Abstract
Spinal cord injury (SCI) affects thousands of people every year in the USA, and most patients are left with some permanent paralysis. Therapeutic options are limited and only modestly affect outcome. To address this issue, we used magnetic resonance imaging-guided focused ultrasound (MRgFUS) as a non-invasive approach to increase permeability in the blood-spinal cord barrier (BSCB). We hypothesize that localized, controlled sonoporation of the BSCB by MRgFUS will aid delivery of therapeutics to the injury. Here, we report our preliminary findings for the ability of MRgFUS to increase BSCB permeability in the thoracic spinal cord of a normal rat model. First, an excised portion of normal rat spinal column was used to characterize the acoustic field and to estimate the insertion losses that could be expected in an MRgFUS blood spinal cord barrier opening. Then, in normal rats, MRgFUS was applied in combination with intravenously administered microbubbles to the spinal cord region. Permeability of the BSCB was indicated as signal enhancement by contrast administered prior to T1-weighted magnetic resonance imaging and verified by Evans blue dye. Neurological testing using the Basso, Beattie, and Breshnahan scale and the ladder walk was normal in 8 of 10 rats tested. Two rats showed minor impairment indicating need for further refinement of parameters. No gross tissue damage was evident by histology. In this study, we have opened successfully the blood spinal cord barrier in the thoracic region of the normal rat spine using magnetic resonance-guided focused ultrasound combined with microbubbles.
Keywords: focused ultrasound, spinal cord, magnetic resonance imaging, contrast-enhanced, blood-spinal cord barrier
Introduction
Spinal cord injury (SCI) affects thousands of people every year in the USA, and most patients are left with some permanent paralysis. Therapeutic options are limited and only modestly effect outcome (Rolls et al., 2009). An important barrier to the success of therapies to repair or regenerate damaged spinal cord axons is the formation of the glial scar. Acutely, the reactive process that results in the scar helps to stabilize the physical and chemical integrity of the injury by filling in the breach in the blood-spinal cord barrier (BSCB), thereby reducing infiltration of non-central nervous system (CNS) elements and minimizing subsequent tissue damage. Thus, it is becoming increasingly recognized that therapies to reduce or prevent the glial scar formation may actually hamper endogenous healing mechanisms (Silver and Miller, 2004; Rolls et al., 2009; Lukovic et al., 2015). However, in a chronic state, the glial scar forms a physical and chemical inhibitory barrier to spinal cord regeneration (Silver and Miller, 2004).
To address this issue, we propose to use magnetic resonance imaging (MRI)-guided focused ultrasound (MRgFUS) as a non-invasive approach to increase permeability at the site of subacute injury after the scar is formed, with the goal of encouraging axonal sprouting and regeneration. We hypothesize that localized, controlled sonoporation of the BSCB by MRgFUS will aid delivery of therapeutics to the injury. Here, we report our preliminary findings for the ability of MRgFUS to increase BSCB permeability in the thoracic spinal cord of a normal rat model.
Currently MRgFUS is used to treat benign (McDannold et al., 2006b) and malignant tumors (Tempany et al., 2011), and neurological disorders such as essential tremor (Elias et al., 2011). It has also been used to control localized drug delivery (Rapoport et al., 2011), and affect nerve functionality (Foley et al., 2008; Colucci et al., 2009; King et al., 2014). It has been shown through numerous preclinical studies (Hynynen et al., 2005; McDannold et al., 2006a; O’Reilly et al., 2010) that MRgFUS combined with systemically injected ultrasound microbubbles can transiently open the blood-brain barrier with no long-term side effects. When the ultrasound wave interacts with the microbubbles that are contained in the vasculature, the bubbles oscillate, locally opening the blood-brain barrier. Because these microbubbles provide stable cavitation sites, opening the blood- brain barrier can be accomplished at relatively low ultrasound intensities (Hynynen et al., 2001; Kobus et al., 2016) when compared to the intensity required for thermal ablation.
Using the principles established with blood-brain barrier opening, one study demonstrated that MRgFUS can open the BSCB in the cervical region with the potential application of gene delivery (Weber-Adrian et al., 2015). In this study, we have built upon that work, hypothesizing that MRgFUS could be used to target to the thoracic spinal cord in rats and increase BSCB permeability without significant tissue damage. Future studies will apply this method to a model of spinal cord injury.
Materials and Methods
Acoustic field characterization
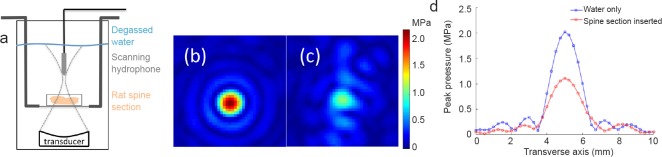
The large acoustic impedance mismatch between bone and soft tissues can result in reflection, refraction and scattering of the ultrasound beam. In transcranial MRgFUS applications, there is known to be significant inter- and intra-patient variability in the pressure field distribution due to the skull (Vyas et al., 2016). To evaluate these effects for the rat spine, we performed a hydrophone experiment with an excised portion of a normal rat (~250 g) spinal column and surrounding skin and musculature to estimate the insertion losses that could be expected in an MRgFUS blood-spinal cord barrier opening procedure. The excised rat spine was surrounded by degassed water and enclosed in a cylindrical tissue holder that had mylar membranes on both sides (Figure 1a). This holder was suspended in a degassed water bath with a hydrophone (HNR-500, Onda Corporation, Sunnyvale, CA, USA) that was rastered with stepper motors (NRT150, Thorlabs Inc., Newton, NJ, USA) in two-dimensional plane through the transducer's geometric focus (256-element phased array, f = 1 MHz, f# = 0.84, 13 cm focal length). Pressure patterns were obtained for conditions with the excised spine section in place and with water only (0.25 mm isotropic spacing). The acoustic power of the transducer was 3 W.
Figure 1.
Acoustic insertion loss through an excised normal rat spinal column.
(a) Experimental setup of the scanning hydrophone showing the rat spinal section in the near field of the focused ultrasound beam. (b, c) Two-dimensional pressure pattern in (b) water only and (c) excised rat spine section conditions. (d) Line plots along the dotted lines for the water only and with the excised spine section inserted.
Animals for in vivo experiments
Sprague-Dawley rats (n = 14, 200–235 g, all female, 12 treated, 2 sham procedures, Charles River Laboratories, Wilmington, MA, USA) were used for the in vivo experiments. All animals were kept on a normal 12-hour light/dark cycle and had free access to food and water. All procedures were approved by the University of Utah's Institutional Animal Care and Use Committee.
Sonications and MRI
Under isoflurane anesthesia combined with medical air, rats were depilated with Nair over the target region and positioned on a preclinical MRgFUS system (Image Guided Therapy, Inc., Pessac, France) in a 3T MR scanner (PrismaFit, Siemens Healthcare, Erlangen, Germany). The MRgFUS system was fitted with a custom holder with an integrated MRI radiofrequency coil that allowed supine positioning of the animal over the transducer. A Solidworks design schematic is shown in Figure 2. For the MR imaging, 3D T1W high-resolution MR images (3D VIBE, FOV = 162 mm × 162 mm × 45 mm, resolution = 0.4 mm × 0.4 mm × 0.8 mm interpolated to 0.2 mm × 0.2 mm × 0.4 mm, TR/TE = 6.21/2.94 ms, FA = 10°) were used to both position the rat and to assess the efficacy of the BSCB opening, which was achieved using a 256-element phased-array transducer (f = 940 kHz, focal depth = 10 cm, intensity full-width-half-maximum = 1.8 mm × 2.5 mm × 10.9 mm). Each rat received 1–3 sonications consisting of four locations spaced 2 mm apart (20 ms bursts applied at a 1 Hz pulse repetition frequency for 3 minutes at 1.0–2.1 MPa peak pressure). Both cervical (n = 2) and thoracic (n = 11, 1 rat was targeted in both regions) spine regions were targeted. Optison microbubbles (GE Healthcare) was injected intravenously immediately prior to each sonication. The initial 4 rats received a 0.06 mL/kg dose. The subsequent 10 rats received 0.02 mL/kg dose. BSCB opening was confirmed by an injection of gadolinium (Prohance [Gadoteridol], 0.25 mL/kg followed by 0.2 mL saline) and several contrast-enhanced T1W MR images were acquired (same parameters as listed above). Six rats received a 1% Evans blue dye injection (10 μL/g) immediately after the injection of contrast to confirm BSCB opening. Sham animals received equivalent procedures with no ultrasound power applied.
Figure 2.
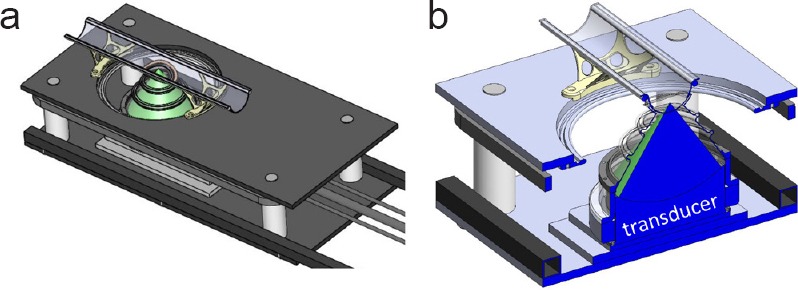
Solid model schematic of the MRgFUS device with custom rat holder.
(a) Modified pre-clinical large animal MRgFUS device with rat holder was installed. A semi-circular positioning trough was secured over the focused ultrasound transducer with a custom MRI radiofrequency coil (9 cm × 5 cm ellipse) permanently fixed to the trough under the animal. (b) Cross section through the center of the focused ultrasound transducer. The geometric focus of the ultrasound beam was approximately 1 cm above the bottom of the positioning trough.
MRI data analysis
All MRI data analysis was performed in Osirix and Matlab. Signal changes in the spinal cord were measured across the 8 mm insonified region, centered on the enhancing spot. The signal change was normalized using a non-sonicated area in the spinal cord. For all measurements, the mean normalized enhancement and the standard deviation is reported.
Neurological function
Rats (n = 10) were observed for gross neurological function. To assess motor function, each animal was allowed to move around freely in a plastic wading pool (height: 19 cm, diameter: 88 cm) for 3 minutes. A blinded observer scored each animal on a scale from 0–21 based on the Basso, Beattie, and Breshnahan (BBB) scale (Basso et al., 1995).
Histology
Evans blue dye
Rats (n = 6) that received Evans blue dye were euthanized 4 days post-MRgFUS by transcardial perfusion with phosphate buffered saline (PBS) only. Three of six samples were damaged in the processing. The remaining 3 spinal cords were dissected from the base of the cerebellum to the cauda equina. The tissue was frozen in OCT (Scigen Scientific, Gardena, CA, USA) 7 days after dissection and stored at –80°C.
Hematoxylin and eosin staining
All rats that did not receive Evans blue dye (n = 8) were euthanized 4 days post-MRgFUS by transcardial perfusion with PBS and 4% paraformaldehyde (Acros Organics). Spinal cords were dissected from the base of the brainstem to the cauda equina. The region of interest on the spinal cords was assessed using the MRI and was between 4–5 cm from the base of the brainstem. The tissue was cut at either 2.5 cm or 3 cm from the base of the brainstem and another cut was made at 6 cm from the base of the brainstem to encompass the entire region of interest. Sections were sliced at 20 μm and stained with hematoxylin and eosin (Poly Scientific, Bay Shore, NY, USA).
Statistical analysis
Data are expressed as the mean ± SD. Animals were assessed 2–4 days post-MRgFUS and scores were compared statistically using a paired t-test with a P-value ≤ 0.05 to determine significance.
Results
The measured pressure patterns of water only and excised spinal column insertion cases are shown (Figure 1). The spatial patterns at the transverse plane are indicated in Figure 1b and c while line traces directly comparing the two measurements through the focus are indicated in Figure 1d. A peak pressure of 2.2 MPa was seen in the water only case at an acoustic power of 3.1 W. When the excised insertion column was placed in the near field of the ultrasound beam, a peak pressure of 1.1 MPa was measured at the focal point, representing an acoustic insertion loss of approximately 50% at 1 MHz.
An increase in normalized signal intensity on the MRI CE-T1w image was seen in 10/12 rats at 4–5 minutes post-MRgFUS sonication (31.5 ± 23.1%, range: 7.5–83.5%). The animals that received a sham procedure had no increase in normalized signal intensity in the MRI CE-T1W image. The contrast enhanced T1W MR images in a sagittal and axial view and the excised spinal cord for animal 14 are shown (Figure 3). The signal-enhancing region in the sonicated area is indicated by the yellow arrow in the sagittal image. Axial slices at both an enhancing and non-enhancing region are also shown. Evans blue dye penetration was seen in up to a 4 cm length of spinal cord.
Figure 3.
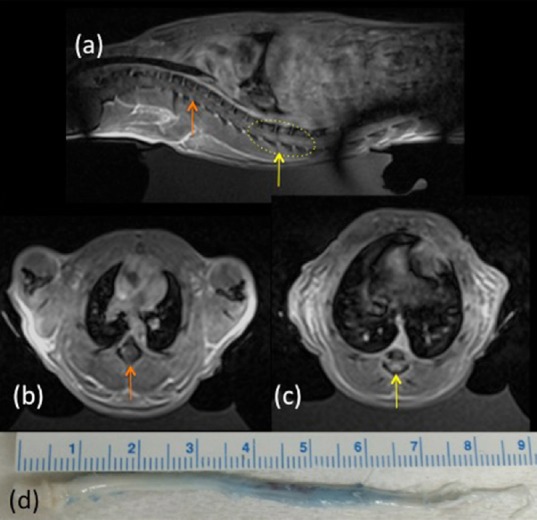
Example of a thoracic blood-spinal cord barrier opening.
(a) CE-T1W MR images showing spinal cord enhancement in sagittal (left side rostral and right side caudal to sonication region). MRgFUS sonications were applied in the yellow dashed region. (b, c) Axial views of (b) non-enhancement (orange arrow) and (c) enhancement (yellow arrow) regions of the spinal cord. (d) Excised spinal cord showing extensive Evans blue dye penetration through 3 cm of the cord. Cord orientation is the same as seen in (a). Note the banding artifact in (a) was due to a B1 transmit shim decoupling failure.
Evans blue dye was given to rats immediately after the MRgFUS sonication and dye accumulation was seen in 3 of the excised spinal cords, corresponding to in the areas sonicated (the spinal cords of the remaining 3 rats were damaged in the processing) (Figure 4).
Figure 4.
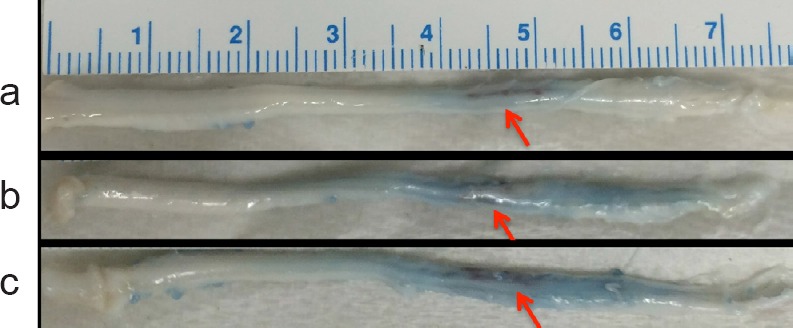
Evans blue dye infiltration in excised spinal cords.
(a–c) Rats underwent identical MRgFUS blood spinal cord barrier opening procedures resulting in the length of the blue region in the cord ranging from approximately 15 to 40 mm. In all rats a 1 cm length of the thoracic spinal cord was targeted. The ruler indicates scale. Arrows indicate dye accumulation at the region of sonication.
Ten rats were tested for neurological function using the BBB and ladder walk assessment pre and post-MRgFUS. Eight rats scored a normal BBB (21), while 2 showed impairment (scores of 14 and 15 out of 21) at 3–4 days post. These 2 rats also had impairment on the ladder walk. Both of these impaired subjects had received Evans blue dye, so we were unable to perform a histological examination on the excised cords. The 8 rats with normal BBB scores did not have significant differences in the ladder walk pre- and post-MRgFUS (1.90 ± 0.05 vs. 1.82 ± 0.17 and 1.87 ± 0.09 vs. 1.81 ± 0.22 score/step, for left and right respectively).
Hematoxylin and eosin staining in the region of MRgFUS did not reveal any gross morphological tissue injury in the spinal cords that were sampled (from subjects not receiving Evan's blue dye). Representative images from a sham and MRgFUS-treated rat are shown (Figure 5).
Figure 5.
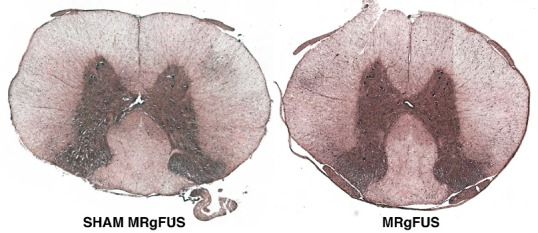
Hematoxylin and eosin staining indicates no gross neuronal injury from MRgFUS.
Representative samples from 2 rats (SHAM and MRgFUS) taken from the region of the MRgFUS application (or SHAM MRgFUS).
Discussion
Extensive research is present in the literature demonstrating that MRgFUS combined with ultrasound microbubbles can transiently open the blood-brain barrier with no long-term side effects (Hynynen et al., 2005). While Weber-Adrian et al. (2015) extended this work to open the BSCB in the cervical region demonstrating the potential application of gene delivery, our study demonstrates the potential to increase the permeability of the BSCB in the thoracic region of the rat spinal cord. Ultimately, the goal of this research is to use MRgFUS to treat spinal cord injuries by controllably disrupting the cellular structure of the glial scar as well as open the BSCB to facilitate delivery of therapeutics. This study represents a first step towards that objective. Our results show the application of MRgFUS to the normal rat spinal cord resulted in significantly increased contrast enhancement on T1w MRI of the targeted region of the BSCB when compared to a non-sonicated region.
Because it has been shown that potential permanent damage can occur with the application of MRgFUS (Oakden et al., 2014), this study assessed neurological function following the MRgFUS procedure to evaluate potential injury to the normal rat spinal cord. Two rats out of 10 had minor alterations in neurological function after undergoing MRgFUS. These subjects did not have histological examinations because they were in the group receiving Evans blue dye. Therefore, it is not known if the neurological impairment was due to tissue damage or transient swelling around the spinal cord. Of the samples that were processed with Hematoxylin and eosin staining, no tissue damage was observed. In addition, the survival post-MRgFUS was only 4 days and the impairment in these 2 rats may have resolved if given a longer recovery time. These issues will be addressed in future studies. We will further optimize the MRgFUS parameters to prevent any post-procedure impairment and we will survive subjects longer to evaluate longer-term effects on outcome. The parameters used in this present study will be considered an “upper limit” and we will seek to decrease the energy deposition near the cord while still increasing permeability of the BSCB.
In this initial study, we used a single sample of excised rat spinal column to characterize the acoustic pressure patterns as well as the insertion losses to estimate the MRgFUS parameters needed to accomplish BSCB opening through extrapolating other BSCB and BBB opening works in the literature. While the rats in the study were roughly equivalent in size (216 ± 12 g), the variability in spinal column anatomy as a function of size is unknown. In addition, the phased array transducer was electronically steered to achieve the sonication pattern across a 1-cm section of spinal column, resulting in some variability in the pressure distribution both between and within animals. Therefore, while equivalent sonication parameters were applied between animals, the actual pressure distribution achieved in situ will have varied between animals. A limitation of this study was the lack of real-time procedure monitoring. While confirmation of blood brain barrier opening has been observed using both contrast-enhanced MRI and dual-focal microscopy (Hynynen et al., 2005, 2006; Cho et al., 2011), these measurements were obtained after the application of sonications, and therefore cannot be considered “real time”. Indeed, some irreversible tissue damage has been observed and correlated with increased pressure (Hynynen et al., 2006) or high microbubble dosage (Kovacs et al., 2017). Monitoring of focused ultrasound blood brain barrier opening through real time analysis of the acoustic emissions during sonications has been investigated. Acoustic emissions can emanate from the interaction of the ultrasound and the microbubbles as well as the surrounding tissues or the acoustic coupling media (McDannold et al., 2006a). One study demonstrated that monitoring acoustic emissions using a hydrophone embedded in the therapeutic transducer and adjusting the ultrasound sonication parameters based on the subharmonic frequency response resulted in repeatable blood brain barrier disruption (O’Reilly and Hynynen, 2012). Histologically there were no apparent tissue changes with only mild extravasation of red blood cells exhibited in a few cases. Similarly, another investigator monitored the harmonic frequencies and wideband emissions using a passive cavitation detector mounted in close proximity to the target site (McDannold et al., 2017). In general, monitoring the stable cavitation activity with passive cavitation detectors has demonstrated varying degrees of correlation between the recorded emissions and the resulting blood brain barrier permeability (Arvanitis et al., 2012; Sun et al., 2015) indicating the potential of this method as a monitoring technique. We will incorporate real-time monitoring in future studies.
This study has several limitations. First, the sonication parameters were constant for all rats and were not tailored for rat weight or variations in anatomy observed on MRI. Second, as indicated above, no real time feedback was used during the MRgFUS sonication besides the CE-T1w images that were acquired after the sonications were performed. Third, at this time, we do not know why 2 subjects suffered slight neurological impairment. This may have been a result of the application of Evans Blue at the time of MRgFUS. In future studies, some animals will receive MRg-FUS only, without Evans Blue to determine the root cause of such impairments. These subjects were only survived for 4 days and, because they had Evans blue dye, we were unable to perform a histological examination of the spinal cord. Last, we only performed H&E stain in this preliminary study, not luxol fast blue. Future studies will further investigate and refine MRgFUS parameters to avoid all neurological impairments as well as monitor rats longer to evaluate the long-term sequelae of sonication and correlate with histology.
In this initial study, we have opened successfully the blood spinal cord barrier in the thoracic region of the normal rat spine using MR-guided focused ultrasound combined with microbubbles. This procedure was accomplished with no or very minimal effect on neurological function and no overt evidence of tissue damage. Future studies will further refine the MRgFUS parameters as well as apply the technique to treat spinal cord injuries.
Acknowledgments
The authors would like to acknowledge Greg Garwin for his assistance with this research.
Footnotes
Conflicts of interest: None declared.
Research ethics: The study protocol was approved by the University of Utah's Institutional Animal Care and Use Committee.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review report:
Reviewer: Lukas Grassner, Trauma Center Murnau, Germany.
Comments to authors: The authors present a very interesting manuscript investigating manipulation of the BSCB. In my opinion, the role of the BSCB is one major oversight in the SCI field. This is a first step towards a better understanding and most importantly with this method the BSCB integrity might be influenced.
Funding: This work was supported by the University of Utah Radiology and Neuroscience Initiative Pilot grant and the Department of Neurosurgery pilot fund.
References
- Arvanitis CD, Livingstone MS, Vykhodtseva N, McDannold N. Controlled ultrasound-induced blood-brain barrier disruption using passive acoustic emissions monitoring. PLoS One. 2012;7:e45783. doi: 10.1371/journal.pone.0045783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Cho EE, Drazic J, Ganguly M, Stefanovic B, Hynynen K. Two-photon fluorescence microscopy study of cerebrovascular dynamics in ultrasound-induced blood-brain barrier opening. J Cereb Blood Flow Metab. 2011;31:1852–1862. doi: 10.1038/jcbfm.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci V, Strichartz G, Jolesz F, Vykhodtseva N, Hynynen K. Focused ultrasound effects on nerve action potential in vitro. Ultrasound Med Biol. 2009;35:1737–1747. doi: 10.1016/j.ultrasmedbio.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias J, Huss D, Khaled M, Monteith S, Frysinger R, Loomba J. Washington, DC, USA: Congress of Neurological Surgeons; 2011. MR-guided focused ultrasound lesioning for the treatment of essential tremor -- a new paradigm for noninvasive lesioning and neuromodulation. [Google Scholar]
- Foley JL, Little JW, Vaezy S. Effects of high-intensity focused ultrasound on nerve conduction. Muscle Nerve. 2008;37:241–250. doi: 10.1002/mus.20932. [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24:12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA, Sheikov N. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg. 2006;105:445–454. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- King RL, Brown JR, Pauly KB. Localization of ultrasound-induced in vivo neurostimulation in the mouse model. Ultrasound Med Biol. 2014;40:1512–1522. doi: 10.1016/j.ultrasmedbio.2014.01.020. [DOI] [PubMed] [Google Scholar]
- Kobus T, Vykhodtseva N, Pilatou M, Zhang Y, McDannold N. Safety validation of repeated blood-brain barrier disruption using focused ultrasound. Ultrasound Med Biol. 2016;42:481–492. doi: 10.1016/j.ultrasmedbio.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs ZI, Kim S, Jikaria N, Qureshi F, Milo B, Lewis BK, Bresler M, Burks SR, Frank JA. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci U S A. 2017;114:E75–E84. doi: 10.1073/pnas.1614777114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukovic D, Stojkovic M, Moreno-Manzano V, Jendelova P, Sykova E, Bhattacharya SS, Erceg S. Concise review: reactive astrocytes and stem cells in spinal cord injury: good guys or bad guys. Stem Cells. 2015;33:1036–1041. doi: 10.1002/stem.1959. [DOI] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood-brain barrier with focused ultrasound: association with cavitation activity. Phys Med Biol. 2006a;51:793–807. doi: 10.1088/0031-9155/51/4/003. [DOI] [PubMed] [Google Scholar]
- McDannold N, Zhang Y, Vykhodtseva N. The effects of oxygen on ultrasound-induced blood-brain barrier disruption in mice. Ultrasound Med Biol. 2017;43:469–475. doi: 10.1016/j.ultrasmedbio.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Tempany CM, Fennessy FM, So MJ, Rybicki FJ, Stewart EA, Jolesz FA, Hynynen K. Uterine leiomyomas: MR imaging-based thermometry and thermal dosimetry during focused ultrasound thermal ablation. Radiology. 2006b;240:263–272. doi: 10.1148/radiol.2401050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MA, Hynynen K. Blood-brain barrier: real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions-based controller. Radiology. 2012;263:96–106. doi: 10.1148/radiol.11111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MA, Huang Y, Hynynen K. The impact of standing wave effects on transcranial focused ultrasound disruption of the blood-brain barrier in a rat model. Phys Med Biol. 2010;55:5251–5267. doi: 10.1088/0031-9155/55/18/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakden W, Kwiecien JM, O’Reilly MA, Lake EM, Akens MK, Aubert I, Whyne C, Finkelstein J, Hynynen K, Stanisz GJ. A non-surgical model of cervical spinal cord injury induced with focused ultrasound and microbubbles. J Neurosci Methods. 2014;235:92–100. doi: 10.1016/j.jneumeth.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Rapoport N, Nam KH, Gupta R, Gao Z, Mohan P, Payne A, Todd N, Liu X, Kim T, Shea J, Scaife C, Parker DL, Jeong EK, Kennedy AM. Ultrasound-mediated tumor imaging and nanotherapy using drug loaded, block copolymer stabilized perfluorocarbon nanoemulsions. J Control Release. 2011;153:4–15. doi: 10.1016/j.jconrel.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009;10:235–241. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sun T, Samiotaki G, Wang S, Acosta C, Chen CC, Konofagou EE. Acoustic cavitation-based monitoring of the reversibility and permeability of ultrasound-induced blood-brain barrier opening. Phys Med Biol. 2015;60:9079–9094. doi: 10.1088/0031-9155/60/23/9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempany CM, McDannold NJ, Hynynen K, Jolesz FA. Focused ultrasound surgery in oncology: overview and principles. Radiology. 2011;259:39–56. doi: 10.1148/radiol.11100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas U, Ghanouni P, Halpern CH, Elias J, Pauly KB. Predicting variation in subject thermal response during transcranial magnetic resonance guided focused ultrasound surgery: Comparison in seventeen subject datasets. Med Phys. 2016;43:5170. doi: 10.1118/1.4955436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Adrian D, Thévenot E, O’Reilly MA, Oakden W, Akens MK, Ellens N, Markham-Coultes K, Burgess A, Finkelstein J, Yee AJ, Whyne CM, Foust KD, Kaspar BK, Stanisz GJ, Chopra R, Hynynen K, Aubert I. Gene delivery to the spinal cord using MRI-guided focused ultrasound. Gene Ther. 2015;22:568–577. doi: 10.1038/gt.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]