SUMMARY
Background & aims
The purpose of the study was to compare the effects of the parenteral emulsion SMOFlipid®, with 15% fish oil, with Clinoleic® on retinopathy of prematurity (ROP) and other morbidities and growth, and to compare their impact on longitudinal serum levels of fatty acids. Retinopathy of prematurity, other morbidity and growth were correlated with each parenteral lipid supplement.
Methods
Ninety infants born at gestational age <28 weeks were randomized to treatment with SMO-Flipid® or Clinoleic®. Two thirds (66%) of the infants received parenteral nutrition for up to 14 days birth (median 8, range 2–14 days), and additional 25% of the infants received for up to 28 days after birth (median 21, range 15–28 days). Cord blood samples and then venous blood samples were obtained at ages 1, 7, 14, and 28 days and at postmenstrual age (PMA) 32, 36, and 40 weeks. Breastmilk was collected at postnatal day 7, and at PMA 32 and 40 weeks. Serum phospholipid and breastmilk total fatty acids were analyzed by gas chromatography–mass spectrometry. Treatment groups were compared with regard to ROP, bronchopulmonary dysplasia, necrotizing enterocolitis, patent ductus arteriosus sepsis and growth between birth and 36 weeks.
Results
Infants on SMOFlipid® had higher fractions of omega-3 LCPUFA eicosapentaenoic acid (EPA) and slightly higher omega-3 LCPUFA docosahexaenoic acid (DHA) fraction and a decreased arachidonic acid (AA) to DHA ratio from one week after birth up to 32 postmenstrual weeks compared to infants on Clinoleic®. Treatment groups did not differ in morbidities or growth.
Conclusion
Supplementation with SMOFlipid® containing 15% fish oil during parenteral nutrition increased EPA substantially, DHA marginally, reduced AA and decreased AA to DHA ratio. It did not reduce morbidity or affect growth. Since extremely preterm infants accumulate a large deficit of DHA and AA, studies on more prolonged or different levels of DHA and AA supplementation are warranted.
Keywords: Preterm, Parenteral nutrition, Long-chain polyunsaturated fatty acids, Morbidities Growth
1. Introduction
Extremely preterm infants are at increased risk of poor growth and development and prone to develop morbidities and dysfunctions both short and long term. These infants miss the third trimester of gestation in utero and the supply of nutrients, hormones, and other factors that are normally provided in amounts appropriate for developmental stage. Instead, they rely on parenteral nutrition for the first weeks after birth with lipid solutions lacking important components. During gestation, long-chain polyunsaturated fatty acids (LCPUFAs), which are structural constituents of most cell membranes, and play functional roles in fetal development, are selectively transferred from the mother to the fetus [1,2]. The omega-3 LCPUFA docosahexaenoic acid (DHA) is an important component in cell membranes in the CNS including the retina. In addition, omega-3 LCPUFAs influence the immune response and are precursors of resolvins and protectins which promote resolution of inflammation [3]. Dietary DHA is mainly derived from oily fish, which in turn obtain the LCPUFAs from microalgae [4]. Preterm infants can synthesize small amounts DHA [5] but not enough to meet their developmental needs. During the third trimester, 80% of fetal brain DHA accumulates, and large amounts are accumulated in adipose tissue [6]. Very preterm infants develop a large deficit in DHA as well as in the omega-6 LCPUFA arachidonic acid (AA) during parenteral nutrition with commonly used soybean- [7] and olive oil-based lipid emulsions [8] as well as during enteral nutrition even with breastfeeding [9,10]. Lapillonne et al. have estimated that an infant born at gestational age (GA) 27 weeks weighing 1000 g will have a DHA deficit of 600 mg/kg at age 4 weeks [11] which is thought to contribute to preterm morbidities such as bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC) and white matter injury and sepsis [12]. Supplementation of omega-3 LCPUFA to preterm children was recently reviewed by Zhang et al. [13]. No randomized controlled trials had targeted a population with exclusively extremely preterm infants (born at a GA <28 weeks). In a systematic review of omega-3 supplementation to infants born at GA ≤32 weeks a reduction in the incidence of NEC and a trend of decreased risk of BPD was found [13]. Dietary omega-3 LCPUFAs reduces pathologic retinal neovascularization in oxygen-induced retinopathy in mice [14–17]. With regard to ROP, studies of fish oil supplementation have reported a reduction in the need for laser therapy [18,19], less ROP but no difference in the need for treatment [20] as well as no benefit [21,22].
The most immature infants develop the largest DHA deficit and are most likely to benefit from supplementation [23]. The aims of this study were to determine and compare serum LCPUFA (DHA, eicosapentaenoic acid (EPA) and AA) profiles, ROP, BPD, NEC, patent ductus arteriosus (PDA), sepsis and growth in extremely preterm infants receiving parenteral nutrition with an olive oil-based lipid solution (Clinoleic®, Baxter) or a solution containing 15% fish oil with omega-3 LCPUFAs (SMOFlipid®, Fresenius Kabi).
2. Patients and methods
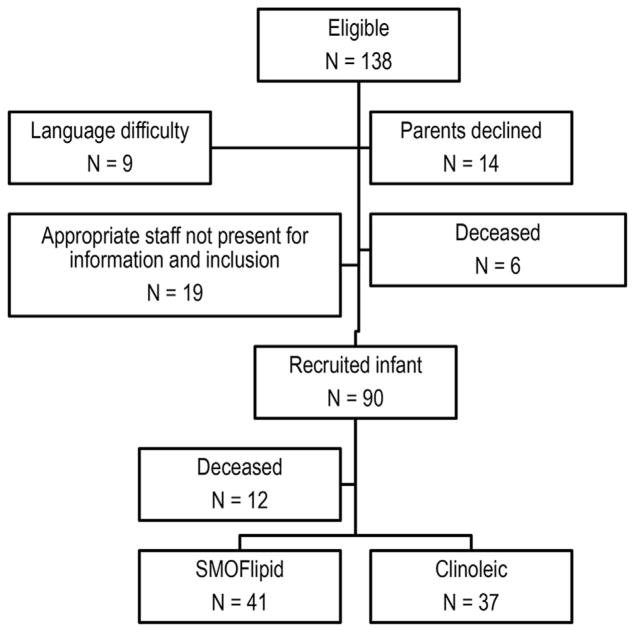
Included were infants with GA <28 weeks admitted to the neonatal intensive care unit at Sahlgrenska University Hospital in Gothenburg, Sweden, from 04/04/13 to 09/22/15. Exclusion criteria were major congenital malformations. Of the 138 infants born at GA <28 weeks during this period, parents of 90 eligible infants agreed to participation after informed consent (Fig. 1, Table 1). Randomization was in blocks of 20 infants, adjusting for GA to ensure equal numbers of Clinoleic®- and SMOFlipid®-treated infants in each GA group. Twins were randomized to the same lipid solution due to ethical concerns. The treating nurse/doctor received the randomization online. Type of lipid emulsion was blinded for data analysis and the screening ophthalmologists.
Fig. 1.
Patient enrollment flow chart.
Table 1.
Clinical characteristics of 78 infants completing the study, Clinoleic® n = 37 and SMOFlipid® n = 41 *n = 39.
| Clinoleic® (n = 37) | SMOFlipid® (n = 41) | |
|---|---|---|
| Male, n (%) | 19 (51) | 24 (59) |
| Gestational age (wks), mean (SD) | 25.6 (1.6) | 25.5 (1.3) |
| Birth weight (g), mean (SD) | 799 (225) | 799 (225) |
| Birth weight SDS, mean (SD) | −0.83 (1.0) | −0.81 (1.4) |
| Birth weight small for gestational age, n (%) | 5 (14) | 6 (15) |
| Birth length (cm), mean (SD) | 33.3 (3.4) | 33.2 (3.1)* |
| Birth length SDS, mean (SD) | −1.04 (1.8) | −1.14 (2.0)* |
| Birth head circumference (cm), mean (SD) | 23.4 (2.2) | 23.7 (2.8)* |
| Birth head circumference SDS, mean (SD) | −0.50 (0.7) | −0.40 (1.5)* |
SDS, standard deviation scores.
2.1. Routine nutritional management
2.1.1. Nutritional strategy
The nutritional strategy has been described previously [24]. Briefly, parenteral nutrition was initiated as soon as possible after birth with a standard solution containing Vaminolac and 10% Glucose (total protein content 2 g/100 mL) aiming at 80–90 mL/kg/d of the resulting solution during the first 24 h. Lipid solution (Clinoleic or SMOFLipid) was normally started at 6–12 h after birth at a rate of 1 g/kg/d with daily increases up to 2 g/kg/d. The resulting intake is recorded in Table 2. Enteral nutrition used either maternal or donor breastmilk with individualized fortification based on results from breastmilk analysis using a commercial bovine milk fortifier (Nutriprem, Breast Milk Fortifier, Nutricia, France). Minimal enteral feeding was started within 3 h of birth and administered every 2–3 h (1–2 mL/meal) with a gradual increase in volume. Administered breastmilk was analyzed at day 7 and then weekly for lipids, protein, carbohydrates and energy content in a 2 h sample until a PMA of at least 35 weeks. Daily intakes of protein (g/kg/d) and energy (kcal/kg/d) were prospectively registered from birth during the first 2 weeks of life (Table 2). All infants received parenteral and enteral nutrition according to clinical routine. Daily intakes of fatty acids AA, eicosapentaenoic acid (EPA) and DHA (mg/kg/d) were prospectively registered from birth during the first 2 weeks of life (Table 2).
Table 2.
Nutritional intake of 78 infants completing the study.
| Variables | Clinoleic® (n = 37) Postnatal week |
SMOFlipid® (n = 41) Postnatal week |
||
|---|---|---|---|---|
|
|
|
|
||
| Total parenteral and enteral intake | 1 Mean (SD)/Median (range) | 2 Mean (SD)/Median (range) | 1 Mean (SD)/Median (range) | 2 Mean (SD)/Median (range) |
| Energy (kcal/kg/d) | 82.42 (7.48) | 122.64 (16.94) | 88.58 (8.34) | 120.94 (18.94) |
| Carbohydrate (g/kg/d) | 10.33 (1.25) | 13.98 (1.44) | 10.91 (1.36) | 14.15 (1.59) |
| Protein (g/kg/d) | 3.06 (0.47) | 4.16 (0.61) | 3.17 (0.66) | 4.26 (0.74) |
|
| ||||
| Total enteral intake | ||||
| Lipids (g/kg/d) | 1.91 (0.82) | 4.52 (1.67) | 1.56 (0.84) | 4.09 (1.75) |
| DHA (mg/kg/d) | 6.66 (0.5–52.5) | 15.92 (0.0–107.4) | 5.00 (0.3–19.8) | 13.23 (0.0–55.2) |
| EPA (mg/kg/d) | 1.39 (0.0–5.2) | 2.85 (0.0–10.6) | 0.98 (0.0–4.8) | 2.27 (0.0–13.6) |
| AA (mg/d) | 8.49 (2.1–29.8) | 16.95 (1.8–54.0) | 6.27 (0.31–20.8) | 15.57 (0.0–3.8) |
| Total parenteral intake | ||||
| Lipids (g/kg/d) | 1.20 (0.44) | 0.58 (0.61) | 1.40 (0.39) | 0.91 (0.79) |
| DHA (mg/kg/d) | – | – | 45.16 (6.5–61.4) | 39.20 (0–80.7) |
| EPA (mg/kg/d) | – | – | 47.93 (6.9–65.2) | 41.60 (0–85.7) |
| AA (mg/kg/d) | 1.7 (0.4–2.7) | 0.75 (0–3.5) | 4.61 (0.7–6.3) | 4.00 (0–8.2) |
AA arachidonic acid, DHA: docosahexaenoic acid, EPA: Eicosapentaenoic acid.
2.2. Parenteral and enteral lipid administration
The parenteral lipid dosing strategy was to deliver a dose of 2–3 g/kg body weight every 24 h. The fatty acid compositions of SMOFlipid® and Clinoleic® were analyzed by gas chromatography–mass spectrometry, and the chromatograms are shown in Supplementary Fig. 1. The main difference between the two lipid supplements was that the omega-3 LCPUFAs EPA and DHA were found only in the SMOFlipid® solution. The LCPUFA content in the parental solutions are shown in Supplementary Table 1. The total parenteral intake of LCPUFAs was calculated using a set content 0.2 mg/L AA in Clinoleic® and 0.5 mg/L AA, 5.2 mg/L EPA and 4.9 mg/L AA in SMOFlipid®. The total enteral intake of LCPUFAs AA, EPA and DHA was calculated using an average fat content of 3.5% in breastmilk. LCPUFA content was analyzed in five collected donor breastmilk samples and a mean value was calculated for AA (125 mg/L), EPA (32.5 mg/L) and DHA (81 mg/L), which was used for all donor breastmilk samples. Mothers from 74 infants gave breastmilk at 7 and 14 days postnatal age, these samples were analyzed and used in calculation. Total amount of LCPUFA from breastmilk and donor milk was calculated (Table 2). The type of lipid emulsion given was not blinded.
3. Eye examinations
ROP screening started at 5–6 weeks of age but not before 31 weeks postmenstrual age (PMA). Retinal examinations through dilated pupils were performed biweekly to twice a week depending on ROP severity, until the retina was fully vascularized or the condition was considered stable. ROP was classified according to the international classification [25]. Severe ROP was defined as stage 3 or more.
3.1. Other morbidities and growth
Diagnoses such as BPD, NEC, PDA, and sepsis as well as growth variables (weight, length and head circumference) measured weekly were retrieved from clinical records. BPD was defined as the need for supplemental oxygen at 36 weeks of PMA, NEC was diagnosed by clinical signs and radiologic findings (Bell’s stages 2–3), and PDA was registered when the infant had clinical symptoms that required either pharmacological or surgical treatment. Sepsis was diagnosed by clinical symptoms accompanied by a positive blood culture. When the culture contained Staphylococcus epidermidis, an elevated C-reactive protein (>20 mg/L) was required for diagnosis. Suspected sepsis was defined as clinical symptoms with C-reactive protein elevated >20 mg/L, but without positive cultures. Cholestasis was defined as conjugated bilirubin blood level of >50 μmol/L for at least 2 weeks at any time during the follow up, unrelated to sepsis.
3.2. Blood sampling and laboratory analyses
Blood samples were taken at birth (cord blood), at postnatal days 1, 7, 14, and 28 and at PMA 32, 36, and 40 weeks. The serum phospholipids were extracted [26] and then fractionated on a single SEP-PAK aminopropyl cartridge from Waters Corp.
The FA methyl esters derived from serum phospholipids were analyzed on an Agilent 7820 GC coupled to an Agilent 5975 mass selective detector using a 30 m, 0.25 mm DB-23 column (Agilent). One microliter of sample was injected in pulsed (40 psi for 1 min) splitless mode and separated using helium as the carrier gas at a constant flow of 0.62 mL/min. Oven temperature was raised by 2 °C/min from 135 °C to 210 °C and held for 2 min. The detector was operated in scan mode at 70 eV with the transfer line heated to 220 °C. FA methyl esters were identified by comparison of retention time and mass spectra to authentic standards (Me 100, Me 81, and individual FA methyl esters, Larodan, Solna, Sweden). FAs were quantified and expressed as molar %. Longitudinal phospholipid fatty acid profiles for AA, EPA and DHA were analyzed.
4. Statistics
The study was powered on the risk of ROP. ROP classified as no ROP or ROP stage 1, 2, 3, or 3+. On this ordinal scale, a higher number indicates a more severe outcome. The frequency of outcome was based on data from the Swedish national register for ROP (SWEDROP) registrar data (www.swedrop.se). For infants born extremely preterm in the register 38% of infants have ROP 0, 12% ROP 1, 19% ROP 2, 19% ROP stage 3 and 12% ROP stage 3+. The estimated preventive treatment effect was based on the following assumptions: 25% of the infants will not benefit from the fish oil treatment; 25% will have one stage less ROP; and 50% will have two stages less ROP. To achieve 80% power at alpha 0.05, a sample of 40 subjects per group was needed (80 total).
Statistical analysis was performed using SPSS 23 for Microsoft Windows (IBM, Armonk, NY). p values < 0.05 were considered significant. For variables without normal distribution, the Spearman rank correlation coefficient or Mann–Whitney U test was used. For comparison of frequencies, the Chi-square test or Fisher exact test was used. For repeated measurements we used the SAS-procedure mixed with patient as subject and the option lsmeans in order to calculate the means and the differences between the means. The SAS-procedure multtest was used to perform the Bonferroni–Holm correction. Population marginal means with their confidence intervals were calculated for each group and each time point. Differences between the means of the groups at each time point with their confidence intervals are given. p values were calculated for the differences both uncorrected and corrected. Corrections were performed by the Bonferroni–Holm procedure. SAS version 9.3 was used (SAS Institute Inc., Cary, N.C., USA).
5. Ethics
The study was approved by the Regional Ethical Board, Gothenburg (Dnr 303-11) (Clinical trial NCT02760472).
6. Results
6.1. Clinical characteristics of the patients
Among the 90 infants recruited for the study, 78 (87%) survived the study period, 41 of whom were randomized to receive SMO-Flipid® and 37 to Clinoleic® (Fig. 1). The clinical characteristics of the study infants are reported in Table 1, Supplemental Table 2.
Total nutritional intake during the time period in which a majority received parenteral nutrition (days 1–14) was similar in the treatment groups (Table 2). Infants on Clinoleic® received a median (min–max) of 72 (15–1558) mL fat given for a median (min–max) of 12 (2–92) days. Infants with SMOFlipid® received a median (min–max) of 92 (9–1384) mL parenteral fats given for a median (min–max) of 12 (2–72) days. The treatment groups did not differ in either amount or duration of parenteral FA supplementation.
6.2. Longitudinal serum LCPUFAs in relation to parenteral lipid supplementation
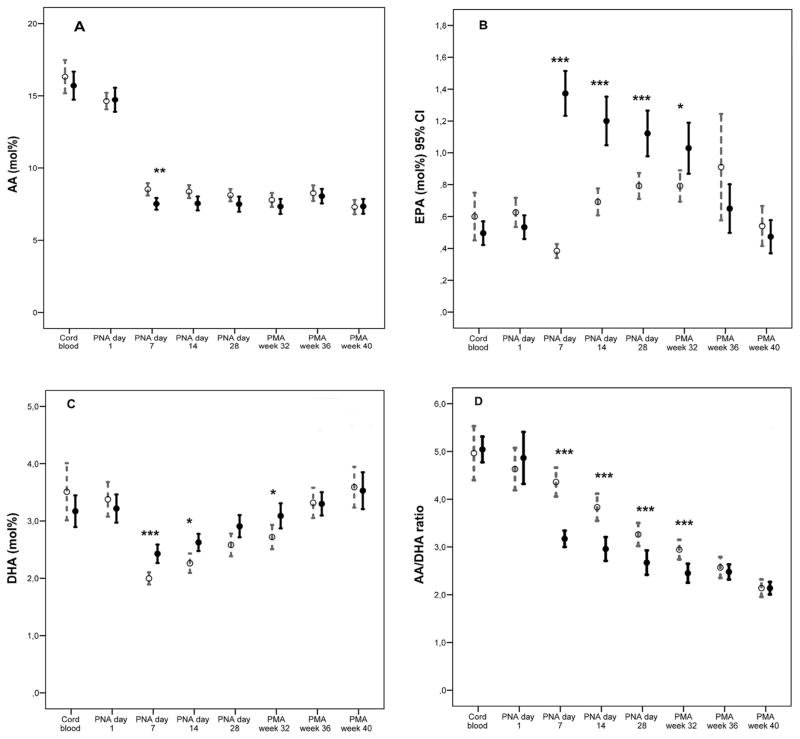
We found a decrease in serum AA fractions to approximately 50% of that in cord serum from one week after birth and no increase thereafter with either solution. In addition, infants receiving SMOFlipid® had a significantly lower fraction of AA at postnatal days 7 and 14 compared to those receiving Clinoleic®. The SMO-Flipid® group had a higher relative proportion of omega-3 LCPUFAs EPA and DHA at postnatal days 7, 14, and 28 and at PMA 32 weeks compared to the Clinoleic® group (Fig. 2A–C).
Fig. 2.
Longitudinal development of AA, EPA, and DHA in infants receiving SMOFlipid® (n = 41) and Clinoleic® (n = 37), infants on SMOFlipid depicted with solid dots and infants on Clinoleic depicted with open dots. P-values were calculated for the differences both uncorrected and corrected for repeated measurements. Corrections performed by the Bonferroni–Holm procedure are given in the figures. *p-value< 0.05; **p-value <0.01; ***p-value <0.001 (A) Decreased AA levels from birth to one week after birth by approximately 50% with no subsequent increase. Infants on SMOFlipid® had significantly lower levels at 7 days after birth compared to infants receiving Clinoleic®. (B) Infants on SMOFlipid® had significantly increased EPA levels (between 30% and 350% times higher) than infants on Clinoleic® from one week after birth up to a postmenstrual age corresponding to 32 weeks. (C) Decreased DHA levels from birth to one week after birth by approximately 50%; cord blood levels were not reached until an age corresponding to postmenstrual age 36 weeks. Infants on SMOFlipid® had significantly higher levels (between 12% and 20% times higher) at 7 and 14 days after birth and at postmenstrual age corresponding to 32 weeks compared to infants receiving Clinoleic®. Significantly lower omega-6 AA to omega-3 DHA ratio in infants receiving SMOFlipid® was seen from first week of life to PMA 32 weeks (Fig. 2D).
6.3. Relations between serum DHA, EPA and AA
Inverse correlations were found between EPA and AA fraction at day 7 (r = −0.38, p = 0.001) and at day 14 (r = −0.33, p = 0.003) in the SMOFlipid® group only. We found a more pronounced drop in omega-6 AA to omega-3 DHA ratio in infants on SMOFlipid® than in those on Clinoleic® from one week after birth to PMA 32 weeks (Fig. 2D). Mean AA to DHA ratio in the SMOFlipid® groups at 7 days was 3.17 (0.54) versus 4.36 (0.91) (p < 0.001) in the Clinoleic® group and 2.96 (0.78) in the SMOFlipid® groups versus 3.83 (0.85) in the Clinoleic® group at 14 days.
6.4. Morbidity and growth in relation to lipid solution
Treatment groups did not differ for any or severe ROP (Table 3). They also did not differ for BPD, NEC, or sepsis, and the reduction in the incidence of PDA in the SMOFlipid® group (p = 0.08) did not reach the predetermined level of statistical significance. Furthermore, there was no difference in postnatal gain of weight, length or head circumference between birth and PMA 36 weeks in infants receiving SMOFlipid® or Clinoleic®.
Table 3.
Outcome measurements of 78 infants completing the study, Clinoleic® n = 37 and SMOFlipid® n = 41.
| Clinoleic® | SMOFlipid® | p-value | |
|---|---|---|---|
| BPD n (%) | 17 (42) | 22 (58) | 0.18 |
| NEC (%) | 1 (3) | 4 (10) | 0.21 |
| PDA (%) | 29 (79) | 25 (61) | 0.08 |
| Any ROP (%) | 28 (78) | 33 (80) | 0.40 |
| Severe ROP (%) | 13 (35) | 18 (44) | 0.29 |
| SEPSIS (%) | 11 (30) | 19 (46) | 0.10 |
| Cholestasis (%) | 2 (5.7) | 4 (9.8) | 0.39 |
| Weight change SDS mean (SD) (n = 65) | −0.38 (1.2) | −0.22 (1.1) | 0.91 |
| Height change SDS mean (SD) (n = 60) | −1.62 (1.9) | −0.75 (1.9) | 0.25 |
| Head circumference change mean (SD) (n = 58) | −0.59 (1.2) | −0.64 (1.4) | 0.79 |
BPD: Bronchopulmonary dysplasia; NEC: Necrotizing enterocolitis; PDA, patent ductus arteriosus; Any ROP: Any stage of retinopathy of prematurity, Severe ROP: ROP stage 3 and or treated for ROP, SDS, standard deviation scores, SEPSIS: Sepsis verified by culture and or C-Reactive Protein. Chi-square and Mann–Whitney test used.
7. Discussion
Serum DHA levels of infants who received SMOFlipid® were higher than those of infants receiving Clinoleic® that lacked DHA and EPA. However, this study also shows that a lipid emulsion containing 15% fish oil does not prevent a large decrease in plasma DHA during the first weeks of life when provided at a mean rate of 1.7 (0.27.7) mg/d (Table 2). In both groups, serum AA decreased by approximately 50% after birth. Administration of SMOFlipid® resulted in increased EPA and even further decreased AA compared to Clinoleic® despite three times higher AA fraction in SMOFlipid® than in Clinoleic® (Supplement Table 1).
Infants on SMOFlipid® had a lower AA/DHA ratio than infants on Clinoleic®. Both DHA and AA are selectively transferred from the mother to the fetus during the third trimester. Bernhard et al. recently reported that the relative proportion of AA in fetal plasma phosphatidylcholine is two-fold higher than that in maternal serum during the third trimester, and that fetal DHA increases above maternal levels after approximately 33 weeks of gestation resulting in an early high AA to DHA ratio (4.9:1) at 24–27 weeks PMA with a decrease to term age in AA/DHA (2.5:1). In addition, they found an association between low AA to DHA ratio and BPD severity and suggested that AA needs to be supplied in higher doses than previously thought and especially to the most immature infants [27]. Interestingly, it has been proposed by Kuipers et al. that the placenta regulates the fetal DHA and AA balance such that at high maternal DHA levels, a too high transfer of DHA to the fetus is inhibited [28]. That high DHA suppresses AA was illustrated in full term baboons fed 0.67% AA and 0.33 or 1.0% DHA. The higher level of DHA reduced AA levels in red blood cells (RBC) and in two areas of the brain (superior colliculus and globus pallidus) [29]. There appears to be a certain cutting point in the influence of DHA on AA levels. In red blood cells with DHA less than approximately 6 wt%, AA was synergistically increased while with increasing DHA levels, AA decreased [28].
Preterm infants on parenteral nutrition lack both DHA and AA [7], presumably because of inadequate levels of DHA and AA and a high linoleic acid supply in parenteral lipid solutions [8]. Supplementation with enteral DHA but no AA to prevent BPD in infants with GA <29 weeks is currently being studied in a large randomized controlled trial [30]. The role of arachidonic acid in preterm infants is poorly studied. Like DHA, AA is an important component of cell membranes where a change in composition results in changed function [31]. AA is particularly enriched in the vasculature, and its metabolites can both stimulate and inhibit angiogenesis as well as inflammation [32]. During fetal development, AA is related to growth [33], and birth weight is correlated with plasma triglyceride content of AA [34]. Furthermore, astrocytes release AA products with proangiogenic effects on cerebral and retinal microvascular endothelial cells in culture [35,36]. Thus, AA is an important precursor of factors that appear to be essential for angiogenesis. Giving human milk supplemented with both DHA and AA to infants with BW < 1500 g resulted in better short but not long term cognitive development than un-supplemented human milk [37,38], and providing a formula with an AA/DHA ratio of 2/1 compared to 1/1 resulted in better psychomotor development in very preterm infants [39].
We found a negative correlation between EPA and AA levels in the infants treated with SMOFlipid®. Omega-3 and omega-6 fatty acids (e.g. LA and ALA) compete with each other for common desaturation and elongation enzymes in the synthesis of LCPUFAs. The two series of fatty acids including LCPUFAs also compete for incorporation into tissue phospholipids.
Low AA levels are associated with poor growth [30]. A recent review on the safety and efficacy of parenteral fish oil for preterm infants concluded that fish oil treatments increase EPA and decrease AA and that the safety of this supplement remains to be proven [40]. With increasing EPA, an association between low AA concentrations and late-onset sepsis has been reported in preterm infants [7]. We have not found any studies addressing the consequences of high EPA serum levels.
We found no association between the type of parenteral lipid emulsion and either postnatal morbidity or growth in extremely preterm infants. This might in part be due to the limited time period of parenteral nutrition and the variability in omega-3 LCPUFA content reported in breastmilk. In our study the two groups did not differ with regard to enteral DHA and AA exposure (Table 2).
Currently, no lipid solutions for parenteral use supply DHA to normal intrauterine levels in preterm infants. However, most formulas for preterm infants contain DHA. Recent reports indicate that early gavage feeding of oil with DHA from algae [41] or low EPA fish oil [42] and algae-derived DHA plus AA from fungi [34] successfully raises blood DHA concentrations without adverse effects.
In conclusion, after birth, extremely preterm infants developed large deficits of both DHA and AA. Parenteral nutrition containing 15% fish oil did not affect the frequency of ROP or other morbidities or postnatal growth in the present study. Despite higher AA content in SMOFlipid®, infants on this solution had lower fractions of AA than those on Clinoleic®. The AA to DHA ratio was significantly decreased with SMOFlipid®. Studies on preventing the large DHA and AA deficit while maintaining an AA to DHA ratio which is optimal for developmental stage in extremely preterm infants are needed, and supplementation of these LCPUFA’s with more AA than DHA may be a promising alternative (37).
Supplementary Material
Key notes
Parenteral nutrition with a lipid solution containing omega-3 long chain polyunsaturated fatty acids in fish oil (SMO-Flipid®) compared to an olive oil based solution (Clinoleic®) to extremely preterm infants increased the serum fraction of EPA substantially and that of DHA marginally, and decreased that of AA as well as of AA/DHA ratio. Morbidities and growth were not affected.
Clinical Relevancy Statement
Long chain polyunsaturated fatty acids (LCPUFAs) are essential for normal structural and functional development of the fetus, especially the retina and CNS. These fatty acids are lacking in commonly used lipid solutions for parenteral use in preterm infants. The resultant deficiency is thought to contribute to prematurity related morbidities. Newer lipid solutions provide LCPUFAs derived from fish oil. Compared to olive oil based Clinoleic® SMOFlipid® containing 15% fish oil did not affect morbidities or growth but resulted in significant changes in longitudinal serum fatty acid composition and a decreased arachidonic to docosahexaenoic acid ratio. Alternative strategies to provide LCPUFAs to the preterm infant need to be investigated.
Acknowledgments
Funding source
This study is supported by grants provided by the Swedish Research Council (DNR# 2011-2432) and Gothenburg County Council (ALFGBG-426531) long-term support by De Blindas Vänner and Kronprinsessan Margaretas Arbetsnämnd för synskadade, Stiftelsen Handlanden Herman Svenssons fond för blinda och synsvaga, Carmen och Bertil Regnérs Stiftelse, NIH EY024864, EY017017, P01 HD18655, Lowy Medical Research Institute. SMO-Flipid® was generously provided by Kabi Fresenius.
The authors thank all participants, the study team led by Carola Pfeiffer-Mosesson and Anne Rosenqvist for very valuable help with retrieving data for the study, Berit Holmberg for skillfully deriving the FA methyl esters derived from serum phospholipids and Kjell Pettersson at The Health Metrics unit the Sahlgrenska Academy at University of Gothenburg for statistical advice on analyzing repeated measurements.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.clnesp.2017.04.004.
Footnotes
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Conflict of interest
The authors have no potential conflicts of interest relevant to this article to disclose.
Contributors’ statement
Dr Najm enrolled the majority of study subjects, reviewed the manuscript, and approved the final manuscript as submitted.
Dr Löfqvist analyzed the data and outlined the results, wrote the manuscript, and approved the final manuscript as submitted.
Dr Hellgren organized sample collection, reviewed the manuscript, and approved the final manuscript as submitted.
Dr Lundgren performed data collection, reviewed the manuscript, and approved the final manuscript as submitted.
Dr Engström coordinated enrollment of study subjects, reviewed the manuscript, and approved the final manuscript as submitted.
Dr Hård designed the study, evaluated the results, drafted and wrote the manuscript, and approved the final manuscript as submitted.
Prof Lapillonne contributed to the analyses of data, critically reviewed the manuscript, and approved the final manuscript as submitted.
Dr Sävman contributed to the design of the study, coordinated enrollment of study subjects, reviewed the manuscript, and approved the final manuscript as submitted.
Dr Nilsson validated the GS/MS analyses, interpreted results, reviewed the manuscript, and approved the final manuscript as submitted.
Dr Andersson carried out the GS/MS analyses, interpreted results, reviewed the manuscript, and approved the final manuscript as submitted.
Prof Smith contributed to the design of the study, critically reviewed the manuscript, and approved the final manuscript as submitted.
Prof Hellström conceptualized and designed the study, supervised data collection, interpreted the results, drafted and wrote the manuscript, and approved the final manuscript as submitted.
References
- 1.Crawford M. Placental delivery of arachidonic and docosahexaenoic acids: implications for the lipid nutrition of preterm infants. Am J Clin Nutr. 2000;71:275S–84S. doi: 10.1093/ajcn/71.1.275S. [DOI] [PubMed] [Google Scholar]
- 2.Haggarty P. Placental regulation of fatty acid delivery and its effect on fetal growth-a review. Placenta. 2002;23:S28–38. doi: 10.1053/plac.2002.0791. [DOI] [PubMed] [Google Scholar]
- 3.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adarme-Vega TC, Thomas-Hall SR, Schenk PM. Towards sustainable sources for omega-3 fatty acids production. Curr Opin Biotechnol. 2014;26:14–8. doi: 10.1016/j.copbio.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Uauy R, Mena P, Wegher B, Nieto S, Salem N., Jr Long chain polyunsaturated fatty acid formation in neonates: effect of gestational age and intrauterine growth. Pediatr Res. 2000;47:127–35. doi: 10.1203/00006450-200001000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Kuipers RS, Luxwolda MF, Offringa PJ, Boersma ER, Dijck-Brouwer DA, Muskiet FA. Fetal intrauterine whole body linoleic, arachidonic and docosahexaenoic acid contents and accretion rates. Prostagl Leukot Essent Fat Acids. 2012;86:13–20. doi: 10.1016/j.plefa.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Martin CR, Dasilva DA, Cluette-Brown JE, Dimonda C, Hamill A, Bhutta AQ, et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J Pediatr. 2011;159:743–9. doi: 10.1016/j.jpeds.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernhard W, Raith M, Koch V, Kunze R, Maas C, Abele H, et al. Plasma phospholipids indicate impaired fatty acid homeostasis in preterm infants. Eur J Nutr. 2014;53:1533–47. doi: 10.1007/s00394-014-0658-3. [DOI] [PubMed] [Google Scholar]
- 9.Lapillonne A, Jensen CL. Reevaluation of the DHA requirement for the premature infant. Prostagl Leukot Essent Fat Acids. 2009;81:143–50. doi: 10.1016/j.plefa.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Sabel KG, Lundqvist-Persson C, Bona E, Petzold M, Strandvik B. Fatty acid patterns early after premature birth, simultaneously analysed in mothers’ food, breast milk and serum phospholipids of mothers and infants. Lipids Health Dis. 2009;8:20. doi: 10.1186/1476-511X-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapillonne A, Eleni dit Trolli S, Kermorvant-Duchemin E. Postnatal docosahexaenoic acid deficiency is an inevitable consequence of current recommendations and practice in preterm infants. Neonatology. 2010;98:397–403. doi: 10.1159/000320159. [DOI] [PubMed] [Google Scholar]
- 12.Lapillonne A, Moltu SJ. Long-chain polyunsaturated fatty acids and clinical outcomes of preterm infants. Ann Nutr Metab. 2016;69(Suppl 1):35–44. doi: 10.1159/000448265. [DOI] [PubMed] [Google Scholar]
- 13.Zhang P, Lavoie PM, Lacaze-Masmonteil T, Rhainds M, Marc I. Omega-3 long-chain polyunsaturated fatty acids for extremely preterm infants: a systematic review. Pediatrics. 2014;134:120–34. doi: 10.1542/peds.2014-0459. [DOI] [PubMed] [Google Scholar]
- 14.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–73. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stahl A, Sapieha P, Connor KM, Sangiovanni JP, Chen J, Aderman CM, et al. Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy. Circ Res. 2010;107:495–500. doi: 10.1161/CIRCRESAHA.110.221317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of {omega}-3 polyunsaturated fatty acids. Sci Transl Med. 2011;3:69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu Z, Lofqvist CA, Shao Z, Sun Y, Joyal JS, Hurst CG, et al. Dietary omega-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectin. Am J Clin Nutr. 2015;1:879–88. doi: 10.3945/ajcn.114.099291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pawlik D, Lauterbach R, Turyk E. Fish-oil fat emulsion supplementation may reduce the risk of severe retinopathy in VLBW infants. Pediatrics. 2011;127:223–8. doi: 10.1542/peds.2010-2427. [DOI] [PubMed] [Google Scholar]
- 19.Pawlik D, Lauterbach R, Walczak M, Hurkala J, Sherman MP. Fish-oil fat emulsion supplementation reduces the risk of retinopathy in very low birth weight infants: a prospective, randomized study. J Parenter Enter Nutr. 2014;38:711–6. doi: 10.1177/0148607113499373. [DOI] [PubMed] [Google Scholar]
- 20.Beken S, Dilli D, Fettah ND, Kabatas EU, Zenciroglu A, Okumus N. The influence of fish-oil lipid emulsions on retinopathy of prematurity in very low birth weight infants: a randomized controlled trial. Early Hum Dev. 2014;90:27–31. doi: 10.1016/j.earlhumdev.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 21.D’Ascenzo R, Savini S, Biagetti C, Bellagamba MP, Marchionni P, Pompilio A, et al. Higher docosahexaenoic acid, lower arachidonic acid and reduced lipid tolerance with high doses of a lipid emulsion containing 15% fish oil: a randomized clinical trial. Clin Nutr. 2014;33:1002–9. doi: 10.1016/j.clnu.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Vlaardingerbroek H, Vermeulen MJ, Carnielli VP, Vaz FM, van den Akker CH, van Goudoever JB. Growth and fatty acid profiles of VLBW infants receiving a multicomponent lipid emulsion from birth. J Pediatr Gastroenterol Nutr. 2014;58:417–27. doi: 10.1097/MPG.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 23.Lapillonne A, Groh-Wargo S, Gonzalez CH, Uauy R. Lipid needs of preterm infants: updated recommendations. J Pediatr. 2013;162:S37–47. doi: 10.1016/j.jpeds.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 24.Hansen-Pupp I, Lofqvist C, Polberger S, Niklasson A, Fellman V, Hellström A, et al. Influence of insulin-like growth factor I and nutrition during phases of postnatal growth in very preterm infants. Pediatr Res. 2011;69:448–53. doi: 10.1203/PDR.0b013e3182115000. [DOI] [PubMed] [Google Scholar]
- 25.The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 26.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 27.Bernhard W, Raith M, Koch V, Maas C, Abele H, Poets CF, et al. Developmental changes in polyunsaturated fetal plasma phospholipids and feto-maternal plasma phospholipid ratios and their association with bronchopulmonary dysplasia. Eur J Nutr. 2016;55:2265–74. doi: 10.1007/s00394-015-1036-5. [DOI] [PubMed] [Google Scholar]
- 28.Kuipers RS, Luxwolda MF, Sango WS, Kwesigabo G, Dijck-Brouwer DA, Muskiet FA. Maternal DHA equilibrium during pregnancy and lactation is reached at an erythrocyte DHA content of 8 g/100 g fatty acids. J Nutr. 2011;141:418–27. doi: 10.3945/jn.110.128488. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh AT, Anthony JC, Diersen-Schade DA, Rumsey SC, Lawrence P, Li C, et al. The influence of moderate and high dietary long chain polyunsaturated fatty acids (LCPUFA) on baboon neonate tissue fatty acids. Pediatr Res. 2007;61:537–45. doi: 10.1203/pdr.0b013e318045bec9. [DOI] [PubMed] [Google Scholar]
- 30.Collins CT, Gibson RA, Makrides M, McPhee AJ, Sullivan TR, Davis PG, et al. N3RO Investigative Team. The N3RO trial: a randomised controlled trial of docosahexaenoic acid to reduce bronchopulmonary dysplasia in preterm infants < 29 weeks’ gestation. BMC Pediatr. 2016;16:72. doi: 10.1186/s12887-016-0611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hadley KB, Ryan AS, Forsyth S, Gautier S, Salem N. The essentiality of arachidonic acid in infant development. Nutrients. 2016;8:216. doi: 10.3390/nu8040216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bogatcheva NV, Sergeeva MG, Dudek SM, Verin AD. Arachidonic acid cascade in endothelial pathobiology. Microvasc Res. 2005;69:107–27. doi: 10.1016/j.mvr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Carlson SE, Werkman SH, Peeples JM, Cooke RJ, Tolley EA. Arachidonic acid status correlates with first year growth in preterm infants. Proc Natl Acad Sci U S A. 1993;90:1073–7. doi: 10.1073/pnas.90.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koletzko B, Braun M. Arachidonic acid and early human growth: is there a relation? Ann Nutr Metab. 1991;35:128–31. doi: 10.1159/000177636. [DOI] [PubMed] [Google Scholar]
- 35.Munzenmaier DH, Harder DR. Cerebral microvascular endothelial cell tube formation: role of astrocytic epoxyeicosatrienoic acid release. Am J Physiol Heart Circ Physiol. 2000;278:H1163–7. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- 36.Capozzi ME, McCollum GW, Penn JS. The role of cytochrome P450 epoxygenases in retinal angiogenesis. Investig Ophthalmol Vis Sci. 2014;55:4253–60. doi: 10.1167/iovs.14-14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henriksen C, Haugholt K, Lindgren M, Aurvåg AK, Rønnestad A, Grønn M, et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics. 2008;121:1137–45. doi: 10.1542/peds.2007-1511. [DOI] [PubMed] [Google Scholar]
- 38.Henriksen C, Almaas AN, Westerberg AC, Drevon CA, Iversen PO, Nakstad B. Growth, metabolic markers, and cognition in 8-year old children born prematurely, follow-up of a randomized controlled trial with essential fatty acids. Eur J Pediatr. 2016;175:1165–74. doi: 10.1007/s00431-016-2755-1. [DOI] [PubMed] [Google Scholar]
- 39.Alshweki A, Muñuzuri AP, Baña AM, de Castro MJ, Andrade F, Aldamiz-Echevarría L, et al. Effects of different arachidonic acid supplementation on psychomotor development in very preterm infants; a randomized controlled trial. Nutr J. 2015;14:101. doi: 10.1186/s12937-015-0091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Y, Wu Y, Pei J, Chen Z, Wang Q, Xiang B. Safety and efficacy of parenteral fish oil-containing lipid emulsions in premature neonates. J Pediatr Gastroenterol Nutr. 2015;60:708–16. doi: 10.1097/MPG.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 41.Baack ML, Puumala SE, Messier SE, Pritchett DK, Harris WS. Daily enteral DHA supplementation alleviates deficiency in premature infants. Lipids. 2016;51:423–33. doi: 10.1007/s11745-016-4130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins CT, Sullivan TR, McPhee AJ, Stark MJ, Makrides M, Gibson RA. A dose response randomised controlled trial of docosahexaenoic acid (DHA) in pre-term infants. Prostagl Leukot Essent Fat Acids. 2015;99:1–6. doi: 10.1016/j.plefa.2015.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.