Amyloid peptide (Aβ) oligomers are considered one of the primary causal factors for the synaptic loss characteristic of Alzheimer's disease (AD) (Karran and De Strooper, 2016). However, Aβ is generated in normal brains and accumulates at synaptic sites, which raises the question whether Aβ plays a physiological role in synapses. This unresolved issue is especially relevant in view of the recent AD therapeutic strategies aimed at blocking or reducing Aβ production. Here, we justify the use of the Drosophila adult neuromuscular junction (NMJ) as a model to address this question, describe our recent results that indicate that Aβ regulates synapse dynamics, and suggest future approaches to understand Aβ synaptic function.
Aβ comprises a heterogeneous mixture of peptides of various lengths, which are generated by proteolytic processing of the amyloid precursor protein (APP). The type I transmembrane protein APP can go through different complex proteolytic pathways to generate a variety of proteolytic fragments (Müller et al., 2017). Two types of proteases, the α- and β-secretases, cleave the protein at the juxtamembrane/extracellular sequence, generating a soluble ectodomain and a transmembrane C-terminal fragment. The latter can undergo intramembranous processing by the γ-secretase complex, which requires presenilin activity. In the non-amyloidogenic pathway, cleavage by the α-secretase occurs within the Aβ sequence, precluding the formation of Aβ. The amyloidogenic pathway involves the sequential action of the β- and γ-secretases, and generates Aβ peptides with different C-terminal endings. During this process, Aβ40 is abundantly produced, mostly as soluble monomers. The longer Aβ forms are produced at lower levels and are aggregation-prone. However, mutations in APP and presenilins genetically linked to dominant familial AD (FAD) alter intramembranous cleavage, increasing production of the longer peptides, such as Aβ42, and resulting in a qualitative shift in Aβ profile towards the aggregation-prone forms. These findings strongly support a central role for Aβ aggregates in AD, and have stimulated research on their neurotoxic effects (Ferreira et al., 2015).
Because the key clinical symptom of AD is impaired acquisition of episodic memories, and synaptic loss is the strongest quantitative morphological correlate of dementia in AD, much effort has been directed towards understanding how Aβ affects synaptic plasticity, the physiological substrate for learning and memory. Synaptic effects of acute application of Aβ or of genetic modifications of FAD-linked and related genes, have been analysed in cell culture, brain slices, or animal models (Ferreira et al., 2015). These studies have shown that Aβ inhibits long-term potentiation (LTP) and enhances long-term depression, two acute forms of activity-dependent modification of synaptic strength that underlie learning and memory. Furthermore, persistent Aβ exposure causes structural synaptic changes, resulting in decrease synaptic density and altered spine dynamics which in turn, lead to robust deficits in learning and memory. The soluble oligomeric aggregates, particularly Aβ42, seem to be synaptotoxic, and associated with weakening of excitatory transmission.
The large body of evidence indicates that abnormal synaptic plasticity triggered by an increase in soluble Aβ oligomers contribute to early memory loss in AD (Ferreira et al., 2015). But the question remains whether this synaptotoxicity is an anomalous, non-physiological action of the Aβ aggregates, or a dysregulation of synaptic physiological activities of Aβ. In other words, does Aβ play a normal role in synaptic function? Indeed, several studies strongly suggest that Aβ is not only involved but required for normal synaptic plasticity (Puzzo et al., 2015). A low, close-to-physiological concentration of Aβ42 enhances LTP and memory, while reduction of endogenous Aβ, by neutralizing antibodies or through genetic or pharmacological inhibition of amyloidogenic APP processing, has the opposite effect. Aβ production and secretion is regulated by neuronal activity, further supporting a physiological role for Aβ.
Exactly which Aβ isoforms and how they are relevant to the normal process of synaptic plasticity are still unresolved (Ferreira et al., 2015; Karran and De Strooper, 2016). In vitro experiments allow for strict control of the length of the Aβ peptide, but can only be used to address acute, short-term effects. Long-term, age-dependent effects of Aβ have been analysed in classical in vivo models, but they have the disadvantage of producing a complex mixture of Aβ peptides. The absence of the Aβ sequence in the invertebrate APP homologs has prompted the use of transgenic, invertebrate models, which have provided relevant insights onto the mechanisms of action of Aβ. Transgenic Drosophila melanogaster is one such model. Expression of exogenous AD-related human proteins in the fly brain recapitulates many of the symptoms of the disease, including early cognitive decline and late, progressive neurodegeneration and amyloid deposition. Moreover, treatments known to ameliorate AD-like pathology in mammalian models and in humans are also effective in the AD transgenic flies (Iijima-Ando and Iijima, 2010).
In the last decades, the Drosophila larval NMJ has been demonstrated to be a successful model to study the molecular bases of synaptic formation and physiology (Harris and Littleton, 2015). The molecular composition and physiology of this NMJ is most similar to mammalian glutamatergic excitatory synapses. In contrast to mammalian central synapses, in the Drosophila NMJ each presynaptic motor neuron and postsynaptic muscle cell can be, however, easily identified and visualized and has a segmental stereotypical morphology with minimum inter-individual variability. This allows for accurate quantitative determination of the in vivo effects of multiple parameters, such as altered genes, training paradigms, or drug application, on a single glutamaergic synapse. Moreover, powerful genetic tools can be utilized to understand the cellular and molecular mechanisms underlying synaptic changes. Indeed, essential synaptic players, such as Dynamin, have been first characterized in the Drosophila larval NMJ. However, synaptic processes extending over long-time periods and modifications related to aging can only be studied in adult synapses. For these reasons, we recently turned to the NMJ of the adult fly as the experimental setup to study the effects of specific Aβ peptides on synapses (López-Arias et al., 2017). Morphometric parameters were measured at specific age points in the ventral abdominal adult NMJ.
In the adult insect brain, the first days post-eclosion comprise a critical period of experience-dependent, developmental structural plasticity (Golovin and Broadie, 2016). For example, in young Drosophila flies, social interaction changes fiber number in the mushroom bodies and synaptic elaboration of circadian clock neurons, olfactory stimulus modifies the volume of olfactory glomeruli, and light exposure alters photoreceptor terminal size. Similarly, we have documented that in the NMJ of wild type adult flies, there is a progressive increase in the number of active zones (AZs) during the first two weeks of adult life (Figure 1; López-Arias et al., 2017). Thereafter, AZs decrease. This point of transition from synapse addition to synapse elimination coincides with the onset of fly behavioural and synaptic senescence, which has been revealed by reduced motor activity, sensory acuity, sleep, learning and memory, between others (Iliadi and Boulianne, 2010). Thus, the adult NMJ allows for the assessment of the effects of Aβ on synaptic dynamics during synaptic maturation and aging.
Figure 1.
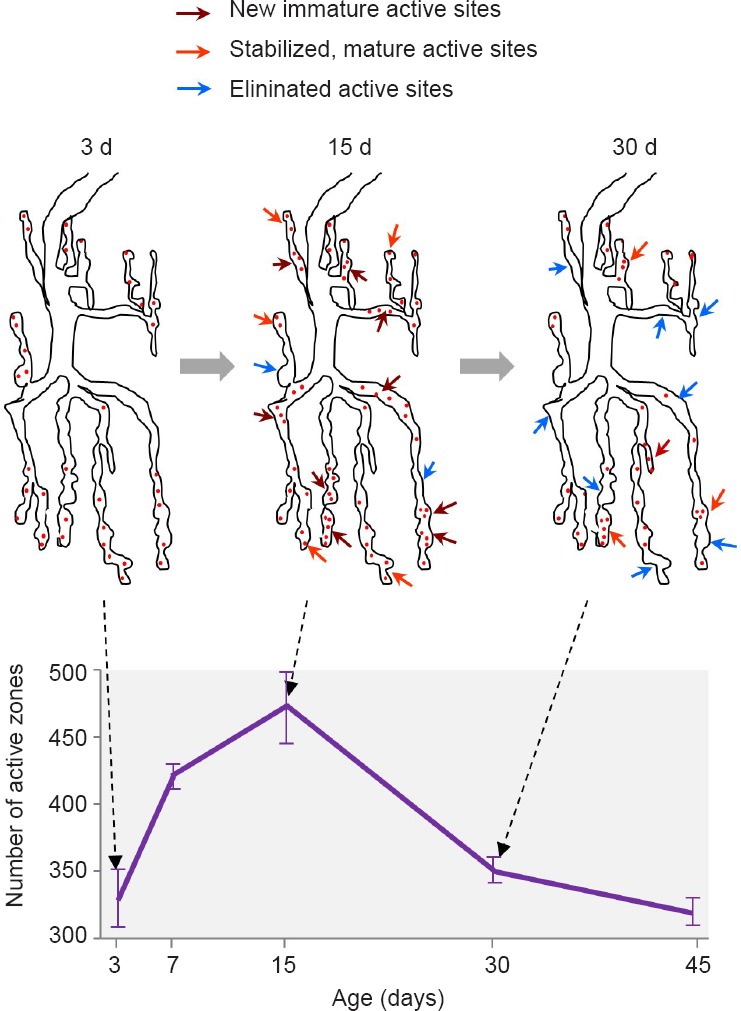
Age-dependent changes in synaptic active zones at the fly abdominal neuromuscular junction (NMJ).
(A) Schematic diagram of the process of active zone addition (dark red arrows), stabilization (orange arrows) and elimination (blue arrows) occurring in the adult abdominal ventral NMJ during the first month of adult life. Up to 15 days (d), net active zone addition occurs. Thereafter, net active zone elimination takes place. (B) Graph depicting the age-dependent changes in the average number (± SEM) of active zones measured in wild type NMJs. Active zones were revealed by the presence of the presynaptic scaffold protein Bruchpilot (ELKS/CAST protein). Data were extracted from López-Arias et al., 2017.
By expressing single human Aβ peptides in Drosophila motor neurons, we have revealed synaptic effects specific for three different Aβ species (Figure 2): Aβ40, Aβ42, and Aβ42arc, a FAD mutant peptide with enhanced aggregation properties (López-Arias et al., 2017). We predicted that expression of synaptotoxic amyloid species would elicit net synaptic elimination, and consequently reduce the number of AZs while maintaining the biphasic temporal pattern seen in wild type NMJs. This was indeed the case for Aβ42arc, which induced a dramatic reduction of the number of AZs with respect to normal age-matched NMJs, but displayed the expected early rise and subsequent drop in AZs. Surprisingly, expression of Aβ40 rendered NMJs with an invariable number of AZs from 3 up to 30 days of age. Thus, these NMJs did not undergo the increase in AZs characteristic of young flies nor the decrease in AZs typically observed at older ages. For Aβ42, the situation was intermediate. Up to 15 days of age, Aβ42 NMJs had a phenotype similar to Aβ40 without significant variations in the number of AZs. From 15 to 30 days, the age-dependent reduction in AZs occurred as in controls, but the number of AZs was significantly lower at every age tested. Linear regression analyses were used to statistically compare the age-dependent rate of AZ change (Figure 2; López-Arias et al., 2017). Up to 15 days of age, this rate was positive for control and Aβ42arc NMJs, but 0 for Aβ40 and Aβ42 NMJs. From 15 days on, this rate remained 0 for Aβ40 NMJs, while AZs decreased in Aβ42 and Aβ42arc expressing NMJs at a rate similar to controls.
Figure 2.
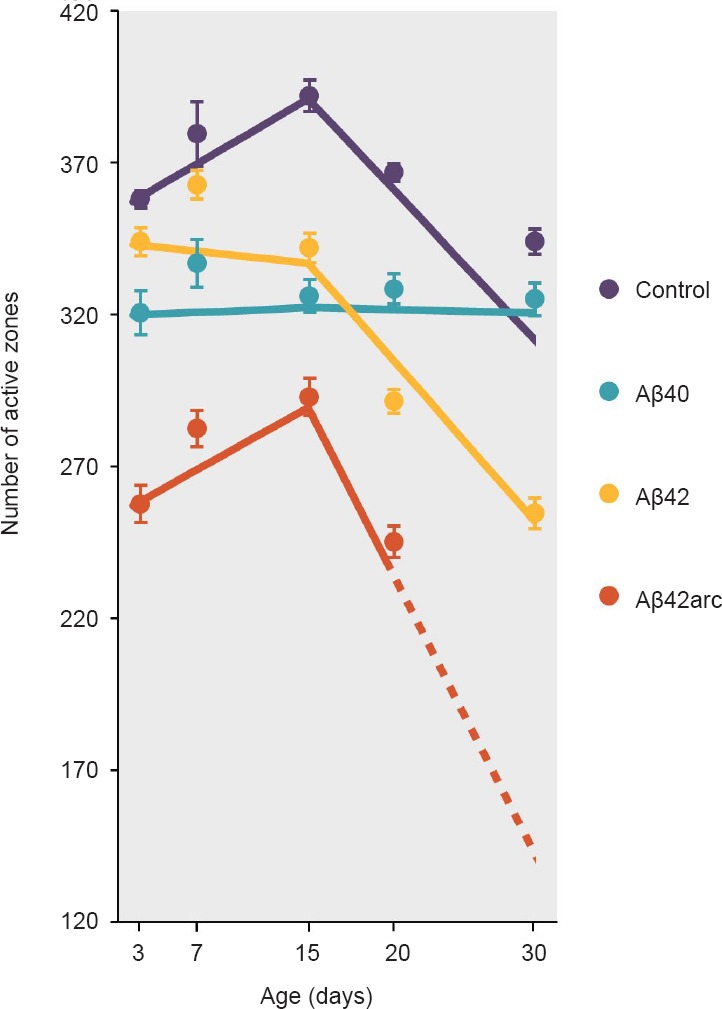
Age-dependent rate of active zone changes induced by different Aβ species.
Lines show the rate of change of the number of active zones, calculated by applying linear regression analysis to the actual data (see López-Arias et al., 2017 for details on data and statistical analyses). Dots depict the average number ± SEM of active zones measured from 10 neuromuscular junctions for each genotype and age. Aβ42arc-expressing flies died before reaching 30 days of age, and therefore the dotted line was inferred from the slope obtained between 15 and 20 days.
The most striking finding from this study was the fact that presynaptic expression of Aβ40 prevented the early rise of AZs, while Aβ42arc did not. This suggests that in flies, Aβ40, and probably Aβ42, constrains synapse addition. Surprisingly and contrary to Aβ42arc and Aβ42 in aged flies, late AZ reduction did not occur in Aβ40-expressing NMJs. A coherent interpretation of these results is that Aβ40 alters synapse turnover, which has been shown to directly correlate with new learning and synaptic plasticity in vertebrates (Caroni et al., 2012). This could explain why pan-neuronal expression of Aβ40 has been shown to impair associative olfactory learning and memory already in young flies (Iijima-Ando and Iijima, 2010), while it does not cause motor dysfunction, neurodegeneration, or reduced lifespan.
The remarkable specificity of the Aβ40 phenotype points to this peptide as a possible physiological regulator of synapse dynamics (López-Arias et al., 2017). We can now use this fly model to understand how Aβ40 acts. The appropriate balance between synapse assembly, stabilization, and disassembly will determine the direction of AZ changes. The use of fluorescently-tagged pre- and post-synaptic molecules and high-resolution microscopy has enabled a detailed characterization of the mechanisms of neurodevelopmental synapse dynamics during the growth of the larval NMJ (Harris and Littleton, 2015). As in mammals, differential trafficking of ionotropic glutamate receptors (GluR) is essential for this process. Newly formed immature synapses contain mostly postsynaptic ionotropic GluRIIA-containing receptors, which desensitize slowly. These synapses are highly dynamic and are easily disassembled, but they can be stabilized by activity-induced recruitment of ionotropic GluRIIB to their postsynaptic fields. Thus, immature and mature synapses can be differentiated by their relative GluRIIA/IIB content. In fact, Aβ has been reported to alter ionotropic GluR trafficking in mammalian synapses (Ferreira et al., 2015). Because similar mechanisms are likely to operate in the adult NMJ, analogous approaches can be used for understanding the temporal sequence of events underlying age-dependent synapse dynamics in wild type flies and how these processes are influenced by Aβ.
One of the most evasive questions in the AD field refers to the molecular nature of the receptors that mediate Aβ synaptic actions. Many have been proposed (Ferreira et al., 2015), but their physiological relevance is unknown. The fly NMJ offers a highly accessible and relatively easy setup for genetically manipulating these putative receptors and study how they modify Aβ action. APP itself is a very good candidate: it binds Aβ at physiological concentrations; it is implicated in the formation and stabilization of synapses during neurodevelopment; and knock out adult mice show reduced spine turnover and inability to increase synaptic density upon environmental enrichment (Müller et al., 2017). Indeed, APP has been proposed to act as a molecular switch between synaptoblastic and synaptocastic conditions by means of proteolytic processing. In Drosophila, the single APP-like protein (APPL) induces synapse formation at the larval NMJ, while reduced APPL causes learning and memory defects that are exacerbated by Aβ (Cassar and Kretzschmar, 2016). The hypothesis that APPL mediates Aβ40 synaptic effect can be tested by altering APPL levels in fly motoneurons, and by performing rescue experiments with different APPL or human APP proteolytic fragments.
A relevant conclusion derived from our results is that Aβ40, Aβ42, and Aβ42arc have very different synaptic effects, and thus are likely to act through different receptors. Interestingly, Aβ42 action seems to transform with age, from an Aβ40-like activity to an Aβ42arc-like action. A previous study in Drosophila found a strong correlation between Aβ toxicity and the propensity of the Aβ to form soluble oligomeric aggregates (Speretta et al., 2012). This propensity was shown to be significantly higher for Aβ42 than for Aβ40. Therefore, we hypothesize that monomeric forms, which would be enriched in Aβ40 and young Aβ42 flies, act physiologically regulating synapse dynamics while oligomeric forms, which would prevail in Aβ42arc and aged Aβ42 flies, induce net synapse elimination. This proposition could be tested using Aβ40 and Aβ42 forms with altered aggregation kinetics. A recently identified APP mutation in the Aβ sequence reduces its aggregation, and is linked with lower risk of clinical AD (Karran and De Strooper, 2016). In Drosophila, tandem dimeric Aβ constructs with varying linker lengths show different aggregation kinetics (Speretta et al., 2012). These Aβ constructs can be tested in vivo for their synaptic, age-dependent effects in the adult NMJ.
Studies about the normal function of Aβ will benefit from focussing on the Aβ40 peptide, which predominates in the human brain. Using the fly adult NMJ, we have provided additional support for a physiological synaptic role for Aβ40, that is distinct from the synaptotoxic actions of Aβ42 aggregates. Once we clarify Aβ40 mechanisms, we can look further into Aβ42 to understand its physiological and pathological action at synapses. This type of studies in invertebrate models will continue granting information essential for the development of successful AD therapies.
The work was supported Fundación Reina Sofía Grant PI0006-08 to LT and by Ministerio de Ciencia y Tecnología (ES) grant BFU2008-04683-C02-02 to LT.
Footnotes
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review report:
Reviewer: Anindita Banerjee, ICARE Institute of Medical Science & Research & Dr.Bidhan Chandra Roy Hospital, India.
Comments to authors: The perspective is very well composed and relevant in the present context of re-understanding Alzheimer's pathogenesis. The dissected analysis of physiological and pathological role of Aβ in the synaptic functions and alterations along with its biological implications are finely discussed in an organized manner with necessary references. Functions and expressions of the proteins in Drosophila NM junctions which are homologous to the human counterpart are nicely mentioned. Differential role of Aβ40 and Aβ42 on synaptic dynamics are also discussed well in the manuscript along with the schematic diagram. A concise and informative writing focusing on the potential pathologic mechanisms of early signs of AD.
References
- Caroni P, Donato F, Muller D. Structural plasticity upon learning: regulation and functions. Nat Rev Neurosci. 2012;13:478–490. doi: 10.1038/nrn3258. [DOI] [PubMed] [Google Scholar]
- Cassar M, Kretzschmar D. Analysis of Amyloid Precursor Protein Function in Drosophila melanogaster. Front Mol Neurosci. 2016;9:61. doi: 10.3389/fnmol.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira ST, Lourenco MV, Oliveira MM, De Felice FG. Soluble amyloid-beta oligomers as synaptotoxins leading to cognitive impairment in Alzheimer's disease. Front Cell Neurosci. 2015;9:191. doi: 10.3389/fncel.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovin RM, Broadie K. Developmental experience-dependent plasticity in the first synapse of the Drosophila olfactory circuit. J Neurophysiol. 2016;116:2730–2738. doi: 10.1152/jn.00616.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KP, Littleton JT. Transmission, development, and plasticity of synapses. Genetics. 2015;201:345–375. doi: 10.1534/genetics.115.176529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima-Ando K, Iijima K. Transgenic Drosophila models of Alzheimer's disease and tauopathies. Brain Struct Funct. 2010;214:245–262. doi: 10.1007/s00429-009-0234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliadi KG, Boulianne GL. Age-related behavioral changes in Drosophila. Ann N Y Acad Sci. 2010;1197:9–18. doi: 10.1111/j.1749-6632.2009.05372.x. [DOI] [PubMed] [Google Scholar]
- Karran E, De Strooper B. The amyloid cascade hypothesis: are we poised for success or failure? J Neurochem. 2016;139(Suppl 2):237–252. doi: 10.1111/jnc.13632. [DOI] [PubMed] [Google Scholar]
- López-Arias B, Turiégano E, Monedero I, Canal I, Torroja L. Presynaptic Aβ40 prevents synapse addition in the adult Drosophila neuromuscular junction. PLoS One. 2017;12:e0177541. doi: 10.1371/journal.pone.0177541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller UC, Deller T, Korte M. Not just amyloid: physiological functions of the amyloid precursor protein family. Nat Rev Neurosci. 2017;18:281–298. doi: 10.1038/nrn.2017.29. [DOI] [PubMed] [Google Scholar]
- Puzzo D, Gulisano W, Arancio O, Palmeri A. The keystone of Alzheimer pathogenesis might be sought in Abeta physiology. Neuroscience. 2015;307:26–36. doi: 10.1016/j.neuroscience.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speretta E, Jahn TR, Tartaglia GG, Favrin G, Barros TP, Imarisio S, Lomas DA, Luheshi LM, Crowther DC, Dobson CM. Expression in drosophila of tandem amyloid beta peptides provides insights into links between aggregation and neurotoxicity. J Biol Chem. 2012;287:20748–20754. doi: 10.1074/jbc.M112.350124. [DOI] [PMC free article] [PubMed] [Google Scholar]


