Abstract
B lymphocytes develop from hematopoietic stem cells (HSCs) in specialized bone marrow niches composed of rare mesenchymal-lineage stem/progenitor cells (MSPCs) and sinusoidal endothelial cells. These niches are defined by function and location: MSPCs are mostly perisinusoidal cells that together with a small subset of sinusoidal endothelial cells, express stem cell factor (SCF), Interleukin-7 (IL-7), IL-15, and the highest amounts of CXCL12 in bone marrow. Though rare, MSPCs are morphologically heterogeneous, highly reticular, and form a vast cellular network in the bone marrow parenchyma capable of interacting with large numbers of hematopoietic cells. HSCs, downstream multipotent progenitor cells (MPPs) and common lymphoid progenitor cells (CLPs) utilize CXCR4 to fine-tune access to critical short-range growth factors provided by MSPCs for their long-term maintenance and/or multilineage differentiation. In later stages, developing B lymphocytes use CXCR4 to navigate the bone marrow parenchyma, and predominantly Cannabinoid receptor −2 for positioning within bone marrow sinusoids, prior to being released into peripheral blood circulation. In the final stages of differentiation, transitional B cells migrate to the spleen where they preferentially undergo further rounds of differentiation until selection into the mature B cell pool occurs. This bottleneck purges up to 97% of all developing B cells in a peripheral selection process that is heavily controlled not only by the intensity of BCR signaling and access to BAFF, but also by the proper functioning of the B cell motility machinery.
Keywords: CXCR4, CXCL12, IL-7, HSC niche, GPCR, B lymphocyte
1. Introduction
As if tuned to the popular Clash song “Should I stay or should I go?” lymphocytes are constantly on the move as they develop in primary lymphoid organs and survey secondary lymphoid organs. Over the last two decades, remarkable progress has been made in the understanding of guidance cues that lymphocytes follow in peripheral lymphoid organs during homeostasis and immune responses. Seminal and elegant studies have enabled a near complete temporal and spatial understanding of where lymphocytes reside and how they interact during the course of an immune response (Pereira, Kelly and Cyster, 2010;Qi, Kastenmuller and Germain, 2014). Lagging considerably behind is an understanding of where hematopoietic stem and progenitor cells (HSPC), from which these lymphocytes develop, reside and with what cells they interact as they make the lineage commitment to the lymphoid fate.
While obvious anatomic features like a red and a white pulp, or follicular structures, characterize secondary lymphoid organs, there are no obvious landmarks in the bone marrow environment other than bone and blood vessels. Furthermore, lymphocytes can be easily distinguished by immunostaining with antibodies against single epitopes, whereas HSPCs required for many years complex combinations of antibodies against a variety of cell surface receptors in order to allow their accurate visualization by flow cytometry or microscopy. A major breakthrough was the finding that the cell surface receptor CD150 (or Signaling Lymphocytic Activation Molecule Family member 1; SLAMF1) is expressed on hematopoietic stem cells (HSCs), and can be used in combination with a cocktail of antibodies recognizing receptors associated with cell lineage differentiation to identify a reasonably pure population of HSCs in bone tissue sections using only two fluorescence channels (Kiel et al., 2005). This finding enabled researchers to identify the microenvironments inhabited by HSCs within bone marrow (often called HSC niches), and helped define the migratory and positional cues followed by quiescent and active HSCs (Boulais and Frenette, 2015;Morrison and Scadden, 2014).
Of the many chemoattractants that are produced in bone marrow (Nevius, Gomes and Pereira, 2016), CXCL12 is not only the most abundant but also the most important chemokine for regulating HSC quiescence and differentiation known to date (Nie, Han and Zou, 2008;Sugiyama et al., 2006). CXCR4, the receptor for CXCL12, is expressed by more than 95% of hematopoietic cells in bone marrow including HSCs and hematopoietic progenitors, and is a pharmacological target for HSC mobilization from bone marrow into blood, in both mice and humans (Broxmeyer et al., 2005;Liles et al., 2003). CXCL12 is expressed by heterogeneous populations of cells: Mesenchymal stem and progenitor cells and sinusoidal endothelial cells, which express the highest amounts, as well as osteoblasts and certain hematopoietic cells, which express 100-1000 fold lower amounts. CXCL12 production is regulated by a variety of external cues ranging from circadian signals such as adrenergic hormones (e.g. norepinephrine) to inflammatory mediators (e.g. cytokines, proteases). Importantly, fluctuations in CXCL12 production such as those seen during infection or sterile inflammation result in massive alterations in HSPC distribution between bone marrow and blood, and correlate with shifts in hematopoietic lineage output (Ueda, Kondo and Kelsoe, 2005;Ueda et al., 2004).
Do migratory cues regulate hematopoietic cell lineage decisions? If so, how? In this review we aim to present an integrated model of how chemotactic cues act together with differentiation, proliferation and survival factors to support the generation of mature B lymphocytes. We will start by reviewing the niches and signals regulating HSC maintenance and differentiation into lymphoid lineages in bone marrow, and then consider how cell movement within bone marrow might enable proB and preB cells to balance cell proliferation with RAG-mediated immunoglobulin V(D)J gene recombination. We will also discuss the mechanisms and routes B-lineage cells (and other hematopoietic cells) utilize for exiting bone marrow. Finally, we will review the role played by chemoattractant receptors and components of the B cell motility machinery in the differentiation of immature, transitional B cells into the mature B cell compartment.
2. Hematopoietic stem cell niches
HSCs are responsible for sustaining the lifelong production of blood cells in the process known as hematopoiesis. In order to sustain lifelong hematopoiesis, HSCs have to be maintained in a largely quiescent state in order to be protected from genotoxic harm, while dividing occasionally to self-renew or differentiate. Over the past two decades intense research has been devoted to the characterization of extracellular signals promoting HSC maintenance, bone marrow niches colonized by HSCs, and cells involved in the regulation of HSC quiescence and activation. The cells that provide essential factors for HSC maintenance constitute what is called the “HSC niche,” which is defined by both location and function. The niche influence on HSCs is thought to be highly dependent on proximity and even direct HSC-niche cell interaction. This is because several signals produced by niche cells that are required for the long-term maintenance of HSCs act as short-range signals. Earlier studies suggested that HSCs tend to localize near the endosteum and are regulated by bone-producing osteoblasts (Calvi et al., 2003;Zhang et al., 2003). However, recent studies from several laboratories made compelling observations that are incompatible with a major role played by osteoblasts, and support an alternative model, which is described below. For an historical overview of the bone marrow niche hypothesis initially proposed by Schofield, and of the data in favor of and against the osteoblastic niche model, the reader is referred to other reviews that have been written on the subject (Boulais and Frenette, 2015;Morrison and Scadden, 2014).
Two factors, CXCL12 and SCF, play vital roles in the long-term maintenance of HSCs. Nagasawa and Morrison exploited this fact to identify HSC niche cells by generating CXCL12 and SCF fluorescent reporter mouse strains (Ding and Morrison, 2013;Ding et al., 2012;Sugiyama et al., 2006). This simple and elegant approach enabled the identification of a relatively rare but highly interconnected network of perisinusoidal cells of mesenchymal lineage, and sinusoidal endothelial cells, that express high amounts of SCF and CXCL12. Furthermore, the mesenchymal lineage cells also express Leptin receptor (LEPR), which allowed the use of the Lepr-cre transgene to enforce genetic deletion of Scf or Cxcl12 from these cells. Together with the use of mice expressing cre recombinase in endothelial cells, these experiments demonstrated that HSC niche function is predominantly carried out by perisinusoidal mesenchymal cells and a small subset of sinusoidal endothelial cells (Ding and Morrison, 2013;Ding et al., 2012;Greenbaum et al., 2013), a conclusion supported by another study which used a different promoter to target mesenchymal cells (Greenbaum 2013). These results agreed with prior (and subsequent) studies showing a preferential localization of HSCs in the perisinusoidal compartment (Acar et al., 2015;Chen et al., 2016;Kiel et al., 2005;Sugiyama et al., 2006). Further elegant studies by Nagasawa and Frenette revealed that the mesenchymal-lineage cells with HSC niche activity are in fact mesenchymal stem/progenitor cells (MSPC) capable of undergoing differentiation into adipocytic, osteoblastic, and chondrocytic cell lineages (Mendez-Ferrer et al., 2010;Omatsu et al., 2010). In agreement with these findings, fate mapping of Lepr-Cre+ cells also revealed MSPC activity in HSC niche cells (Zhou et al., 2014). Interestingly, the MSPC differentiation potential is partly co-regulated with SCF and CXCL12 expression by the transcription factor FOXC1 (Omatsu et al., 2014).
2.1 A quiescent niche for quiescent HSCs?
In multicellular organisms, cellular differentiation is often preceded by cell division. This is certainly the case with hematopoiesis, where HSC differentiation takes place after cell cycle activation (Li and Clevers, 2010). Which signals instruct HSCs to self-renew and which promote HSC differentiation still remains poorly understood, but it has been postulated that distinct bone marrow niches might regulate distinct HSC fates. Evidence for this model came from the identification of two distinct Nestin-GFP positive mesenchymal lineage cell populations that differed in positional distribution, quiescence, and expression of HSC maintenance genes—an extremely rare population of Nestin-GFPbright cells (< 0.002%) that is periarteriolar and expresses pericyte markers (Neural/Glial antigen-2, NG2, and alpha smooth muscle actin), also named Nesperi, and a more abundant but less quiescent population of perisinusoidal and reticular Nestin-GFPdim cells, also named Nesretic, that does not express NG2 but expresses LEPR (Kunisaki et al., 2013). In situ analyses of quiescent 5-ethynyl-2′-deoxyuridine (EdU)-retaining HSCs revealed that EdU+ HSCs reside in the vicinity of Nesperi cells. In contrast, active HSCs (EdU− HSCs, or that expressed Ki67) were found in non-defined areas farther away from arterioles, raising the possibility that distinct bone marrow niches control HSC quiescence and activation. In support of the model “quiescent niche for quiescent HSCs,” selective ablation of the Nesperi population led to a reduction in HSC number. Furthermore, nearly 50% of the remaining HSCs were in active cell cycle, suggesting a loss of quiescence. This finding is consistent with results from RNA-sequencing analysis showing that HSC maintenance genes (e.g. CXCL12, SCF) are more abundantly expressed in Nesperi cells than in Nesretic cells (Kunisaki et al., 2013). A careful analysis of the gene expression profile of NG2+ Nesperi and NG2− Nesretic cells revealed, however, that NG2 (gene symbol Cspg4) expression is in fact higher in samples named Nesretic than in those named Nesperi, and reciprocally, that Lepr expression is considerably higher in samples named as Nesperi than in those named Nesretic (our unpublished analyses of gene expression dataset deposited in Gene Expression Omnibus under accession number GSE48764). This discrepancy between gene expression profile and phenotype of Nesperi and Nesretic cells suggests that samples named Nesperi are in fact Nesretic cells, and vice-versa. Furthermore, the conditional deletion of Scf or Cxcl12 from Nesperi cells using the NG2-CreER transgene did not affect HSC numbers in bone marrow (Acar et al., 2015), thus questioning the physiological relevance of SCF and CXCL12 produced by Nesperi cells. Two recent studies that used mouse reporters for α-catulin (gene symbol Ctnnal1) and Hoxb5, which allowed deep imaging of HSCs in optically cleared long-bone bone marrow by confocal microscopy, have further challenged the “quiescent niche for quiescent HSCs” model. HSCs marked by α-catulin-GFP are highly enriched for long-term HSCs and when α-catulin-GFP+ and cKit+ HSCs were visualized in situ they were predominantly found within < 5 μm of both CXCL12+ and Lepr+ cells (Acar et al., 2015). In agreement with this study, HSCs marked by triple mCherry expression driven by Hoxb5 regulatory elements, an HSC subset that is also highly enriched for long-term, presumably quiescent, HSCs, were also predominantly found in direct contact with bone marrow endothelial cells but without an obvious preference for arterioles (Chen et al., 2016). Hence, the exact contribution of periarteriolar pericytes to HSC maintenance remains unclear.
It is possible that the cell ablation strategy used in the Kunisaki et al. study caused indirect effects that in turn affected HSC maintenance. This could have occurred in at least three ways: 1) inflammation, 2) HSC niche alterations, and 3) changes in nutrient access or availability. 1) Tissue inflammation is likely to occur when large numbers of apoptotic cells are generated in a somewhat synchronous manner such that tissue resident phagocytes are unable to clear them effectively. Apoptotic debris is often inflammatory (Nagata, Hanayama and Kawane, 2010) as it contains a variety of ligands for cell extrinsic and intrinsic sensors, (e.g., Toll like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-, leucine-rich repeat–containing receptors, RIG I Like Receptors, and other cytosolic sensors of nucleic acids) that lead to the production of type I and type II interferons (Nathan and Ding, 2010). Given the normal physiological response of HSCs to inflammatory mediators (Baldridge et al., 2010;Essers et al., 2009), it can be difficult to deduce the role of the ablated cell population(s) in HSC maintenance simply by studying changes in HSC numbers and activation status. 2) Alterations in non-targeted HSC niches may also occur when cell ablation strategies are used (e.g. global gene expression changes may have occurred in Nesretic cells when Nesperi cells were ablated.), which in turn could affect HSC numbers in bone marrow. Finally, 3) HSC maintenance and fitness is dependent on essential amino acids, such as Valine (Taya et al., 2016), and it is at present unclear whether cell ablation strategies directly or indirectly affect access to specific nutrients in bone marrow.
2.2 HSC niche: a multilayered cellular network
Besides SCF and CXCL12, several other factors, not necessarily produced by perisinusoidal mesenchymal and sinusoidal endothelial cells, have been proposed to regulate HSC numbers in bone marrow. Glial fibrillary acidic protein (GFAP)-expressing non-myelinating Schwann cells express integrin β8, which converts latent TGFβ into active TGFβ that signals through TGFβR2 expressed on HSPCs to promote quiescence (Yamazaki et al., 2011). GFAP+ cells ensheath sympathetic nerves in bone marrow, are found in close association with Nesperi cells (Kunisaki et al., 2013), and are in direct contact with a significant proportion of HSCs (Yamazaki et al., 2011). While non-myelinating Schwann cells do not seem to express TGFβ, megakaryocytes have been identified as the main source of TGFβ for HSCs in bone marrow (Zhao et al., 2014). Besides TGFβ, megakaryocytes express platelet factor 4 (PF4, also known as CXCL4) that regulates the HSC cell cycle and acts to promote HSC quiescence and long-term maintenance, though the receptor on HSCs for PF4 remains to be identified (Bruns et al., 2014). Megakaryocytes also express Thrombopoietin (THPO), which is important for maintaining HSC quiescence, and possibly retention at HSC niches (Nakamura-Ishizu et al., 2014;Nakamura-Ishizu et al., 2015;Qian et al., 2007;Yoshihara et al., 2007). In humans, mutations in the c-mpl gene (encodes the THPO receptor MPL) lead to the disease congenital amegakaryocytic thrombocytopenia, characterized not only by hypomegakaryocytic thrombocytopenia, but also by a reduction in CD34+ hematopoietic progenitor cells in bone marrow and defective multilineage differentiation capacity (Ballmaier et al., 2003). As THPO plays an important role in megakaryocyte development it is unclear the extent to which its effects on HSC maintenance are direct or indirect. In any case, THPO, PF4 and TGFβ, likely act as short-range signals for HSC maintenance given the fact that many HSCs are often found in non-random and close proximity to megakaryocytes under homeostasis (Bruns et al., 2014;Zhao et al., 2014).
It should be mentioned, however, that several of the studies described above relied on a combination of experimental strategies that warrant concern (specifically, megakaryocyte ablation in vivo, and conditional deletion of Tgfb1 using a Pf4-cre transgene). As previously discussed, cell ablation strategies may cause indirect effects that affect HSC quiescence. Furthermore, the specificity of Pf4-cre activity is questionable given the fact that Pf4 expression is readily detected at high amounts in Scf-GFP+ cells and in Nestin-GFP+ cells (our unpublished analyses of gene expression dataset deposited in Gene Expression Omnibus under accession numbers GSE48764 and GSE33158). This raises the concern that Pf4-cre not only ablated megakaryocytes (or deleted Tgfb1 in megakaryocytes) but also mesenchymal-lineage HSC niche cells.
2.3 CXCR4 as a rheostat for HSC quiescence versus differentiation
Of all extracellular signals that regulate HSC homeostasis, membrane-bound SCF (mSCF) is arguably the most important cytokine (Driessen, Johnston and Nilsson, 2003;Flanagan, Chan and Leder, 1991), and requires direct cell-cell interactions for engaging cKit on HSCs. On the other hand, secreted factors (e.g. THPO, TGFβ, PF4), while not requiring HSCs to be in direct contact with niche cells, presumably form concentration gradients due to a combination of diffusion and consumption. Hence, we argue that the function of CXCL12 is primarily to enable HSCs to position close to HSC niches in order to access critical niche factors that enforce quiescence. Based on this hypothesis, we propose a mechanism by which HSC quiescence (G0) or activation (G1 + S + G2/M), which may lead to self-renewal or differentiation, can be regulated: CXCL12 expression in bone marrow fluctuates in a circadian manner, leading to HSC release from, and homing back to the bone marrow niche (Mendez-Ferrer et al., 2008). Decreases in bone marrow CXCL12 during the day lead to HSC release from bone marrow niches and hence uncoupling from quiescence signals (G0), allowing them to enter into the G1 stage of the cell cycle, while increases in CXCL12 at the end of the day draw HSCs back to their bone marrow niches, where they return to a quiescent G0 state. In this model, an inability of HSCs to leave HSC niches can be just as detrimental as the inability of HSCs to home to the niche, as these HSCs will not be able to escape from quiescence signals to divide and differentiate. In favor of this model, patients with WHIM syndrome (Warts, Hypogammaglobulinemia, Infection, Myelokathexis), a disease caused by mutations in CXCR4 desensitization that leads to constitutive signaling, are characterized by pan-leukopenia that is only attributed to increased bone marrow retention of differentiated leukocytes (Balabanian et al., 2005;Gorlin et al., 2000;Hernandez et al., 2003). Interestingly, the loss of the heterozygous CXCR4 mutant allele in a single HSC of a WHIM patient, in a serendipitous chromothriptic event, allowed the recovery of the patient’s hematopoietic system, with the exception of lymphocytes (McDermott et al., 2015). This strongly suggested that this CXCR4 haploinsufficient HSC gained a significant competitive advantage over CXCR4 mutant HSCs in both self-renewal and differentiation, besides accelerated egress from bone marrow. Thus, we propose at least one additional role for the physiologic recirculation of HSCs (Wright et al., 2001)—that it enables a balance between HSC quiescence, activation, and possibly differentiation, in order to maintain lifelong hematopoiesis in organisms. This model may seem incompatible with recent observations that point to a very limited number of cell divisions that HSCs undergo during an organism’s lifetime (Bernitz et al., 2016), but is consistent with the widespread notion that more than 10% of HSCs are Ki67+ (and thus not in G0) at any given time (Kunisaki et al., 2013;Sugiyama et al., 2006).
Finally, it should be mentioned that besides mediating the homing, retention and maintenance of bone marrow HSCs, CXCR4 signaling itself has also been suggested to control cell cycle gene expression and in this manner to regulate HSC quiescence and proliferation. The deletion of CXCR4 in hematopoietic cells resulted in increased proliferation of HSCs in vivo (Nie, Han and Zou, 2008;Sugiyama et al., 2006), and HSCs cultured in vitro in the presence of CXCL12 were more quiescent than HSCs cultured without it (Nie, Han and Zou, 2008;Tzeng et al., 2011). In conflict with these findings, however, other studies showed that deletion of CXCL12-expressing cells in bone marrow resulted in increased HSC quiescence, while in vitro culture of HSCs with CXCL12 increased the expression of genes associated with cell cycle entry (Omatsu et al., 2010). It should be noted that in vitro studies on HSC quiescence are difficult to interpret considering the vastly different environment that tissue culture represents in contrast to the bone marrow microenvironment. Hence, it remains unclear if CXCR4 signaling itself plays any direct role in regulating HSC quiescence.
3. Hematopoietic Multipotent Progenitor niches
While there has been an abundance of studies investigating the HSC niche in bone marrow, studies looking into the niches for MPP maintenance and differentiation lag considerably behind. Early studies suggested that HSCs and their immediate progeny colonize distinct bone marrow niches upon transplantation, leading to the suggestion that separate niches might orchestrate HSC maintenance and differentiation (Czechowicz et al., 2007; Lo Celso et al., 2009). Which niches are colonized by short-term HSCs and MPPs, and what signals are provided therein, were not examined. But, like long-term HSCs, ST-HSCs and MPPs express surface receptors for the same critical signals that govern the homeostasis of LT-HSCs (e.g. cKit, MPL, CXCR4), and are likely dependent on proper signaling from these receptors for their development, maintenance and retention. Evidence for this model came from analyses of mice conditionally deficient in CXCR4 in MPP cells. Because MPPs express FLT3 (encoded by Flk2) while HSCs do not, MPPs and essentially all downstream hematopoietic cells can be genetically manipulated with a Flk2-cre transgene (Boyer et al., 2011). It was observed that when MPPs lack CXCR4 they were significantly reduced in bone marrow, and differentiated poorly into downstream hematopoietic cells (Cordeiro Gomes et al., 2016). The numerical reduction of MPPs observed in bone marrow was probably a consequence of reduced retention and reduced access to niche signals. Indeed, MPPs reside in very close proximity to mesenchymal lineage cells marked by IL-7 expression, a subset of LEPR+ cells that not only expresses significant amounts of mSCF but also the highest amounts of CXCL12 in bone marrow. This subset of IL-7-expressing LEPR+ cells also exhibits multilineage differentiation potential (e.g. osteoblasts, osteocytes and adipocytes) in vivo, giving strength to the model proposed by Frenette and colleagues, and discussed earlier in this chapter, that the HSC niche is a partnership between two types of stem cells (Frenette et al., 2013). Besides MPPs, HSCs could also be found in significant proximity to IL-7+ cells, and occasionally an MPP and an HSC were found in the vicinity of the same IL-7+ cell (Cordeiro Gomes et al., 2016). Both HSCs and MPPs are dependent on the short-range signals, mSCF and CXCL12 provided by IL-7+ cells for their maintenance, suggesting that overlapping niches control HSC maintenance and multilineage differentiation (Figure 1). Consistent with this model, MPPs require FLT3L for development, maintenance, or multilineage differentiation (McKenna et al., 2000;Sitnicka et al., 2002), and although the physiological source of FLT3L for MPPs has not been defined, Nestin-GFP+, IL-7+ LEPR+, and Scf-GFP+ mesenchymal cells express Flt3l mRNA (our unpublished observations).
Figure 1. HSC niches in bone marrow control HSC homeostasis and lymphopoiesis.
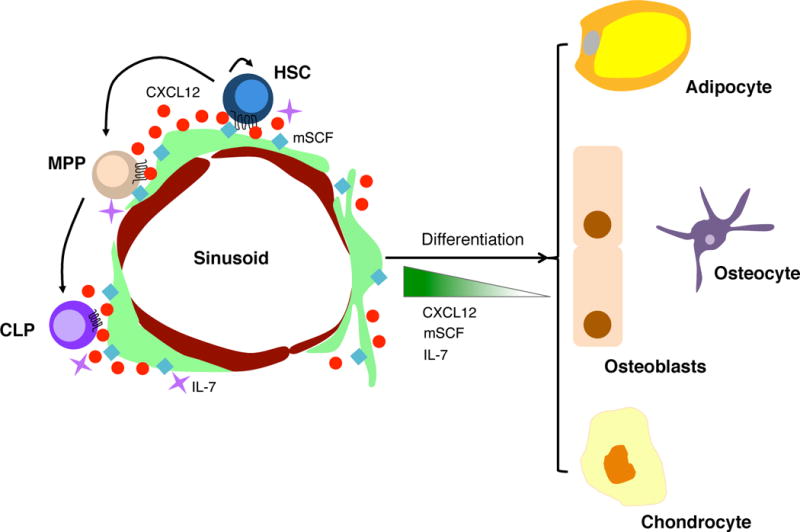
MSPCs, together with some sinusoidal endothelial cells, express soluble and membrane-bound SCF, CXCL12, and IL-7 that not only regulate HSC and MPP numbers, but also sustain lymphopoiesis. MSPCs in the bone marrow are mostly perisinusoidal cells that can differentiate into osteoblasts, osteocytes, chondrocytes and adipocytes. MSPC differentiation is coordinated with the downregulation of SCF, CXCL12 and IL-7 expression.
4. Bone marrow niches controlling lymphoid-lineage commitment
MPPs can give rise to the myeloid or lymphoid lineages by differentiating into common myeloid progenitors (CMPs) or common lymphoid progenitors (CLPs) respectively. CLPs can be divided into Ly6D− CLPs, which give rise to all lymphoid lineages, and Ly6D+ CLPs, which differentiate from Ly6D− CLPs and together with CD93+ CLPs mark the earliest B-lineage committed progenitors (Inlay et al., 2009;Li et al., 1996).
4.1 IL-7 acts as B cell lineage commitment factor
In adult mice, and possibly in adult humans as well, B and T lymphocyte development are highly dependent on IL-7. The critical importance of IL-7 for B cell development can be seen by the severe lymphopenia and complete lack of B lymphopoiesis in the bone marrow of Il7−/− or Il7ra−/− adult mice (Carvalho et al., 2001;Kikuchi et al., 2005;von Freeden-Jeffry et al., 1995). The developmental block in B lymphopoiesis occurs at the Ly6D− to Ly6D+ transition, as Il7−/− mice have a two-fold reduction in Ly6D− CLPs, but have an over twenty-fold reduction in Ly6D+ CLPs (Tsapogas et al., 2011).
In order to commit to the B-lymphoid fate, the transcription factors PU.1, Ikaros, E2A, EBF1 and Pax5 are critical. PU.1 and Ikaros are important at the earliest stages for commitment to the lymphoid lineage, while E2A, EBF1 and Pax5 are the critical transcription factors for the commitment of CLPs to the B cell fate (Busslinger, 2004;Medina et al., 2004). E2A-deficient and EBF1-deficient mice have a very early block in B lymphopoiesis, prior to D-J rearrangement of the IgH locus, and expression of B-lineage associated genes like Pax5, Rag1 and mb1 in fetal liver and bone marrow was significantly reduced compared to WT (Bain et al., 1994;Lin and Grosschedl, 1995;Zhuang, Soriano and Weintraub, 1994). In addition, EBF1 seems to also play a role in maintaining B lineage commitment as deletion of EBF1 in pro-B cells led to loss of repression of genes associated with alternative lymphoid fates (Nechanitzky et al., 2013). PAX5 acts downsteam of EBF1 and E2A, and its expression and function are contingent on EBF1 (Medina et al., 2004). PAX5-deficient mice were blocked at a later stage of B lymphopoiesis, with largely normal D-J rearrangements, but no detectable V-DJ rearrangements (Nutt, Thevenin and Busslinger, 1997). PAX5 is critical for establishing B cell commitment by restricting alternative lineage choices in early progenitors and initiating the B cell transcriptional program, such as expression of CD19 and λ5, and Pax5−/− proB cells were blocked from further B lineage development, but retained ability to differentiate into alternative lineages, such as natural killer (NK) cells, macrophages, and granulocytes (Nutt, Rolink and Busslinger, 1999;Nutt, Thevenin and Busslinger, 1997).
Ebf1 and Pax5 expression are highly dependent on IL-7 and intact IL-7Ra signaling (Dias et al., 2005;Kikuchi et al., 2005;Tsapogas et al., 2011), and the few remaining Ly6D+ CLPs in Il7−/− mice retain NK and dendritic cell (DC)-differentiation potential but have severely reduced B-cell differentiation potential (Dias et al., 2005). Importantly, overexpression of Ebf1 rescues the B cell differentiation defect in Il7−/− CLPs (Dias et al., 2005), and constitutive activation of STAT5, a downstream target of IL-7R signaling, restores Ebf1 and Pax5 expression (Kikuchi et al., 2005). Hence, it appears that IL-7R signaling acts to drive Ebf1 expression, which then activates Pax5 expression to reinforce commitment to the B cell fate. EBF1 also drives increased expression of Il7ra through FOXO1 (Dengler et al., 2008), and therefore perpetuates a feedforward cycle to enforce the B cell fate. IL-7 is therefore an instructive signal that controls differentiation of CLPs to the B cell fate.
4.2 IL-7-producing cells in bone marrow
Given the critical importance of IL-7 in commitment to the B cell fate, cells that form the bone marrow niche for early B lymphopoiesis must express IL-7. Multiple studies have suggested that osteoblasts and/or osteolineage cells form the niche for B lymphopoiesis in bone marrow. Ablation of osteoblasts in mice expressing herpes virus thymidine kinase under control of the 2.3Kb rat collagen α1 type 1 promoter (Col2.3) by treatment with ganciclovir resulted in significant reductions in B cell precursors in bone marrow (Zhu et al., 2007), while ablation of Osx-cre-expressing osteolineage cells resulted in significantly reduced CLP numbers in bone marrow (Terashima et al., 2016). Furthermore, deletion of CXCL12 from osteoblasts using Col2.3-driven cre recombinase (Ding and Morrison, 2013), or IL-7 using tamoxifen-inducible Osx-creER and Ocn-cre (Terashima et al., 2016), also resulted in a significant reduction in the number of bone marrow CLPs. Deletion of the small G alpha stimulatory (Gαs) protein from osteolineage cells using Osx-cre also resulted in significant reductions in total B cells in bone marrow (Wu et al., 2008).
It has to be noted, however, that many of the cell targeting strategies used in these studies may have targeted MSPCs in addition to osteoblasts. Firstly, Col2.3-driven cre recombinase may be active in a small percentage of MSPCs as Col1a1 transcripts are abundant in MSPCs. Second, tamoxifen-induced Osx-driven creER recombinase activity marks MSPCs in both fetal and perinatal bone marrow, but is restricted to osteoblasts in adult mice treated with tamoxifen at 8 weeks of age (Mizoguchi et al., 2014). Importantly, Osx-driven creER recombinase activity is barely detected in osteoblasts in mice treated with tamoxifen at 6 weeks of age (Yu et al., 2016). Therefore, deletion of Gαs from Osx-cre expressing cells would have deleted Gαs in MSPCs, while tamoxifen-induced Osx-driven creER-mediated deletion of Il7 in 6 week old mice is also likely to have deleted Il7 from MSPCs. Furthermore, Ocn-cre has also been shown to target about 70% of CXCL12-expressing cells in bone marrow (Zhang and Link, 2016), and this undesired effect most likely explains the phenotype seen in mice carrying a conditional Il7 deletion in Ocn-expressing cells (Terashima et al., 2016). Finally, as discussed before, the reductions in bone marrow B cells upon osteoblast ablation could be the result of inflammatory signals due to synchronized death of large populations of cells. Furthermore, inflammation is known to inhibit B cell development in bone marrow (Ueda et al., 2004).
Of all the hematopoietic lineages, B lymphocytes seem to be the most critically dependent on CXCR4 and CXCL12 for efficient development in bone marrow. Inducible deletion of Cxcr4 in HSCs and downstream differentiated hematopoietic cells led to a profound reduction in B cell production (Nie, Han and Zou, 2008;Sugiyama et al., 2006). This defect was presumably at an early developmental stage given the fact that conditional Cxcr4 deletion in proB and subsequent developing B cell stages did not cause major differences in total B cell production (Nie et al., 2004). Evidence for this model came from a recent study that looked at the effects of Cxcr4 deletion at the CLP stage using the Il7ra-cre transgene. Deletion of CXCR4 at the CLP stage caused a profound reduction in Ly6D+ CLPs in bone marrow, which resulted in a significant reduction of B, T and NK cells in the periphery (Cordeiro Gomes et al., 2016), a phenotype that remarkably resembles that of IL-7-deficient mice, with the exception of NK cell reduction. Although these results raised the possibility that CXCR4 deficiency prevents CLPs from gaining sufficient access to IL-7, a previous study examining IL-7 and CXCL12 expression in situ suggested that distinct stromal cells either expressed IL-7 or CXCL12, but not both (Tokoyoda et al., 2004). Thus, to gain further insight into CLP positioning in bone marrow, the positioning of CXCR4-sufficient and –deficient CLPs relative to IL-7-expressing cells in bone marrow was analyzed. CXCR4-sufficient Ly6D+ CLPs in bone marrow were found in close proximity to IL-7-expressing cells, while CXCR4-deficient Ly6D− CLPs were positioned further away. Similarly, treatment with the CXCR4 antagonist AMD3100 shifted Ly6D+ cells away from IL-7-expressing cells. In support of these findings, phosphorylated STAT5α, a downstream mediator of IL-7R signaling, was reduced in Cxcr4−/− Ly6D+ CLPs compared to WT Ly6D+ CLPs (Cordeiro Gomes et al., 2016). Furthermore, when Il7GFP/+ mice were crossed with Cxcl12DsRed/+ mice, it was observed that the IL-7-expressing cells comprise the highest producers of CXCL12 in vivo. Although we could not detect IL-7-expressing cells in situ using an anti-IL-7 antibody (a polyclonal antibody that blocks the biological activity of IL-7), detection of Il7-GFP+ cells was possible albeit difficult, and required signal amplification with anti-GFP antibodies, most likely because IL-7 is expressed in very low amounts in vivo, and is likely rapidly consumed by IL-7R-expressing cells. Hence, positioning close to cellular sources of IL-7 is critical for CLPs to receive sufficient IL-7R signaling. Therefore, the role of CXCL12 and CXCR4 in early B lymphopoiesis appears to be for the guidance of CLPs to IL-7-expressing cells in bone marrow (Figure 1), in order for IL-7R signaling to drive lymphoid precursor commitment to the B cell fate. As previously discussed, NK development is independent of IL-7 but requires IL-15 (Kennedy et al., 2000). Of note, CXCR4-CXCL12 are required for NK cell development presumably because CXCL12-producing mesenchymal cells express IL-15 (Noda et al., 2011). Similar to IL-7 and SCF, IL-15 also acts in a short-range manner given that its biological effects require trans presentation through binding to IL15Ra (Dubois et al., 2002).
5. Transition from proB to preB to immature B cell
5.1 ProB and PreB cell niches in bone marrow
As B cells develop from CLPs they undergo several differentiation stages that are characterized by the cell cycle activity, the status of RAG-mediated V(D)J recombination, and cell surface receptor expression. Over the past several decades, a few studies examined the positional distribution of developing B cell subsets in the bone marrow. Landmark studies by Osmond and Batten using in vivo perfusion of radiolabelled antibodies against IgM showed that about 70% of IgM+ cells were located inside the bone marrow parenchyma and that 30% resided in intravascular niches in bone marrow (Batten and Osmond, 1984;Osmond and Batten, 1984). Subsequent studies used immunofluorescent microscopy to characterize the distribution of proB, preB and immature B cells by tracking cells that express nuclear terminal deoxynucleotidyl transferase (TdT, which are mainly proB), or that express the immunoglobulin (Ig) heavy but not the kappa light chain (mostly preB cells), or that express heavy and kappa light chains (a combination of immature and mature B cells). It was noted that all TdT+ proB cells and preB cells resided in the parenchyma, with an apparent enrichment near the sub-endosteal area (Hermans, Hartsuiker and Opstelten, 1989). In contrast, immature and mature B cells were more centrally located near the large collecting central sinusoid, and up to 30% of these cells were inside bone marrow sinusoids, the latter observation in agreement with Osmond and Batten’s observations (Hermans, Hartsuiker and Opstelten, 1989).
5.2 Control of RAG gene expression by bone marrow niches
As studies on molecular requirements for B cell development led to the finding that IL-7 is an essential cytokine for B and T lymphopoiesis (Grabstein et al., 1993; Namen et al., 1988a;Namen et al., 1988b;Peschon et al., 1994;von Freeden-Jeffry et al., 1995), and that ProB cells are particularly dependent on IL-7 for survival and proliferation (Milne and Paige, 2006), it led to studies on the positional distribution of proB and preB cells in relationship to IL-7-producing cells in bone marrow. Taking advantage of a mouse reporter for CXCL12, Nagasawa and colleagues identified two types of stromal cells that either expressed IL-7 or CXCL12 but not both. While proB cells identified by co-expression of B220 and cKit were mostly found in proximity to IL-7+ cells, preB cells identified by B220 and IL-7R expression were found predominantly away from both IL-7+ cells and CXCL12+ cells (Tokoyoda et al., 2004). This model of a stage-dependent niche for B lymphopoiesis gained further traction with the finding that in preB cells, high levels of IL-7 receptor signaling negatively regulated RAG-mediated Ig light chain gene recombination. As preBCR signaling induced activation of IRF4 and CXCR4 expression, it was postulated that an increase in CXCR4 would allow preB cells to migrate away from IL-7 producing cells and possibly towards CXCL12 producing cells (Johnson et al., 2008;Ochiai et al., 2012).
Although structural studies predicted that the preBCR is unable to recognize antigens (Bankovich et al., 2007), a study proposed that the preBCR can physically interact with the stromal-associated lectin Galectin-1 and activate signal transduction (Elantak et al., 2012;Gauthier et al., 2002). In situ analyses of sort-purified preB cells that were transferred into recipient mice showed a close association between preB cells and Galectin-1+ stromal cells that did not express IL-7 but expressed CXCL12 in bone marrow (Mourcin et al., 2011). However, the strategy used by the Tokoyoda study for distinguishing proB (B220 and cKit) from preB (B220 and IL7R) cells would hardly resolve between these two subsets because proB cells express significantly higher amounts of IL-7R than preB cells. Furthermore, given the heterogeneity, broad distribution, and highly reticular morphology of IL-7 and CXCL12 expressing cells it is unclear whether proB and preB cell proximity to these mesenchymal cells is random or specifically enforced by chemotactic and adhesion mechanisms. This is an especially relevant concern given the finding that ~89% of randomly selected cells in bone marrow parenchyma are within < 5μm from the nearest Cxcl12-dsredHi cell (Acar et al., 2015). Finally, as mentioned previously, the large majority of IL-7+ cells express the highest amounts of CXCL12 in bone marrow (Cordeiro Gomes et al., 2016), and so it would seem plausible that a preBCR-driven increase in CXCR4 expression would enforce preB cells to position in the vicinity of IL-7+ cells.
5.3 Is there a bone marrow niche for preB cell leukemias?
The strict regulation of RAG expression and activity likely protects developing lymphocytes from the potentially catastrophic genomic instability that can be brought about from genome-wide RAG activity (Teng et al., 2015). As IL-7R signaling suppresses Rag1 and Rag2 gene expression (Amin and Schlissel, 2008;Herzog et al., 2008;Llorian et al., 2007;Verkoczy et al., 2007), it suggests that preB cell interactions with bone marrow niches may act as a rheostat for the regulation of Rag1 and Rag2 expression. The transcription factor Ikaros is a major regulator of B cell development with a strong impact at the preB to immature B cell transition. Ikaros regulates the expression of several genes required for Ig light chain gene recombination, including the expression of genes in IL-7 receptor and preBCR signal transduction pathways (Heizmann, Kastner and Chan, 2013). Ikaros also regulates the expression of a variety of genes involved in cell migration and adhesion to the extracellular matrix, such that Ikaros-deficient B lineage cells are unable to migrate towards CXCL12 and are aberrantly adherent to VCAM-1-coated surfaces in vitro (Joshi et al., 2014;Schwickert et al., 2014). The impact of aberrant communication between early B-lineage cells and bone marrow niches is unclear, but is particularly relevant given the recent findings of RAG-mediated oncogenic translocations driving the development of preB acute lymphoblastic leukemias (Nussenzweig and Nussenzweig, 2010;Swaminathan et al., 2015), as it suggests that certain bone marrow niches may favor the onset of some preB acute lymphoblastic leukemias. In support of this model, an association between Cxcr4 polymorphisms and chronic lymphocytic leukemia has been proposed (Crowther-Swanepoel et al., 2009). Thus, understanding the positional distribution of Rag-expressing proB and preB cells in relationship to bone marrow mesenchymal-lineage cells is of utmost importance as it remains controversial and largely unresolved.
6. Egress from bone marrow
6.1 Retention mechanisms
Developing B cells, and most prominently immature B cells, are highly dependent on integrin α4β1-mediated adhesion to VCAM-1 (and possibly fibronectin) for movement within parenchyma and retention inside bone marrow sinusoids (Beck et al., 2014). Integrin α4β1 requires chemokine receptor signaling for inside-out integrin transactivation, and in developing B cells this is predominantly controlled by CXCR4 signaling (Glodek et al., 2003). CXCL12 induces integrin α4β1 transactivation and promotes strong adhesion to VCAM-1, possibly through phosphorylation and activation of focal adhesion kinase (FAK) (Glodek et al., 2003). FAK is a cytoplasmic protein tyrosine kinase that accumulates within the cell at sites of attachment to the extracellular matrix and its phosphorylation and activation are predominantly mediated by integrin signaling (Schaller, 2001). In B cells, FAK deficiency causes a mild reduction of developing B cell subsets in bone marrow (Park et al., 2013), a phenotype that is somewhat reminiscent of that seen in mice lacking integrin β1 in developing B cells (Pereira et al., 2009a). Within the femur, proB cells (identified by a non-selective method that relied on co-expression of B220 and CD43) were found enriched in the metaphysis, while in the diaphysis proB cells tended to localize in proximity to the endosteal region (Park et al., 2013). Such bone-proximal positioning was dependent on B-cell-intrinsic FAK (Park et al., 2013) and differed from the perisinusoidal distribution of mature B cells (Cariappa et al., 2005;Park et al., 2013). In rats, TdT-expressing cells also localized in sub-endosteal niches in bone marrow (Hermans, Hartsuiker and Opstelten, 1989), which suggests a non-random localization of proB cells near bone, possibly due to differential expression of α4β1 integrin ligands. A study suggested that Suppressor Of Cytokine Signaling-3 (SOCS3) associates with and negatively regulates CXCR4 signaling in a human B cell line (Soriano et al., 2002). Furthermore, short-term treatment with CXCL12 promoted the association between SOCS3 and FAK, and SOCS3 over-expression in a B cell line prevented CXCR4-mediated chemotaxis (Le et al., 2007). These studies suggested a model in which CXCR4-FAK-α4β1pathway is negatively regulated by SOCS3. In vivo, conditional SOCS3 deletion in a variety of cells, including B-lineage cells, resulted in an accumulation of immature B cells in bone marrow parenchyma, which lent support to that model (Le et al., 2007). However, two independent studies on the effects of B-lineage cell intrinsic deficiency in SOCS3 failed to detect measurable alterations in B cell development. Furthermore, careful analyses of B-lineage positioning, migration, or egress from bone marrow failed to reveal significant differences between Socs3-deficient and wild-type B cells (Jones et al., 2011;Nadrah, Beck and Pereira, 2015).
As developing B cells reach the immature IgM-expressing stage they continue to downregulate CXCR4 expression and reduce their motility within bone marrow, a process that is prevented by BCR signaling (Beck et al., 2014). This change in CXCR4 expression and dynamic behavior alters the B cell morphology from an amoeboid and elongated form to a rounded cell that is often seen loosely adherent to the bone marrow extracellular matrix. BCR signaling can also induce FAK phosphorylation (Mlinaric-Rascan and Yamamoto, 2001), thus implicating a direct relationship between BCR signaling and developing B cell motility in bone marrow. Therefore, attenuation of BCR signaling with concomitant downregulation of CXCR4 expression, and possibly FAK phosphorylation, are the most likely explanation for reduced adhesion and motility of late-stage immature B cells within bone marrow. This form of integrin-dependent motility, however, also informs about the biophysical properties of the bone marrow microenvironment. When leukocytes are exposed to shear stress, such as when facing fluid flow as they move along the lumen of endothelial barriers, cells become highly dependent on integrin-mediated adhesion (also described as haptokinetic motility). In contrast, cell motility in shear-free environments (e.g. densely packed niches in secondary lymphoid organs) is largely independent of integrins and is predominantly controlled by coordinated actin-based leading edge protrusion and myosin-regulated rear end contraction. Thus, developing B cells and possibly hematopoietic cells are exposed to significant shear stress that is presumably caused by plasma exudation from fenestrated sinusoidal endothelium. Consistent with this possibility, during the course of extensive intravital microscopy studies on B cell migration within bone marrow we observed intense leakage of intravascular dyes of high molecular weight (ranging between 500-2,000 KDa) into the bone marrow parenchyma within minutes after injection. An estimate of the interstitial fluid flow rate (calculated as the rate of fluorescent voxel accumulation from vascular dye leakage into parenchyma) suggested that the entire femur parenchymal compartment is perfused with exudate plasma in less than 1 minute (Beck et al., 2014).
6.2 S1P and S1PR pathway in lymphocyte egress from lymphoid organs
Leukocytes are genetically programmed to recirculate between lymphoid organs and circulatory fluids. While leukocyte entry into lymphoid organs has been studied extensively over the past 3 decades, the mechanisms controlling their egress into circulation have been under intense investigation over the past 10-15 years. Insight into this mechanism came from an elegant study using transgenic mice that express the catalytically active S1 subunit of pertussis toxin (PTX) driven by the Lck promoter specifically in T cells (Chaffin and Perlmutter, 1991). PTX is an exotoxin enzyme that catalyzes ADP-ribosylation of G alpha inhibitory (Gαi) proteins, which leaves Gαi proteins in a permanently inactive GDP-bound state (Gilman, 1987). As chemoattractant receptor signaling is dependent on intact Gαi protein-coupled receptor signaling, PTX-expressing thymocytes were effectively unable to respond to chemoattractants and to migrate out of the thymus, despite being capable of undergoing a seemingly normal developmental program. Although the upstream Gαi protein coupled receptor(s) remained unidentified for a while, insight into this question came from the discovery of an immunosupressant small molecule, FTY720 (2-amino-2-[2-(4-octylphenyl) ethyl] propane-1,3-diol hydrochloride) (Fujita et al., 1996). In vivo administration of FTY720 caused a rapid and profound lymphopenia, in which T and B lymphocyte numbers were reduced by 10-100 fold within a few hours (Budde et al., 2003;Chiba et al., 1998;Suzuki et al., 1996;Yagi et al., 2000). The bioactivity of FTY720 is dependent on its phosphorylation by sphingosine kinases, and p-FTY720 is not only structurally similar to sphingosine 1-phosphate (S1P) but also acts as a ligand for four of the five S1P receptors (Brinkmann et al., 2002;Mandala et al., 2002). B and T lymphocytes express high amounts of S1PR1, whereas S1PR2, 3 and 4 are expressed at substantially lower levels (Cinamon et al., 2004;Graler and Goetzl, 2004). S1PR1 is also abundant in endothelial cells where it plays important roles in blood vessel development and permeability (Xiong and Hla, 2014). Some studies suggested that lymphocyte egress is primarily controlled by endothelial cells at exit sites (Rosen et al., 2007). In contrast, other studies provided compelling evidence showing lymphocyte intrinsic S1PR1 signals egress from thymus and lymph nodes (Cyster and Schwab, 2011). For S1PR1 to signal lymphocyte egress, a sharp gradient of S1P between lymphoid organ interstitium, where its concentration is in the subnanomolar range, and circulatory fluids (plasma and lymph), where it ranges from hundreds of nanomolar to micromolar (Schwab et al., 2005), is required. Essentially all cells generate S1P intracellularly as an intermediate metabolite of membrane sphingolipid turnover. Sphingosine is phosphorylated by sphingosine kinase 1 and 2 to form S1P, which is subsequently converted into phosphoethalonamine and 2-hexadecanal by S1P lyase (Serra and Saba, 2010;Spiegel and Milstien, 2011). Hematopoietic cells secrete roughly 90% of plasma S1P, whereas lymphatic endothelial cells are the major producers of S1P in the lymph (Pham et al., 2010). But S1P cannot diffuse across the plasma membrane and requires an active transport mechanism. Lymphatic endothelial cells secrete S1P via a mechanism dependent on SPNS2, a member of the spinster family of transporters, but another unidentified transporter is required for plasma S1P (Fukuhara et al., 2012;Hisano et al., 2012;Mendoza et al., 2012). However, the S1P gradients formed across lymphoid interstitium and circulatory fluids are generated through active degradation mediated by Lipid phosphate phosphatase 3 and S1P lyase (Breart et al., 2011;Schwab et al., 2005). While all leukocytes produce S1P, not all leukocytes express S1P degrading enzymes. Erythrocytes lack all S1P degrading enzymes and are, thus, the major contributor of plasma S1P (Ito et al., 2007;Pappu et al., 2007). Even though S1P is abundant in the cytoplasm of cells that also express S1P receptors, it is only the extracellular S1P that acts as a ligand for S1P receptors.
In thymus or in the lymph node, lymphocytes express high amounts of S1PR1 on the surface, but after exiting into circulatory fluids S1PR1 is desensitized within few minutes of exposure to high concentrations of S1P (Lo et al., 2005). S1PR1 desensitization is nearly complete, such that it can no longer be detected on the surface of T and B lymphocytes in the blood or lymph (Pappu et al., 2007). This unique feature of S1PR1 has been exploited as a read-out of the abundance of S1P in the interstitium of various lymphoid organs. Mature naïve T cells in the thymus exhibit the highest amount of S1PR1, whereas it is detected at lower but still high levels in naïve T cells in the spleen or the lymph node. Surface S1PR1 expression inversely correlates with mass spectrometry measurements of S1P in interstitium, where it is nearly undetectable in the thymus, and increasingly detected in lymph node and spleen (Schwab et al., 2005). The bone marrow is perhaps the organ containing the highest concentration of interstitial S1P of all lymphoid organs (Jenne et al., 2009), likely as a consequence of its highly fenestrated sinusoidal endothelium that allows free passage of plasma constituents into the parenchyma and abundance of red blood cells that cannot degrade interstitial S1P.
Even though immature B cells already express high amounts of the chemoattractant receptor Sphingosine 1-phosphate (S1P) receptor-1 (S1PR1), their dependency on S1PR1 and S1P for exiting the bone marrow is relatively minor. Indeed, in mice that cannot express S1PR1 in developing B cells, or that lack S1P, there is only a 2-3-fold reduction in immature B cell egress from bone marrow (Allende et al., 2010;Pereira, Cyster and Xu, 2010). These findings were consistent with a mild egress defect in mice deficient in the S1P transporter SPNS2 (Fukuhara et al., 2012). This minor dependency on the S1P pathway for bone marrow exit contrasts with the dependency on S1P and S1PR1 for T cell egress from thymus, and T and B cell egress from lymph nodes, where S1P or S1PR1 deficiency essentially blocks cell egress thus resulting in 100-1000 fold reduction in lymphocyte numbers in blood and peripheral lymphoid organs (Matloubian et al., 2004;Pappu et al., 2007). Such strong dependency on the S1P pathway for cell egress from essentially all lymphoid organs with the exception of bone marrow is consistent with a subtle S1P gradient between the bone marrow parenchyma and sinusoids, as measured by surface S1PR1 expression (Jenne et al., 2009;Matloubian et al., 2004;Pappu et al., 2007).
Besides developing B lymphocytes, other hematopoietic cells also use the S1P pathway for exiting from bone marrow. NK cells express a dedicated S1P receptor (S1PR5) that facilitates egress from bone marrow and is essential for lymph node egress (Jenne et al., 2009;Walzer et al., 2007). Eosinophil egress from bone marrow is also partially sensitive to FTY720, likely through effects on S1PR1, S1PR3 and S1PR4 (Sugita et al., 2010). Monocytic cells expressing MCSF receptor and that contain osteoclast differentiation potential are also sensitive to FTY720 treatment, but utilize S1PR1 for bone marrow egress (Ishii et al., 2009). This defect in egress, even though partial, is sufficient to accelerate monocyte/osteoclast precursor differentiation into bone resorbing mature osteoclasts (Ishii et al., 2009), likely as a result of increased chemoattraction to osteoclastogenic niches in the endosteum mediated by oxysterols and EBI2 (Nevius, Gomes and Pereira, 2016;Nevius et al., 2015). At later stages of monocytic cell differentiation, inflammatory monocytes are guided out of the bone marrow mostly in a CCR2 dependent manner (Serbina and Pamer, 2006;Shi et al., 2011). It is unclear whether S1P receptors, or other receptors, synergize with CCR2 to promote monocyte egress from bone marrow. Neutrophil egress is facilitated by a balanced responsiveness to CXCR2 and CXCR4 (Eash et al., 2010), although the exact role played by CXCR4 in neutrophil retention in bone marrow is less clear (Devi et al., 2013).
6.3 Bone marrow egress: an active or a passive mechanism?
An interesting feature of all the pathways identified to date that mediate leukocyte egress from bone marrow is that none seem to be essential for egress to occur. For example, CCR2 is clearly a major regulator of monocyte egress from bone marrow but CCR2 deficiency reveals a mere 6-8 fold reduction in inflammatory monocyte numbers in blood. This contrasts with a 100-1000 fold reduction in lymphocyte numbers in blood of mice conditionally deficient in S1P or S1PR1. These observations led to experiments that aimed at testing whether leukocyte egress is an active process regulated by synergy between several Gαi protein-coupled receptors, or a passive process that can occur independently of chemoattractant receptors. Importantly, when lymphoid-lineage or myeloid-lineage cells were engineered to express PTX, leukocytes were by and large numerically reduced in the bone marrow and increased in peripheral blood, indicating that leukocyte egress from bone marrow is remarkably efficient when cells cannot follow guidance cues (Beck et al., 2014). These findings were in sharp contrast with prior observations that PTX expression in developing thymocytes led to a near complete block in thymic egress, and revealed a fundamental difference in cell export mechanisms between both primary lymphoid organs (Chaffin and Perlmutter, 1991;Willinger et al., 2014). So how can leukocytes migrate through the bone marrow parenchyma and across endothelial barriers? Insight into this question came from intravital microscopy analyses of control and PTX-expressing developing B lymphocytes using a Rag1-GFP reporter mouse strain. It was noted that B-lineage cells exit preferably through sinusoidal fenestrations that leaked considerable amounts of large intravascular dyes (~ 2,000 KDa). Furthermore, the few B-lineage cells that were observed in the process of exiting bone marrow were mostly characterized by a non-amoeboid, rounded morphology reminiscent of red blood cells (Beck et al., 2014). Indeed, millions of newly generated red blood cells exit bone marrow every day, and yet these cells lack intrinsic cell motility mechanisms. Early studies of erythrocyte migration across blood vessels in bone marrow by electron microscopy revealed fenestrations in the sinusoidal endothelium that were nearly as wide as erythrocytes (Hudson and Yoffey, 1966). Thus, the highly fenestrated bone marrow sinusoidal network is presumably an acquired feature that evolved to facilitate export of a variety of hematopoietic cells with a wide range of motility capabilities, from fast moving neutrophils, to essentially non-motile erythrocytes.
Mature thymocytes gain egress competence when TCR signaling strength is reduced, which reduces AKT signaling and FOXO1 phosphorylation, increases KLF2 expression and KLF2-target genes, including S1PR1 (Carlson et al., 2006). This type of molecular circuit most likely ensures that only non-self-reactive T cells become competent for exiting the thymus, given the fact that premature S1PR1 expression in developing thymocytes caused severe autoimmunity (Zachariah and Cyster, 2010), reminiscent of that seen in Aire-deficient mice (Anderson et al., 2002). In the bone marrow, however, self-reactive developing B cells increase CXCR4 expression, which promotes their retention (Beck et al., 2014). Despite this, even when immature B cells are exposed to high affinity cognate antigens, a few cells can still readily escape the bone marrow environment and accumulate in the spleen. This suggests that CXCL12 abundance in the bone marrow parenchyma and the highly fenestrated sinusoidal network are still insufficient to prevent the escape of potentially damaging autoreactive cells. Indeed, these features are presumably similar in mice and in humans given the fact that as many as 75% of developing immature B lymphocytes express antigen receptors that are significantly autoreactive and cross-reactive (Wardemann et al., 2003). Thus, we suggest that the leaky nature of the bone marrow environment imposed additional stages of peripheral B cell development that are controlled by the quality and intensity of tonic and/or self-antigen-driven BCR signaling.
7. Transitional differentiation in periphery
Once immature B cells leave the bone marrow and enter the blood circulation, they reach the spleen where they proceed through their final developmental stages to become mature B cells. Immature B cells can either become marginal zone (MZ) B cells, resident B cells of the splenic marginal zone, which are specialized to respond quickly to blood-borne pathogens, or follicular (FO) B cells, which are circulatory cells that constitute the main B cell compartment capable of mounting T-dependent immune responses. While a detailed understanding of the migratory cues influencing mature B cell recirculation through SLOs has emerged over the last decades, there is still limited understanding of the chemoattractants and niches that regulate immature B cell differentiation into mature B cells in the spleen. Immature B cells are termed transitional (T)1 B cells once they have entered the spleen. Importantly, only about 3% of newly generated bone marrow immature B cells differentiate into circulatory mature B cells (Allman et al., 1993), which illustrates the importance of understanding where and how this bottleneck occurs.
7.1 BAFF receptor and BCR signaling mediate transitional B cell differentiation
The two fundamental and non-redundant signals required for the survival of mature B cells in the periphery are tonic BCR signaling (Kraus et al., 2004;Lam, Kuhn and Rajewsky, 1997;Torres et al., 1996) and activation of B cell activating factor receptor (BAFFR) signaling by BAFF (Gorelik et al., 2003;Mackay et al., 2010;Schiemann et al., 2001;Thompson et al., 2000). These pathways are also critically involved in transitional B cell differentiation into the mature recirculating B cell compartment (Mackay et al., 2010). Over the past couple of decades an impressive body of work elucidated signaling molecules activated by the BCR and BAFFR, and characterized stages of transitional B cell differentiation in the spleen, and these topics have been elegantly reviewed. The focus of this chapter is on the niches that may influence BCR and BAFFR signaling under homeostasis and how movement may influence transitional differentiation.
Although it remains debatable whether tonic BCR signaling is ligand-dependent or independent (Monroe, 2004;Wienands, Larbolette and Reth, 1996;Zikherman and Weiss, 2008), there is no evidence to suggest that tonic BCR signaling is anatomically restricted to the spleen, lymph node, or any other lymphoid or non-lymphoid organ. In contrast, activation of BAFFR by BAFF is thought to be influenced by B cell access to niches with high local BAFF concentrations. The major source of BAFF in vivo is radio-resistant cells (Gorelik et al., 2003), with fibroblastic reticular cells (FRCs), perhaps, being the most important source of BAFF in lymph nodes (Cremasco et al., 2014). However, transitional B cells hardly migrate to lymph nodes and so development into the mature B cell compartment presumably requires an alternative source of BAFF. Splenic stromal cells express higher BAFF amounts than non-stromal splenocytes and may constitute a physiologically important source of BAFF for transitional B cells (Lesley et al., 2004). However, B cells lacking CXCR5, the B cell follicle homing receptor, differentiate into the mature B cell stage efficiently (Forster et al., 1996;Gunn et al., 1998), and even B cells deficient in chemokine receptors required for homing to niches within follicles and T cell zone were also competent in transitional differentiation (Gatto, Wood and Brink, 2011;Pereira et al., 2009b). Whether the stromal cells producing BAFF are in the red or white pulp of the spleen remains unclear, but genetic deficiency in chemoattractant receptors, such as CB2, CXCR4, S1PR1 and S1PR3, which can guide B cells to various niches in the red pulp or the marginal zone, also did not seem to impair transitional B cell differentiation (Donovan, Pelanda and Torres, 2010;Nie et al., 2004;Pereira et al., 2009a;Pereira, Cyster and Xu, 2010). Interestingly, peripheral B cell development has been found to depend on soluble but not membrane-bound BAFF (Bossen et al., 2011), and soluble BAFF is abundant in plasma and interstitium (Kreuzaler et al., 2012). Taken together these findings suggest that transitional B cell differentiation may occur anywhere in the body, a possibility that is consistent with the observation that transitional B cells develop into mature B cells in mice lacking essentially all secondary lymphoid organs (Cariappa et al., 2007).
7.2 Components of the cell motility machinery regulate transitional B cell differentiation
Even though transitional B cell differentiation can occur outside of secondary lymphoid organs and is largely independent of several major chemoattractant receptors, it is counter-intuitively dependent on the proper functioning of the B cell migration machinery. Cell migration is a highly coordinated process requiring the dynamic organization of the actin cytoskeleton controlled by chemoattractant receptor signaling through Gαi proteins, and small GTPases of the Rho family. B cells express Gαi2 and Gαi3 proteins, and both are required for proper B cell migration and transitional B cell differentiation (Hwang et al., 2013). The defect transitional B cell differentiation is unlikely to be due to poor access to follicular stromal cells because transitional B cell differentiation was unaffected in mice lacking CXCR5, CCR7 and EBI2, even though these mice lacked recognizable follicular structures in the spleen (Gatto, Wood and Brink, 2011). Instead, calcium flux elicited by anti-IgM stimulatory antibodies was partially reduced in B cells lacking Gnai2 and Gnai3, which suggested a direct involvement of Gαi2 and Gαi3 in BCR signal transduction (Hwang et al., 2013).
Downstream of Gαi2 and Gαi3 protein signaling, various Rho family GTPases work to promote cell migration and adhesion, in addition to other cellular functions (i.e. cell cycle, survival) (Tybulewicz and Henderson, 2009). Rho GTPases cycle between an active GTP-bound form and an inactive GDP-bound form. Their activity is positively controlled by guanine nucleotide exchange factors (GEFs) and negatively regulated by GTPase-activating proteins (GAPs). Cdc42, a major regulator of membrane trafficking and leading edge protrusion, is a prototypical member of the Rho family and is abundantly expressed in B cells beginning from early developmental stages in bone marrow. Deletion of Cdc42 in B cells causes a partial block at the T1 to T2 transition and a consequent reduction of the mature FO B cell pool (Burbage et al., 2015;Guo et al., 2009). Cdc42 is known to be required for directed movement of migratory cells (Raftopoulou and Hall, 2004), and Cdc42-deficient B cells were impaired in their ability to migrate within lymph nodes in vivo (Burbage et al., 2015). Besides its involvement in cell migration, Cdc42 seems also to be required for proper BCR signaling (Burbage et al., 2015;Guo et al., 2009), and possibly for BAFFR signaling (Guo et al., 2009). It remains unclear if the defect in transitional B cell differentiation was caused by defective synergy between BCR and BAFFR signaling (Stadanlick et al., 2008).
A downstream effector of Cdc42 is Wiskott-Aldrich syndrome protein (WASp). WASp is required for integrin activation, immunological synapse formation, for B cell homing to secondary lymphoid organs, and for B cell migration towards CXCL13 in vitro (Meyer-bahlburg et al., 2008;Westerberg et al., 2005;Westerberg et al., 2012). WASp deficiency in B cells diminishes all peripheral B cell populations, with a possible defect at the transitional to mature B cell differentiation step (Meyer-bahlburg et al., 2008). Spleens contain smaller B cell follicles and lack the marginal zone but have an otherwise preserved splenic architecture (Recher et al., 2012;Westerberg et al., 2005). However, the defect in B cell populations in Cdc42 deficiency is substantially more severe than WASp deficiency, suggesting the involvement of other downstream effectors.
The main Rho GTPases coordinating polarization and cytoskeletal rearrangements during migration are Rho and Rac proteins, which have been described to act in an antagonistic manner (Cain and Ridley, 2009). Rac1 and Rac2 are both abundant in B-lineage cells and when both are simultaneously knocked out in B cells, peripheral B cell development comes to a halt at the transitional B cell stage, due to survival defects (Henderson et al., 2010;Walmsley et al., 2003). Initially, this survival defect was thought to be caused by defective BCR-induced activation of PI3K and impaired BAFF engagement on BAFFR (Walmsley et al., 2003). However, a later study showed that reduced migratory capacity of transitional B cells in the spleen also contributes to reduced B cell survival (Henderson et al., 2010). Early transitional B cells were excluded from the white pulp, which was explained by failure to respond to PTX-sensitive Gαi protein-coupled receptors. Rac1/2 deficiency led to impaired migration towards several chemokines in vitro, and it was suggested that the survival defect in T1 B cells was caused by exclusion from white pulp survival factors, such as BAFF.
In B and T lymphocytes, chemoattractant receptor-mediated activation of Rac1 and Rac2 is largely dependent on Dedicator of cytokinesis −2 (DOCK2) (Fukui et al., 2001). Similarly to Rac1/2 deficient B cells, DOCK2-deficient B cells are also impaired in migration towards several chemokines, and are strongly impaired in homing to and movement within secondary lymphoid organs (Nombela-Arrieta et al., 2004). Importantly, DOCK2-deficiency also causes a substantial reduction in the number of mature B cells. However, it is unclear if defective development in bone marrow, differentiation in the spleen, or simply defective B cell survival in the periphery caused this overall reduction in the B cell compartment. Combined, these studies suggest that transitional B cells depend on intact cytoskeletal organization for productive BCR signaling to allow transition through BCR-dependent checkpoints (Figure 2A). Perhaps the most tantalizing evidence supporting an intimate cross-regulation between the B cell cytoskeleton and BCR signaling is a study by Batista and colleagues showing that inhibitors of actin polymerization, such Latrunculin A and Cytochalasin D, were able to stimulate calcium flux in B cells in a manner similar to BCR stimulation (Treanor et al., 2010). Remarkably, this stimulatory effect on BCR signaling caused by inhibitors of actin polymerization was entirely dependent on cell surface expression of the BCR (Mattila et al., 2013). The B cell sensitivity to low amounts of high affinity cognate antigens was improved upon CXCR5 signaling in vitro (Saez de Guinoa et al., 2011), lending support to a model where components of the cellular motility machinery influence BCR signaling.
Figure 2. Interactions between Gαi protein–coupled receptors and BCR signaling.
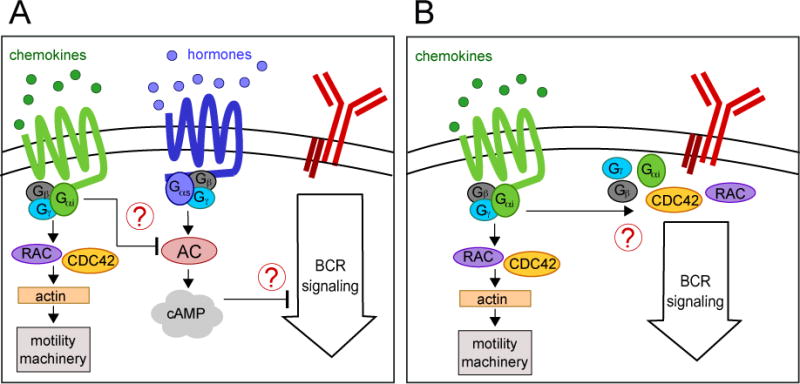
(A) Indirect regulation of BCR signaling through cAMP. Activation of Gαi proteins by chemoattractant sensing may inhibit adenylyl cyclase (AC) activity counteracting the effects of Gαs-coupled receptors and thus lower cytoplasmic concentration of the secondary messenger cAMP. In turn, by yet unidentified mechanisms, cAMP may inhibit BCR signaling.
(B) Interaction between heterotrimeric G proteins and components of the BCR signal transduction pathway (i.e. Cdc42 and RAC proteins.)
An alternative explanation for how the defects in B cell motility machinery described above impact B cell differentiation and homeostasis is that they all cause a substantial reduction in signaling from chemoattractant Gαi protein-coupled receptors. While it still remains possible that Gαi proteins are directly linked to BCR signaling, an alternative (but not mutually exclusive) possibility is that Gαi, Gβ and Gγ proteins activate signal transduction pathways that, in turn, not only control cell migration but also promote B cell transitional differentiation and/or survival. For example, Gαi proteins can inhibit adenylyl cyclase activity and in this manner reduce the intracellular concentration of the second messenger 3′,5′-cyclic AMP (cAMP) (Gilman, 1987). Indeed, B cells encounter ligands that signal through Gαs protein-coupled receptors in the periphery. The βeta-adrenergic receptor-2 (Adrb2), a catecholamine receptor, has been shown to enhance B cell responsiveness to chemokines, and to control B cell retention in lymph nodes, providing a link between the sympathetic nervous system and peripheral B cell recirculation (Nakai et al., 2014;Suzuki et al., 2016). Thus, a balance between inhibitory signals from Gαi proteins, and stimulatory signals from Gαs proteins may regulate adenylyl cyclase activity in lymphocytes (Figure 2B). Importantly, some evidence suggests that high cAMP concentrations are inhibitory to lymphocyte function and TCR signaling (Bourne et al., 1974;Kammer, 1988), and so it is reasonable to consider that fluctuations in cAMP production may also contribute to modulate tonic BCR signaling.
8. Concluding remarks
B lymphocytes use a variety of chemoattractants and receptors to navigate primary and secondary lymphoid organs at multiple stages of development and differentiation. Migratory cues, and most important CXCL12, play a vital role in early stages of hematopoietic progenitor differentiation, particularly in stages prior to commitment into the B lineage. CXCR4 is critically important for ensuring that MPPs and CLPs achieve sustained IL-7R-mediated STAT5 phosphorylation, and accumulate sufficient amounts of B-lineage transcription factors, such as EBF1. In subsequent stages of development, proB and preB cells remain dependent on CXCR4 signaling for retention in bone marrow, but these cells are no longer critically dependent on CXCR4 or other PTX-sensitive GPCRs to develop into the immature B cell stage. At this stage, cells undergo a bottleneck-like selection process dependent on the intensity of BCR signaling and on BAFFR signaling as they differentiate into the mature B cell stage. Paradoxically, defects in a variety of proteins involved in cell migration also perturb transitional B cell differentiation, and implicate PTX-sensitive GPCR signaling as an important and unexpected pathway in B cell homeostasis. Whether GPCR signaling components directly regulate key components of the BCR and/or BAFFR signal transduction pathway through the actin cytoskeleton, or indirectly modulate BCR and/or BAFFR signaling thresholds via second messengers (e.g. cAMP) remains to be determined.
Acknowledgments
VYL was supported by A*STAR, Singapore, and SZ was supported by a fellowship from German Research Foundation (DFG) ZE1060/1-1. This work was funded by the NIH (RO1AI113040).
References
- Acar M, Kocherlakota KS, Murphy MM, Peyer JG, Oguro H, Inra CN, Jaiyeola C, Zhao Z, Luby-Phelps K, Morrison SJ. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526:126. doi: 10.1038/nature15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allende ML, Tuymetova G, Lee BG, Bonifacino E, Wu YP, Proia RL. S1P1 receptor directs the release of immature B cells from bone marrow into blood. J Exp Med. 2010;207:1113. doi: 10.1084/jem.20092210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman DM, Ferguson SE, Lentz VM, Cancro MP. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. Journal of immunology (Baltimore, Md: 1950) 1993;151:4431. [PubMed] [Google Scholar]
- Amin RH, Schlissel MS. Foxo1 directly regulates the transcription of recombination-activating genes during B cell development. Nat Immunol. 2008;9:613. doi: 10.1038/ni.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Bain G, Maandag EC, Izon DJ, Amsen D, Kruisbeek AM, Weintraub BC, Krop I, Schlissel MS, Feeney AJ, van Roon M, et al. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell. 1994;79:885. doi: 10.1016/0092-8674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Balabanian K, Lagane B, Pablos JL, Laurent L, Planchenault T, Verola O, Lebbe C, Kerob D, Dupuy A, Hermine O, Nicolas JF, Latger-Cannard V, Bensoussan D, Bordigoni P, Baleux F, Le Deist F, Virelizier JL, Arenzana-Seisdedos F, Bachelerie F. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105:2449. doi: 10.1182/blood-2004-06-2289. [DOI] [PubMed] [Google Scholar]
- Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmaier M, Germeshausen M, Krukemeier S, Welte K. Thrombopoietin is essential for the maintenance of normal hematopoiesis in humans: development of aplastic anemia in patients with congenital amegakaryocytic thrombocytopenia. Ann N Y Acad Sci. 2003;996:17. doi: 10.1111/j.1749-6632.2003.tb03228.x. [DOI] [PubMed] [Google Scholar]
- Bankovich AJ, Raunser S, Juo ZS, Walz T, Davis MM, Garcia KC. Structural insight into pre-B cell receptor function. Science. 2007;316:291. doi: 10.1126/science.1139412. [DOI] [PubMed] [Google Scholar]
- Batten SJ, Osmond DG. The localization of B lymphocytes in mouse bone marrow: radioautographic studies after in vivo perfusion of radiolabelled anti-IgM antibody. J Immunol Methods. 1984;72:381. doi: 10.1016/0022-1759(84)90007-3. [DOI] [PubMed] [Google Scholar]
- Beck TC, Gomes AC, Cyster JG, Pereira JP. CXCR4 and a cell-extrinsic mechanism control immature B lymphocyte egress from bone marrow. J Exp Med. 2014;211:2567. doi: 10.1084/jem.20140457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernitz JM, Kim HS, MacArthur B, Sieburg H, Moore K. Hematopoietic Stem Cells Count and Remember Self-Renewal Divisions. Cell. 2016;167:1296. doi: 10.1016/j.cell.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossen C, Tardivel A, Willen L, Fletcher CA, Perroud M, Beermann F, Rolink AG, Scott ML, Mackay F, Schneider P. Mutation of the BAFF furin cleavage site impairs B-cell homeostasis and antibody responses. European Journal of Immunology. 2011;41:787. doi: 10.1002/eji.201040591. [DOI] [PubMed] [Google Scholar]
- Boulais PE, Frenette PS. Making sense of hematopoietic stem cell niches. Blood. 2015;125:2621. doi: 10.1182/blood-2014-09-570192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne HR, Lichtenstein LM, Melmon KL, Henney CS, Weinstein Y, Shearer GM. Modulation of inflammation and immunity by cyclic AMP. Science (New York, NY) 1974;184:19. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- Boyer SW, Schroeder AV, Smith-Berdan S, Forsberg EC. All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells. Cell Stem Cell. 2011;9:64. doi: 10.1016/j.stem.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breart B, Ramos-Perez WD, Mendoza A, Salous AK, Gobert M, Huang Y, Adams RH, Lafaille JJ, Escalante-Alcalde D, Morris AJ, Schwab SR. Lipid phosphate phosphatase 3 enables efficient thymic egress. J Exp Med. 2011;208:1267. doi: 10.1084/jem.20102551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, Scheiermann C, Schiff L, Poncz M, Bergman A, Frenette PS. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med. 2014;20:1315. doi: 10.1038/nm.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde K, R LS, Nashan B, Brunkhorst R, P WL, Mayer T, Brookman L, Nedelman J, Skerjanec A, Bohler T, Neumayer HH. Pharmacodynamics of single doses of the novel immunosuppressant FTY720 in stable renal transplant patients. Am J Transplant. 2003;3:846. doi: 10.1034/j.1600-6143.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- Burbage M, Keppler SJ, Gasparrini F, Martínez-Martín N, Gaya M, Feest C, Domart MC, Brakebusch C, Collinson L, Bruckbauer A, Batista FD. Cdc42 is a key regulator of B cell differentiation and is required for antiviral humoral immunity. The Journal of experimental medicine. 2015;212 doi: 10.1084/jem.20141143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger M. Transcriptional control of early B cell development. Annu Rev Immunol. 2004;22:55. doi: 10.1146/annurev.immunol.22.012703.104807. [DOI] [PubMed] [Google Scholar]
- Cain RJ, Ridley AJ. Phosphoinositide 3-kinases in cell migration. Biology of the Cell. 2009;101:13. doi: 10.1042/BC20080079. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Cariappa A, Chase C, Liu H, Russell P, Pillai S. Naive recirculating B cells mature simultaneously in the spleen and bone marrow. Blood. 2007;109:2339. doi: 10.1182/blood-2006-05-021089. [DOI] [PubMed] [Google Scholar]
- Cariappa A, Mazo IB, Chase C, Shi HN, Liu H, Li Q, Rose H, Leung H, Cherayil BJ, Russell P, von Andrian U, Pillai S. Perisinusoidal B cells in the bone marrow participate in T-independent responses to blood-borne microbes. Immunity. 2005;23:397. doi: 10.1016/j.immuni.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(−/)− mice. J Exp Med. 2001;194:1141. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin KE, Perlmutter RM. A pertussis toxin-sensitive process controls thymocyte emigration. Eur J Immunol. 1991;21:2565. doi: 10.1002/eji.1830211038. [DOI] [PubMed] [Google Scholar]
- Chen JY, Miyanishi M, Wang SK, Yamazaki S, Sinha R, Kao KS, Seita J, Sahoo D, Nakauchi H, Weissman IL. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature. 2016;530:223. doi: 10.1038/nature16943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, Hoshino Y. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol. 1998;160:5037. [PubMed] [Google Scholar]
- Cinamon G, Matloubian M, Lesneski MJ, Xu Y, Low C, Lu T, Proia RL, Cyster JG. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol. 2004;5:713. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- Cordeiro Gomes A, Hara T, Lim VY, Herndler-Brandstetter D, Nevius E, Sugiyama T, Tani-Ichi S, Schlenner S, Richie E, Rodewald HR, Flavell RA, Nagasawa T, Ikuta K, Pereira JP. Hematopoietic Stem Cell Niches Produce Lineage-Instructive Signals to Control Multipotent Progenitor Differentiation. Immunity. 2016;45:1219. doi: 10.1016/j.immuni.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremasco V, Woodruff MC, Onder L, Cupovic J, Nieves-Bonilla JM, Schildberg FA, Chang J, Cremasco F, Harvey CJ, Wucherpfennig K, Ludewig B, Carroll MC, Turley SJ. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat Immunol. 2014;15:973. doi: 10.1038/ni.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther-Swanepoel D, Qureshi M, Dyer MJ, Matutes E, Dearden C, Catovsky D, Houlston RS. Genetic variation in CXCR4 and risk of chronic lymphocytic leukemia. Blood. 2009;114:4843. doi: 10.1182/blood-2009-07-235184. [DOI] [PubMed] [Google Scholar]
- Cyster JG, Schwab SR. Sphingosine-1-Phosphate and Lymphocyte Egress from Lymphoid Organs. Annu Rev Immunol. 2011;30:69. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler HS, Baracho GV, Omori SA, Bruckner S, Arden KC, Castrillon DH, DePinho RA, Rickert RC. Distinct functions for the transcription factor Foxo1 at various stages of B cell differentiation. Nat Immunol. 2008;9:1388. doi: 10.1038/ni.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi S, Wang Y, Chew WK, Lima R, N AG, Mattar CN, Chong SZ, Schlitzer A, Bakocevic N, Chew S, Keeble JL, Goh CC, Li JL, Evrard M, Malleret B, Larbi A, Renia L, Haniffa M, Tan SM, Chan JK, Balabanian K, Nagasawa T, Bachelerie F, Hidalgo A, Ginhoux F, Kubes P, Ng LG. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J Exp Med. 2013;210:2321. doi: 10.1084/jem.20130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias S, Silva H, Jr, Cumano A, Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J Exp Med. 2005;201:971. doi: 10.1084/jem.20042393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan EE, Pelanda R, Torres RM. S1P3 confers differential S1P-induced migration by autoreactive and non-autoreactive immature B cells and is required for normal B-cell development. Eur J Immunol. 2010;40:688. doi: 10.1002/eji.200939858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen RL, Johnston HM, Nilsson SK. Membrane-bound stem cell factor is a key regulator in the initial lodgment of stem cells within the endosteal marrow region. Exp Hematol. 2003;31:1284. doi: 10.1016/j.exphem.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120:2423. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elantak L, Espeli M, Boned A, Bornet O, Bonzi J, Gauthier L, Feracci M, Roche P, Guerlesquin F, Schiff C. Structural basis for galectin-1-dependent pre-B cell receptor (pre-BCR) activation. J Biol Chem. 2012;287:44703. doi: 10.1074/jbc.M112.395152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Chan DC, Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991;64:1025. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285. doi: 10.1146/annurev-immunol-032712-095919. [DOI] [PubMed] [Google Scholar]
- Fujita T, Hirose R, Yoneta M, Sasaki S, Inoue K, Kiuchi M, Hirase S, Chiba K, Sakamoto H, Arita M. Potent immunosuppressants, 2-alkyl-2-aminopropane-1,3-diols. J Med Chem. 1996;39:4451. doi: 10.1021/jm960391l. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T, Sunden Y, Arai Y, Moriwaki K, Ishida J, Uemura A, Kiyonari H, Abe T, Fukamizu A, Hirashima M, Sawa H, Aoki J, Ishii M, Mochizuki N. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest. 2012;122:1416. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y, Hashimoto O, Sanui T, Oono T, Koga H, Abe M, Inayoshi A, Noda M, Oike M, Shirai T, Sasazuki T. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412:826. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- Gatto D, Wood K, Brink R. EBI2 operates independently of but in cooperation with CXCR5 and CCR7 to direct B cell migration and organization in follicles and the germinal center. J Immunol. 2011;187:4621. doi: 10.4049/jimmunol.1101542. [DOI] [PubMed] [Google Scholar]
- Gauthier L, Rossi B, Roux F, Termine E, Schiff C. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci U S A. 2002;99:13014. doi: 10.1073/pnas.202323999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Glodek AM, Honczarenko M, Le Y, Campbell JJ, Silberstein LE. Sustained activation of cell adhesion is a differentially regulated process in B lymphopoiesis. J Exp Med. 2003;197:461. doi: 10.1084/jem.20021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. The Journal of experimental medicine. 2003;198:937. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlin RJ, Gelb B, Diaz GA, Lofsness KG, Pittelkow MR, Fenyk JR., Jr WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. Am J Med Genet. 2000;91:368. [PubMed] [Google Scholar]
- Grabstein KH, Waldschmidt TJ, Finkelman FD, Hess BW, Alpert AR, Boiani NE, Namen AE, Morrissey PJ. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J Exp Med. 1993;178:257. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature. 1998;391:799. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- Guo F, Velu CS, Grimes HL, Zheng Y. Rho GTPase Cdc42 is essential for B-lymphocyte development and activation. Blood. 2009;114:2909. doi: 10.1182/blood-2009-04-214676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann B, Kastner P, Chan S. Ikaros is absolutely required for pre-B cell differentiation by attenuating IL-7 signals. J Exp Med. 2013;210:2823. doi: 10.1084/jem.20131735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson RB, Grys K, Vehlow A, de Bettignies C, Zachacz A, Henley T, Turner M, Batista F, Tybulewicz VL. A novel Rac-dependent checkpoint in B cell development controls entry into the splenic white pulp and cell survival. J Exp Med. 2010;207:837. doi: 10.1084/jem.20091489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans MH, Hartsuiker H, Opstelten D. An in situ study of B-lymphocytopoiesis in rat bone marrow. Topographical arrangement of terminal deoxynucleotidyl transferase-positive cells and pre-B cells. J Immunol. 1989;142:67. [PubMed] [Google Scholar]
- Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, Diaz GA. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34:70. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- Herzog S, Hug E, Meixlsperger S, Paik JH, DePinho RA, Reth M, Jumaa H. SLP-65 regulates immunoglobulin light chain gene recombination through the PI(3)K-PKB-Foxo pathway. Nat Immunol. 2008;9:623. doi: 10.1038/ni.1616. [DOI] [PubMed] [Google Scholar]
- Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One. 2012;7:e38941. doi: 10.1371/journal.pone.0038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G, Yoffey JM. The passage of lymphocytes through the sinusoidal endothelium of guinea-pig bone arrow. Proc R Soc Lond B Biol Sci. 1966;165:486. doi: 10.1098/rspb.1966.0079. [DOI] [PubMed] [Google Scholar]
- Hwang IY, Park C, Luong T, Harrison KA, Birnbaumer L, Kehrl JH. The Loss of Gnai2 and Gnai3 in B Cells Eliminates B Lymphocyte Compartments and Leads to a Hyper-IgM Like Syndrome. PLoS One. 2013;8:e72596. doi: 10.1371/journal.pone.0072596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inlay MA, Bhattacharya D, Sahoo D, Serwold T, Seita J, Karsunky H, Plevritis SK, Dill DL, Weissman IL. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev. 2009;23:2376. doi: 10.1101/gad.1836009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL, Germain RN. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Anada Y, Tani M, Ikeda M, Sano T, Kihara A, Igarashi Y. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem Biophys Res Commun. 2007;357:212. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP, Xu Y, Roots CM, Beilke JN, Banerjee A, Reiner SL, Miller SA, Weinmann AS, Goodnow CC, Lanier LL, Cyster JG, Chun J. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206:2469. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K, Hashimshony T, Sawai CM, Pongubala JM, Skok JA, Aifantis I, Singh H. Regulation of immunoglobulin light-chain recombination by the transcription factor IRF-4 and the attenuation of interleukin-7 signaling. Immunity. 2008;28:335. doi: 10.1016/j.immuni.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Jones SA, White CA, Robb L, Alexander WS, Tarlinton DM. SOCS3 deletion in B cells alters cytokine responses and germinal center output. J Immunol. 2011;187:6318. doi: 10.4049/jimmunol.1102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi I, Yoshida T, Jena N, Qi X, Zhang J, Van Etten RA, Georgopoulos K. Loss of Ikaros DNA-binding function confers integrin-dependent survival on pre-B cells and progression to acute lymphoblastic leukemia. Nat Immunol. 2014;15:294. doi: 10.1038/ni.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer GM. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunology today. 1988;9:222. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Lai AY, Hsu CL, Kondo M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J Exp Med. 2005;201:1197. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of Resting Mature B Lymphocytes Depends on BCR Signaling via the Igα/β Heterodimer. Cell. 2004;117:787. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Kreuzaler M, Rauch M, Salzer U, Birmelin J, Rizzi M, Grimbacher B, Plebani A, Lougaris V, Quinti I, Thon V, Litzman J, Schlesier M, Warnatz K, Thiel J, Rolink AG, Eibel H. Soluble BAFF levels inversely correlate with peripheral B cell numbers and the expression of BAFF receptors. J Immunol. 2012;188:497. doi: 10.4049/jimmunol.1102321. [DOI] [PubMed] [Google Scholar]
- Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Le Y, Zhu BM, Harley B, Park SY, Kobayashi T, Manis JP, Luo HR, Yoshimura A, Hennighausen L, Silberstein LE. SOCS3 protein developmentally regulates the chemokine receptor CXCR4-FAK signaling pathway during B lymphopoiesis. Immunity. 2007;27:811. doi: 10.1016/j.immuni.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YS, Wasserman R, Hayakawa K, Hardy RR. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996;5:527. doi: 10.1016/s1074-7613(00)80268-x. [DOI] [PubMed] [Google Scholar]
- Liles WC, Broxmeyer HE, Rodger E, Wood B, Hubel K, Cooper S, Hangoc G, Bridger GJ, Henson GW, Calandra G, Dale DC. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- Lin H, Grosschedl R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 1995;376:263. doi: 10.1038/376263a0. [DOI] [PubMed] [Google Scholar]
- Llorian M, Stamataki Z, Hill S, Turner M, Martensson IL. The PI3K p110delta is required for down-regulation of RAG expression in immature B cells. J Immunol. 2007;178:1981. doi: 10.4049/jimmunol.178.4.1981. [DOI] [PubMed] [Google Scholar]
- Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Cote D, Rowe DW, Lin CP, Scadden DT. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CG, Xu Y, Proia RL, Cyster JG. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J Exp Med. 2005;201:291. doi: 10.1084/jem.20041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237:205. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Mattila PK, Feest C, Depoil D, Treanor B, Montaner B, Otipoby KL, Carter R, Justement LB, Bruckbauer A, Batista FD. The actin and tetraspanin networks organize receptor nanoclusters to regulate B cell receptor-mediated signaling. Immunity. 2013;38:461. doi: 10.1016/j.immuni.2012.11.019. [DOI] [PubMed] [Google Scholar]
- McDermott DH, Gao JL, Liu Q, Siwicki M, Martens C, Jacobs P, Velez D, Yim E, Bryke CR, Hsu N, Dai Z, Marquesen MM, Stregevsky E, Kwatemaa N, Theobald N, Long Priel DA, Pittaluga S, Raffeld MA, Calvo KR, Maric I, Desmond R, Holmes KL, Kuhns DB, Balabanian K, Bachelerie F, Porcella SF, Malech HL, Murphy PM. Chromothriptic cure of WHIM syndrome. Cell. 2015;160:686. doi: 10.1016/j.cell.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, Roux ER, Teepe M, Lyman SD, Peschon JJ. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489. [PubMed] [Google Scholar]
- Medina KL, Pongubala JM, Reddy KL, Lancki DW, Dekoter R, Kieslinger M, Grosschedl R, Singh H. Assembling a gene regulatory network for specification of the B cell fate. Dev Cell. 2004;7:607. doi: 10.1016/j.devcel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza A, Breart B, Ramos-Perez WD, Pitt LA, Gobert M, Sunkara M, Lafaille JJ, Morris AJ, Schwab SR. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Rep. 2012;2:1104. doi: 10.1016/j.celrep.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-bahlburg A, Becker-herman S, Humblet-baron S, Khim S, Bouma G, Thrasher AJ, Batista FD, Rawlings DJ. peripheral homeostasis Wiskott-Aldrich Syndrome protein deficiency in B cells results in impaired peripheral homeostasis. 2008;112:4158. doi: 10.1182/blood-2008-02-140814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne CD, Paige CJ. IL-7: a key regulator of B lymphopoiesis. Semin Immunol. 2006;18:20. doi: 10.1016/j.smim.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, Mendelson A, Ono N, Kronenberg HM, Frenette PS. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29:340. doi: 10.1016/j.devcel.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlinaric-Rascan I, Yamamoto T. B cell receptor signaling involves physical and functional association of FAK with Lyn and IgM. FEBS Lett. 2001;498:26. doi: 10.1016/s0014-5793(01)02474-7. [DOI] [PubMed] [Google Scholar]
- Monroe JG. Ligand-independent tonic signaling in B-cell receptor function. Curr Opin Immunol. 2004;16:288. doi: 10.1016/j.coi.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourcin F, Breton C, Tellier J, Narang P, Chasson L, Jorquera A, Coles M, Schiff C, Mancini SJ. Galectin-1-expressing stromal cells constitute a specific niche for pre-BII cell development in mouse bone marrow. Blood. 2011;117:6552. doi: 10.1182/blood-2010-12-323113. [DOI] [PubMed] [Google Scholar]
- Nadrah K, Beck TC, Pereira JP. Immature B Cell Egress from Bone Marrow Is SOCS3 Independent. PLoS One. 2015;10:e0136061. doi: 10.1371/journal.pone.0136061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through beta2-adrenergic receptors. J Exp Med. 2014;211:2583. doi: 10.1084/jem.20141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Ishizu A, Takubo K, Fujioka M, Suda T. Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin. Biochem Biophys Res Commun. 2014;454:353. doi: 10.1016/j.bbrc.2014.10.095. [DOI] [PubMed] [Google Scholar]
- Nakamura-Ishizu A, Takubo K, Kobayashi H, Suzuki-Inoue K, Suda T. CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. J Exp Med. 2015;212:2133. doi: 10.1084/jem.20150057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namen AE, Lupton S, Hjerrild K, Wignall J, Mochizuki DY, Schmierer A, Mosley B, March CJ, Urdal D, Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988a;333:571. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- Namen AE, Schmierer AE, March CJ, Overell RW, Park LS, Urdal DL, Mochizuki DY. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J Exp Med. 1988b;167:988. doi: 10.1084/jem.167.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Nechanitzky R, Akbas D, Scherer S, Gyory I, Hoyler T, Ramamoorthy S, Diefenbach A, Grosschedl R. Transcription factor EBF1 is essential for the maintenance of B cell identity and prevention of alternative fates in committed cells. Nat Immunol. 2013;14:867. doi: 10.1038/ni.2641. [DOI] [PubMed] [Google Scholar]
- Nevius E, Gomes AC, Pereira JP. Inflammatory Cell Migration in Rheumatoid Arthritis: A Comprehensive Review. Clin Rev Allergy Immunol. 2016;51:59. doi: 10.1007/s12016-015-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevius E, Pinho F, Dhodapkar M, Jin H, Nadrah K, Horowitz MC, Kikuta J, Ishii M, Pereira JP. Oxysterols and EBI2 promote osteoclast precursor migration to bone surfaces and regulate bone mass homeostasis. J Exp Med. 2015;212:1931. doi: 10.1084/jem.20150088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205:777. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y, Waite J, Brewer F, Sunshine MJ, Littman DR, Zou YR. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J Exp Med. 2004;200:1145. doi: 10.1084/jem.20041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Omatsu Y, Sugiyama T, Oishi S, Fujii N, Nagasawa T. CXCL12-CXCR4 chemokine signaling is essential for NK-cell development in adult mice. Blood. 2011;117:451. doi: 10.1182/blood-2010-04-277897. [DOI] [PubMed] [Google Scholar]
- Nombela-Arrieta C, Lacalle RA, Montoya MaC, Kunisaki Y, Megías D, Marqués M, Carrera AC, Mañes S, Fukui Y, Martínez-A C, Stein JV. Differential Requirements for DOCK2 and Phosphoinositide-3-Kinase γ during T and B Lymphocyte Homing. Immunity. 2004;21:429. doi: 10.1016/j.immuni.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Nussenzweig A, Nussenzweig MC. Origin of chromosomal translocations in lymphoid cancer. Cell. 2010;141:27. doi: 10.1016/j.cell.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt SL, Rolink AG, Busslinger M. The molecular basis of B-cell lineage commitment. Cold Spring Harb Symp Quant Biol. 1999;64:51. doi: 10.1101/sqb.1999.64.51. [DOI] [PubMed] [Google Scholar]
- Nutt SL, Thevenin C, Busslinger M. Essential functions of Pax-5 (BSAP) in pro-B cell development. Immunobiology. 1997;198:227. doi: 10.1016/S0171-2985(97)80043-5. [DOI] [PubMed] [Google Scholar]
- Ochiai K, Maienschein-Cline M, Mandal M, Triggs JR, Bertolino E, Sciammas R, Dinner AR, Clark MR, Singh H. A self-reinforcing regulatory network triggered by limiting IL-7 activates pre-BCR signaling and differentiation. Nat Immunol. 2012;13:300. doi: 10.1038/ni.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omatsu Y, Seike M, Sugiyama T, Kume T, Nagasawa T. Foxc1 is a critical regulator of haematopoietic stem/progenitor cell niche formation. Nature. 2014;508:536. doi: 10.1038/nature13071. [DOI] [PubMed] [Google Scholar]
- Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The Essential Functions of Adipo-osteogenic Progenitors as the Hematopoietic Stem and Progenitor Cell Niche. Immunity. 2010;33:387. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Osmond DG, Batten SJ. Genesis of B lymphocytes in the bone marrow: extravascular and intravascular localization of surface IgM-bearing cells in mouse bone marrow detected by electron-microscope radioautography after in vivo perfusion of 125I anti-IgM antibody. Am J Anat. 1984;170:349. doi: 10.1002/aja.1001700310. [DOI] [PubMed] [Google Scholar]
- Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- Park SY, Wolfram P, Canty K, Harley B, Nombela-Arrieta C, Pivarnik G, Manis J, Beggs HE, Silberstein LE. Focal adhesion kinase regulates the localization and retention of pro-B cells in bone marrow microenvironments. J Immunol. 2013;190:1094. doi: 10.4049/jimmunol.1202639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JP, An J, Xu Y, Huang Y, Cyster JG. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat Immunol. 2009a;10:403. doi: 10.1038/ni.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JP, Cyster JG, Xu Y. A role for S1P and S1P1 in immature-B cell egress from mouse bone marrow. PLoS One. 2010;5:e9277. doi: 10.1371/journal.pone.0009277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JP, Kelly LM, Cyster JG. Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int Immunol. 2010;22:413. doi: 10.1093/intimm/dxq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JP, Kelly LM, Xu Y, Cyster JG. EBI2 mediates B cell segregation between the outer and centre follicle. Nature. 2009b;460:1122. doi: 10.1038/nature08226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR, Cyster JG. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2010;207:17. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Kastenmuller W, Germain RN. Spatiotemporal basis of innate and adaptive immunity in secondary lymphoid tissue. Annu Rev Cell Dev Biol. 2014;30:141. doi: 10.1146/annurev-cellbio-100913-013254. [DOI] [PubMed] [Google Scholar]
- Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Mansson R, Thoren LA, Ekblom M, Alexander WS, Jacobsen SE. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1:671. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Developmental Biology. 2004;265:23. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Recher M, Burns SO, De La Fuente MA, Volpi S, Dahlberg C, Walter JE, Moffitt K, Mathew D, Honke N, Lang PA, Patrizi L, Falet H, Keszei M, Mizui M, Csizmadia E, Candotti F, Nadeau K, Bouma G, Delmonte OM, Frugoni F, Ferraz Fomin AB, Buchbinder D, Lundequist EM, Massaad MJ, Tsokos GC, Hartwig J, Manis J, Terhorst C, Geha RS, Snapper S, Lang KS, Malley R, Westerberg L, Thrasher AJ, Notarangelo LD. B cell-intrinsic deficiency of the Wiskott-Aldrich syndrome protein (WASp) causes severe abnormalities of the peripheral B-cell compartment in mice. Blood. 2012;119:2819. doi: 10.1182/blood-2011-09-379412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 2007;28:102. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Saez de Guinoa J, Barrio L, Mellado M, Carrasco YR. CXCL13/CXCR5 signaling enhances BCR-triggered B-cell activation by shaping cell dynamics. Blood. 2011;118:1560. doi: 10.1182/blood-2011-01-332106. [DOI] [PubMed] [Google Scholar]
- Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An Essential Role for BAFF in the Normal Development of B Cells Through a BCMA-Independent Pathway. Science (New York, NY) 2001;293:2111. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Schwickert TA, Tagoh H, Gultekin S, Dakic A, Axelsson E, Minnich M, Ebert A, Werner B, Roth M, Cimmino L, Dickins RA, Zuber J, Jaritz M, Busslinger M. Stage-specific control of early B cell development by the transcription factor Ikaros. Nat Immunol. 2014;15:283. doi: 10.1038/ni.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- Serra M, Saba JD. Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function. Adv Enzyme Regul. 2010;50:349. doi: 10.1016/j.advenzreg.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone Marrow Mesenchymal Stem and Progenitor Cells Induce Monocyte Emigration in Response to Circulating Toll-like Receptor Ligands. Immunity. 2011;34:590. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnicka E, Bryder D, Theilgaard-Monch K, Buza-Vidas N, Adolfsson J, Jacobsen SE. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 2002;17:463. doi: 10.1016/s1074-7613(02)00419-3. [DOI] [PubMed] [Google Scholar]
- Soriano SF, Hernanz-Falcon P, Rodriguez-Frade JM, De Ana AM, Garzon R, Carvalho-Pinto C, Vila-Coro AJ, Zaballos A, Balomenos D, Martinez AC, Mellado M. Functional inactivation of CXC chemokine receptor 4-mediated responses through SOCS3 up-regulation. J Exp Med. 2002;196:311. doi: 10.1084/jem.20012041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadanlick JE, Kaileh M, Karnell FG, Scholz JL, Miller JP, Quinn WJ, 3rd, Brezski RJ, Treml LS, Jordan KA, Monroe JG, Sen R, Cancro MP. Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nat Immunol. 2008;9:1379. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita K, Kabashima K, Sakabe J, Yoshiki R, Tanizaki H, Tokura Y. FTY720 regulates bone marrow egress of eosinophils and modulates late-phase skin reaction in mice. Am J Pathol. 2010;177:1881. doi: 10.2353/ajpath.2010.100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Hayano Y, Nakai A, Furuta F, Noda M. Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. J Exp Med. 2016;213:2567. doi: 10.1084/jem.20160723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Enosawa S, Kakefuda T, Shinomiya T, Amari M, Naoe S, Hoshino Y, Chiba K. A novel immunosuppressant, FTY720, with a unique mechanism of action, induces long-term graft acceptance in rat and dog allotransplantation. Transplantation. 1996;61:200. doi: 10.1097/00007890-199601270-00006. [DOI] [PubMed] [Google Scholar]
- Swaminathan S, Klemm L, Park E, Papaemmanuil E, Ford A, Kweon SM, Trageser D, Hasselfeld B, Henke N, Mooster J, Geng H, Schwarz K, Kogan SC, Casellas R, Schatz DG, Lieber MR, Greaves MF, Muschen M. Mechanisms of clonal evolution in childhood acute lymphoblastic leukemia. Nat Immunol. 2015;16:766. doi: 10.1038/ni.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taya Y, Ota Y, Wilkinson AC, Kanazawa A, Watarai H, Kasai M, Nakauchi H, Yamazaki S. Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation. Science. 2016;354:1152. doi: 10.1126/science.aag3145. [DOI] [PubMed] [Google Scholar]
- Teng G, Maman Y, Resch W, Kim M, Yamane A, Qian J, Kieffer-Kwon KR, Mandal M, Ji Y, Meffre E, Clark MR, Cowell LG, Casellas R, Schatz DG. RAG Represents a Widespread Threat to the Lymphocyte Genome. Cell. 2015;162:751. doi: 10.1016/j.cell.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima A, Okamoto K, Nakashima T, Akira S, Ikuta K, Takayanagi H. Sepsis-Induced Osteoblast Ablation Causes Immunodeficiency. Immunity. 2016;44:1434. doi: 10.1016/j.immuni.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Thompson JS, Schneider P, Kalled SL, Wang L, Lefevre EA, Cachero TG, MacKay F, Bixler SA, Zafari M, Liu ZY, Woodcock SA, Qian F, Batten M, Madry C, Richard Y, Benjamin CD, Browning JL, Tsapis A, Tschopp J, Ambrose C. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J Exp Med. 2000;192:129. doi: 10.1084/jem.192.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Torres RM, Flaswinkel H, Reth M, Rajewsky K. Aberrant B Cell Development and Immune Response in Mice with a Compromised BCR Complex. Science. 1996;272:1804. doi: 10.1126/science.272.5269.1804. [DOI] [PubMed] [Google Scholar]
- Treanor B, Depoil D, Gonzalez-Granja A, Barral P, Weber M, Dushek O, Bruckbauer A, Batista FD. The membrane skeleton controls diffusion dynamics and signaling through the B cell receptor. Immunity. 2010;32:187. doi: 10.1016/j.immuni.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapogas P, Zandi S, Ahsberg J, Zetterblad J, Welinder E, Jonsson JI, Mansson R, Qian H, Sigvardsson M. IL-7 mediates Ebf-1-dependent lineage restriction in early lymphoid progenitors. Blood. 2011;118:1283. doi: 10.1182/blood-2011-01-332189. [DOI] [PubMed] [Google Scholar]
- Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol. 2009;9:630. doi: 10.1038/nri2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng YS, Li H, Kang YL, Chen WC, Cheng WC, Lai DM. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 2011;117:429. doi: 10.1182/blood-2010-01-266833. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J Exp Med. 2004;199:47. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkoczy L, Duong B, Skog P, Ait-Azzouzene D, Puri K, Vela JL, Nemazee D. Basal B cell receptor-directed phosphatidylinositol 3-kinase signaling turns off RAGs and promotes B cell-positive selection. J Immunol. 2007;178:6332. doi: 10.4049/jimmunol.178.10.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley MJ, Ooi SK, Reynolds LF, Smith SH, Ruf S, Mathiot A, Vanes L, Williams DA, Cancro MP, Tybulewicz VL. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459. doi: 10.1126/science.1089709. [DOI] [PubMed] [Google Scholar]
- Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, Garrigue-Antar L, Jacques Y, Baratin M, Tomasello E, Vivier E. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8:1337. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Westerberg L, Larsson M, Hardy SJ, Fernandez C, Thrasher AJ, Severinson E. Wiskott-Aldrich syndrome protein deficiency leads to reduced B-cell adhesion, migration, and homing, and a delayed humoral immune response. Blood. 2005;105:1144. doi: 10.1182/blood-2004-03-1003. [DOI] [PubMed] [Google Scholar]
- Westerberg LS, Dahlberg C, Baptista M, Moran CJ, Detre C, Keszei M, Eston MA, Alt FW, Terhorst C, Notarangelo LD, Snapper SB. Wiskott-Aldrich syndrome protein (WASP) and N-WASP are critical for peripheral B cell development and function. Blood. 2012;119:3966. doi: 10.1182/blood-2010-09-308197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienands J, Larbolette O, Reth M. Evidence for a preformed transducer complex organized by the B cell antigen receptor. Proc Natl Acad Sci U S A. 1996;93:7865. doi: 10.1073/pnas.93.15.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willinger T, Ferguson SM, Pereira JP, De Camilli P, Flavell RA. Dynamin 2-dependent endocytosis is required for sustained S1PR1 signaling. J Exp Med. 2014;211:685. doi: 10.1084/jem.20131343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- Wu JY, Purton LE, Rodda SJ, Chen M, Weinstein LS, McMahon AP, Scadden DT, Kronenberg HM. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci U S A. 2008;105:16976. doi: 10.1073/pnas.0802898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Hla T. S1P control of endothelial integrity. Curr Top Microbiol Immunol. 2014;378:85. doi: 10.1007/978-3-319-05879-5_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Kamba R, Chiba K, Soga H, Yaguchi K, Nakamura M, Itoh T. Immunosuppressant FTY720 inhibits thymocyte emigration. Eur J Immunol. 2000;30:1435. doi: 10.1002/(SICI)1521-4141(200005)30:5<1435::AID-IMMU1435>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K, Miyazaki H, Takahashi T, Suda T. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Yu VW, Lymperi S, Oki T, Jones A, Swiatek P, Vasic R, Ferraro F, Scadden DT. Distinctive Mesenchymal-Parenchymal Cell Pairings Govern B Cell Differentiation in the Bone Marrow. Stem Cell Reports. 2016;7:220. doi: 10.1016/j.stemcr.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science. 2010;328:1129. doi: 10.1126/science.1188222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Link DC. Targeting of Mesenchymal Stromal Cells by Cre-Recombinase Transgenes Commonly Used to Target Osteoblast Lineage Cells. J Bone Miner Res. 2016;31:2001. doi: 10.1002/jbmr.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC, Ahamed J, Li L. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med. 2014;20:1321. doi: 10.1038/nm.3706. [DOI] [PubMed] [Google Scholar]
- Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15:154. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, Joe GJ, Hexner E, Choi Y, Taichman RS, Emerson SG. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- Zhuang Y, Soriano P, Weintraub H. The helix-loop-helix gene E2A is required for B cell formation. Cell. 1994;79:875. doi: 10.1016/0092-8674(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Zikherman J, Weiss A. Alternative splicing of CD45: the tip of the iceberg. Immunity. 2008;29:839. doi: 10.1016/j.immuni.2008.12.005. [DOI] [PubMed] [Google Scholar]


