Abstract
Background
The contemporary risk classification criteria of gastrointestinal stromal tumors (GISTs) may still have room to improve. The aim of our research was to analyze the impact factors for GIST patients’ relapse-free survival (RFS). Furthermore, we explore whether gastrointestinal (1) bleeding will be a valuable indicator to predict GIST patients’ prognosis.
Material/Methods
R0 resection GISTs patients were retrospectively enrolled during an 8-year period at West China Hospital of Sichuan University, and all patients’ data were from the WCHSU-GIST database. Of a total of 333 GIST patients, 164 patients had GI bleeding. Univariate analysis and Cox regression analysis were used to calculate the survival and recurrence rates.
Results
Compared with non-GI-bleeding patients, GI-bleeding patients had a shorter relapse-free survival (RFS, P=0.003), but among the different risk groups, GI bleeding only affected the RFS rate of the high-risk group. A Cox regression analysis illustrated that tumor site (P<0.001), tumor size (P=0.009), mitotic index (P<0.001), tumor rupture (P<0.001), and GI-bleeding (P=0.01) were independent indicators for GIST patients’ RFS.
Conclusions
Our study demonstrates that the RFS of GIST patients with GI bleeding was significantly shorter than that of non-GI-bleeding patients, and GI bleeding was an independent negative factor predicting RFS, while GI bleeding had more influence among high-risk patients.
MeSH Keywords: Gastrointestinal Hemorrhage, Gastrointestinal Stromal Tumors, Prognosis
Background
Gastrointestinal stromal tumors (GISTs), which are the most common mesenchymal tumor in the gastrointestinal tract [1], show a rising trend in incidence worldwide over the past 3 decades [2,3], accounting for 0.1–3% of all GI malignancies [4]. GISTs can be observed in the entire GI tract up to the esophagus and down to the anus [1], and they can also be found in other organs or systems such as: prostate, pancreas, spleen, retroperitoneum, mesocolon, mesentery of the small intestine, mediastinum, and in the pelvis as vulvovaginal/rectovaginal septal masses [5–8], and some paraneoplastic syndromes also can be found in GIST patients [9,10]. Since the mutations in c-KIT and the platelet-derived growth factor receptor α (PDGFRA) were discovered, GISTs have been classified as a new disease with malignant potential [11–13]. Endoscopy and computerized tomography (CT) are frequently used before surgery for GI-bleeding GIST patients, while preoperative biopsy is seldom used because of the submucosal location and the possibility of rupture of the tumor [14,15]. Completely surgical resection once was the only way to treat GISTs, while other interventions, such as chemotherapy and radiotherapy, helped a little to prolong relapse-free survival (RFS) [16]. With the application of imatinib mesylate (IM, Gleevec, Novartis), a small-molecule tyrosine kinase inhibitor, RFS of GIST patients has been significantly extended [17–24].
The progress in GIST treatment enabled by IM shows how important it is to determine the risk of GIST recurrence and to select patients who will most likely benefit from using IM. According to National Institutes of Health (NIH) consensus criteria [25], Armed Forces Institute of Pathology (AFIP) criteria [26], and modified NIH consensus criteria [27], tumor site, size, and mitotic index are only used for postoperative patients to estimate the risk of prognosis; however, improvement may still be possible for all of these evaluation systems.
GI bleeding is common in GIST patients, and it is reported that approximately 23–33% manifest this symptom early in the course of their illness [4,28,29]. Many years of clinical observation show that GISTs patients who have GI bleeding have a more serious prognosis. Few studies [30–33] have focused on this manifestation, and we still know little about the correlation between GI bleeding and GIST recurrence. In the present study, we retrospectively studied GIST patients who were diagnosed and treated at West China Hospital of Sichuan University, and found that many of them had GI bleeding as the primary symptom. We analyzed factors that were listed in previous criteria that affect the recurrence of GIST and explored whether GI bleeding could be a potential new indicator to predict GIST clinical behavior and prognosis.
Material and Methods
Patients
In our research, from December 2009 to January 2017, we retrospectively assessed a total of 596 GIST patients who were diagnosed and treated at West China Hospital of Sichuan University, and all their data were obtained from the WCHSU-GIST database. All these patients were confirmed by immunohistochemistry to be KIT (CD117)- positive. Of all these patients, 395 received tumor R0 resection by open surgery or laparoscopic surgery. The inclusion criteria of this study included: (1) patients without recurrent or metastatic GISTs; (2) patients without other malignant diseases or those diseases that may cause GI bleeding; (3) patients without preoperative treatment such as chemotherapy, radiotherapy, and imatinib. Thus, 395 patients were enrolled in this research with complete clinicopathological data and meeting the modified NIH criteria, which is now widely used as a risk stratification scheme for GISTs. These patients were divided into 4 sub-groups: very low-risk, low-risk, moderate-risk, and high-risk.
The patients who had GI bleeding had the following symptoms: melena, hematochezia, and hematemesis. In our study, until the final follow-up date, 164 patients had GI bleeding and 169 patients did not have GI bleeding.
Follow-up
Follow-up data were collected by professional researchers via telephone and regular clinical re-checks. The follow-up period started from the date on which patients had surgical resection and ended on the date that recurrence or metastasis occurred, or on the latest follow-up date. RFS is more suitable than overall survival for GIST patients in survival analysis because of the use of IM, which prolongs survival time. During our research, until March 2017, we lost contact with 62 patients, and 333 patients were finally enrolled in our study.
Statistical analysis
All data were analyzed using SPSS 20.0 for MAC (IBM, Chicago, USA). The chi-square test was used to examine categorical data, and the t test or ANOVA was used to compare continuous data. The RFS rate was calculated by the Kaplan-Meier method and log-rank test, and the log-rank test was also used in subgroup analysis to distinguish differences. Multivariate survival analysis was performed using the Cox proportional hazards model to predict independent risk factors for RFS. The confidence interval was 5–95% and P<0.05 was considered as indicating a significant statistical difference.
Results
Clinical and pathological characteristics
Among the 333 GIST patients, 176 (41.6%) were males and 157 (58.4%) were females (male-to-female ratio 0.712: 1). Patient ages ranged from 3 to 81 years, with an average age of 54.9±11.4 years and median age of 57.0 years; 125 (37.5%) patients were over 60 years of age and were the largest age group. Primary clinical presentation included abdominal pain or distention (n=105), obstruction (n=29), GI bleeding (n=164), gastrointestinal perforation (n=23), and imaging tests (n=12). We found that the most frequent locations for GISTs were in the stomach (n=179, 53.8%), followed by the small intestine (n=83, 24.9%), and the colorectum (n=32, 9.6%). There were 191 (57.4%) patients who underwent open surgery, and all the others underwent laparoscopic resection. Tumor sizes ranged from 4 mm to 350 mm, with an average size of 75.1 mm. CD117, CD34, DOG-1, SMA, and S-100 were expressed in 317 (95.2%), 320 (96.1%), 320 (96.1%), 91 (27.3%), 43 (12.9%) patients, respectively. According to the modified NIH criteria, the risk categories of GIST patients were very low-risk (n=16, 4.8%), low-risk (n=63, 18.9%), intermediate-risk (n=59, 17.7%), and high-risk (n=195, 47.7%). Only 6 patients had undergone genetic testing: 5 showed a mutation in c-Kit exon 11 and 1 had a mutation in exon 9. All characteristics and details are summarized in Table 1.
Table 1.
Comparison between the GI-bleeding group and the non-GI bleeding group.
| Factors | GI bleeding (n=164) | Not GI bleeding (n=169) | P |
|---|---|---|---|
| Age | 54.0±12.8 | 55.9±9.9 | 0.15 |
| Gender (M/F, n) | 97/67 | 79/90 | 0.53 |
| Tumor size (cm) | 7.5±5.8 | 7.5±6.3 | 0.41 |
| Mitotic (/50HPF) | 0.33 | ||
| <5 | 46 | 66 | |
| 5–10 | 48 | 54 | |
| >10 | 70 | 43 | |
| Tumor sites (n)* | 0.16 | ||
| Gastric | 83 | 96 | |
| Non-gastric | 81 | 73 | |
| Tumor rupture | 0.31 | ||
| No | 104 | 121 | |
| Yes | 60 | 48 | |
| Risk** | 0.27 | ||
| Very low and low | 30 | 49 | |
| Intermediate | 29 | 30 | |
| High | 105 | 90 |
RFS – relapse-free survival; HPF – high power field; GI – gastrointestinal. The χ2 test was used to exam categorical data and the t test or ANOVA was applied to compare continuous data. P values <0.05 was considered statistical significance.
In non-gastric group, GISTs were located in small intestine, colorectum, and esophagus.
A risk category was assigned to all patients based on the application of the modified NIH criteria (2008 Edition).
GI bleeding occurred in164 patients out of the total 333 GIST patients, and there was no significant difference between the baseline data of the GI bleeding group and the non-GI bleeding group (Table 1).
Follow-up
The follow-up time for patients who were free of recurrence or metastasis ranged from 4 months to 100 months, and the median follow-up time was 41 months. Of all these patients, the cumulative 1-, 3-, and 5-year rates of RFS were 91.6% (n=305), 79.9% (n=266), and 70.9% (n=236), respectively. There were 119 (35.7%) patients who received adjuvant therapy by IM after the operation, with a dose of 400 mg/day, for 12–55 months.
Relapse-free survival analysis
Of the total of 333 GIST patients, during the follow-up period, 97 patients had tumor recurrence or metastasis, 58 patients had GI bleeding, and 178 did not have GI bleeding group. The recurrence or metastasis times ranged from 3 to 100 months (median, 26 months) after the operation. Univariate survival analysis illustrated that RFS of GIST patients was affected by tumor site (P<0.001), tumor size (P<0.001), mitotic index (P<0.001), tumor rupture (P<0.001), and GI-bleeding (P=0.003), while other clinicopathological and immunohistochemical factors had no correlation with the RFS (Table 2).
Table 2.
Univariate survival analysis of RFS using the Kaplan-Meier method for 333 GIST patients.
| Factors | No. of recurrences or metastases/total | P |
|---|---|---|
| Age (years) | 0.143 | |
| ≤50 | 40/114 | |
| 51~60 | 21/94 | |
| >60 | 36/125 | |
| Gender | 0.114 | |
| Male | 57/176 | |
| Female | 40/157 | |
| Tumor size (cm) | <0.001 | |
| ≤5 cm | 15/138 | |
| 5~10 cm | 54/150 | |
| >10 cm | 28/45 | |
| Mitotic (/50HPF) | <0.001 | |
| <5 | 15/128 | |
| 5~10 | 11/86 | |
| >10 | 71/119 | |
| Tumor sites (n)* | <0.001 | |
| Gastric | 30/179 | |
| Non-gastric | 67/154 | |
| Tumor rupture | <0.001 | |
| No | 31/225 | |
| Yes | 66/108 | |
| GI-bleeding | 0.003 | |
| No | 39/169 | |
| Yes | 58/164 | |
| Risk** | <0.001 | |
| Very low and low | 7/79 | |
| Intermediate | 5/59 | |
| High | 85/195 |
RFS – relapse-free survival; HPF – high power field; GI – gastrointestinal. In total, The RFS rates were calculated by the Kaplan-Meier method and were compared by the long-rank test model. P values <0.05 was considered statistical significance.
In non-gastric group, GISTs were located in small intestine, colorectum, and esophagus.
A risk category was assigned to all patients based on the application of the modified NIH criteria (2008 Edition).
The log-rank test was used to confirm the prognostic factors, showing that the patients who did not have GI bleeding had a longer RFS than those with GI bleeding (P=0.003, Figure 1). Furthermore, in different risk groups, GI bleeding showed a varied influence on RFS. In the very low-risk group and the low-risk group, GI bleeding showed no influence on RFS (P=0.25, Figure 2), while in the high-risk group, the RFS was much longer patients without GI bleeding (P=0.02, Figure 3). The intermediate-risk group showed an uncertain result, but suggested that GI bleeding may have shortened the RFS of GIST patients (P=0.07, Figure 4). Cox regression analysis demonstrates that GI bleeding [HR=0.573 (95% CI 0.372–0.885), P=0.01], GIST size (P=0.009), tumor location (P<0.001), rupture (P<0.001), and mitotic index (P<0.001) were statistically significant independent predictors of RFS and other parameters (Table 3). Furthermore, GI bleeding was a negative predictor for RFS.
Figure 1.
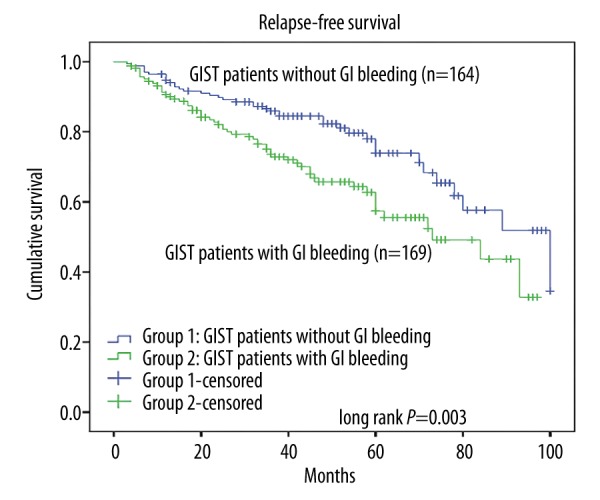
Kaplan-Meier estimates of the RFS of 333 patients in the GI bleeding group and the non-GI bleeding group.
Figure 2.
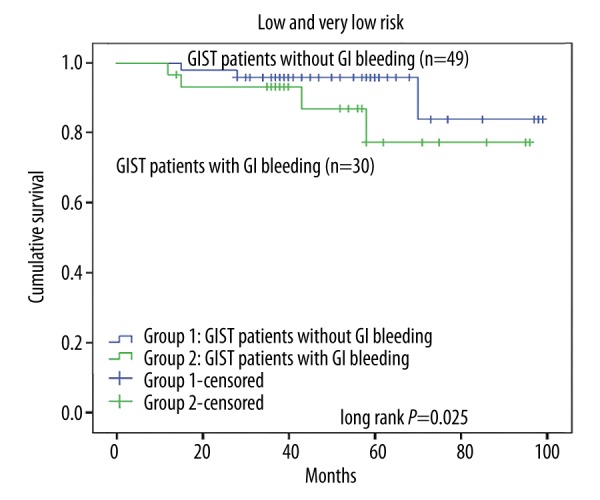
Relapse-free survival analysis of 333 patients with primary GISTs in different risk groups: Kaplan-Meier curve analysis demonstrated no differences in relapse-free survival for patients presenting with gastrointestinal (GI) bleeding in the very low-risk and low-risk group.
Figure 3.
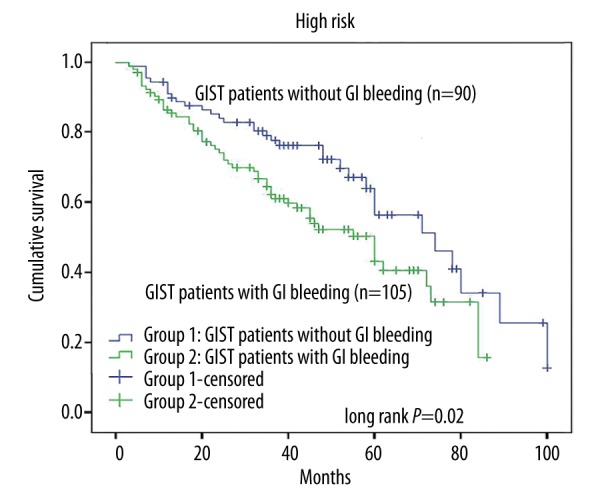
Kaplan-Meier curve analysis demonstrated worse relapse-free survival for patients presenting with gastrointestinal (GI) bleeding in the high-risk group.
Figure 4.
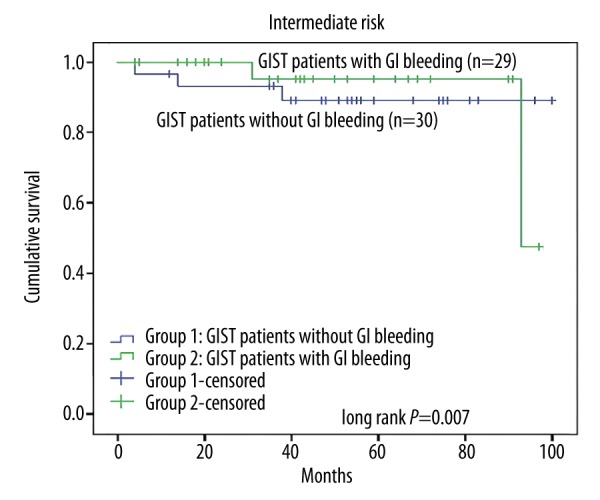
Kaplan-Meier curve analysis demonstrated an uncertain result in relapse-free survival for patients presenting with gastrointestinal (GI) bleeding in the intermediate-risk group.
Table 3.
Cox regression of RFS for the 333 GIST patients.
| Factors | HR | 95%CI | P | |
|---|---|---|---|---|
| Time of drug | −0.936 | 0.906 | 0.967 | <0.001 |
| Size | 1.045 | 1.011 | 1.079 | 0.009 |
| Rupture | 2.811 | 1.740 | 4.541 | <0.001 |
| Site | 2.271 | 1.456 | 3.543 | <0.001 |
| Motoric | 1.025 | 1.015 | 1.035 | <0.001 |
| GI bleeding | 0.573 | 0.372 | 0.885 | 0.01 |
RFS – relapse-free survival; HR – hazard ratio; GI – gastrointestinal; CI – confidence intervals. A multivariate survival analysis was performed by the Cox hazards model and the confidence interval was 5–95%. P values <0.05 was considered statistical significance.
Discussion
While clinical manifestations, endoscopy and CT scan results of GIST patients were not statistically significant predictors of RFS, in most cases they can be useful in diagnosis and in prediction of postoperative prognosis. In our retrospective research, we focused on the GIST patients with GI bleeding due to its high incidence. We found that: (1) RFS of GIST patients with GI bleeding was significantly shorter than that of non-GI-bleeding patients; (2) GI bleeding was an independently negative factor predicting RFS; and (3) GI bleeding had more influence on RFS among high-risk patients.
As GI bleeding often occurs in GIST patients, previous reports suggest the incidence is approximately 23–33% [4,28,29], but a long-term follow-up study for 1765 patients with GISTs [34] showed a much higher incidence (54.5%) of GI bleeding in GIST patients, and our findings shows the incidence of GI bleeding in GIST patients was 30.9%. Most studies found there is no sex bias or age-related differences in patients with GIST, and this was confirmed by the present study. Approximately 55–70% of GIST tumors are discovered in the stomach [35,36]; in patients with GI bleeding, we found most tumors were located in the stomach (50.6%) and intestine (32.9%), and this can explain why most patients with GI bleeding had melena (74.3%), followed by hematochezia (13.9%), and hematemesis (11.8%). Severe studies have indicated that tumor primary site, tumor size, tumor rupture, and mitotic index are significant independently predictors of GIST patient prognosis [8,25,27,37], and modified NIH criteria and other criteria have widely used these indicators to predict the prognosis and guide treatment of GIST patients. In our research, GI bleeding had been confirmed as a new independent predictor of RFS for GIST patients, which has been shown in some other studies [30,31], and a study from China suggested using GI bleeding to re-modify the GIST risk stratification system [30]. However, some studies reported that GI bleeding did not seem to be associated with risk of recurrence [32]. A study from Huazhong University of Science and Technology, China, reported that GI bleeding was a protective factor for GISTs relapse, and found that patients with GI bleeding had better prognosis [33].
GI bleeding was once considered as tumor rupture. Joensuu [27] firstly defined the concept of tumor rupture; GIST tumor rupture was proved to be an independent indicator of poor RFS [27,37] and tumor rupture was reported to increase the sensitivity of recurrence prediction [8]. However, whether GI bleeding indicates tumor rupture is uncertain. Most GIST tumors originate from the bowel wall and grow to both the serosal layer side and mucosa side, causing ulceration and mucosal invasion of the digestive tract. GI bleeding is caused by ulceration and mucosal invasion, and mucosal ulceration is seen in 39.6% of GIST patients [38], while mucosal invasion is rare [39]. Both of these may reveal a high progression of GIST tumors [38,39]. In our research, GI bleeding was an independent predictor for unfavorable RFS, but log-rank testing in different risk groups revealed that GI bleeding only influenced the RFS of the high-risk group, and there might be a higher risk group that could be explained by the contour maps for estimating the risk of GIST recurrence after surgery [40], and should therefore not be considered as tumor rupture [32]. Therefore, GI bleeding should not be defined as tumor rupture, or perhaps there is an overlap between GI bleeding and tumor rupture, and a more comprehensive study is needed.
Our study has several limitations. First, the sample size was limited because some patients were lost to follow-up. Secondly, this was a retrospective, single-center study, and we only focused on Chinese GIST patients, without considering patients from other ethnic groups. Thirdly, melena, hematochezia, or hematemesis may not be the only GI bleeding symptoms. Thus, a large, multicenter, prospective study with long-term follow-up is needed in the near future.
Conclusions
GI-bleeding often occurs in GIST patients and can be a common clinical manifestation at the beginning of the illness. In our research, GI bleeding was an independent prognostic predictor for poor RFS of GIST patients; and it especially influenced GIST patients with high risk and it can also influence the treatment of those patients. We suggest that these topics require further study in more patients and more institutions.
Acknowledgments
The authors thank the nursing and support staff at West China Hospital of Sichuan University.
Footnotes
Conflict of interest
None.
Source of support: The study was supported by the Sichuan Provincial Science and Technology Support Project (grant no. 2016SZ0047)
References
- 1.Barros A, Linhares E, Valadao M, et al. Extragastrointestinal stromal tumors (EGIST): A series of case reports. Hepatogastroenterology. 2011;58(107–108):865–68. [PubMed] [Google Scholar]
- 2.Steigen SE, Eide TJ. Trends in incidence and survival of mesenchymal neoplasm of the digestive tract within a defined population of northern Norway. APMIS. 2006;114(3):192–200. doi: 10.1111/j.1600-0463.2006.apm_261.x. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson B, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: The incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era – a population-based study in western Sweden. Cancer. 2005;103(4):821–29. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 4.Scarpa M, Bertin M, Ruffolo C, et al. A systematic review on the clinical diagnosis of gastrointestinal stromal tumors. J Surg Oncol. 2008;98(5):384–92. doi: 10.1002/jso.21120. [DOI] [PubMed] [Google Scholar]
- 5.Devanand B, Vadiraj P. The cytology of the benign extra-gastrointestinal stromal tumour in the pouch of Douglas: A case report. J Clin Diagn Res. 2011;5:1659–61. [Google Scholar]
- 6.Abedalthagafi M. Gastrointestinal stromal tumour originating from the hepatic falciform ligament. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr-03-2012-6136. pii: bcr0320126136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munoz M, Ramirez PT, Echeverri C, et al. Gastrointestinal stromal tumors as an incidental finding in patients with a presumptive diagnosis of ovarian cancer. J Gynecol Oncol. 2012;23(1):48–52. doi: 10.3802/jgo.2012.23.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: Recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231(1):51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson JM, Ginsberg J, Cutts K, Urban S. A case of non-islet cell tumor hypoglycemia (NICTH) associated with gastrointestinal stromal tumor (GIST) Am J Case Rep. 2017;18:984–88. doi: 10.12659/AJCR.904753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singhal A, Hadi R, Mehrotra K, et al. Paraneoplastic hypoglycaemia: A rare manifestation of pelvic gastrointestinal stromal tumour. J Clin Diagn Res. 2017;11(2):Xd01–xd2. doi: 10.7860/JCDR/2017/17146.9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joensuu H. Gastrointestinal stromal tumor (GIST) Ann Oncol. 2006;17(Suppl 10):x280–86. doi: 10.1093/annonc/mdl274. [DOI] [PubMed] [Google Scholar]
- 12.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science (New York, NY) 1998;279(5350):577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 13.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science (New York, NY) 2003;299(5607):708–10. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 14.Isik A, Soyturk M, Suleyman S, et al. Correlation of bowel wall thickening seen using computerized tomography with colonoscopies: A preliminary study. Surg Laparosc Endosc Percutan Tech. 2017;27(3):154–57. doi: 10.1097/SLE.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 15.Vander Noot MR, 3rd, Eloubeidi MA, Chen VK, et al. Diagnosis of gastrointestinal tract lesions by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer. 2004;102(3):157–63. doi: 10.1002/cncr.20360. [DOI] [PubMed] [Google Scholar]
- 16.Joensuu H. Adjuvant treatment of GIST: Patient selection and treatment strategies. Nat Rev Clin Oncol. 2012;9(6):351–58. doi: 10.1038/nrclinonc.2012.74. [DOI] [PubMed] [Google Scholar]
- 17.Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344(14):1052–56. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 18.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 19.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26(4):620–25. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 20.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial. Lancet (London, England) 2004;364(9440):1127–34. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 21.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26(4):626–32. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 22.Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: A randomised, double-blind, placebo-controlled trial. Lancet (London, England) 2009;373(9669):1097–104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frolov A, Chahwan S, Ochs M, et al. Response markers and the molecular mechanisms of action of Gleevec in gastrointestinal stromal tumors. Mol Cancer Ther. 2003;2(8):699–709. [PubMed] [Google Scholar]
- 24.Heinrich MC, Blanke CD, Druker BJ, Corless CL. Inhibition of KIT tyrosine kinase activity: A novel molecular approach to the treatment of KIT-positive malignancies. J Clin Oncol. 2002;20(6):1692–703. doi: 10.1200/JCO.2002.20.6.1692. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33(5):459–65. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 26.Miettinen M, Lasota J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23(2):70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39(10):1411–19. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 28.Lopes LF, Ojopi EB, Bacchi CE. Gastrointestinal stromal tumor in Brazil: Clinicopathology, immunohistochemistry, and molecular genetics of 513 cases. Pathol Int. 2008;58(6):344–52. doi: 10.1111/j.1440-1827.2008.02235.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang M, Xu J, Zhang Y, et al. Gastrointestinal stromal tumor: 15-years’ experience in a single center. BMC Surg. 2014;14:93. doi: 10.1186/1471-2482-14-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Chen P, Liu XX, et al. Prognostic impact of gastrointestinal bleeding and expression of PTEN and Ki-67 on primary gastrointestinal stromal tumors. World J Surg Oncol. 2014;12:89. doi: 10.1186/1477-7819-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yacob M, Inian S, Sudhakar CB. Gastrointestinal stromal tumours: Review of 150 cases from a single centre. Indian J Surg. 2015;77(Suppl 2):505–10. doi: 10.1007/s12262-013-0899-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmebakk T, Bjerkehagen B, Boye K, et al. Definition and clinical significance of tumour rupture in gastrointestinal stromal tumours of the small intestine. Br J Surg. 2016;103(6):684–91. doi: 10.1002/bjs.10104. [DOI] [PubMed] [Google Scholar]
- 33.Yin Z, Gao J, Liu W, et al. Clinicopathological and prognostic analysis of primary gastrointestinal stromal tumor presenting with gastrointestinal bleeding: A 10-year retrospective study. J Gastrointest Surg. 2017;21(5):792–800. doi: 10.1007/s11605-017-3385-2. [DOI] [PubMed] [Google Scholar]
- 34.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: A clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29(1):52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 35.Lamba G, Gupta R, Lee B, et al. Current management and prognostic features for gastrointestinal stromal tumor (GIST) Exp Hematol Oncol. 2012;1(1):14. doi: 10.1186/2162-3619-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steigen SE, Eide TJ. Gastrointestinal stromal tumors (GISTs): A review. APMIS. 2009;117(2):73–86. doi: 10.1111/j.1600-0463.2008.00020.x. [DOI] [PubMed] [Google Scholar]
- 37.Rutkowski P, Bylina E, Wozniak A, et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour – the impact of tumour rupture on patient outcomes. Eur J Surg Oncol. 2011;37(10):890–96. doi: 10.1016/j.ejso.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am. 2013;42(2):399–415. doi: 10.1016/j.gtc.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trupiano JK, Stewart RE, Misick C, et al. Gastric stromal tumors: A clinicopathologic study of 77 cases with correlation of features with nonaggressive and aggressive clinical behaviors. Am J Surg Pathol. 2002;26(6):705–14. doi: 10.1097/00000478-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265–74. doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]


