Abstract
Background: Lactic acid bacteria (LAB) are normal flora of the mouth, intestines and the female genital tract. They are also frequently found in meat, vegetables, and dairy products. Most of probiotic bacteria belong to the LAB group. Some probiotic LAB are useful in prevention and treatment of diarrheal diseases. The aim of this study was to investigate the antimicrobial properties of LAB isolated from traditional yogurt and milk against Shigella strains.
Materials and methods: Forty LAB strains were isolated from traditional yogurt and milk. The antimicrobial activity of LAB against Shigella strains (eight S. flexneri, four S. sonnei) was examined using the agar-well diffusion assay. LAB strains with antimicrobial effect against all Shigella strains were identified by 16S rRNA gene sequencing.
Results: Six LAB strains inhibited the growth of all 12 Shigella strains. Lb. paracasei Y1-3, Lb. paracasei Y8-1 and Lb. fermentum Y2-2 were isolated from yogurt. Lb. paracasei M18-1, Lb. parelimentarius M4-3 and Lb. plantarum M19-1 were isolated from milk.
Conclusion: This study showed that Lactobacillus strains with good inhibitory activity against S. flexneri and S. sonnei could be isolated from traditional yogurt and milk.
Keywords: lactic acid bacteria, yogurt, milk, antimicrobial properties, Shigella
Zusammenfassung
Hintergrund: Milchsäurebakterien (LAB) gehören zur normalen Flora der Mundhöhle, des Darmtrakts und des weiblichen Genitaltrakts. Sie sind häufig in Fleisch, Gemüse und Milchprodukten nachweisbar. Die meisten probiotischen Bakterien gehören zu den LAB. Einige probiotische LAB sind zur Prävention und Behandlung infektiöser Diarrhoen geeignet. Zielsetzung der Studie war die Untersuchung antimikrobieller Eigenschaften von aus traditionellem Joghurt und Milch isolierten LAB gegen Shigella-Stämme.
Materialien und Methode: 40 LAB-Stämme wurden aus traditionellem Joghurt und Milch isoliert. Die antimikrobielle Wirkung der LAB gegen die Shigella-Stämme (acht S. flexneri, vier S. sonnei) wurde im Agardiffusionstest ermittelt. Antimikrobiell wirksame LAB Stämme wurden mittels 16S rRNA Gensequenzing identifiziert.
Ergebnisse: Sechs LAB Stämme hemmten die Vermehrung der geprüften 12 Shigella Stämme. Lactobacillus (Lb.) paracasei Y1-3, Lb. paracasei Y8-1 und Lb. fermentum Y2-2 wurden aus Joghurt, Lb. paracasei M18-1, Lb. parelimentarius M4-3 und Lb. plantarum M19-1 aus Milch isoliert.
Schlussfolgerung: Aus Joghurt und Milch konnten Lactobacillus-Stämme mit guter Hemmwirkung gegen S. flexneri und S. sonnei isoliert werden.
Background
Lactic acid bacteria (LAB) are normal, physiological flora of the mouth, intestines and female genital tract. They are also frequently found in meat, vegetables, and dairy products, such as milk and yogurt. The Lactobacillus genus is one of the most important genera in the group of LAB. Lactobacillus strains are Gram-positive, catalase-negative, non-spore-forming and non-motile bacilli [1], [2], [3]. LAB have protective effects in fermented food preservation, because they produce organic acids in food during their growth. Conversion of carbohydrates to organic acids and reduction of pH is the reason for the increased half-life and good quality of such food products [1], [4].
Probiotics are living microorganisms which, when consumed in adequate amounts, confer health benefits to the host by altering the indigenous microflora [5]. Most probiotic bacteria belong to the LAB group [6]. The presence of LAB in food has beneficial effects on human health, including effects on the natural gut microflora equilibrium, reducing blood cholesterol, decreasing intestinal tumors, facilitating calcium absorption in the intestines, reducing lactose intolerance, and preventing and treating diarrheal diseases [7], [8], [9]. Several mechanisms have been suggested for the inhibitory activity of LAB against pathogenic bacteria, especially Gram-negative pathogens. These mechanisms include production of organic acids, hydrogen peroxide and bacteriocin, and competition for colonization sites with pathogenic bacteria [10], [11], [12].
Shigella spp. are common intestinal Gram-negative pathogens which cause diarrheal diseases and dysentery in many countries [13]. Shigella spp. are a leading cause of gastroenteritis-induced deaths in 3–5 million children under five years old in developing countries [14]. In several studies, the probiotic potential of various Lactobacillus strains has been demonstrated. Some Lactobacillus strains commonly used as probiotics are efficaceous especially against acute diarrhea in children [11], [15], [16], [17]. The purpose of this study was to investigate the antimicrobial effect of LAB isolated from traditional yogurt and milk against Shigella spp.
Methods
Samples collection and culture
Twenty samples of traditional yogurt and milk were collected in sterile containers and transferred to the microbiology laboratory of Babol University of Medical Sciences, Iran. Two grams of yogurt and 500 µl of milk were inoculated into 15 ml de Man, Rogosa and Sharp (MRS) broth medium (Merck, Germany), and cultured for 48 h in anaerobic jars at 37°C. Then the MRS broth was subcultured on MRS agar (Merck, Germany) plates, and inoculated for 48 h in anaerobic jars at 37°C. The suspicious colonies were tested using Gram staining and catalase reaction. Gram-positive and catalase-negative bacteria were purified by streaking on MRS agar, and stocked in MRS broth containing 20% glycerol at –20°C.
Evaluation of antimicrobial effect of LAB against Shigella strains
The antimicrobial activity of LAB against Shigella strains was tested using the agar well diffusion assay [18]. Twelve Shigella strains (eight S. flexneri, four S. sonnei), which were previously isolated from children with diarrhea, were included in this study. The LAB were cultured in 3 ml MRS broth medium in anaerobic jars and incubated for 24 h at 37°C. The MRS broth tubes were subsequently centrifuged (10,000 g, 10 min) to prepare cell-free culture supernatants (CFCS). Shigella strains were grown on nutrient agar medium (Merck, Germany) and incubated for 24 h at 37°C. A suspension of 107 colony forming units (CFU)/ml of Shigella strains was then prepared and spread onto the nutrient agar, into which 5-mm-deep wells had been dug. About 100 µl of CFCS was poured into each well, and nutrient agar plates were incubated for 18 h at 37°C. Lb. rhamnosus GG was used as the positive control. Finally, inhibition zone diameter was measured. LAB strains with clear zones <11 mm, 11–16 mm, 17–22 mm and >23 mm, were classified as negative (–), mild (+), strong (++), and very strong (+++) inhibitors, respectively.
Production of bacteriocin-like compounds
Two main mechanisms of antimicrobial activity are the production of organic acids, which reduce the pH, and the production of hydrogen peroxide. Some Lactobacillus spp. also produce bacteriocin, which is antimicrobially active [19]. For these reasons, the pH of the CFCS was measured and adjusted to 6.5 with NaOH (Merck, Germany, 2.5M); catalase (1 mg/ml, Sigma-Aldrich, Germany) was then added to the CFCS and incubated at 25°C for 1 h [12]. The antimicrobial activity of these CFCS was investigated using the agar well diffusion assay.
Identification of LAB species by 16S rRNA gene sequencing
LAB strains with antimicrobial efficacy against all Shigella strains were identified by 16S rRNA gene sequencing. DNA extraction of strains was performed using the boiling method [20]. PCR was performed with primers 27F (5'-CTCGTTGCGGGACTTAA-3') and 1522R (5'-GCAGCAGTAGGGAATCTTC-3') (Bioneer, Korea) [21]. The reaction mixture consisted of 1.5 mM MgCl2, 0.2 mM dNTPs, 2.75 ml of genomic DNA, 5 ml 10X PCR buffer, 3 pmol of each primer and 3 U of Taq DNA polymerase (Jena Bioscience, Germany) in a final volume of 50 ml. The PCR protocol started with an initial denaturation at 95°C for 2 min, followed by 35 cycles of 94°C for 1 min, 55°C for 1 min and 72°C for 10 min. PCR products were electrophoretically separated on agarose gel (1.5% w/v) and visualized by staining with safe stain (Yekta Tajhiz Azma, Iran). Finally, PCR products were sent for sequencing (Bioneer, Korea). Species of LAB were identified after comparison of the obtained sequences by nucleotide-nucleotide BLAST (https://www.ncbi.nlm.nih.gov/blast).
Results
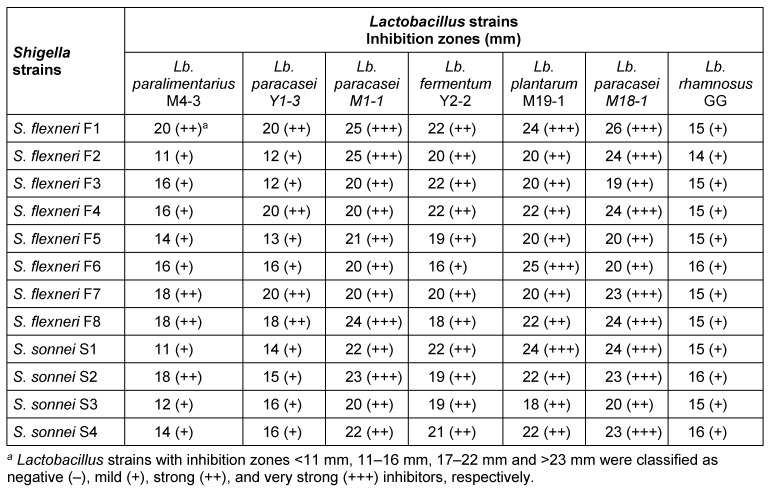
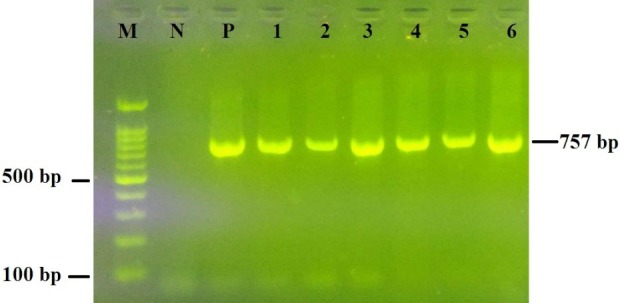
Forty LAB strains were isolated from 20 samples of traditional yogurt and milk (10 samples of milk and 10 samples of yogurt). Of the 40 LAB strains, 22 were obtained from milk and 18 strains from yogurt. Six LAB strains in the well diffusion assay inhibited the growth of all 12 Shigella strains (Table 1 (Tab. 1)). The species of these six LAB strains were identified by 16s rRNA gene sequencing PCR (Figure 1 (Fig. 1)). Three strains belonged to the species Lb. paracasei. Lb. paracasei Y1-3 (GenBank accession number KY552923.1) and Lb. paracasei Y8-1 (GenBank accession number: KY552924.1) were isolated from yogurt, and Lb. paracasei M18-1 (GenBank accession number: KY552927.1) was obtained from milk. Strain M4-3, which was isolated from milk, was identified as Lb. parelimentarius (GenBank accession number: KY552927.1). Lb. fermentum Y2-2 (GenBank accession number: KY552925.1) was isolated from yogurt. Lb. plantarum M19-1 (GenBank accession number: KY552926.1) was isolated from milk.
Table 1. Inhibitory activity of cell-free culture supernatants (CFCS) of Lactobacillus strains against Shigella strains.
Figure 1. 16s rRNA gene PCR. Lane M, 100 bp DNA ladder (Yekta Tajhiz Azma, Iran); Lane N, Negative control; Lane P, Positive control (Lb. rhamnosus GG); Lanes 1–6, Lactobacillus strains.
Lb. rhamnosus GG has mild inhibitory activity against Shigella strains, while Lb. paracasei M18-1, Lb. paracasei Y8-1 and Lb. plantarum M19-1 have strong or very strong inhibitory activity against Shigella strains (Table 1 (Tab. 1)).
Among the other 34 strains of lactic acid bacteria, 6 inhibited the growth of 11 Shigella strains (91.6%), 9 inhibited the growth of 10 Shigella strains (83.3%), 5 inhibited the growth of nine Shigella strains (75%), 3 inhibited the growth of eight Shigella strains (66.6%), and 2 inhibited the growth of seven Shigella strains (58.3%) (data not shown).
The antimicrobial activity of Lactobacillus strains disappeared when the CFCS was adjusted to pH 6.5 and treated with catalase. The Lactobacillus strains did not produce bacteriocin-like compounds.
Discussion
Shigellosis is usually a self-limiting infection, but in severe cases of the infection, antibiotic therapy may be required. Quinolones and cephalosporins are the drugs of choice. However, the worldwide emergence of antimicrobial resistance among Shigella species has limited the choice of antimicrobial agents for treating the infection. Resistance to different antibiotics such as sulfonamides, ampicillin, tetracyclines and co-trimoxazole has been described among Shigella species worldwide, and the causes of treatment failure can be attributed to these antibiotic resistances [13]. Because there are concerns about the increase in drug-resistant pathogenic bacteria, probiotic LAB are being used as a preventive treatment alternative [22]. Probiotic bacteria have been widely studied over the past decades in the prevention and treatment of diarrheal diseases. The most commonly used probiotic microorganisms for prevention and treatment of diarrheal diseases are Lactobacillus GG, Lb. acidophilus, Lb. casei, Bifidobacterium spp., Streptococcus spp., and the yeast Saccharomyces boulardii [23].
In this study, six Lactobacillus strains (three Lb. paracasei, one Lb. parelimentarius, one Lb. fermentum, and one Lb. plantarum) with inhibitory activity against all S. flexneri and S. sonnei strains were isolated from traditional yogurt and milk. Some of these Lactobacillus strains, such as Lb. paracasei M18-1, Lb. paracasei Y8-1 and Lb. plantarum M19-1 have strong or very strong inhibitory activity against Shigella strains.
Mirnejad et al. [14] reported that CFCS from Lb. casei strongly inhibits the growth of multiple drug resistance (MDR) clinical samples of S. sonnei and S. flexneri in vitro. They suggested that Lb. casei is a good probiotic candidate. Zhang et al. [10] isolated 91 lactobacilli from human fecal samples and screened these lactobacilli for inhibitory activity against S. sonnei. Their results showed that 15 lactobacilli have strong inhibitory activity. Similar to our study, Hütt et al. [24] showed that Lb. rhamnosus GG has inhibitory activity against S. sonnei ATCC 25931. In addition, Zhihui Yu et al. [25] isolated Lb. plantarum S4-1 from naturally-fermented Chinese sauerkraut, and showed that this strain possesses inhibitory activity against S. flexneri CMCC. They suggested that Lb. plantarum S4-1 have potential as an excellent probiotic candidate for use in functional products.
In the present study, when the CFCS of Lactobacillus was adjusted to pH 6.5 and treated with catalase, the antimicrobial activity disappeared. These results suggest that the production of bacteriocin-like compounds did not play a role in the mechanism of antimicrobial activity. The inhibition of Shigella strains appeared to be the result of organic acids or hydrogen peroxide production by the Lactobacillus strains. Several studies have previously shown that a pH-dependent mechanism was involved in the antimicrobial activity of Lactobacillus strains [26], [27].
Conclusions
Lactobacillus strains with good inhibitory activity against S. flexneri and S. sonnei were isolated from traditional Iranian yogurt and milk. These Lactobacillus strains may be useful as potential novel and effective probiotic strains for the prevention or treatment of diarrhea, but further in vitro and in vivo investigations on these strains are still required.
Notes
Competing interests
The authors declare that they have no competing interests.
Acknowledgment
This work was supported by the Student Research Committee of Babol University of Medical Science, Babol, Iran (Grant no. 3976).
Erratum
The first name of the author Davoodabadi was originally mispelled (Aboldfazl).
References
- 1.Voss P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman W. Bergey's Manual of Systematic Bacteriology. 2nd ed. New York: Springer-Verlag; 2009. pp. 465–511. [Google Scholar]
- 2.Beasley S. Isolation, identification and exploitation of lactic acid bacteria from human and animal microbiota [dissertation] Helsinki: University of Helsinki; 2004. [Google Scholar]
- 3.Soltan Dallal MM, Zamaniahari S, Davoodabadi A, Hosseini M, Rajabi Z. Identification and characterization of probiotic lactic acid bacteria isolated from traditional persian pickled vegetables. GMS Hyg Infect Control. 2017 Sep 28;12:Doc15. doi: 10.3205/dgkh000300. doi: 10.3205/dgkh000300. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mokoena MP, Mutanda T, Olaniran AO. Perspectives on the probiotic potential of lactic acid bacteria from African traditional fermented foods and beverages. Food Nutr Res. 2016 Mar 8;60:29630. doi: 10.3402/fnr.v60.29630. doi: 10.3402/fnr.v60.29630. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouwehand AC, Kirjavainen PV, Shortt C, Salminen S. Probiotics: mechanisms and established effects. Int Dairy J. 1999;9(1):43–52. doi: 10.1016/S0958-6946(99)00043-6. doi: 10.1016/S0958-6946(99)00043-6. Available from: [DOI] [Google Scholar]
- 6.Axelsson L, Ahrné S. Lactic acid bacteria. In: Priest FG, editor. Applied microbial systematics. Dordrecht: Springer; 2000. pp. 367–388. [Google Scholar]
- 7.Soomro A, Masud T, Anwaar K. Role of lactic acid bacteria (LAB) in food preservation and human health – a review. PJN. 2002;1(1):20–24. doi: 10.3923/pjn.2002.20.24. doi: 10.3923/pjn.2002.20.24. Available from: [DOI] [Google Scholar]
- 8.Sullivan A, Nord CE. The place of probiotics in human intestinal infections. Int J Antimicrob Agents. 2002 Nov;20(5):313–319. doi: 10.1016/S0924-8579(02)00199-1. doi: 10.1016/S0924-8579(02)00199-1. Available from: [DOI] [PubMed] [Google Scholar]
- 9.Sullivan A, Nord CE. Probiotics in human infections. J Antimicrob Chemother. 2002 Nov;50(5):625–627. doi: 10.1093/jac/dkf194. doi: 10.1093/jac/dkf194. Available from: [DOI] [PubMed] [Google Scholar]
- 10.Zhang YC, Zhang LW, Ma W, Yi HX, Yang X, Du M, Shan YJ, Han X, Zhang LL. Screening of probiotic lactobacilli for inhibition of Shigella sonnei and the macromolecules involved in inhibition. Anaerobe. 2012 Oct;18(5):498–503. doi: 10.1016/j.anaerobe.2012.08.007. doi: 10.1016/j.anaerobe.2012.08.007. Available from: [DOI] [PubMed] [Google Scholar]
- 11.Davoodabadi A, Soltan Dallal MM, Rahimi Foroushani A, Douraghi M, Sharifi Yazdi MK, Amin Harati F. Antibacterial activity of Lactobacillus spp. isolated from the feces of healthy infants against enteropathogenic bacteria. Anaerobe. 2015 Aug;34:53–58. doi: 10.1016/j.anaerobe.2015.04.014. doi: 10.1016/j.anaerobe.2015.04.014. Available from: [DOI] [PubMed] [Google Scholar]
- 12.Davoodabadi A, Soltan Dallal MM, Lashani E, Tajabadi Ebrahimi M. Antimicrobial Activity of Lactobacillus spp. Isolated From Fecal Flora of Healthy Breast-Fed Infants Against Diarrheagenic Escherichia coli. Jundishapur J Microbiol. 2015 Dec 26;8(12):e27852. doi: 10.5812/jjm.27852. doi: 10.5812/jjm.27852. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niyogi SK. Shigellosis. J Microbiol. 2005 Apr;43(2):133–143. [PubMed] [Google Scholar]
- 14.Mirnejad R, Vahdati AR, Rashidiani J, Erfani M, Piranfar V. The antimicrobial effect of lactobacillus casei culture supernatant against multiple drug resistant clinical isolates of Shigella sonnei and Shigella flexneri in vitro. Iran Red Crescent Med J. 2013 Feb;15(2):122–126. doi: 10.5812/ircmj.7454. doi: 10.5812/ircmj.7454. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdenelli MC, Ghelfi F, Silvi S, Orpianesi C, Cecchini C, Cresci A. Probiotic properties of Lactobacillus rhamnosus and Lactobacillus paracasei isolated from human faeces. Eur J Nutr. 2009 Sep;48(6):355–363. doi: 10.1007/s00394-009-0021-2. doi: 10.1007/s00394-009-0021-2. Available from: [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Yun HS, Cho KW, Oh S, Kim SH, Chun T, Kim B, Whang KY. Evaluation of probiotic characteristics of newly isolated Lactobacillus spp.: immune modulation and longevity. Int J Food Microbiol. 2011 Aug;148(2):80–86. doi: 10.1016/j.ijfoodmicro.2011.05.003. doi: 10.1016/j.ijfoodmicro.2011.05.003. Available from: [DOI] [PubMed] [Google Scholar]
- 17.Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003 Oct;16(4):658–672. doi: 10.1128/CMR.16.4.658-672.2003. doi: 10.1128/CMR.16.4.658-672.2003. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rammelsberg M, Radler F. Antibacterial polypeptides of Lactobacillus species. J Appl Microbiol. 1990;69(2):177–184. doi: 10.1111/j.1365-2672.1990.tb01507.x. doi: 10.1111/j.1365-2672.1990.tb01507.x. Available from: [DOI] [Google Scholar]
- 19.González L, Sandoval H, Sacristán N, Castro JM, Fresno JM, Tornadijo ME. Identification of lactic acid bacteria isolated from Genestoso cheese throughout ripening and study of their antimicrobial activity. Food Control. 2007;18(6):716–722. doi: 10.1016/j.foodcont.2006.03.008. doi: 10.1016/j.foodcont.2006.03.008. Available from: [DOI] [Google Scholar]
- 20.Antonsson M, Molin G, Ardö Y. Lactobacillus strains isolated from Danbo cheese as adjunct cultures in a cheese model system. Int J Food Microbiol. 2003 Aug 15;85(1-2):159–169. doi: 10.1016/S0168-1605(02)00536-6. doi: 10.1016/S0168-1605(02)00536-6. Available from: [DOI] [PubMed] [Google Scholar]
- 21.Yun JH, Lee KB, Sung YK, Kim EB, Lee HG, Choi YJ. Isolation and characterization of potential probiotic lactobacilli from pig feces. J Basic Microbiol. 2009 Apr;49(2):220–226. doi: 10.1002/jobm.200800119. doi: 10.1002/jobm.200800119. Available from: [DOI] [PubMed] [Google Scholar]
- 22.Lin PP, Hsieh YM, Tsai CC. Antagonistic activity of Lactobacillus acidophilus RY2 isolated from healthy infancy feces on the growth and adhesion characteristics of enteroaggregative Escherichia coli. Anaerobe. 2009 Aug;15(4):122–126. doi: 10.1016/j.anaerobe.2009.01.009. doi: 10.1016/j.anaerobe.2009.01.009. Available from: [DOI] [PubMed] [Google Scholar]
- 23.Guandalini S. Probiotics for prevention and treatment of diarrhea. J Clin Gastroenterol. 2011 Nov;45 Suppl:S149–S153. doi: 10.1097/MCG.0b013e3182257e98. doi: 10.1097/MCG.0b013e3182257e98. Available from: [DOI] [PubMed] [Google Scholar]
- 24.Hütt P, Shchepetova J, Lõivukene K, Kullisaar T, Mikelsaar M. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J Appl Microbiol. 2006 Jun;100(6):1324–1332. doi: 10.1111/j.1365-2672.2006.02857.x. doi: 10.1111/j.1365-2672.2006.02857.x. Available from: [DOI] [PubMed] [Google Scholar]
- 25.Yu Z, Zhang X, Li S, Li C, Li D, Yang Z. Evaluation of probiotic properties of Lactobacillus plantarum strains isolated from Chinese sauerkraut. World J Microbiol Biotechnol. 2013 Mar;29(3):489–498. doi: 10.1007/s11274-012-1202-3. doi: 10.1007/s11274-012-1202-3. Available from: [DOI] [PubMed] [Google Scholar]
- 26.Juárez Tomás MS, Saralegui Duhart CI, De Gregorio PR, Vera Pingitore E, Nader-Macías ME. Urogenital pathogen inhibition and compatibility between vaginal Lactobacillus strains to be considered as probiotic candidates. Eur J Obstet Gynecol Reprod Biol. 2011 Dec;159(2):399–406. doi: 10.1016/j.ejogrb.2011.07.010. doi: 10.1016/j.ejogrb.2011.07.010. Available from: [DOI] [PubMed] [Google Scholar]
- 27.Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004 Oct;28(4):405–440. doi: 10.1016/j.femsre.2004.01.003. doi: 10.1016/j.femsre.2004.01.003. Available from: [DOI] [PubMed] [Google Scholar]