Injuries to the nervous system manifest in various forms ranging from stroke to trauma (i.e., motor vehicle accidents, combats) to diabetic neuropathy as well as many other neurological diseases. Nerve regeneration remains a complex biological process that is challenging to address clinically. There is no effective medical treatment for central nervous system repair. For a peripheral injury, the current gold standard for treating critically-sized neve defects (> 10 mm) is to use autologous nerve grafts, which have only shown 80% functional recovery. These grafts have significant drawbacks including the availability of donor source, size of donor nerve, and morbidity and scarring occurring at the donor site. Nerve tissue engineering provides a new alternative to neural recovery. Specifically, biomaterials integrate natural or synthetic biomimetic materials and biological systems to facilitate regeneration of diseased tissues and organs. Conductive polymers in particular provide a platform to manipulate cell behavior through electrical stimulation in addition to the existing biophysical and biochemical cues presented in the microenvironment (George et al., 2017). The ability to fabricate advanced materials allows us to re-create physiologic conditions that more dynamically interact with the nervous system and improve neural repair.
Electrically conductive polymers such as polypyrrole (PPy), polyaniline (PANI), and poly(3,4-ethylenedioxythiophene) (PEDOT) share common chemical structures. In these conductive polymers, a series of alternating single and double bonds with overlapping pi-bonds allows for free movement of electrons between atoms. By introducing a dopant in the solution, polymers can be oxidized and their backbones are disrupted to allow passage of electrons under an applied electrical potential. Different concentrations and types of doping agents can significantly alter the material properties of conductive polymers during the polymerization process. For example, the roughness of chondroitin sulphate incorporated PPy increases as more chondroitin sulphate is added. Polystyrene sulfonate doped PPy is more flexible compared to the hyaluronic acid doped PPy. Among all electrically conductive polymers, PPy is the most extensively studied and has emerged as a promising biomaterial that maintains reasonable conductivity (1–75 S/m) under physiological conditions. PPy has shown great biocompatibility both in vitro and in vivo (George et al., 2005). PPy is also dissolvable in many types of solvents such as water and is therefore easily synthesizable with oxidants and electrochemical processes (George et al., 2017). Traditionally to study the effect of electrical stimulation on neural behavior, PC12 cells have been cultured on electrically stimulated PPy films. Research has shown that PC12 cells with unfunctionalized PPy showed more than 90% enhancement in the length of neurite outgrowth when electrically stimulated. Subsequent implantation of PPy nerve conduits in the rat sciatic nerve model demonstrated regenerated myelinated nerve fibers after four weeks (George et al., 2009). To improve the mechanical strength and cytocompatibility of the polymer, PPy has been further incorporated with other polymers (e.g., poly(D, L-lactide-co-epsilon-caprolactone), poly(lactic-co-glycolic acid)) for better modulation of adhesion and growth of PC12 cells (Zhang et al., 2007; Xu et al., 2014). However, PC12 cells may not be the ideal cell line to study the neural behavior including neuronal differentiation and the function of neurotrophic factors. Because PC12 cells are derived from a neural tumor, they may represent altered signaling proteins and pathways. Specifically, the p46/53Shc and SNT, parts of the neurotrophin/Trk signaling pathway, are expressed highly in PC12 cells but are minimally expressed in mouse embryonic and adult brains (Kaplan, 1998).
With advances in stem cell technology, human neural progenitor cells (hNPCs) have developed into potential therapeutic cells for nerve regeneration. hNPCs have generated increases in synapse formation, axonal and dendritic expansion, and angiogenesis in neural applications. Intriguing questions exist on the use of electrical current to affect the behavior of hNPCs in vitro and in vivo. Indeed, past research has demonstrated that electrical stimulation guides endogenous neural progenitor cells (NPCs) migration in vivo (Cao et al., 2013) and leads to differentiation into neurons ex vivo (Li et al., 2008). In addition, many of the previous studies focused on how electrical field direct migration of hNPCs in a voltage or time-dependent manner. Studies investigated the signaling pathway particularly involved with cell mobilization as a result of electrical influence (e.g., Wnt/GSK3β pathway, P13K/Akt pathway, and NMDAR/Rac2/actin pathway). However, the underlying mechanism of hNPCs response to external electrical stimuli and utilizing electrically stimulated hNPCs to treat nerve injury are not well-investigated. Conductive polymers offer an interesting platform to further understand the interaction of electrical stimulation on hNPCs for nerve recovery applications.
In our recent work, a conductive PPy scaffold was utilized to electrically stimulate hNPCs in vitro. We further investigate how these electronically-conditioned cells can be used for enhancing functional recovery of rats with induced cerebral stroke (George et al., 2017). Prior conductive scaffold systems only allowed for in vitro manipulation of cells and not in vivo implantation of the system. In our system, the PPy can be versatilely sandwiched with chamber slides to allow seeding of hNPCs with direct electrical stimulation. This creates an in vitro conductive scaffold system that can be separated for in vivo implantation of cell-seeded PPy scaffolds. Specifically, hNPCs were first electrically stimulated on the PPy films with a +1 V to –1 V square wave at 1 kHz for one hour. This paradigm showed no change in cell survival 1 day after stimulation. To identify the pathways that may be altered by electrical stimulation, we screened the difference in gene expression between the stimulated and unstimulated groups. The vascular endothelial growth factor A (VEGF-A) pathway and other secreted factors associated with the pathway such as matrix metallopeptidase 9 (MMP-9) were significantly upregulated (Figure 1). The addition of bevacizumab (a human monoclonal antibody that blocks VEGF-A) during electrical stimulation blocked any increase in VEGF-A gene expression. This showed that the activation of VEGF-A pathway is a direct result of electrical stimulation on hNPCs. Our data matches well with previous research which showed that an electric field induces VEGF receptor signaling in endothelial cells in culture (Zhao et al., 2004). Additionally, electric fields have been demonstrated to upregulate brain-derived neurotrophic factor (BDNF) and VEGF signaling pathways in the neurogenesis of neuronal stem cells (Kim et al., 2014). Interestingly, we found that not all factors of the VEGF-A pathway were significantly changed in their gene expression after electrical stimulation. For example, thrombospondin 1 (THBS1) and transforming growth factor (TFG-β), key mediators of cell survival and angiogenesis, showed no significant difference between pre- and post-electrical stimulation with gene analysis (George et al., 2017). With an increase in electrical field strength (250 mV/mm) and longer duration of stimulation (three hours), researchers previously reported that 68% of the migrating NPCs generate immature neurons (Li et al., 2008). However, we observed no change in hNPC differentiation pre- and post-stimulation given the stimulation period was brief. The ability to manipulate hNPCs electrically provides a unique paradigm to understand the important pathways for hNPC-mediated neural recovery.
Figure 1.
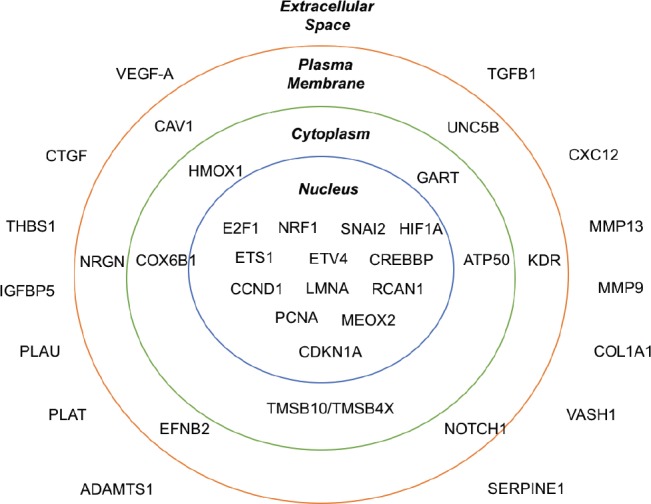
An illustration of significantly altered in the VEGF-A pathway after electrical stimulation (George et al., 2017).
VEGF-A: Vascular endothelial growth factor A; CTGF: connective tissue growth factor; THBS1: thrombospondin 1; IGFBP5: insulin-like growth factor binding protein 5; PLAU: plasminogen activator urokinase; PLAT: tissue plasminogen activator; ADAMTS1: a disintegrin and metalloproteinase with thrombospondin motifs 1; TGFB1: transforming growth factor beta 1; CXC12: chemokine 12; MMP13: matrix metallopeptidase 13; MMP9: matrix metallopeptidase 9; COL1A1: collagen type I alpha 1; VASH1: vasohibin 1; SERPINE1: serine proteinase inhibitor family E member 1; CAV1: caveolin 1; NRGN: neurogranin; EFNB2: ephrin B2; UNC5B: un-coordinated-5 homolog B; KDR: kinase domain region receptor; HMOX1: heme oxygenase 1; COX6B1: cytochrome c oxidase subunit 6B1; TMSB10: thymosin beta 10; TMSB4X: thymosin beta 4X; ATP5O: ATP synthase subunit O; GART: glycinamide ribonucleotide transformylase; NRF1: nuclear respiratory factor 1; HIF1A: hypoxia-inducible factor 1 alpha; ETV4: ETS translocation variant 4; CREBBP: cAMP-response element binding protein; CCND1: cyclin D1; LMNA: lamin A/C; RCAN1: regulator of calcineurin 1; PCNA: proliferating cell nuclear antigen; MEOX2: mesenchyme homeobox 2; CDKN1A: cyclin dependent kinase inhibitor 1A.
The electrically-conditioned hNPCs on the PPy scaffold were then placed on the peri-infarct cortical surface in rats with distal middle cerebral artery occlusion strokes. Based on behavioral models (the neurological severity scale and vibrissae-forepaw model), we observed that the electrically pre-conditioned groups outperformed the other groups including the unstimulated hNPCs and polymer alone starting at 1–2 weeks post implantation (George et al., 2017). We also observed that there was an increase in blood vessel density surrounding the peri-infarct area in animals that received electrically preconditioned hNPCs compared to the other groups. This trend undoubtedly correlates well with the increase in VEGF-A expression as a result of electrical stimulation, because VEGF-A is known to be linked to angiogenesis and cell survival in stroke recovery (Horie et al., 2011). The gene expression profile of the peri-infarct region from rats that received electrical pre-conditioned hNPCs was compared with the ones implanted with unstimulated cells. Results showed that 42 overlapping genes of the endogenous rat cortical tissues were upregulated with no alterations from exogenous human genes from hNPCs. It is likely that the endogenous VEGF-A pathway was re-enforced by the exogenous VEGF-A through secreted factors (Knizetova et al., 2008) from the electrical pre-conditioning hNPCs. The electrically pre-conditioned stem cells produce trophic factors (Kim et al., 2014) more efficiently which then act upon remote targets to improve stroke recovery.
Electrically conductive polymers offer a novel platform to interact with hNPCs and the nervous system. Our recent work developed a stand-alone PPy scaffold suitable for in vitro stimulation and in vivo implantation and cell delivery. Furthermore, we found increased angiogenesis and functional recovery of the animals due to the upregulated VEGF-A pathway and secreted factors via electrical stimulation. These results not only provide us with insight on the importance of electrophysiology on stem cell function but also help us understand the mechanism upon which the electrically-conditioned transplanted stem cells enhance long-term function. These exciting findings reinforce the concept that neural cells respond to chemical and electrical signals which could offer unique avenues for neural therapies. It is also important to consider other factors in designing conductive scaffolds such as incorporating all microenvironmental cues to further enhance the material property for better cell, material, and host interaction. Conductive polymer scaffolds create the ability to manipulate the nervous system during repair to investigate essential recovery mechanisms such as angiogenesis, cell survival, and migration. As tissue engineered methods of interacting with the nervous system advance, better methods from three dimensional printing and microfabrication can be developed for advanced functional materials to improve neural regeneration.
The work was supported in part by the American Brain Foundation/Academy of Neurology and NIH grant K08NS089976.
Footnotes
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review reports:
Reviewer 1: Mahboubeh Kabiri, University of Tehran, Iran.
Reviewer 2: Jinghui Li, Kunming Medical University, China.
Comments to authors: Nerve regeneration remains a complex biological process that is challenging to address clinically. There is no effective medical treatment for central nervous system repair. Conductive polymer scaffolds also create the ability to manipulate the nervous system during repair to investigate essential recovery mechanisms such as angiogenesis, cell survival, and migration. As tissue engineered methods of interacting with the nervous system advance, better methods can be developed to improve neural regeneration. Conductive polymer scaffolds are excellent candidates for promoting neural recovery, thus a promising research directions, ideas and strategy for future neural regeneration applications. In sum, as tissue engineered methods of interacting with the nervous system advance, better methods can be developed to improve neural regeneration Their facing challenges and future developments are significance to promote their further clinical treatment of nervous system injury.
References
- Cao L, Wei D, Reid B, Zhao S, Pu J, Pan T, Yamoah E, Zhao M. Endogenous electric currents might guide rostral migration of neuroblasts. EMBO Rep. 2013;14:184–190. doi: 10.1038/embor.2012.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George PM, Bliss TM, Hua T, Lee A, Oh B, Levinson A, Mehta S, Sun G, Steinberg GK. Electrical preconditioning of stem cells with a conductive polymer scaffold enhances stroke recovery. Biomaterials. 2017;142:31–40. doi: 10.1016/j.biomaterials.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George PM, Lyckman AW, LaVan DA, Hegde A, Leung Y, Avasare R, Testa C, Alexander PM, Langer R, Sur M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials. 2005;26:3511–3519. doi: 10.1016/j.biomaterials.2004.09.037. [DOI] [PubMed] [Google Scholar]
- George PM, Saigal R, Lawlor MW, Moore MJ, LaVan DA, Marini RP, Selig M, Makhni M, Burdick JA, Langer R, Kohane DS. Three-dimensional conductive constructs for nerve regeneration. J Biomed Mater Res A. 2009;91:519–527. doi: 10.1002/jbm.a.32226. [DOI] [PubMed] [Google Scholar]
- Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, Shamloo M, Hamilton SA, Jiang K, Huhn S, Palmer TD, Bliss TM, Steinberg GK. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29:274–285. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR. Studying signal transduction in neuronal cells: the Trk/NGF system. Prog Brain Res. 1998;117:35–46. doi: 10.1016/s0079-6123(08)64005-4. [DOI] [PubMed] [Google Scholar]
- Kim YR, Kim HN, Ahn SM, Choi YH, Shin HK, Choi BT. Electroacupuncture promotes post-stroke functional recovery via enhancing endogenous neurogenesis in mouse focal cerebral ischemia. PLoS One. 2014;9:e90000. doi: 10.1371/journal.pone.0090000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knizetova P, Ehrmann J, Hlobilkova A, Vancova I, Kalita O, Kolar Z, Bartek J. Autocrine regulation of glioblastoma cell cycle progression, viability and radioresistance through the VEGF-VEGFR2 (KDR) interplay. Cell Cycle. 2008;7:2553–2561. doi: 10.4161/cc.7.16.6442. [DOI] [PubMed] [Google Scholar]
- Li L, El-Hayek YH, Liu B, Chen Y, Gomez E, Wu X, Ning K, Li L, Chang N, Zhang L, Wang Z, Hu X, Wan Q. Direct-current electrical field guides neuronal stem/progenitor cell migration. Stem Cells. 2008;26:2193–2200. doi: 10.1634/stemcells.2007-1022. [DOI] [PubMed] [Google Scholar]
- Xu H, Holzwarth JM, Yan Y, Xu P, Zheng H, Yin Y, Li S, Ma PX. Conductive PPY/PDLLA conduit for peripheral nerve regeneration. Biomaterials. 2014;35:225–235. doi: 10.1016/j.biomaterials.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Rouabhia M, Wang Z, Roberge C, Shi G, Roche P, Li J, Dao LH. Electrically conductive biodegradable polymer composite for nerve regeneration: electricity-stimulated neurite outgrowth and axon regeneration. Artif Organs. 2007;31:13–22. doi: 10.1111/j.1525-1594.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Zhao M, Bai H, Wang E, Forrester JV, McCaig CD. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci. 2004;117:397–405. doi: 10.1242/jcs.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]


