Abstract
BACKGROUND:
Globally, amoebic liver abscess, a common extraintestinal complication of intestinal amoebiasis. Diagnosis of hepatic amoebiasis is based on the detection of anti-Entamoeba histolytica immunoglobulin G (IgG) antibody using enzyme-linked immunosorbent assay (ELISA), because of its technique's relatively higher sensitivity and specificity (90%).
AIM:
The aim of the present study was to determine the seroprevalence of hepatic amoebiasis in a referral tertiary care hospital in North India.
MATERIALS AND METHODS:
The blood samples were tested specifically for anti-E. histolytica IgG antibody using commercially available ELISA kit (RIDASCREEN® E. histolytica IgG [K1721] kit).
RESULTS:
A total of 879 patients (n = 879) were evaluated, of which 78.49% (690/879) were positive for anti-E. histolytica IgG antibody. The seroprevalence rates showed a declining trend from 2010 to 2015 with rates falling from 91.4% to 66.7%. He present a study showed the decreasing trend of seroprevalence of hepatic amoebiasis from 2010 to 2015.
CONCLUSIONS:
This decrease may be attributed to several factors such as increase in awareness, improved hygienic practices, use of safe drinking water, better socioeconomic condition, and perhaps early treatment sought for intestinal amoebiasis.
Keywords: Hepatic amoebiasis, intestinal amoebiasis, seroprevalence
Introduction
Amoebiasis caused by Entamoeba histolytica prevalent in developing nations represents a major health problem and is the second leading cause of morbidity and mortality worldwide followed by malaria.[1] Amoebiasis can present as asymptomatic cyst passer (90%) and infected individuals serve as carriers. However, in 10% cases amoebiasis may develop to invasive amoebiasis such as amoebic dysentery, liver abscesses, rarely lung and brain abscesses, heart, urinary tract, and skin infections.[2,3,4,5] Globally, amoebic liver abscess, the most common extraintestinal complication of amoebiasis is noted in around 50 million cases with a mortality rate of 100,000 deaths every year.[6] Diagnosis of amoebiasis is based on microscopic examination, xenic and axenic in vitro cultures of clinical samples, serology, and molecular techniques. Stool microscopy has never been useful in the diagnosis of extraintestinal amoebiasis. Diagnosis of extraintestinal amoebiasis primarily based on microscopic examination of clinical samples such as pus and aspirated fluids, however, it is time-consuming, requires expertise, and has a sensitivity of 60%.[7,8,9] Molecular techniques such as polymerase chain reaction assay and DNA probes for dot-blot hybridization can accurately differentiate the species, have greater sensitivity and specificity than microscopy. However, considering their high cost and need for technical expertise these molecular techniques often limits its applications in the routine diagnostics in many resource limited country.[10,11] Serological techniques such as enzyme-linked immunosorbent assay (ELISA), indirect hemagglutination assay are helpful in the diagnosis; however, ELISA is the most preferred cost-effective serological method with both sensitivity and specificity of 90%.[7] The aim of the present study was to determine the seroprevalence of hepatic amoebiasis in a referral tertiary care hospital in North India.
Materials and Methods
Study area, population, and period
The present study was carried out in the Deparment of Microbiology, All India Institute of Medical Sciences, New Delhi, India. This was primarly laboratory-based study. Between the year 2010 and 2015, the patients with clinically suspected hepatic amoebiasis who attended our outpatient department/clinic for consultation and/or admitted to the Departments of Gastroenterology and Human Nutrition and Internal Medicine and Pediatrics of our hospital were retrospectively analyzed. The details of these patients were analysed as per a well-structured pro forma that included clinical (types and duration of fever, pain in the epigastrium, enlargement of liver, presence of jaundice, and history of treatment), relevant radiological examinations, and microbiological examinations.
Collection and processing of samples
About 4–5 ml of venous blood without anticoagulant was collected from all patients taking aseptic measures and after obtaining consent of all the patients. Serum was separated as per standard protocol.
Serological evaluation
The qualitative determination of anti-E. histolytica specific immunoglobulin G (IgG) antibodies was carried out using commercially available ELISA kit (RIDASCREEN® E. histolytica IgG (K1721)-R-Biopharm AG, An der neuen Bergstraße 17, D-64297 Darmstadt, Germany). The ELISA test was performed as per manufacturer's instructions.
Statistical analysis
The data collected were analyzed using STATA/SE version 14.0 statistical software (Stata Corp, Texas, USA). Categorical data were described using numbers and percentages. Data generated from the present study have been presented in the form of tables and all descriptive analyses have been shown in percentages. P value has been calculated to analyze statistically significance.
Results
A total of 879 adult patients (n = 879) were included in the present study. Of these 879 patients, 78.49% (690/879) were seropositive for anti-E. histolytica specific IgG antibodies. Among these 879 patients, 80.31% (706/879) were males and 19.68% (173/879) were females and the male-to-female ratio was 4.08:1. Association of gender with that of E. histolytica infection has been shown in Table 1. The age ranged from 2 to 98 years with mean age of 40.53 ± 17.14 years and median value of 40 years. Of the total 879, 823 (95.90%) were adults and 56 (76.78%) were children ≤15 years of age [Table 1]. The mean age of children was 10.21 with interquartile range value 15. Most of the patients were adults followed by children ≤15 years (X2 = 146.11, P = 0.00). A higher seroprevalence was observed in males compared to females (X2 = 16.7196, P = 0.00). Seasonal variation was noted in seroprevalence rate and was highest during monsoon 354/690 (51.34%) (June–September) season, followed by 196/690 (28.40%) premonsoon (February–May) and 140/690 (20.28%) postmonsoon (October–January) season [Figure 1]. Overall, seroprevalence rate was higher in indoor patients 63.33% (437/690) than outdoor patients 36.74% (253/690). The seroprevalence varied from 66.66% to 91.4% between 2015 and 2010, with a mean of 78.49%. The decreasing trend of seroprevalence was noted from the year 2010 to 2015 [Figure 2].
Table 1.
Sociodemographic characteristics of Entamoeba histolytica

Figure 1.
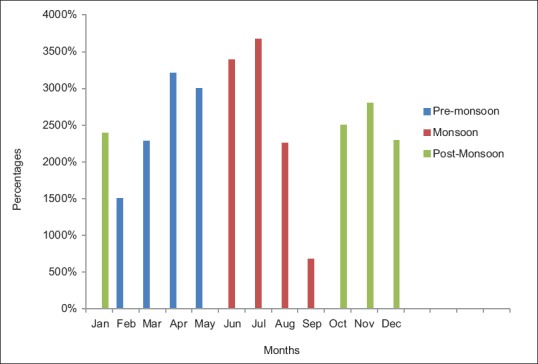
Seasonal variation of Entamoeba histolytica infection
Figure 2.
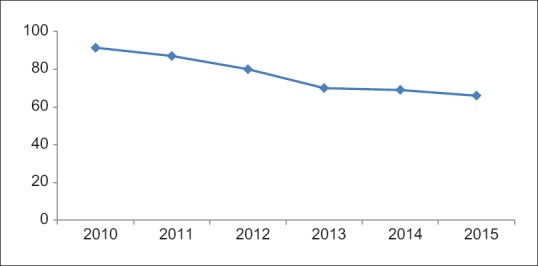
Decreasing trend of seroprevalence of Entamoeba histolytica infection
Discussion
E. histolytica infection predominates in developing countries and represents a major health problem.[12] There is limited information regarding the seroprevalence of extraintestinal amoebiasis in Indian population. In the present study, we observed a seroprevalence of 78.49% in our patients with hepatic amoebiasis and the seropositivity rate observed in our study was much higher than earlier studies.[13,14] Furthermore, another interesting observation that was noted in the present study was a definite decreasing trend in the seroprevalence of hepatic amoebiasis in our referral tertiary care center which receives a significant number of patients with such illnesses. This decrease may be attributed to several factors such as increase in awareness, improved hygienic practices, use of safe drinking water, better socioeconomic condition, and perhaps early treatment sought for intestinal amoebiasis. Gender variation in seroprevalence, i.e., with higher antibody prevalence observed in males (81.16%) (P< 0.05) is in consistent with other studies.[15,16] As hypothesized earlier, such observation may be due to estrogen stimulating effect on the phagocytic system leading to a better humoral and cellular response against E. histolytica infection among women.[17,18] The seasonal variation was observed in this study, we observed higher seroprevalence rate of E. histolytica infection during monsoon season, which is in consistent with some of the earlier studies and higher rate of fecal-oral contamination may be implicated during monsoon season.[19]
Conclusions
We conclude that serological test like ELISA is useful for seroprevalence study to determine the problem load particularly in resource limited countries. This can help in better patient management and reducing the transmission improving socioeconomic condition and better hygienic practices. The seroprevalence rate has shown a significant fall over the years; however, hepatic amoebiasis still remains an important public health problem, which needs to be diagnosed to allow specific treatment. Considering the scarcity of information available in our country, more region/province-wise studies on seroprevalence of hepatic amoebiasis are required to improve our understanding of the actual burden.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dinoop KP, Parija SC, Mandal J, Swaminathan RP, Narayanan P. Comparison of nested-multiplex, Taqman & SYBR Green real-time PCR in diagnosis of amoebic liver abscess in a tertiary health care institute in India. Indian J Med Res. 2016;143:49–56. doi: 10.4103/0971-5916.178592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chacín-Bonilla L. An update on amebiasis. Rev Med Chil. 2013;141:609–15. doi: 10.4067/S0034-98872013000500009. [DOI] [PubMed] [Google Scholar]
- 3.Wuerz T, Kane JB, Boggild AK, Krajden S, Keystone JS, Fuksa M, et al. A review of amoebic liver abscess for clinicians in a nonendemic setting. Can J Gastroenterol. 2012;26:729–33. doi: 10.1155/2012/852835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petri WA, Haque R. Entamoeba histolytica brain abscess. Handb Clin Neurol. 2013;114:147–52. doi: 10.1016/B978-0-444-53490-3.00009-1. [DOI] [PubMed] [Google Scholar]
- 5.Tengku SA, Norhayati M. Public health and clinical importance of amoebiasis in Malaysia: A review. Trop Biomed. 2011;28:194–222. [PubMed] [Google Scholar]
- 6.Bansal D, Sehgal R, Chawla Y, Malla N, Mahajan RC. Multidrug resistance in amoebiasis patients. Indian J Med Res. 2006;124:189–94. [PubMed] [Google Scholar]
- 7.Parija SC, Khairnar K. Detection of excretory Entamoeba histolytica DNA in the urine, and detection of E. histolytica DNA and lectin antigen in the liver abscess pus for the diagnosis of amoebic liver abscess. BMC Microbiol. 2007;7:41. doi: 10.1186/1471-2180-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haque R, Faruque AS, Hahn P, Lyerly DM, Petri WA Jr. Entamoeba histolytica and Entamoeba dispar infection in children in Bangladesh. J Infect Dis. 1997;175:734–6. doi: 10.1093/infdis/175.3.734. [DOI] [PubMed] [Google Scholar]
- 9.Parija SC. Progress in the research on diagnosis and vaccines in amebiasis. Trop Parasitol. 2011;1:4–8. doi: 10.4103/2229-5070.72108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhanalakshmi S, Parija SC. Seroprevalence of Entamoeba histolytica from a tertiary care hospital, South India. Trop Parasitol. 2016;6:78–81. doi: 10.4103/2229-5070.175116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalkan IH, Daǧli U. What is the most accurate method in the diagnosis of amebic dysentery? Turk J Gastroenterol. 2010;21:87–90. doi: 10.4318/tjg.2010.0062. [DOI] [PubMed] [Google Scholar]
- 12.Ralston KS, Petri WA Jr. Tissue destruction and invasion by Entamoeba histolytica. Trends Parasitol. 2011;27:254–63. doi: 10.1016/j.pt.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan AH, Ghosh PK, Das SR. A seroepidemiological survey of amoebiasis in Lucknow. Trop Gastroenterol. 1985;6:30–6. [PubMed] [Google Scholar]
- 14.Manjula, Mateen MA, Habibullah CM. Seroepidemiology of E. histolytica infection in Hyderabad. Trop Gastroenterol. 1986;7:173–7. [PubMed] [Google Scholar]
- 15.Jamaiah I, Shekhar KC. Amoebiasis: A 10 year retrospective study at the University Hospital, Kuala Lumpur. Med J Malaysia. 1999;54:296–302. [PubMed] [Google Scholar]
- 16.Stauffer W, Abd-Alla M, Ravdin JI. Prevalence and incidence of Entamoeba histolytica infection in South Africa and Egypt. Arch Med Res. 2006;37:266–9. doi: 10.1016/j.arcmed.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Caballero-Salcedo A, Viveros-Rogel M, Salvatierra B, Tapia-Conyer R, Sepulveda-Amor J, Gutierrez G, et al. Seroepidemiology of amebiasis in Mexico. Am J Trop Med Hyg. 1994;50:412–9. doi: 10.4269/ajtmh.1994.50.412. [DOI] [PubMed] [Google Scholar]
- 18.Ansar Ahmed S, Penhale WJ, Talal N. Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action. Am J Pathol. 1985;121:531–51. [PMC free article] [PubMed] [Google Scholar]
- 19.Nath J, Ghosh SK, Singha B, Paul J. Molecular epidemiology of amoebiasis: A cross-sectional study among North East Indian population. PLoS Negl Trop Dis. 2015;9:e0004225. doi: 10.1371/journal.pntd.0004225. [DOI] [PMC free article] [PubMed] [Google Scholar]


