-1. [DOI] [PubMed] [Google Scholar]
- 15.Albrechtsen R, Nielsen M, Wewer U, Engvall E, Ruoslahti E. Basement membrane changes in breast cancer detected by immunohistochemical staining for laminin. Cancer Res. 1981;41:5076–81. [PubMed] [Google Scholar]
- 16.Nielsen M, Christensen L, Albrechtsen R. The basement membrane component laminin in breast carcinomas and axillary lymph node metastases. Acta Pathol Microbiol Scand A. 1983;91:257–64. doi: 10.1111/j.1699-0463.1983.tb02755.x. [DOI] [PubMed] [Google Scholar]
- 17.Brown E, McKee T, diTomaso E, Pluen A, Seed B, et al. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 18.Gibb L, Matthews D. Two photon microscopy and second harmonic generation. 2006 Unpubl. ms https://pdfs.semanticscholar.org/e189/a6d5eb6b7e92154dfdd6746508801253872f.pdf.
- 19.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 20.Rodemann HP, Müller GA. Characterization of human renal fibroblasts in health and disease. II In vitro growth, differentiation, and collagen synthesis of fibroblasts from kidneys with interstitial fibrosis. Am J Kidney Dis. 1991;17:684–86. doi: 10.1016/s0272-6386(12)80352-0. [DOI] [PubMed] [Google Scholar]
- 21.Chang HY, Chi J-T, Dudoit S, Bondre C, van de Rijn M, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. PNAS. 2002;99:12877–82. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 23.Sieweke MH, Thompson NL, Sporn MB, Bissell MJ. Mediation of wound-related Rous sarcoma virus tumorigenesis by TGF-β. Science. 1990;248:1656–60. doi: 10.1126/science.2163544. [DOI] [PubMed] [Google Scholar]
- 24.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cawston TE, Young DA. Proteinases involved in matrix turnover during cartilage and bone breakdown. Cell Tissue Res. 2010;339:221–35. doi: 10.1007/s00441-009-0887-6. [DOI] [PubMed] [Google Scholar]
- 26.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Pei D, Kang T, Qi H. Cysteine array matrix metalloproteinase (CA-MMP)/MMP-23 is a type II transmembrane matrix metalloproteinase regulated by a single cleavage for both secretion and activation. J Biol Chem. 2000;275:33988–97. doi: 10.1074/jbc.M006493200. [DOI] [PubMed] [Google Scholar]
- 28.Seiki M. Membrane-type matrix metalloproteinases. Acta Pathol Microbiol Immunol Scand. 1999;107:137–43. doi: 10.1111/j.1699-0463.1999.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 29.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–94. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 30.Nabeshima K, Inoue T, Shimao Y, Sameshima T. Matrix metalloproteinases in tumor invasion: role for cell migration. Pathol Int. 2002;52:255–64. doi: 10.1046/j.1440-1827.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 31.Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284:31493–97. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erler JT, Bennewith KL, Nicolau M, Dornhöfer N, Kong C, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–26. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 34.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Baker A, Bird D, Lang G, Cox TR, Erler J. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene. 2013;32:1863–68. doi: 10.1038/onc.2012.202. [DOI] [PubMed] [Google Scholar]
- 36.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rautavuoma K, Takaluoma K, Passoja K, Pirskanen A, Kvist A-P, et al. Characterization of three fragments that constitute the monomers of the human lysyl hydroxylase isoenzymes 1–3: The 30-kDa N-terminal fragment is not required for lysyl hydroxylase activity. J Biol Chem. 2002;277:23084–91. doi: 10.1074/jbc.M112077200. [DOI] [PubMed] [Google Scholar]
- 38.Hyry M, Lantto J, Myllyharju J. Missense mutations that cause Bruck syndrome affect enzymatic activity, folding, and oligomerization of lysyl hydroxylase 2. J Biol Chem. 2009;284:30917–24. doi: 10.1074/jbc.M109.021238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pirskanen A, Kaimio A-M, Myllylä R, Kivirikko KI. Site-directed mutagenesis of human lysyl hydroxylase expressed in insect cells: identification of histidine residues and an aspartic acid residue critical for catalytic activity. J Biol Chem. 1996;271:9398–402. doi: 10.1074/jbc.271.16.9398. [DOI] [PubMed] [Google Scholar]
- 40.Eisinger-Mathason TK, Zhang M, Qiu Q, Skuli N, Nakazawa MS, et al. Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov. 2013;3:1190–205. doi: 10.1158/2159-8290.CD-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilkes DM, Bajpai S, Wong CC, Chaturvedi P, Hubbi ME, et al. Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol Cancer Res. 2013;11:456–66. doi: 10.1158/1541-7786.MCR-12-0629. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Lewis DM, Park KM, Tang V, Xu Y, Pak K, et al. Intratumoral oxygen gradients mediate sarcoma cell invasion. PNAS. 2016;113:9292–97. doi: 10.1073/pnas.1605317113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damaghi M, Wojtkowiak JW, Gillies RJ. pH sensing and regulation in cancer. Front Physiol. 2013;4:370. doi: 10.3389/fphys.2013.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Datta M, Via LE, Kamoun WS, Liu C, Chen W, et al. Anti–vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. PNAS. 2015;112:1827–32. doi: 10.1073/pnas.1424563112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–75. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Policastro LL, Ibáñnez IL, Notcovich C, Duran HA, Podhajcer OL. The tumor microenvironment: characterization, redox considerations, and novel approaches for reactive oxygen species–targeted gene therapy. Antioxid Redox Signal. 2013;19:854–95. doi: 10.1089/ars.2011.4367. [DOI] [PubMed] [Google Scholar]
- 47.Blumenson LE, Bross I. A possible mechanism for enhancement of increased production of tumor angiogenic factor. Growth. 1976;40:205–9. [PubMed] [Google Scholar]
- 48.Young S, Marshall R, Hill R. Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. PNAS. 1988;85:9533–37. doi: 10.1073/pnas.85.24.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van den Brenk HAS, Moore V, Sharpington C, Orton C. Production of metastases by a primary tumour irradiated under aerobic and anaerobic conditions in vivo. Br J Cancer. 1972;26:402–12. doi: 10.1038/bjc.1972.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young S, Hill R. Effects of reoxygenation on cells from hypoxic regions of solid tumors: analysis of transplanted murine tumors for evidence of DNA overreplication. Cancer Res. 1990;50:5031–38. [PubMed] [Google Scholar]
- 51.Heacock C, Sutherland R. Enhanced synthesis of stress proteins caused by hypoxia and relation to altered cell growth and metabolism. Br J Cancer. 1990;62:217–25. doi: 10.1038/bjc.1990.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. PNAS. 1995;92:5510–14. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang B-H, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol Cell Physiol. 1996;271:C1172–80. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 54.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 55.Jain S, Maltepe E, Lu MM, Simon C, Bradfield CA. Expression of ARNT, ARNT2, HIF1α, HIF2αand Ah receptor mRNAs in the developing mouse. Mech Dev. 1998;73:117–23. doi: 10.1016/s0925-4773(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 56.Wiesener MS, Jürgensen JS, Rosenberger C, Scholze CK, Hörstrup JH, et al. Widespread hypoxia-inducible expression of HIF-2αin distinct cell populations of different organs. FASEB J. 2003;17:271–73. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 57.Gilkes DM, Semenza GL. Role of hypoxia-inducible factors in breast cancer metastasis. Future Oncol. 2013;9:1623–36. doi: 10.2217/fon.13.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–47. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 59.Eales K, Hollinshead K, Tennant D. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis. 2016;5:e190. doi: 10.1038/oncsis.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 61.Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomark Prev. 1998;7:1133–44. [PubMed] [Google Scholar]
- 62.Pathak A, Kumar S. Independent regulation of tumor cell migration by matrix stiffness and confinement. PNAS. 2012;109:10334–39. doi: 10.1073/pnas.1118073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong S, Guo W-H, Wang Y-L. Fibroblasts probe substrate rigidity with filopodia extensions before occupying an area. PNAS. 2014;111:17176–81. doi: 10.1073/pnas.1412285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haeger A, Krause M, Wolf K, Friedl P. Cell jamming: collective invasion of mesenchymal tumor cells imposed by tissue confinement. Biochim Biophys Acta. 2014;1840:2386–95. doi: 10.1016/j.bbagen.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 65.Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1–G3BP2 mechanotransduction pathway. Nat Cell Biol. 2015;17:678–88. doi: 10.1038/ncb3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Polet F, Feron O. Endothelial cell metabolism and tumour angiogenesis: glucose and glutamine as essential fuels and lactate as the driving force. J Intern Med. 2013;273:156–65. doi: 10.1111/joim.12016. [DOI] [PubMed] [Google Scholar]
- 67.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 68.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–36. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 69.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 70.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–70. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 71.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–39. [PMC free article] [PubMed] [Google Scholar]
- 72.Siemann DW. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor–vascular disrupting agents. Cancer Treat Rev. 2011;37:63–74. doi: 10.1016/j.ctrv.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dudley AC. Tumor endothelial cells. Cold Spring Harb Perspect Med. 2012;2:a006536. doi: 10.1101/cshperspect.a006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vong S, Kalluri R. The role of stromal myofibroblast and extracellular matrix in tumor angiogenesis. Genes Cancer. 2011;2:1139–45. doi: 10.1177/1947601911423940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lai ES, Huang NF, Cooke JP, Fuller GG. Aligned nanofibrillar collagen regulates endothelial organization and migration. Regen Med. 2012;7:649–61. doi: 10.2217/rme.12.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hielscher AC, Gerecht S. Engineering approaches for investigating tumor angiogenesis: exploiting the role of the extracellular matrix. Cancer Res. 2012;72:6089–96. doi: 10.1158/0008-5472.CAN-12-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stamati K, Priestley JV, Mudera V, Cheema U. Laminin promotes vascular network formation in 3D in vitro collagen scaffolds by regulating VEGF uptake. Exp Cell Res. 2014;327:68–77. doi: 10.1016/j.yexcr.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kelley LC, Lohmer LL, Hagedorn EJ, Sherwood DR. Traversing the basement membrane in vivo: a diversity of strategies. J Cell Biol. 2014;204:291–302. doi: 10.1083/jcb.201311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–90. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 80.García-Caballero M, Poveda BM, Medina MÁ, Quesada AR. Targeting tumor angiogenesis for cancer prevention. In: Chatterjee M, editor. Molecular Targets and Strategies in Cancer Prevention. Berlin: Springer; 2016. pp. 117–49. [Google Scholar]
- 81.Heist RS, Duda DG, Sahani DV, Ancukiewicz M, Fidias P, et al. Improved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer. PNAS. 2015;112:1547–52. doi: 10.1073/pnas.1424024112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sullivan R, Paré GC, Frederiksen LJ, Semenza GL, Graham CH. Hypoxia-induced resistance to anticancer drugs is associated with decreased senescence and requires hypoxia-inducible factor 1 activity. Mol Cancer Ther. 2008;7:1961–73. doi: 10.1158/1535-7163.MCT-08-0198. [DOI] [PubMed] [Google Scholar]
- 83.Rosales AM, Anseth KS. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat Rev Mater. 2016;1:15012. doi: 10.1038/natrevmats.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guvendiren M, Burdick JA. Engineering synthetic hydrogel microenvironments to instruct stem cells. Curr Opin Biotechnol. 2013;24:841–46. doi: 10.1016/j.copbio.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rice JJ, Martino MM, De Laporte L, Tortelli F, Briquez PS, Hubbell JA. Engineering the regenerative microenvironment with biomaterials. Adv Healthc Mater. 2013;2:57–71. doi: 10.1002/adhm.201200197. [DOI] [PubMed] [Google Scholar]
- 86.Park KM, Blatchley MR, Gerecht S. The design of dextran-based hypoxia-inducible hydrogels via in situ oxygen-consuming reaction. Macromol Rapid Commun. 2014;35:1968–75. doi: 10.1002/marc.201400369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park KM, Gerecht S. Hypoxia-inducible hydrogels. Nat Commun. 2014;5:4075. doi: 10.1038/ncomms5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shinde UP, Yeon B, Jeong B. Recent progress of in situ formed gels for biomedical applications. Prog Polym Sci. 2013;38:672–701. [Google Scholar]
- 89.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–70. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 90.Leitinger B, Hohenester E. Mammalian collagen receptors. Matrix Biol. 2007;26:146–55. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 91.Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. J Cell Sci. 2007;120:1955–58. doi: 10.1242/jcs.03453. [DOI] [PubMed] [Google Scholar]
- 92.Girton T, Oegema T, Grassl E, Isenberg B, Tranquillo R. Mechanisms of stiffening and strengthening in media-equivalents fabricated using glycation. J Biomech Eng. 2000;122:216–23. doi: 10.1115/1.429652. [DOI] [PubMed] [Google Scholar]
- 93.Orban JM, Wilson LB, Kofroth JA, El-Kurdi MS, Maul TM, Vorp DA. Crosslinking of collagen gels by transglutaminase. J Biomed Mater Res A. 2004;68:756–62. doi: 10.1002/jbm.a.20110. [DOI] [PubMed] [Google Scholar]
- 94.Zhang X, Chen X, Yang T, Zhang N, Dong L, et al. The effects of different crossing-linking conditions of genipin on type I collagen scaffolds: an in vitro evaluation. Cell Tissue Bank. 2014;15:531–41. doi: 10.1007/s10561-014-9423-3. [DOI] [PubMed] [Google Scholar]
- 95.Liang Y, Jeong J, DeVolder RJ, Cha C, Wang F, et al. A cell-instructive hydrogel to regulate malignancy of 3D tumor spheroids with matrix rigidity. Biomaterials. 2011;32:9308–15. doi: 10.1016/j.biomaterials.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 96.Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng B. 2008;14:199–215. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 97.Blombäck B. Fibrinogen and fibrin proteins with complex roles in hemostasis and thrombosis. Thromb Res. 1996;83:1–75. doi: 10.1016/0049-3848(96)00111-9. [DOI] [PubMed] [Google Scholar]
- 98.Fuss C, Palmaz JC, Sprague EA. Fibrinogen: structure, function, and surface interactions. J Vasc Interv Radiol. 2001;12:677–82. doi: 10.1016/s1051-0443(07)61437-7. [DOI] [PubMed] [Google Scholar]
- 99.Hogg PJ, Jackson CM. Fibrin monomer protects thrombin from inactivation by heparin–antithrombin. III Implications for heparin efficacy. PNAS. 1989;86:3619–23. doi: 10.1073/pnas.86.10.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Del Bufalo F, Manzo T, Hoyos V, Yagyu S, Caruana I, et al. 3D modeling of human cancer: a PEG–fibrin hydrogel system to study the role of tumor microenvironment and recapitulate the in vivo effect of oncolytic adenovirus. Biomaterials. 2016;84:76–85. doi: 10.1016/j.biomaterials.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 101.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266–76. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 102.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13:645–52. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lutolf M, Hubbell J. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules. 2003;4:713–22. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- 104.Darling NJ, Hung Y-S, Sharma S, Segura T, Darling N. Controlling the kinetics of thiol-maleimide Michael-type addition gelation kinetics for the generation of homogenous poly(ethylene glycol) hydrogels. Biomaterials. 2016;101:199–206. doi: 10.1016/j.biomaterials.2016.05.053. [DOI] [PubMed] [Google Scholar]
- 105.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 106.Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6:623–33. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 107.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8:153–70. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dhoot NO, Tobias CA, Fischer I, Wheatley MA. Peptide-modified alginate surfaces as a growth permissive substrate for neurite outgrowth. J Biomed Mater Res A. 2004;71:191–200. doi: 10.1002/jbm.a.30103. [DOI] [PubMed] [Google Scholar]
- 109.Hou J, Li C, Guan Y, Zhang Y, Zhu X. Enzymatically crosslinked alginate hydrogels with improved adhesion properties. Polym Chem. 2015;6:2204–13. [Google Scholar]
- 110.Sun J-Y, Zhao X, Illeperuma WR, Chaudhuri O, Oh KH, et al. Highly stretchable and tough hydrogels. Nature. 2012;489:133–36. doi: 10.1038/nature11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klotz BJ, Gawlitta D, Rosenberg AJ, Malda J, Melchels FP. Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotechnol. 2016;34:394–407. doi: 10.1016/j.tibtech.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Crit Rev Biochem Mol Biol. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- 113.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden micro-engineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–44. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Colosi C, Shin SR, Manoharan V, Massa S, Costantini M, et al. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv Mater. 2015;28:677–84. doi: 10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yue K, Trujillo–de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–71. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Highley CB, Prestwich GD, Burdick JA. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr Opin Biotechnol. 2016;40:35–40. doi: 10.1016/j.copbio.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 117.Allison DD, Grande-Allen KJ. Hyaluronan: a powerful tissue engineering tool. Tissue Eng. 2006;12:2131–40. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 118.Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–92. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 119.Rodell CB, Kaminski AL, Burdick JA. Rational design of network properties in guest–host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules. 2013;14:4125–34. doi: 10.1021/bm401280z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013;12:458–65. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kusuma S, Shen Y-I, Hanjaya-Putra D, Mali P, Cheng L, Gerecht S. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. PNAS. 2013;110:12601–6. doi: 10.1073/pnas.1306562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 123.Gu L, Mooney DJ. Biomaterials and emerging anticancer therapeutics: engineering the microenvironment. Nat Rev Cancer. 2016;16:56–66. doi: 10.1038/nrc.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zaman MH. The role of engineering approaches in analysing cancer invasion and metastasis. Nat Rev Cancer. 2013;13:596–603. doi: 10.1038/nrc3564. [DOI] [PubMed] [Google Scholar]
- 125.Roudsari LC, West JL. Studying the influence of angiogenesis in in vitro cancer model systems. Adv Drug Deliv Rev. 2016;97:250–59. doi: 10.1016/j.addr.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 126.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, et al. Cancer treatment and survivorship statistics, 2014. Cancer J Clin. 2014;64:252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 127.Taubenberger AV. In vitro microenvironments to study breast cancer bone colonisation. Adv Drug Deliv Rev. 2014;79:135–44. doi: 10.1016/j.addr.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 128.Charoen KM, Fallica B, Colson YL, Zaman MH, Grinstaff MW. Embedded multicellular spheroids as a biomimetic 3D cancer model for evaluating drug and drug–device combinations. Biomaterials. 2014;35:2264–71. doi: 10.1016/j.biomaterials.2013.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fisher SA, Anandakumaran PN, Owen SC, Shoichet MS. Tuning the microenvironment: Click-crosslinked hyaluronic acid--based hydrogels provide a platform for studying breast cancer cell invasion. Adv Funct Mater. 2015;25:7163–72. [Google Scholar]
- 130.Taylor BS, Barretina J, Maki RG, Antonescu CR, Singer S, Ladanyi M. Advances in sarcoma genomics and new therapeutic targets. Nat Rev Cancer. 2011;11:541–57. doi: 10.1038/nrc3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Singh SP, Schwartz MP, Lee JY, Fairbanks BD, Anseth KS. A peptide functionalized poly(ethylene glycol) (PEG) hydrogel for investigating the influence of biochemical and biophysical matrix properties on tumor cell migration. Biomater Sci. 2014;2:1024–34. doi: 10.1039/C4BM00022F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lewis DM, Park KM, Tang V, Xu Y, Pak K, et al. Intratumoral oxygen gradients mediate sarcoma cell invasion. PNAS. 2016;113:9292–97. doi: 10.1073/pnas.1605317113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Agnihotri S, Burrell KE, Wolf A, Jalali S, Hawkins C, et al. Glioblastoma: a brief review of history, molecular genetics, animal models and novel therapeutic strategies. Arch Immunol Ther Exp. 2013;61:25–41. doi: 10.1007/s00005-012-0203-0. [DOI] [PubMed] [Google Scholar]
- 134.Rape A, Ananthanarayanan B, Kumar S. Engineering strategies to mimic the glioblastoma microenvironment. Adv Drug Deliv Rev. 2014;79:172–83. doi: 10.1016/j.addr.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang C, Tong X, Yang F. Bioengineered 3D brain tumor model to elucidate the effects of matrix stiffness on glioblastoma cell behavior using PEG-based hydrogels. Mol Pharm. 2014;11:2115–25. doi: 10.1021/mp5000828. [DOI] [PubMed] [Google Scholar]
- 136.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 137.Loessner D, Holzapfel BM, Clements JA. Engineered microenvironments provide new insights into ovarian and prostate cancer progression and drug responses. Adv Drug Deliv Rev. 2014;79:193–213. doi: 10.1016/j.addr.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 138.Fong EL, Martinez M, Yang J, Mikos AG, Navone NM, et al. Hydrogel-based 3D model of patient-derived prostate xenograft tumors suitable for drug screening. Mol Pharmacol. 2014;11:2040–50. doi: 10.1021/mp500085p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 140.Kaemmerer E, Melchels FP, Holzapfel BM, Meckel T, Hutmacher DW, Loessner D. Gelatine methacrylamide–based hydrogels: an alternative three-dimensional cancer cell culture system. Acta Biomater. 2014;10:2551–62. doi: 10.1016/j.actbio.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 141.Asghar W, El Assal R, Shafiee H, Pitteri S, Paulmurugan R, Demirci U. Engineering cancer microenvironments for in vitro 3-D tumor models. Mater Today. 2015;18:539–53. doi: 10.1016/j.mattod.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang L, Li Y, Huang G, Zhang X, Pingguan-Murphy B, et al. Hydrogel-based methods for engineering cellular microenvironment with spatiotemporal gradients. Crit Rev Biotechnol. 2016;36:553–65. doi: 10.3109/07388551.2014.993588. [DOI] [PubMed] [Google Scholar]
- 143.Shen Y-I, Abaci HE, Krupski Y, Weng L-C, Burdick JA, Gerecht S. Hyaluronic acid hydrogel stiffness and oxygen tension affect cancer cell fate and endothelial sprouting. Biomater Sci. 2014;2:655–65. doi: 10.1039/C3BM60274E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van Duinen V, Trietsch SJ, Joore J, Vulto P, Hankemeier T. Microfluidic 3D cell culture: from tools to tissue models. Curr Opin Biotechnol. 2015;35:118–26. doi: 10.1016/j.copbio.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 145.Sung KE, Beebe DJ. Microfluidic 3D models of cancer. Adv Drug Deliv Rev. 2014;79:68–78. doi: 10.1016/j.addr.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jeon JS, Zervantonakis IK, Chung S, Kamm RD, Charest JL. In vitro model of tumor cell extravasation. PLOS ONE. 2013;8:e56910. doi: 10.1371/journal.pone.0056910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Knowlton S, Onal S, Yu CH, Zhao JJ, Tasoglu S. Bioprinting for cancer research. Trends Biotechnol. 2015;33:504–13. doi: 10.1016/j.tibtech.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 148.Zhao Y, Yao R, Ouyang L, Ding H, Zhang T, et al. Three-dimensional printing of HeLa cells for cervical tumor model in vitro. Biofabrication. 2014;6:035001. doi: 10.1088/1758-5082/6/3/035001. [DOI] [PubMed] [Google Scholar]
- 149.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–68. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dickinson LE, Lütgebaucks C, Lewis DM, Gerecht S. Patterning microscale extracellular matrices to study endothelial and cancer cell interactions in vitro. Lab Chip. 2012;12:4244–48. doi: 10.1039/c2lc40819h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Highley CB, Rodell CB, Burdick JA. Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv Mater. 2015;27:5075–79. doi: 10.1002/adma.201501234. [DOI] [PubMed] [Google Scholar]
- 152.Jonas O, Landry HM, Fuller JE, Santini JT, Baselga J, et al. An implantable microdevice to perform high-throughput in vivo drug sensitivity testing in tumors. Sci Transl Med. 2015;7:284ra57. doi: 10.1126/scitranslmed.3010564. [DOI] [PMC free article] [PubMed] [Google Scholar]