Keywords: nerve regeneration, incomplete spinal cord injury, gray matter volume, functional connectivity, sensorimotor areas, functional magnetic resonance imaging, brain plasticity, non-concomitant, anatomical structure, network, imaging biomarker, neural regeneration
Abstract
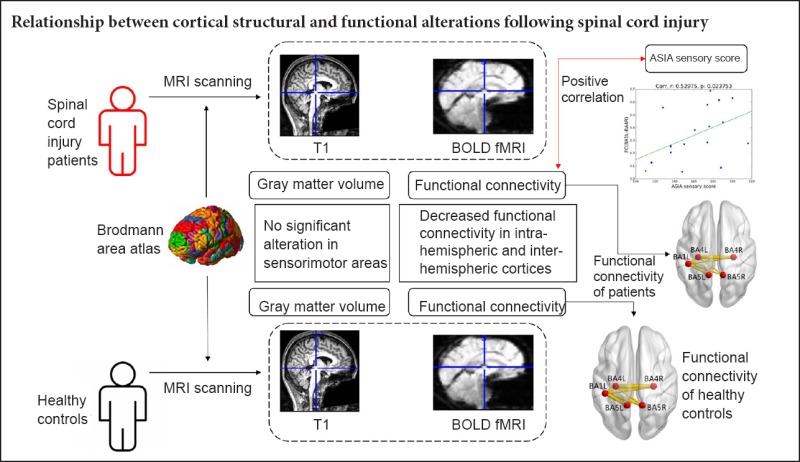
Brain plasticity, including anatomical changes and functional reorganization, is the physiological basis of functional recovery after spinal cord injury (SCI). The correlation between brain anatomical changes and functional reorganization after SCI is unclear. This study aimed to explore whether alterations of cortical structure and network function are concomitant in sensorimotor areas after incomplete SCI. Eighteen patients with incomplete SCI (mean age 40.94 ± 14.10 years old; male:female, 7:11) and 18 healthy subjects (37.33 ± 11.79 years old; male:female, 7:11) were studied by resting state functional magnetic resonance imaging. Gray matter volume (GMV) and functional connectivity were used to evaluate cortical structure and network function, respectively. There was no significant alteration of GMV in sensorimotor areas in patients with incomplete SCI compared with healthy subjects. Intra-hemispheric functional connectivity between left primary somatosensory cortex (BA1) and left primary motor cortex (BA4), and left BA1 and left somatosensory association cortex (BA5) was decreased, as well as inter-hemispheric functional connectivity between left BA1 and right BA4, left BA1 and right BA5, and left BA4 and right BA5 in patients with SCI. Functional connectivity between both BA4 areas was also decreased. The decreased functional connectivity between the left BA1 and the right BA4 positively correlated with American Spinal Injury Association sensory score in SCI patients. The results indicate that alterations of cortical anatomical structure and network functional connectivity in sensorimotor areas were non-concomitant in patients with incomplete SCI, indicating the network functional changes in sensorimotor areas may not be dependent on anatomic structure. The strength of functional connectivity within sensorimotor areas could serve as a potential imaging biomarker for assessment and prediction of sensory function in patients with incomplete SCI. This trial was registered with the Chinese Clinical Trial Registry (registration number: ChiCTR-ROC-17013566).
Introduction
Spinal cord injury (SCI) is a life-changing event that causes characteristic neural reorganization (Fouad et al., 2008; García-Alías et al., 2015; Fink et al., 2016), which brings the opportunity for spontaneous functional recovery or intervention rehabilitation, especially in patients with incomplete SCI (Curt et al., 2008; Kuppuswamy et al., 2011; Silva et al., 2015). Brain reorganization includes changes of anatomical structure and function following SCI (Freund et al., 2013; Hou et al., 2014; Sabre et al., 2016). Resting state functional magnetic resonance imaging (fMRI) is a potent tool for quantitatively evaluating changes of brain structure and functional reorganization and for developing biomarkers for prognosis (Fuerra-Carrillo et al., 2014; Jutzeler et al., 2015; Oni-Orisan et al., 2016; Kaushal et al., 2017). Previous studies have investigated anatomical or functional characteristics and analyzed these data separately to explore cerebral reorganization after SCI (Freund et al., 2011; Min et al., 2015; Zhu et al., 2015). Each imaging technique provides a different view of brain function or structure (Choe et al., 2015; Eippert et al., 2017; Sharp et al., 2017). Voxel-based morphometry is used to detect gray or white matter volume changes (Hou et al., 2014a, b; Ganzola et al., 2017; Lemola et al., 2017). Resting state functional connectivity is used to investigate functional alterations at the brain network level (Ugurbil, 2015; Lefebvre et al., 2017; Palacios et al., 2017). However, separate analysis does not enable examination of joint information between modalities (Sui et al., 2012; Wang et al., 2015; Calhoun et al., 2016). To our knowledge, few studies have combined information of brain anatomical and functional reorganization and explored the internal relationship between them following SCI.
Previous studies have shown reduced gray matter volume (GMV) in patients with SCI in multiple brain regions, such as the bilateral primary motor cortex, primary somatosensory cortex, and supplementary motor areas (Freund et al., 2011, 2013a, b; Hou et al., 2014a, b). However, others have reported no cortical atrophy after SCI (Lundell et al., 2011; Villiger et al., 2015). Cortical reorganization following SCI depends on the extent of the lesion, disease duration, and exposure to rehabilitation (Chen et al., 2012; Isa et al., 2014; Jutzeler et al., 2015). These factors may lead to inconsistent morphometric results. Therefore, single cortical morphometric analysis may only partially reveal the structural reorganization mechanism and overlook other information (i.e., network functional alterations). Sensorimotor network alterations have also been reported at acute and chronic stages in SCI patients (Min et al., 2015; Zhu et al., 2015). Whether the network functional changes following SCI accompany cortical atrophy remains unclear. In addition, fMRI could provide an objective and quantitative method for predicting neurological recovery (Freund et al., 2013a, b; Lee et al., 2017; Morgan et al., 2017). The International Standards for the Neurological Classification of Spinal Cord Injury is routinely used to determine levels of injury and to classify the severity of the injury (Marino et al., 2003; Steeves et al., 2012). However, it is insensitive for evaluating rehabilitation intervention or prognosis (Kirshblum et al., 2014; Kumru et al., 2016).
We aimed to explore whether the alteration of anatomical structure and network function were concomitant in sensorimotor areas after incomplete SCI, and determine the association between altered characteristics of cerebral reorganization and clinical scores. Combined anatomic and network functional information from fMRI may provide more comprehensive descriptions of brain reorganization following incomplete SCI, and may be helpful for identification of accurate and sensitive imaging biomarkers for rehabilitation intervention or prognosis.
Participants and Methods
Participants
Eighteen inpatients with incomplete SCI (7 males, 11 females; mean age of 40.9 ± 14.1 years old) were from the Department of Rehabilitation at the Beijing Tsinghua Changgung Hospital in China. At the time of study enrollment, motor function, sensory function, neurologic level, and injury degree were assessed by American Spinal Injury Association (ASIA) criteria (Marino et al., 2003), the walking index for spinal cord injury II (WISCI II; Dittuno et al., 2001), and the Spinal Cord Independence Measure (SCIM; Catz et al., 2001). Eighteen healthy subjects (seven males, 11 females; mean age of 37.3 ± 11.8 years old) were enrolled from the Department of Rehabilitation at the Beijing Tsinghua Changgung Hospital as controls and had no history of neurologic disorder. The study protocol was approved by the Ethics Committee of Beijing Tsinghua Changgung Hospital of China (IRB No. 2015-002). All participants provided their written informed consent to participate according to the Declaration of Helsinki. This trial was registered with the Chinese Clinical Trial Registry (registration number: ChiCTR-ROC-17013566).
Inclusion criteria of eligible patients
Patients presenting with all of the following criteria were considered for study inclusion: incomplete injury (ASIA C or D; Marino et al., 2003), subacute SCI and chronic SCI (time since injury greater than 1 month and less than 12 months), neurologic level above T12.
Exclusion criteria of eligible patients
Patients with one or more of the following conditions were excluded from this study: brain lesions, mental illness, seizures.
Data acquisition
All MRI data were acquired with a GE 3.0T MR scanner (DISCOVERY MR750 model; General Electric American, Waukesha, WI, USA). Participants were positioned supine and scanned using a standard 32-channel head-coil. Functional MRI parameters for resting state Blood Oxygen Level Dependent (BOLD) images were an “Ax-BOLD-rest” series using a gradient echo planar-imaging sequence with repetition time = 2,000 ms, echo time = 30 ms, flip angle = 90°, pixel space = 3.5 mm2, slice thickness = 3.5 mm, spacing between slices = 4 mm, acquisition matrix = (64, 0, 0, 64) equivalent to in-plane resolution = 64 × 64, reconstruction diameter = 224 mm, 34 axial slices, and 240 temporal positions. T1-weighted images (T1) were a “Sag 3D T1BRAVO” series, with repetition time = 8.21 ms, echo time = 3.18 ms, flip angle = 8°, voxel space = 1 mm3, spacing between slices = 1 mm, acquisition matrix = [0, 256, 256, 0], equivalent to 256 axial slices and 256 coronal slices. The sagittal slice number depended on the head size of each subject, ranging from 156 to 174 mm, corresponding to the 36 subjects in this study. The reconstruction diameter was 256 mm.
Data processing
GMV based on Brodmann area
There were four steps to obtain the GMV. Step 1 was to obtain skull stripped T1 images. First, T1-weighted images were segmented into gray matter, white matter, cerebrospinal fluid, and others in native space, using the “Segment” function of SPM12. To extract brain tissue, only the segmented gray matter, white matter, and cerebrospinal fluid were regrouped in the native space to generate the extraction mask. The skull stripped images were extracted by masking original T1 data. Step 2 was Montreal Neurological Institute (MNI) space normalization. The skull-stripped T1 images were normalized to the MNI space (space size = 181, 217, 181) by using the “normalize” function of SPM12. Step 3 was gray matter segmentation. The normalized T1 in the MNI space was segmented into three types of tissue: gray matter, white matter, and cerebrospinal fluid, using the approach of brain tissue segmentation based on Markov Random Fields (Ruan et al, 2002), to obtain the gray matter in the MNI space. Step 4 was the GMV calculation. GMV was defined as the total voxel number inside a specific area (or volume in 3D). The MNI-template with Brodmann area labels (including left and right hemispheres, total 82 areas) was used to accumulate all gray matter-MNI voxels inside the labeled area to obtain the GMV corresponding to each Brodmann area.
Functional connectivity analysis
There were three steps to obtain functional connectivity: preprocessing, brain network node construction, and network features analysis.
Step 1 was resting state BOLD signal preprocessing, which was performed using DPARSFA version 3.2 (http://rfmri.org/DPARSF). After the first 10 temporal positions of data were discarded, for each remaining piece of temporal position data, slicing timing correction and head motion correction were performed and then normalized to the MNI space with 3 mm isotropic voxel resampling. Preprocessing in the MNI space included smoothing the data with 4 mm FWHM (Full-Width-Half-Maximum), removing the linear trend of time courses and nuisance covariates with global signal regression, and temporally filtering with 0.01–0.08 Hz.
Step 2 was the node value time series calculation. Each Brodmann area was considered a node in the brain network. At each temporal position, within each Brodmann area, the average BOLD signal was assigned to the node as the signal intensity. This was a time series, with each temporal position consisting of values corresponding to each Brodmann area.
Step 3 was the connection matrix calculation. Pearson's correlation coefficient was the covariance of two variables divided by the product of their standard deviations. Pearson's correlation coefficients between the time series were used to measure functional connectivity between two nodes in the brain network. That is, correlation coefficients were used as weights of edges in the network.
Regions of interest
Regions of interest were positioned at the bilateral primary somatosensory cortex (BA1, BA2, BA3), primary motor cortex (BA4), somatosensory association cortex (BA5), and premotor cortex (BA6), which are areas that have connections to the spinal cord via corticospinal and spinothalamic tracts and have been reported to have anatomical structural and functional abnormalities in SCI (Hou et al., 2014a, b; Min et al., 2015). We used bilateral BA1, BA2, BA3, BA4, BA5, and BA6 as seeds to explore internal relationships between anatomical and functional reorganization in patients with SCI.
Outcome measures
Primary outcome measure
Functional connectivity in sensorimotor areas was the major data used to detect functional reorganization.
Secondary outcome measures
GMV was minor data used to assess cerebral anatomical structure. Demographic data including sex, age, time since injury, injury level, and ASIA scale were also minor data. Clinical scores including ASIA motor scores, ASIA sensory scores, WISCI II and SCIM were minor data used to assess motor function, sensory function, ambulation and activity of daily life of SCI patients
Statistical analysis
All data were analyzed using NumPy 1.12.1 software (http://www.numpy.org) and Scipy 0.19.0 software (http://www.scipy.org). Since the GMV, functional connectivity and age were of near normal distribution, their quantitative data were expressed as the mean ± SD. Sex was numerical data expressed as a percentage. Two-tailed independent sample t-tests were performed to evaluate the differences in GMV, functional connectivity and age between SCI patients and healthy subjects. Pearson chi-squared analysis was performed to evaluate sex differences between SCI patients and healthy subjects. Spearman's rank correlation was calculated for analyzing the relationship between clinical score rankings and corresponding functional connectivity. A P-value of less than 0.05 was considered statistically significant for all tests.
Results
Demographics of SCI patients and healthy subjects
No differences were observed between SCI patients and healthy subjects in age (40.94 ± 14.10 vs. 37.33 ± 11.79 years; t = 0.833, P = 0.411, independent sample t-test) or gender (male:female, 7 (38.89%):11 (61.11%) vs. 7 (38.89%):11 (61.11%), P = 1.000, Pearson chi-squared analysis). Time since SCI was 4.28 ± 3.30 months. Demographics and clinical characteristics of 18 SCI patients are listed in Table 1. Severity of SCI was defined using ASIA criteria.
Table 1.
Demographic data and clinical values of patients with spinal cord injury
GMV changes based on Brodmann area in sensorimotor brain areas
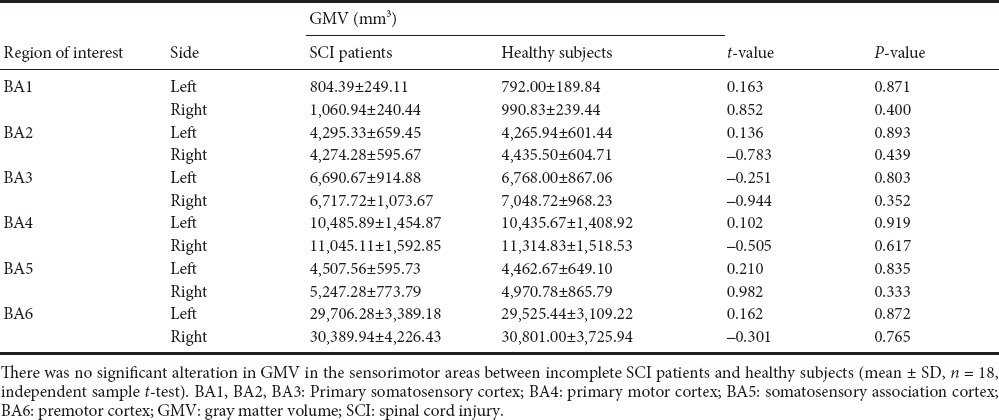
GMVs in the bilateral BA1, BA2, BA3, BA4, BA5, and BA6 were calculated. There was no significant alteration in GMV in the sensorimotor areas in incomplete SCI patients compared with healthy subjects (Table 2).
Table 2.
GMV of sensorimotor areas in SCI patients and healthy subjects
Functional connectivity in sensorimotor brain areas
We selected the bilateral BA1, BA2, BA3, BA4, BA5, and BA6 as seeds to analyze functional connectivity. Intra-hemispheric functional connectivity between left BA1 and left BA4, and left BA1 and left BA5 was decreased, as well as inter-hemispheric functional connectivity between left BA1 and right BA4, left BA1 and right BA5, left BA4 and right BA5. Functional connectivity between both BA4 areas was also decreased (Table 3 and Figure 1).
Table 3.
Functional connectivity of sensorimotor brain areas
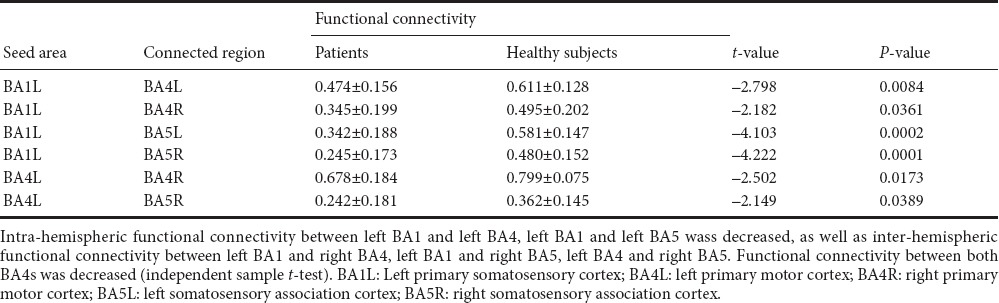
Figure 1.
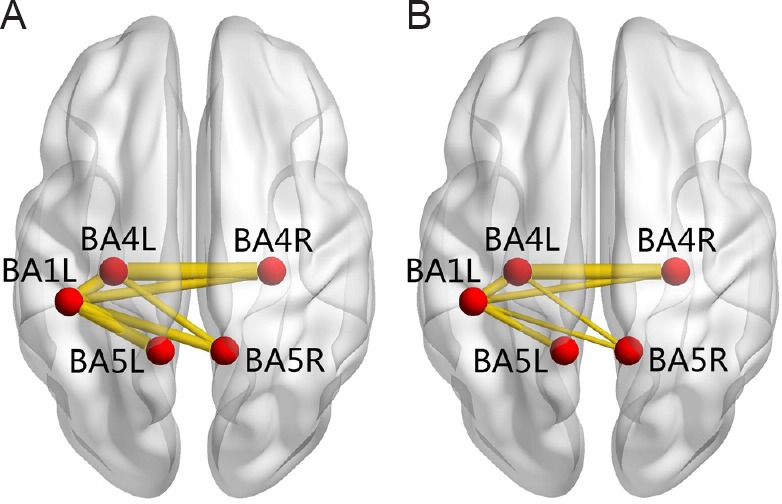
Anatomic replicas show the decreased functional connectivity of sensorimotor brain areas in patients with SCI compared with healthy subjects.
(A) Normal functional connectivity in healthy subjects; (B) decreased functional connectivity in patients with spinal cord injury. The red nodes represent the seed areas and significantly changed areas of functional connectivity. The yellow lines represent decreased functional connectivity in spinal cord injury patients relative to healthy subjects. BA1L: Left primary somatosensory cortex; BA4L: left primary motor cortex; BA4R: right primary motor cortex; BA5L: left somatosensory association cortex; BA5R: right somatosensory association cortex.
Associations between functional connectivity and clinical scores in changed sensorimotor areas
A correlation analysis was performed on the strength of the functional connectivity in abnormal areas and ASIA motor score, ASIA sensory score, WISCI II, and SCIM in SCI patients. The decreased functional connectivity between the left BA1 and the right BA4 positively correlated with ASIA sensory score in SCI patients (r = 0.529, P = 0.023; Figure 2). Correlation analysis with GMV and clinical scores was not performed because of insignificant changes in GMV in sensorimotor areas of SCI patients.
Figure 2.
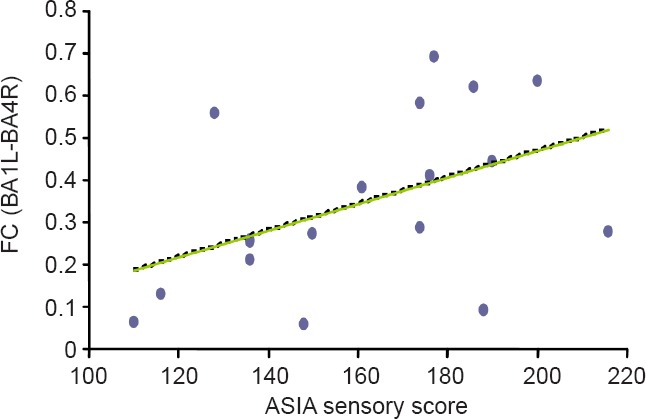
Correlation between functional connectivity (FC) and ASIA sensory score.
The decreased FC between the left BA1 and the right BA4 positively correlated with ASIA sensory score in spinal cord injury patients (r = 0.529, P = 0.023; Spearman's rank correlation). FC (BA1L-BA4R): Functional connectivity between left primary somatosensory cortex and right primary motor cortex; ASIA: American Spinal Injury Association.
Discussion
In this study, we did not find significant alterations in GMV in sensorimotor areas following incomplete SCI. Intra-hemispheric functional connectivity and inter-hemispheric functional connectivity in sensorimotor brain areas were decreased in SCI patients compared with healthy subjects. We also observed the decreased functional connectivity between the left BA1 and the right BA4 positively correlated with ASIA sensory score in SCI patients. Our findings provide evidence that alterations of cortical anatomic structure and network functional integration in sensorimotor areas were non-concomitant in patients with incomplete SCI. The strength of functional connectivity within sensorimotor areas could serve as a potential imaging biomarker for assessment and prediction of sensory function in incomplete SCI patients.
After SCI, cerebral reorganization including structural and functional changes has been shown (Freund et al., 2013a, b; Hou et al., 2014a, b; Moxon, et al., 2014; Sabre et al., 2016). However, the correlation between anatomical structure and network function after SCI is unclear. Henderson et al. (2011) found that functional reorganization of the primary somatosensory area was associated with anatomic changes following SCI. However, alterations of anatomic structure in sensorimotor brain areas were inconsistent in previous studies of SCI (Freund et al., 2011, 2013a, b; Lundell et al., 2011; Villiger et al., 2015), which do not seem to support the mechanism that functional changes are associated with structural changes. Gray matter atrophy in sensorimotor brain areas was observed in complete SCI patients or complete mixed incomplete SCI patients (Freund et al., 2011, 2013a, b; Hou et al., 2014a, b). A few studies reported no structural changes in sensorimotor brain areas following incomplete SCI (Lundell et al., 2011; Villiger et al., 2015), which are consistent with our results. In our study, sixteen patients had incomplete SCI with ASIA D, while the other two patients had ASIA C. SCI duration was 4.28 ± 3.30 months. We speculate that the duration and severity of SCI may affect cortical structure. The time since SCI was relatively short in our study. Patients received rehabilitation therapy for motor and sensory functional recovery. These reasons may contribute to sustaining the normal structure of sensorimotor areas. In our study, alterations of cortical anatomical structure and network function in sensorimotor areas were non-concomitant. Therefore, we speculate that network functional changes in sensorimotor areas may not be dependent on anatomic structure following incomplete SCI.
To our knowledge, few studies have combined cerebral structural and functional MRI data when exploring the brain reorganization mechanism after SCI. The combined analysis of MRI data, called multimodality fusion, has been proven to be more informative in understanding of brain activity and disorders (Sui et al., 2012). Some studies based on combined structural and functional connectivity data demonstrated potentially important variations that were only partially detected by each modality alone. The combination uncovered previously hidden information (Doucet et al., 2016; Hao et al., 2016). In our study, combined structural and network functional MRI data contributed to exploration of the internal relationship between anatomical and functional reorganization. Multimodality fusion could not only be combined with brain structural and functional MRI data, but also with spinal cord and brain MRI information. Rao et al. (2013) reported that there were significant correlations between spinal cord atrophy, the degree of contralateral primary somatosensory cortex reorganization, and the time after SCI. The combined assessment of spinal cord and cerebral structure along with functional reorganization and neurological deficits could be a potential tool to further understand neural plasticity in SCI.
In our study, we observed decreased intra-hemispheric and inter-hemispheric functional connectivity in sensorimotor areas in incomplete SCI patients. We speculate that the decreased functional connectivity implies decreased efficiency of information transfer and collaboration within sensorimotor areas because of injured afferent and efferent spinal pathways. In addition, we observed that the strength of functional connectivity between inter-hemispheric sensorimotor areas was positively correlated with ASIA sensory score in SCI patients. This finding indicates that patients with incomplete SCI with diminished functional connectivity within inter-hemispheric sensorimotor areas may display more significant sensory disability. To our knowledge, ASIA scales are most commonly used to quantify the motor and sensory function of SCI patients in the clinic (Marino et al., 2003; Steeves et al., 2012). However, it is insensitive for assessing slight changes of neurological function (Kirshblum et al., 2014; Kumru et al., 2016) and could be affected by different examiners. The association between network function and neurologic disabilities contributes to providing more sensitive and accurate MRI imaging biomarkers for assessment of disability and rehabilitation intervention.
However, cerebral functional reorganization could be dynamic, with variability depending on the extent of the lesion, disease duration, and exposure to rehabilitation. Aguilar et al., (2010) reported that deafferentation due to SCI can immediately (within minutes) change the state of large cortical networks. Hou et al. (2014a, b) reported that there was decreased inter-hemispheric functional connectivity between the bilateral primary sensorimotor cortex and increased intra-hemispheric functional connectivity within the motor network. Moreover, they found that increased functional connectivity within the sensorimotor cortex and cerebellum negatively correlated with ASIA motor scores. Although there are different insults in association with functional connectivity within sensorimotor areas and neurologic disabilities following SCI, current studies provide evidence that the characteristics of neuronal functional reorganization provided by fMRI could be a promising tool for assessment of prognosis and intervention. Further experiments are needed to explore more characteristics of the neuronal network in incomplete SCI and to understand the mechanism of functional reorganization and its value in functional recovery.
This study has several limitations. Firstly, the duration of SCI in patients was between 1 and 12 months. Such a long time span and differences in rehabilitation could affect GMV. We plan to compare similar patients with regards to injury time and rehabilitation to avoid these biases. Secondly, we analyzed structural and functional connectivity with the Brodmann template, which did not include the brain stem and cerebellum. Therefore, we likely overlooked brain stem and cerebellar information, which is an important region for motor recovery and working memory. Further studies should consider the brain stem and cerebellum to reveal their changes after SCI. Thirdly, we only combined structural and functional connectivity MRI data to explore brain organization in SCI patients. Multimodality fusion could be developed with more modalities to take maximal advantage of cross-information. Multi-level brain networks, including intra-region and inter-region networks based on the graph theory approach should be considered to further study brain activity.
Alterations of cortical anatomical structure and network function in sensorimotor areas were non-concomitant in patients with incomplete SCI. The network functional changes in sensorimotor areas may not be dependent on anatomic structure following incomplete SCI. The strength of the functional connectivity within inter-hemispheric sensorimotor areas could serve as a potential imaging biomarker for assessment and prediction of sensory function in incomplete SCI patients.
Acknowledgments
We appreciate Hai-xiao Du from Tsinghua University, China for his suggestions during this study.
Footnotes
Conflicts of interest: None declared.
Research ethics: The study protocol was approved by the Ethics Committee of Beijing Tsinghua Changgung Hospital of China (IRB No. 2015-002). This trial was registered with the Chinese Clinical Trial Registry (registration number: ChiCTR-ROC-17013566).
Declaration of participant consent: The authors certify that they have obtained all appropriate participant consent forms. In the form, participants have given their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: He Huang, Central South University Xiangya School of Medicine, China; Josue Ordaz, Indiana University School of Medicine, USA.
Copyedited by Turnley A, Raye W, Wang J, Li CH, Qiu Y, Song LP, Zhao M
Funding: This work was supported by a grant from Tsinghua University Initiative Scientific Research Program, No. 2014081266, 20131089382; and the National Natural Science Foundation of China, No. 61171002, 60372023.
References
- Aguilar J, Humanes-Valera D, Alonso-Calviño E, Yague JG, Moxon KA, Oliviero A, Foffani G. Spinal cord injury immediately changes the state of the brain. J Neurosci. 2010;30:7528–7537. doi: 10.1523/JNEUROSCI.0379-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Sui J. Multimodal fusion of brain imaging data: a key to finding the missing link(s) in complex mental illness. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:230–244. doi: 10.1016/j.bpsc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catz A, Itzkovich M, Steinberg F, Philo O, Ring H, Ronen J, Spasser R, Gepstein R, Tamir A. The Catz-Itzkovich SCIM: a revised version of the Spinal Cord Independence Measure. Disabil Rehabil. 2001;23:263–268. doi: 10.1080/096382801750110919. [DOI] [PubMed] [Google Scholar]
- Chen LM1, Qi HX, Kaas JH. Dynamic reorganization of digit representations in somatosensory cortex of nonhuman primates after spinal cord injury. J Neurosci. 2012;32:14649–14663. doi: 10.1523/JNEUROSCI.1841-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe AS, Jones CK, Joel SE, Muschelli J, Belegu V, Caffo BS, Lindquist MA, van Zijl PC, Pekar JJ. Reproducibility and temporal structure in weekly resting-state fMRI over a period of 3.5 years. PLoS One. 2015;10:e0140134. doi: 10.1371/journal.pone.0140134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curt A, Van Hedel HJ, Klaus D, Dietz V EM-SCI Study Group. Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J Neurotrauma. 2008;25:677–685. doi: 10.1089/neu.2007.0468. [DOI] [PubMed] [Google Scholar]
- Dittuno PL, Ditunno JF., Jr Walking index for spinal cord injury (WISCI II): scale revision. Spinal Cord. 2001;39:654–656. doi: 10.1038/sj.sc.3101223. [DOI] [PubMed] [Google Scholar]
- Doucet GE, He X, Sperling M, Sharan A, Tracy JI. Gray Matter abnormalities in temporal lobe epilepsy: relationships with resting-state functional connectivity and episodic memory performance. PLoS One. 2016;11:e0154660. doi: 10.1371/journal.pone.0154660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Kong Y, Winkler AM, Andersson JL, Finsterbusch J, Büchel C, Brooks JC, Tracey I. Investigating resting-state functional connectivity in the cervical spinal cord at 3T. Neuroimage. 2017;147:589–601. doi: 10.1016/j.neuroimage.2016.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink KL, Cafferty WB. Reorganization of intact descending motor circuits to replace lost connections after injury. Neurotherapeutics. 2016;13:370–1381. doi: 10.1007/s13311-016-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Tse A. Adaptive changes in the injured spinal cord and their role in promoting functional recovery. Neurol Res. 2008;30:17–27. doi: 10.1179/016164107X251781. [DOI] [PubMed] [Google Scholar]
- Freund P, Curt A, Friston K, Thompson A. Tracking changes following spinal cord injury: insights from neuroimaging. Neuroscientist. 2013a;19:116–128. doi: 10.1177/1073858412449192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Weiskopf N, Ashburner J, Wolf K, Sutter R, Altmann DR, Friston K, Thompson A, Curt A. MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: a prospective longitudinal study. Lancet Neurol. 2013b;12:873–881. doi: 10.1016/S1474-4422(13)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Weiskopf N, Ward NS, Hutton C, Gall A, Ciccarelli O, Craggs M, Friston K, Thompson AJ. Disability, atrophy and cortical reorganization following spinal cord injury. Brain. 2011;46:1610–1622. doi: 10.1093/brain/awr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzer PD, Manohar A, Shumsky JS, Moxon KA. Therapy induces widespread reorganization of motor cortex after complete spinal transection that supports motor recovery. Exp Neurol. 2016;279:1–12. doi: 10.1016/j.expneurol.2016.01.022. [DOI] [PubMed] [Google Scholar]
- Ganzola R, Duchesne S. Voxel-based morphometry meta-analysis of gray and white matter finds significant areas of differences in bipolar patients from healthy controls. Bipolar Disord. 2017;19:74–83. doi: 10.1111/bdi.12488. [DOI] [PubMed] [Google Scholar]
- García-Alías G, Edgerton VR. Who is who after spinal cord injury and repair? Can the brain stem descending motor pathways take control of skilled hand motor function? Neural Regen Res. 2015;10:1735–1736. doi: 10.4103/1673-5374.165318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Carrillo B, Mackey AP, Bunge SA. Resting-state fMRI: a window into human brain plasticity. Neuroscientist 2014. 2014;20:522–33. doi: 10.1177/1073858414524442. [DOI] [PubMed] [Google Scholar]
- Hao X, Huang Y, Li X, Song Y, Kong X, Wang X, Yang Z, Zhen Z, Liu J. Structural and functional neural correlates of spatial navigation: a combined voxel-based morphometry and functional connectivity study. Brain Behav. 2016;6:e00572. doi: 10.1002/brb3.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LA, Gustin SM, Macey PM, Wrigley PJ, Siddall PJ. Functional reorganization of the brain in humans following spinal cord injury: evidence for underlying changes in cortical anatomy. J Neurosci. 2011;31:2630–2637. doi: 10.1523/JNEUROSCI.2717-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou JM, Sun TS, Xiang ZM, Zhang JZ, Zhang ZC, Zhao M, Zhong JF, Liu J, Zhang H, Liu HL, Yan RB, Li HT. Alterations of resting-state regional and network-level neural function after acute spinal cord injury. Neuroscience. 2014a;277:446–454. doi: 10.1016/j.neuroscience.2014.07.045. [DOI] [PubMed] [Google Scholar]
- Hou JM, Yan RB, Xiang ZM, Zhang H, Liu J, Wu YT, Zhao M, Pan QY, Song LH, Zhang W, Li HT, Liu HL, Sun TS. Brain sensorimotor system atrophy during the early stage of spinal cord injury in humans. Neuroscience. 2014b;266:208–215. doi: 10.1016/j.neuroscience.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Isa T, Nishimura Y. Plasticity for recovery after partial spinal cord injury- hierarchical organization. Neurosci Res. 2014;78:3–8. doi: 10.1016/j.neures.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Jutzeler CR, Curt A, Kramer JL. Relationship between chronic pain and brain reorganization after deafferentation: A systematic review of functional MRI findings. Neuroimage Clin. 2015;9:599–606. doi: 10.1016/j.nicl.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal M, Oni-Orisan A, Chen G, Li W, Leschke J, Ward BD, Kalinosky B, Budde MD, Schmit BD, Li SJ, Muqeet V, Kurpad SN. Evaluation of whole-brain resting-state functional connectivity in spinal cord injury: a large-scale network analysis using network-based statistic. J Neurotrauma. 2017;34:1278–1282. doi: 10.1089/neu.2016.4649. [DOI] [PubMed] [Google Scholar]
- Kirshblum SC, Biering-Sørensen F, Betz R, Burns S, Donovan W, Graves DE, Johansen M, Jones L, Mulcahey MJ, Rodriguez GM, Schmidt-Read M, Steeves JD, Tansey K, Waring W. International standards for neurological classification of spinal cord injury: cases with classification challenges. Top Spinal Cord Inj Rehabil. 2014;20:81–89. doi: 10.1310/sci2002-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumru H, Murillo N, Benito-Penalva J, Tormos JM, Vidal J. Transcranial direct current stimulation is not effective in the motor strength and gait recovery following motor incomplete spinal cord injury during Lokomat(®) gait training. Neurosci Lett. 2016;620:143–147. doi: 10.1016/j.neulet.2016.03.056. [DOI] [PubMed] [Google Scholar]
- Kuppuswamy A, Balasubramaniam AV, Maksimovic R, Mathias CJ, Gall A, Craggs MD, Ellaway PH. Action of 5 Hz repetitive transcranial magnetic stimulation on sensory, motor and autonomic function in human spinal cord injury. Clin Neurophysiol. 2011;122:2452–1261. doi: 10.1016/j.clinph.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Lee J, Park E, Lee A, Chang WH, Kim DS, Kim YH. Recovery-related indicators of motor network plasticity according to impairment severity after stroke. Eur J Neurol. 2017;24:1290–1299. doi: 10.1111/ene.13377. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Dricot L, Laloux P, Desfontaines P, Evrard F, Peeters A, Jamart J, Vandermeeren Y. Increased functional connectivity one week after motor learning and tDCS in stroke patients. Neuroscience. 2017;340:424–435. doi: 10.1016/j.neuroscience.2016.10.066. [DOI] [PubMed] [Google Scholar]
- Lemola S, Oser N, Urfer-Maurer N, Brand S, Holsboer-Trachsler E, Bechtel N, Grob A, Weber P, Datta AN. Effects of gestational age on brain volume and cognitive functions in generally healthy very preterm born children during school-age: a voxel-based morphometry study. PLoS One. 2017;12:e0183519. doi: 10.1371/journal.pone.0183519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundell H, Christensen MS, Barthélemy D, Willerslev-Olsen M, Biering-Sørensen F, Nielsen JB. Cerebral activation is correlated to regional atrophy of the spinal cord and functional motor disability in spinal cord injured individuals. Neuroimage. 2011;54:1254–1261. doi: 10.1016/j.neuroimage.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, Haak M, Hudson LM, Priebe M. International standards for neurological classification of spinal cord Injury. J Spinal Cord Med. 2003;26:S50–56. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- Min YS, Park JW, Jin SU, Jang KE, Nam HU, Lee YS, Jung TD, Chang Y. Alteration of resting-state brain sensorimotor connectivity following spinal cord injury: a resting-state functional magnetic resonance imaging study. J Neurotrauma. 2015;32:1422–1427. doi: 10.1089/neu.2014.3661. [DOI] [PubMed] [Google Scholar]
- Morgan VL, Englot DJ, Rogers BP, Landman BA, Cakir A, Abou-Khalil BW, Anderson AW. Magnetic resonance imaging connectivity for the prediction of seizure outcome in temporal lobe epilepsy. Epilepsia. 2017;58:1251–1260. doi: 10.1111/epi.13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon KA, Oliviero A, Aguilar J, Foffani G. Cortical reorganization after spinal cord injury: always for good. Neuroscience. 2014;283:78–94. doi: 10.1016/j.neuroscience.2014.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone R, Höller Y, Brigo F, Seidl M, Christova M, Bergmann J, Golaszewski S, Trinka Functional brain reorganization after spinal cord injury: systematic review of animal and human studies. Brain Res. 2013;1504:58–73. doi: 10.1016/j.brainres.2012.12.034. [DOI] [PubMed] [Google Scholar]
- Oni-Orisan A, Kaushal M, Li W, Leschke J, Ward BD, Vedantam A, Kalinosky B, Budde MD, Schmit BD, Li SJ, Muqeet V, Kurpad SN. Alterations in cortical sensorimotor connectivity following complete cervical spinal cord injury: a prospective resting-state fMRI study. PLoS One. 2016;11:e0150351. doi: 10.1371/journal.pone.0150351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios EM, Yuh EL, Chang YS1, Yue JK, Schnyer DM, Okonkwo DO, Valadka AB, Gordon WA, Maas AIR, Vassar M, Manley GT, Mukherjee P. Resting-state functional connectivity alterations associated with six-month outcomes in mild traumatic brain injury. J Neurotrauma. 2017;34:1546–1557. doi: 10.1089/neu.2016.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JS, Manxiu M, Zhao C, Xi Y, Yang ZY, Zuxiang L, Li XG. Atrophy and primary somatosensory cortical reorganization after unilateral thoracic spinal cord injury: a longitudinal functional magnetic resonance imaging study. Biomed Res Int. 2013;2013:753061. doi: 10.1155/2013/753061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan S, Bloyet D, Revenu M, Dou WB, Liao QM. Cerebral magnetic resonance image segmentation using fuzzy markov random fields. IEEE Int Symposium Biomed Imaging. 2002;2002:237–240. [Google Scholar]
- Sabre L, Tomberg T, Kõrv J, Kepler J, Kepler K, Linnamägi Ü, Asser T. Brain activation in the chronic phase of traumatic spinal cord injury. Spinal Cord. 2016;54:65–68. doi: 10.1038/sc.2015.158. [DOI] [PubMed] [Google Scholar]
- Sharp KG, Gramer R, Page SJ, Cramer SC. Increased brain sensorimotor network activation after incomplete spinal cord injury. J Neurotrauma. 2017;34:623–631. doi: 10.1089/neu.2016.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva FT, Rêgo JT, Raulino FR, Silva MR, Reynaud F, Egito ES, Dantas PM. Transcranial direct current stimulation on the autonomic modulation and exercise time in individuals with spinal cord injury. A case report. Auton Neurosci. 2015;193:152–155. doi: 10.1016/j.autneu.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Steeves JD, Lammertse DP, Kramer JL, Kleitman N, Kalsi-Ryan S, Jones L, Curt A, Blight AR, Anderson KD. Outcome measures for acute/subacute cervical sensorimotor complete (AIS-A) spinal cord injury during a phase 2 Clinical trial. Top Spinal Cord Inj Rehabil. 2012;18:1–14. doi: 10.1310/sci1801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, Adali T, Yu Q, Chen J, Calhoun VD. A review of multivariate methods for multimodal fusion of brain imaging data. J Neurosci Methods. 2012;204:68–81. doi: 10.1016/j.jneumeth.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K. What is feasible with imaging human brain function and connectivity using functional magnetic resonance imaging. Philos Trans R Soc Lond B Biol Sci. 2015;371:20150361. doi: 10.1098/rstb.2015.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez N, Gall A, Ellaway PH, Craggs MD. Light touch and pin prick disparity in the international standard for neurological classification of spinal cord injury (ISNCSCI) Spinal Cord. 2013;51:375–378. doi: 10.1038/sc.2012.175. [DOI] [PubMed] [Google Scholar]
- Villiger M, Grabher P, Hepp-Reymond MC, Kiper D, Curt A, Bolliger M, Hotz-Boendermaker S, Kollias S, Eng K, Freund P. Relationship between structural brainstem and brain plasticity and lower-limb training in spinal cord injury: a longitudinal pilot study. Front Hum Neurosci. 2015;9:254. doi: 10.3389/fnhum.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Meda SA, Keshavan MS, Tamminga CA, Sweeney JA, Clementz BA, Schretlen DJ, Calhoun VD, Lui S, Pearlson GD. Large-scale fusion of gray matter and resting-state functional mri reveals common and distinct biological markers across the psychosis spectrum in the b-snip cohort. Front Psychiatry. 2015;6:174. doi: 10.3389/fpsyt.2015.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Wu G, Zhou X, Li J, Wen Z, Lin F. Altered spontaneous brain activity in patients with acute spinal cord injury revealed by resting-state functional MRI. PLoS One. 2015;10:e0118816. doi: 10.1371/journal.pone.0118816. [DOI] [PMC free article] [PubMed] [Google Scholar]


