Keywords: nerve regeneration, peripheral nerve injury, peripheral nerve defect, autologous nerve graft, functional recovery, nerve conduction velocity, sural nerve, common peroneal nerve, sleeve bridging suture, neural regeneration
Abstract
Peripheral nerve injury is a serious disease and its repair is challenging. A cable-style autologous graft is the gold standard for repairing long peripheral nerve defects; however, ensuring that the minimum number of transplanted nerve attains maximum therapeutic effect remains poorly understood. In this study, a rat model of common peroneal nerve defect was established by resecting a 10-mm long right common peroneal nerve. Rats receiving transplantation of the common peroneal nerve in situ were designated as the in situ graft group. Ipsilateral sural nerves (10–30 mm long) were resected to establish the one sural nerve graft group, two sural nerves cable-style nerve graft group and three sural nerves cable-style nerve graft group. Each bundle of the peroneal nerve was 10 mm long. To reduce the barrier effect due to invasion by surrounding tissue and connective-tissue overgrowth between neural stumps, small gap sleeve suture was used in both proximal and distal terminals to allow repair of the injured common peroneal nerve. At three months postoperatively, recovery of nerve function and morphology was observed using osmium tetroxide staining and functional detection. The results showed that the number of regenerated nerve fibers, common peroneal nerve function index, motor nerve conduction velocity, recovery of myodynamia, and wet weight ratios of tibialis anterior muscle were not significantly different among the one sural nerve graft group, two sural nerves cable-style nerve graft group, and three sural nerves cable-style nerve graft group. These data suggest that the repair effect achieved using one sural nerve graft with a lower number of nerve fibers is the same as that achieved using the two sural nerves cable-style nerve graft and three sural nerves cable-style nerve graft. This indicates that according to the ‘multiple amplification’ phenomenon, one small nerve graft can provide a good therapeutic effect for a large peripheral nerve defect.
Introduction
Peripheral nerve injury is a serious disease, which leads to life-long disability and long-term dysfunction (Biazar et al., 2010; Gonzalez-Perez et al., 2017). People with such injuries usually lose their ability to work thus becoming a heavy burden to their family (Bastami et al., 2017; Kim et al., 2017). So far, repair of peripheral nerve injury is a major challenge in clinical settings as there is no suitable treatment method especially for long nerve defects (Yan et al., 2017). Previously, we reported that the biological degradable conduit was used to repair peripheral nerve injury using a 2-mm small gap sleeve suture between the two ruptured stumps and found that the repair effect was slightly better than that using traditional epineurial neurorrhaphy (Zhang et al., 2013a). However, few studies have applied this new method to long nerve defects.
According to a previous study, following nerve transection, a single proximal axon generates several lateral buds during nerve repair, and these lateral buds noticeably outnumber the distal endoneurial tubes of the damaged nerve; this phenomenon is known as “multiple amplification”. Therefore, the use of fewer proximal fibers to bridge the distal nerve enabled amplification to be achieved during nerve regeneration (Zhang et al., 2005). The maximum amplification ratio for nerve regeneration is approximately 3.3 (Jiang et al., 2007). When a biodegradable chitin conduit is applied, using a finer nerve as a donor to connect the distal stump of the damaged nerve promotes amplification during nerve repair.
For a small nerve defect, the injured nerve stump could be sutured directly through neural mobilization and translocation (Boyd et al., 2011; Rochkind and Shainberg, 2017). However, the gold standard for repairing long peripheral nerve defects is the use of an autologous nerve graft (Brown and Mackinnon, 2008; Jiang et al., 2016). Sensory and motor disorders can be detected at the site of the autologous nerve. In addition, it is difficult to match the tiny sensory nerves with the large defective composite nerve (Neubauer et al., 2010). The cable-style nerve graft can effectively solve the problem of large anastomotic tension, promote the infiltration of tissue fluid and growth of blood vessels. However, excessive transplanted nerve will lead to problems, including separation of bundles and distortions and dysfunction, finally affecting nerve regeneration and functional recovery (Daly et al., 2012). Therefore, it is important to ensure that a minimum percentage of transplanted nerve attains maximum therapeutic effect after nerve injury.
This study investigated the regeneration and functional recovery following transplantation of different numbers of nerves for the repair of nerve defects in a rat model of common peroneal nerve defect. This method was compared with a standardized method in which a nerve graft was transplanted in situ. This study can help with making the most economical use of donor nerves for nerve graft transplantation.
Materials and Methods
Animals
All procedures conformed to the National Institutes of Health guidelines on animal experimentation and were approved by the Research Ethics Committee at Peking University People's Hospital of China (approval number: 2011-16). Twenty-four healthy specific-pathogen-free male adult Sprague-Dawley rats aged 8–10 weeks and weighing 200–250 g were provided by the Animal Experimental Center of Peking University People's Hospital (Beijing, China; license No. SYXK (Jing) 2011-0010).
Twenty-four rats were equally and randomly divided into four groups. An animal model of right common peroneal nerve defect was established by transecting the right sural nerve in each rat. Rats received a transplantation of the common peroneal nerve in situ (in situ graft group, n = 6), and a cable-style nerve graft with one sural nerve graft (one sural nerve graft group, n = 6), two sural nerves graft (two sural nerves cable-style nerve graft group, n = 6), and three sural nerves graft (three sural nerves cable-style nerve graft group, n = 6). In the experimental groups, a small gap sleeve suture was used at both the proximal and distal common peroneal nerve stumps. A deacetylated chitin biological tube was used as bridging material (8 mm length, 1.5 mm inner diameter and 0.1 mm wall thickness) (jointly developed by Peking University People's Hospital and China Textile Academy; Patent number: 01136314.2).
Establishing an animal model of common peroneal nerve defect
All rats were intraperitoneally anesthetized with 2% pentobarbital sodium (30 mg/kg; Sigma, St. Louis, MO, USA). The skin was shaved and sterilized, then the right hind limb was disinfected. An incision was made along the femoral long axis of the right hind limb. A 10- to 30-mm segment of the sural nerve in right hind limb was taken below the merger of the nerve fascicle. The right common peroneal nerve was cut at 1 cm approximately distal from the separation of sciatic nerve, and a 10-mm segment was resected to establish the common peroneal nerve defect model. For the in situ graft group, the common peroneal nerve segment was sutured in situ. For the cable-style nerve graft groups (one sural nerve graft group, two sural nerves cable-style nerve graft group, three sural nerves cable-style nerve graft group), different numbers of sural nerve segments were transplanted into the defect site. In each group, the small gap sleeve suture technique was used in the proximal and distal common peroneal nerve stumps combined with the nerve graft. The epineurium was sutured to the sleeve with 1–2 stitches by leaving a 2-mm gap (Figure 1).
Figure 1.
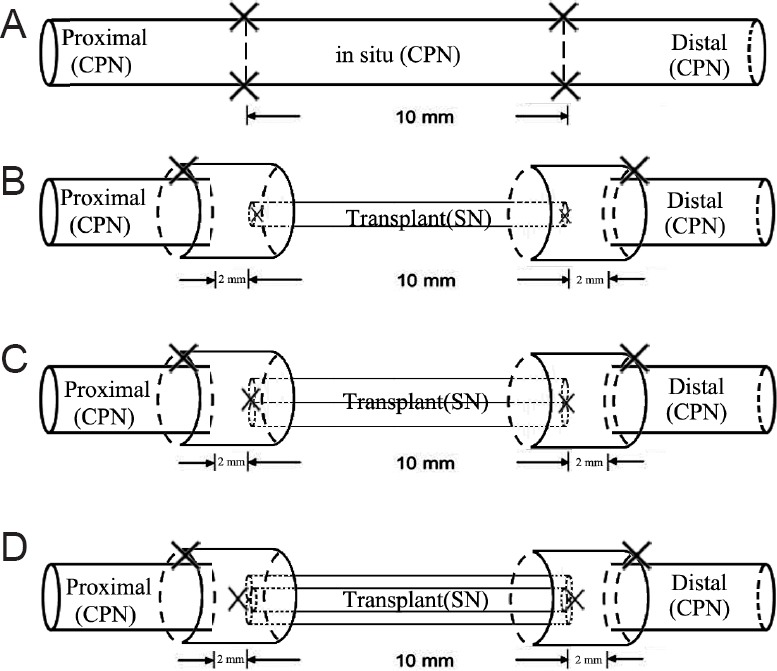
Diagram of repair models in each group.
(A) In situ graft group; (B) one sural nerve graft group; (C) two sural nerves cable-style nerve graft group; (D) three sural nerves cable-style nerve graft group. In one sural nerve graft group, two sural nerves cable-style nerve graft group and three sural nerves cable-style nerve graft group, a small gap sleeve suture was used at proximal and distal anasto-mosis with a gap of 2 mm. Rats underwent a transplantation of the common peroneal nerve in situ (in situ graft group, n = 6), and cable-style nerve graft with one sural nerve graft (one sural nerve graft group, n = 6), two sural nerves graft (two sural nerves cable-style nerve graft group, n = 6) and three sural nerves graft (three sural nerves cable-style nerve graft group, n = 6). SN: Sural nerve; CPN: common peroneal nerve.
General observations
At three months postoperatively, the general conditions of rats were observed regularly, including activities of limbs that were operated upon, wound healing and functional recovery, ulcer formation, and any rottenness for the feet due to self-biting.
Common peroneal nerve function index
At three months postoperatively, the footprints of each group were recorded. The left footprint served as the normal control, while that on right, served as the experimental footprint. Paired footprint parameters for print length (distance from heel to toe, PL), toe spread (distance from first to fifth toe, TS), and intermediary toe spread (distance from second to fourth toe, IT) were recorded in left normal control foot (as NPL, NTS, NIT) and corresponding right experimental foot (as EPL, ETS, EIT) for each rat (Figure 2). The common peroneal nerve function index was calculated according to the Bain-Mackinnon-Hunter formula (Bain et al., 1989; Dijkstra et al., 2000).
Figure 2.
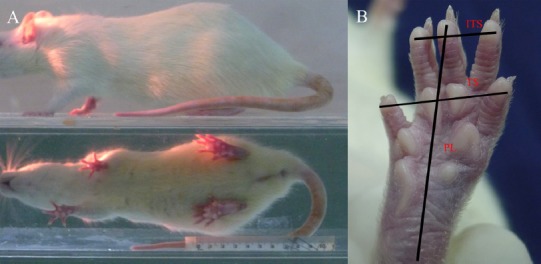
Common peroneal nerve function index.
(A) Walking track analysis was performed in a confined walkway for each rat to obtain footprints from both hind limbs. (B) Paired footprint parameters: index print length (PL), toe spread (TS), and intermediary toe spread (IT) were recorded in the normal control foot (as NPL, NTS, NIT) and the corresponding experimental foot (as EPL, ETS, EIT) for each rat. Common peroneal nerve function index = 174.9 ([EPL–NPL]/NPL)+80.3 ([ETS–NTS]/NTS)–13.4.
Electrophysiological assessment
Electrophysiological (MedlecSynergy; Oxford Instrument Inc., Oxfordshire, UK) assessment proceeded to evaluate the conduction properties of the operated graft nerve prior to sacrifice of the rats at three months postoperatively. Rats were intraperitoneally anesthetized with sodium pentobarbital (30 mg/kg; Sigma), following which, the repaired common peroneal nerve was exposed. Bipolar stimulating electrodes were placed at proximal and distal sites of repaired nerve. The recording electrode was placed in the tibialis anterior muscle. The ground electrode was placed in subcutaneous tissue between the stimulating and recording electrodes. Rectangular pulses (duration 0.1 ms, intensity 0.9 mA, frequency 10 Hz) were set to stimulate the nerves. The motor nerve conduction velocity (m/s) was gained semiautomatically by dividing the distance between the two stimulating sites by the difference in the onset latency. The motor nerve conduction velocity at the contralateral normal common peroneal nerve was recorded using the same method in six randomly selected rats of the experimental groups.
Tetanic muscle contraction strength
Bilateral tibialis anterior muscle strength was measured after electrophysiological assessment. The PCLAB-UE biomedical signal acquisition and processing system (Beijing Microsignal star Inc., Beijing, China) was utilized to record the waveform of the tetanic contraction of tibialis anterior muscle on both sides. Before dissection and isolation of the tibialis anterior muscle, the rats were fully anesthetized at three months postoperatively. The hind limb was fixed on a custom-made holding frame. The distal end of the tibialis anterior muscle was connected to a tension sensor, which was then kept and aligned by using the frame. The initial tension was maintained at a chosen level (0 < F < 0.1 N). The initial electric stimulation was generated by an electrophysiological system with 0.9 mA intensity, 0.1 ms wave length and 1 Hz frequency. The electric current was strengthened subsequently until the tetanic contraction induced waveform began to increase. The ratio of the wave amplitudes of the experimental side to normal control side was considered to represent the overall recovery rate of muscle strength (Shin et al., 2008).
Measurement of wet muscle weight
After experimental rats were sacrificed using an intraarterial overdose of sodium pentobarbital, tibialis anterior muscle from the experimental and normal control sides was isolated from the bone at their origin and terminal point, and weighed with an electronic scale immediately. The conserved muscle-mass ratio was recorded on each side of all rats.
Osmium tetroxide staining and quantification of common peroneal nerve fibers
At three months postoperatively, rats were perfused with 4% paraformaldehyde through the left ventricle. The distal stump of common peroneal nerve of the right hind limb was obtained. The sural nerve and common peroneal nerve on the normal side were obtained in three experimental groups. After 1% osmium tetroxide staining for 12 hours, all specimens were dehydrated and immersed in xylene, and embedded in paraffin. The nerves on the experimental side were cross-sectioned 5 mm away from the distal anastomosis with a 2-μm slice thickness and photographed. The Leica Q550CW analytical system was used to analyze the osmium tetroxide staining results, and the number of myelinated fibers was calculated manually.
Data analysis
All data were statistically analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA) and presented as the mean ± SD. The significance of data in each group was tested using a one-way analysis of variance followed by a least significant difference test. A value of P < 0.05 was considered statistically significant.
Results
General observation of common peroneal nerve after transplantation with different numbers of sural nerves
Three months after surgery, all chitin tubes in the one sural nerve graft group, two sural nerves cable-style nerve graft group and three sural nerves cable-style nerve graft group had good biocompatibility. The chitin tube at the proximal and distal anastomosis of cable-style nerve graft groups had not been fully absorbed. The wounds recovered without infection or ulcers. Toe spread was observed in all rats. Autophagy was observed in the right feet of three rats (one rat from one sural nerve graft group, and two rats from three sural nerves cable-style nerve graft group). Newly generated tissues adhered to the transplanted nerve and its surrounding area. The transplanted nerve regenerated successfully in all groups and its diameter was similar or sometimes exceeded that of the normal common peroneal nerve. No distinct formation of neurofibromas was observed (Figure 3).
Figure 3.
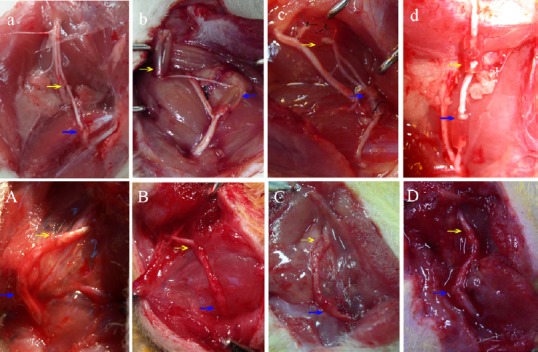
Establishment of rat models and general observation of the transplanted nerve in each group.
(a–d) Preparation of rat models. (a) Common peroneal nerve was sutured in situ; (b) transplantation of one sural nerve segment as the nerve graft; (c) transplantation of two sural nerve segments as the cable-style nerve graft; (d) transplantation of three sural nerve segments as the cable-style nerve graft. (A–D) Observation of the transplanted nerve in each group at three months postoperatively under direct vision. The transplanted nerve regenerated successfully in all groups. The chitin tube at the proximal and distal anastomosis of cable-style nerve graft groups had not been fully absorbed. The nerve diameter was close to the normal common peroneal nerve. Yellow arrows: Proximal suture of the grafts. Blue arrows: Distal suture of grafts. Rats received a trans-plantation of the common peroneal nerve in situ (in situ graft group), and cable-style nerve graft with one sural nerve graft (one sural nerve graft group), two sural nerves graft (two sural nerves cable-style nerve graft group) and three sural nerves graft (three sural nerves cable-style nerve graft group).
Histological changes in the common peroneal nerve after transplantation with different numbers of sural nerves
The fiber number, fiber diameter, axon diameter and myelin thickness of common peroneal nerve on unaffected side were assessed. Microscopic observation at high magnification showed that the uniformity in nerve diameter was lower for the regenerated common peroneal nerve fibers in the experimental groups than for the normal nerve. Necrotic and disorganized connective tissues of the myelin remnants were occasionally visible in the regenerated axons. Moreover, the thickness of the myelin sheath of the regenerated nerve fibers was uneven (Figure 4). The number of regenerated myelinated nerve fibers at the distal anastomosis is listed in Table 1. The number of normal sural nerve was 857 ± 11. The entire difference in the number of distal regenerated common peroneal nerve fibers was not statistically significant among the one sural nerve graft group, two sural nerves cable-style nerve graft group and three sural nerves cable-style nerve graft group (P > 0.05). The myelin thickness and diameters of fiber and axon were calculated at three months postoperatively (Table 1). The values were not significantly different among the one sural nerve graft group, two sural nerves cable-style nerve graft group and three sural nerves cable-style nerve graft group (P > 0.05).
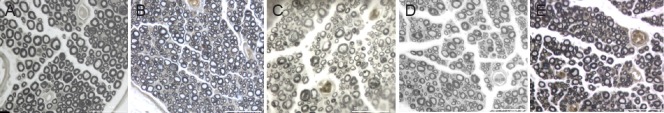
Figure 4.
Osmium tetroxide staining at the distal anastomosis of the common peroneal nerve in the normal and the experimental groups.
Myelinated nerve fiber morphology was observed in each group using a light microscope (osmium tetroxide staining, ×400). The uniformity in nerve diameter was lower for the newly regenerated nerve fibers in each group than for the normal nerve. The thickness of the myelin sheath of regenerated nerve fibers was not uniform. (A) Normal peroneal nerve (unaffected side); (B) in situ graft group; (C) one sural nerve graft group; (D) two sural nerves cable-style nerve graft group; (E) three sural nerves cable-style nerve graft group. Scale bar: 50 μm. Rats underwent a transplantation of the common peroneal nerve in situ (in situ graft group), and cable-style nerve graft with one sural nerve graft (one sural nerve graft group), two sural nerves graft (two sural nerves cable-style nerve graft group) and three sural nerves graft (three sural nerves cable-style nerve graft group).
Table 1.
Fiber number, fiber diameter, axon diameter, and myelin thickness in the distal common peroneal nerve stump
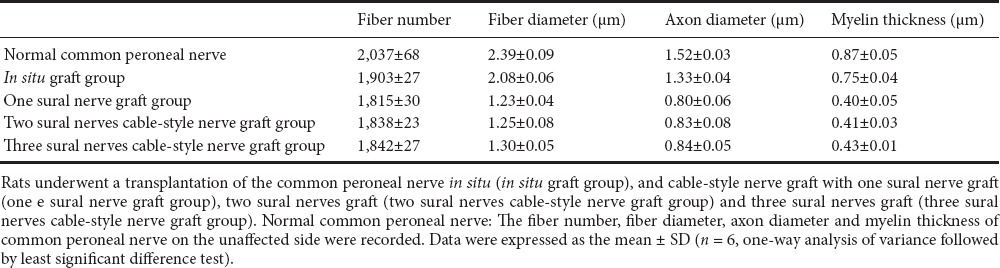
Common peroneal nerve function index after transplantation with different numbers of sural nerves
Common peroneal nerve function indexes of each group were 30.67 ± 4.27 in the in situ graft group, 39.00 ± 2.37 in the one sural nerve graft group, 36.67 ± 1.75 in the two sural nerves cable-style nerve graft group, and 36.33 ± 1.37 in the three sural nerves cable-style nerve graft group. The differences in common peroneal nerve function index among the experimental groups were not significant (P > 0.05; Figure 5).
Figure 5.
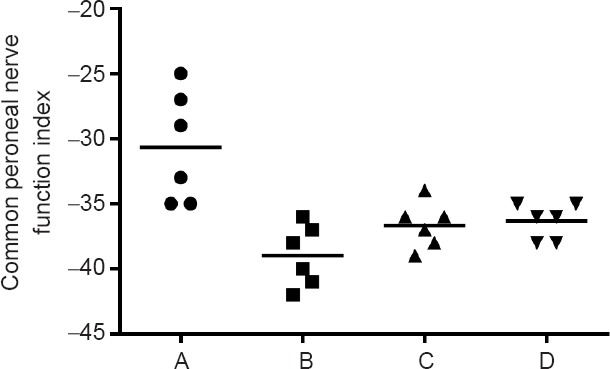
Common peroneal nerve function index in each group.
The index was not significantly different among the one sural nerve graft group (B), two sural nerves cable-style nerve graft group (C), and three sural nerves cable-style nerve graft group (D) (P > 0.05). Rats received a transplantation of the common peroneal nerve in situ (A: in situ graft group, n = 6), and cable-style nerve graft with one sural nerve graft (B: one sural nerve graft group, n = 6), two sural nerves graft (C: two sural nerves cable-style nerve graft group, n = 6) and three sural nerves graft (D: three sural nerves cable-style nerve graft group, n = 6).
Conduction velocity of common peroneal nerve after transplantation with different numbers of sural nerves
The conduction velocity was 48.5 ± 2.8 m/s in the normal common peroneal nerve and 35.6 ± 2.7 m/s in newly generated nerve in the in situ graft group, 25.5 ± 1.0 m/s in the one sural nerve graft group, 26.8 ± 1.2 m/s in the two sural nerves cable-style nerve graft group, and 26.7 ± 0.7 m/s in the three sural nerves cable-style nerve graft group. Although the conduction velocities were lower in the experimental groups than in the in situ graft group, there were no significant differences among the one sural nerve graft group, two sural nerves cable-style nerve graft group and three sural nerves cable-style nerve graft group (P > 0.05; Figure 6).
Figure 6.
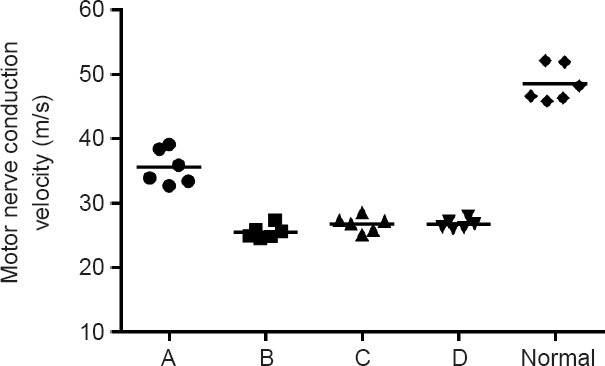
Motor nerve conduction velocity in each group.
Motor nerve conduction velocity was not significantly different among the one sural nerve graft group (B), two sural nerves cable-style nerve graft group (C), and three sural nerves cable-style nerve graft group (D) (P > 0.05). Rats received a transplantation of the common peroneal nerve in situ (A: in situ graft group, n = 6), and cable-style nerve graft with one sural nerve graft (B: one sural nerve graft group, n = 6), two sural nerves graft (C: two sural nerves cable-style nerve graft group, n = 6), and three sural nerves graft (D: three sural nerves cable-style nerve graft group, n = 6).
Tetanic muscle contraction strength after transplantation with different numbers of sural nerves
The maximum tetanic contraction forces of tibialis anterior muscles in each group are shown in Table 2. The value on the left side was set at 100%. The muscle force percentages on the right side (repair side) to the left side were 92.91 ± 7.98% in the in situ graft group, 83.38 ± 5.93% in the one sural nerve graft group, 85.71 ± 8.13% in the two sural nerves cable-style nerve graft group, and 81.60 ± 8.03% in the three sural nerves cable-style nerve graft group (Figure 7). There were no significant differences among the one sural nerve graft group, two sural nerves cable-style nerve graft group and three sural nerves cable-style nerve graft group (P > 0.05).
Table 2.
Maximum tetanic contraction force (N) of bilateral tibialis anterior muscles
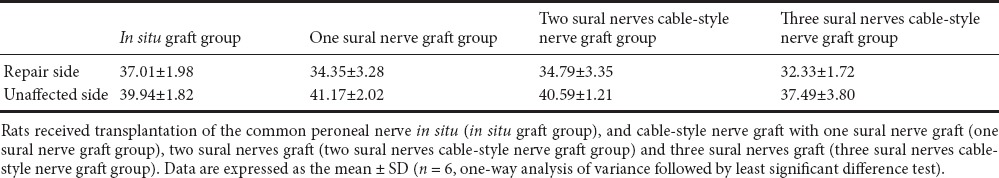
Figure 7.
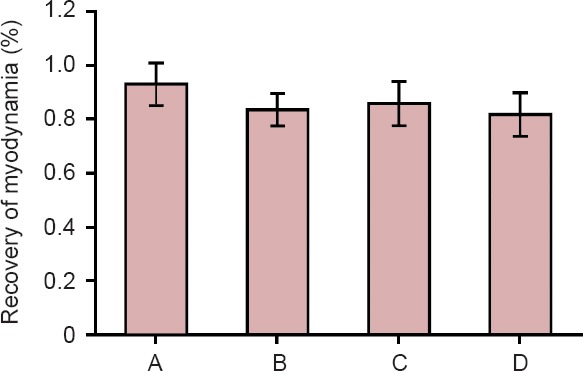
Recovery of myodynamia in each group at three months postoperatively.
There were no significant differences among the one sural nerve graft group (B), two sural nerves cable-style nerve graft group (C), and three sural nerves cable-style nerve graft group (D) Rats received a transplantation of the common peroneal nerve in situ (A: in situ graft group), and cable-style nerve graft with one sural nerve graft (B: one sural nerve graft group), two sural nerves graft (C: two sural nerves cable-style nerve graft group) and three sural nerves graft (D: three sural nerves cable-style nerve graft group). Data are expressed as the mean ± SD (n = 6, one-way analysis of variance followed by least significant difference test).
Wet weight of gastrocnemius muscle after transplantation with different numbers of sural nerves
There were no differences in the body weights of rats before surgery and four weeks after surgery in all groups. The wet weight ratios of tibialis anterior muscles were 74.67 ± 3.98% in the in situ graft group, 54.50 ± 7.42% in the one sural nerve graft group, 56.17 ± 4.26% in the two sural nerves cable-style nerve graft group, 56.83 ± 5.56% in the three sural nerves cable-style nerve graft group. Although the wet weight ratios were lower in the experimental groups than in the in situ graft group, there were no significant differences among the one sural nerve graft group, the two sural nerves cable-style nerve graft group and the three sural nerves cable-style nerve graft group (P > 0.05).
Discussion
Autologous nerve transplantation for the treatment of peripheral nerve injury was first reported (Millesi, 1979) in 1870. Numerous surgeons used autologous nerve transplantation to repair different complex nerve injury and autologous nerve transplantation was regarded as “gold standard” in neurorehabilitation (Schmitte et al., 2010; Bozkurt et al., 2014). However, autologous nerve transplantation inevitably had several drawbacks, including limited quantity, small nerve diameter, and inaccurate connection between motor nerve and sensory neurons. Previous studies reported that the nerve used for transplantation should conform to the following standards (Millesi et al., 1972; Fawcett and Keynes, 1986): the regenerating axons should (1) be able to rearrange in an orderly manner and grow towards remote target zone by autologous nerve; (2) have a normal diameter, myelination and action potential conduction; (3) not have antigenicity; and (4) receive blood supply quickly. So far, only autologous nerve for transplantation can fulfill the above-mentioned criteria (Gaudin et al., 2016; Trehan et al., 2016). Hence, we selected the sural nerve for transplantation in this study. Currently, suture of the common peroneal nerve in situ is the universal and ideal therapeutic procedure in clinical settings, which is a reference standard for observation of repair effects (George and Boyce, 2014). In this study, we compared the different effects obtained by using single transplantation and multiple transplantations to investigate the use of the sural nerve for treatment of common peroneal nerve injury, and to compare the effect obtained with the gold standard repair method. Moreover, it is necessary to highlight that for transplantation, sensory nerve fibers for treating motor nerve or mixed nerve defects in clinical settings. The objective of this study was not to compare the repair effects obtained using sensory nerve fibers between the mixed and motor nerves, but to compare the repair effect obtained by using different numbers of nerve fibers to resolve nerve defects. That is, assessing how the best repair effect can be achieved using an economical number of nerve fibers for transplantation.
Our results showed that the evaluated parameters, including the number of regenerated nerve fibers, common peroneal nerve function index, regenerated motor nerve conduction velocity, osmic acid stained nerve segment, tibialis anterior muscle power, and wet weight were not significantly different among the experimental groups.
Small gap sleeve suture is an innovative nerve repair method that can create enough selective growth space for nerve regeneration and greatly ensure an accurate connection between injured nerves (Kou et al., 2011). Compared with the traditional suture method, the small gap sleeve suture decreases the operation time, reduces nerve injury, and contributes to nerve anastomosis (Zhang et al., 2013b). Meanwhile, our neural research team has already developed a chitin tube, which has proven to be a curative biomaterial for the treatment of peripheral nerve injury, thus making it possible to suture nerves with different diameters (Zhang et al., 2005). Based on this experiment, this novel technique makes it possible to suture nerves with different diameters.
An important feature of peripheral nerve regeneration is “multiple regeneration”, which refers to the number of new axon buds that can far outstrip the number of injured proximal nerve fibers (An et al., 2015). Thus, a single axon might repair a couple of damaged nerve axons (Jianping et al., 2012). Merle and Dautel (1991) reported that compared with other nerve transplantation methods, a single autologous nerve transplantation could greatly improve the number of regenerated nerve fibers and myelination, as well as result in good neurological functional recovery. Millesi et al. (1972) reported a good outcome when autologous nerve transplantation was used to restore median nerve injury and ulnar nerve injury. The transplanted nerve survived easily owing to the small nerve volume used. Additionally, peripheral vessels and tissue fluid regenerated rapidly. However, it was important to distinguish between the different functional clusters to reduce the rate of incorrect nerve connection. For the “multiple amplification” phenomenon, a few studies were undertaken in our laboratory to prove that multiple regeneration of a single axon could restore nerve function (Xu et al., 2014).
Based on the above research and theory, we established a rat model of common peroneal nerve defect (10 mm) and designed an experiment to compare the therapeutic efficacy of different numbers of autologous sural nerve grafts for the repair of nerve defects. Furthermore, to reduce the barrier effect due to invasion by surrounding tissue and connective-tissue overgrowth between neural stumps, small gap sleeve suture was used in both proximal and distal terminals. Therefore, we conclude that one small sural nerve graft could successfully repair a large common peroneal nerve defect. This study offered new supporting evidence that a small nerve segment can repair a large nerve defect. However, due to the limitations encountered with the use of laboratory animals, high-quality clinical studies still need to be conducted in the future. In addition, understanding the process of regenerated peripheral nerve passing through bridged nerves of different diameters, the mechanism of newborn nerve fibers growing into the bridging materials, and the dynamic process of nerve function recovery, still require further investigation.
Footnotes
Funding: This study was supported by the National Basic Research Program of China (973 Program), No. 2014CB542200; a grant from the Ministry of Education Innovation Team, No. IRT1201; the National Natural Science Foundation of China, No. 31271284, 31171150, 81171146, 30971526, 31100860, 31040043, 31640045, 31671246; a grant from the Educational Ministry New Century Excellent Talents Support Project in China, No. BMU20110270; a grant from the National Key Research and Development Program in China, No. 2016YFC1101604.
Conflicts of interest: None declared.
Research ethics: All procedures conformed to the National Institutes of Health guidelines on animal experimentation and were approved by the Research Ethics Committee at Peking University People's Hospital of China (approval number: 2011-16).
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Rogerio Leone Buchaim, Universidade de Sao Paulo, Brazil.
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
References
- An S, Zhang P, Peng J, Deng L, Wang Z, Wang Z, Wang Y, Yin X, Kou Y, Ha N, Jiang B. Motor function recovery during peripheral nerve multiple regeneration. J Tissue Eng Regen Med. 2015;9:415–423. doi: 10.1002/term.1833. [DOI] [PubMed] [Google Scholar]
- Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–138. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- Bastami F, Vares P, Khojasteh A. Healing Effects of Platelet-Rich Plasma on Peripheral Nerve Injuries. J Craniofac Surg. 2017;28:e49–57. doi: 10.1097/SCS.0000000000003198. [DOI] [PubMed] [Google Scholar]
- Biazar E, Khorasani MT, Montazeri N, Pourshamsian K, Daliri M, Mostafa RT, Mahmoud JB, Khoshzaban A, Saeed HK, Jafarpour M, Roviemiab Z. Types of neural guides and using nanotechnology for peripheral nerve reconstruction. Int J Nanomed. 2010;5:839–852. doi: 10.2147/IJN.S11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd KU, Nimigan AS, Mackinnon SE. Nerve reconstruction in the hand and upper extremity. Clin Plast Surg. 2011;38:643–660. doi: 10.1016/j.cps.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Bozkurt A, van Neerven SG, Claeys KG, O’Dey DM, Sudhoff A, Brook GA, Sellhaus B, Schulz JB, Weis J, Pallua N. The proximal medial sural nerve biopsy model: a standardised and reproducible baseline clinical model for the translational evaluation of bioengineered nerve guides. Biomed Res Int. 2014;2014:121452. doi: 10.1155/2014/121452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Mackinnon SE. Nerve transfers in the forearm and hand. Hand Clin. 2008;24:319–340. doi: 10.1016/j.hcl.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Daly W, Yao L, Zeugolis D, Windebank A, Pandit A. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface. 2012;9:202–221. doi: 10.1098/rsif.2011.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra JR, Meek MF, Robinson PH, Gramsbergen A. Methods to evaluate functional nerve recovery in adult rats: walking track analysis, video analysis and the withdrawal reflex. J Neurosci Methods. 2000;96:89–96. doi: 10.1016/s0165-0270(99)00174-0. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Keynes RJ. Muscle basal lamina: a new graft material for peripheral nerve repair. J Neurosurg. 1986;65:354–363. doi: 10.3171/jns.1986.65.3.0354. [DOI] [PubMed] [Google Scholar]
- Gaudin R, Knipfer C, Henningsen A, Smeets R, Heiland M, Hadlock T. Approaches to peripheral nerve repair: generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. Biomed Res Int. 2016;2016:3856262. doi: 10.1155/2016/3856262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SC, Boyce DE. An evidence-based structured review to assess the results of common peroneal nerve repair. Plast Reconstr Surg. 2014;134:302e–311. doi: 10.1097/PRS.0000000000000318. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez F, Cobianchi S, Heimann C, Phillips JB, Udina E, Navarro X. Stabilization, rolling, and addition of other extracellular matrix proteins to collagen hydrogels improve regeneration in chitosan guides for long peripheral nerve gaps in rats. Neurosurgery. 2017;80:465–474. doi: 10.1093/neuros/nyw068. [DOI] [PubMed] [Google Scholar]
- Jiang BG, Yin XF, Zhang DY, Fu ZG, Zhang HB. Maximum number of collaterals developed by one axon during peripheral nerve regeneration and the influence of that number on reinnervation effects. Eur Neurol. 2007;58:12–20. doi: 10.1159/000102161. [DOI] [PubMed] [Google Scholar]
- Jiang CQ, Hu J, Xiang JP, Zhu JK, Liu XL, Luo P. Tissue-engineered rhesus monkey nerve grafts for the repair of long ulnar nerve defects: similar outcomes to autologous nerve grafts. Neural Regen Res. 2016;11:1845–1850. doi: 10.4103/1673-5374.194757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jianping P, Xiaofeng Y, Yanhua W, Zhenwei W, Yuhui K, Chungui X, Peixun Z, Baoguo J. Different multiple regeneration capacities of motor and sensory axons in peripheral nerve. Artif Cells Blood Substit Immobil Biotechnol. 2012;40:309–316. doi: 10.3109/10731199.2012.657205. [DOI] [PubMed] [Google Scholar]
- Kim H, Choi B, Lim H, Min H, Oh JH, Choi S, Cho JG, Park JS, Lee SJ. Polyamidoamine dendrimer-conjugated triamcinolone acetonide attenuates nerve injury-induced spinal cord microglia activation and mechanical allodynia. Molecular pain. 2017 doi: 10.1177/1744806917697006. doi: 10.1177/1744806917697006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou YH, Yin XF, Zhang PX, Jiang BG. Small-gap bridging technology for peripheral nerve injury repair and the new sleeve material. Beijing Da Xue Xue Bao. 2011;3:647–651. [PubMed] [Google Scholar]
- Merle M, Dautel G. Vascularised nerve grafts. J Hand Surg Br. 1991;16:483–488. doi: 10.1016/0266-7681(91)90099-a. [DOI] [PubMed] [Google Scholar]
- Millesi H. Microsurgery of peripheral nerves. World J Surg. 1979;3:67–79. doi: 10.1007/BF01556388. 128-129. [DOI] [PubMed] [Google Scholar]
- Millesi H, Meissl G, Berger A. The interfascicular nerve-grafting of the median and ulnar nerves. J Bone Joint Surg Am. 1972;54:727–750. [PubMed] [Google Scholar]
- Neubauer D, Graham JB, Muir D. Nerve grafts with various sensory and motor fiber compositions are equally effective for the repair of a mixed nerve defect. Exp Neurol. 2010;223:203–206. doi: 10.1016/j.expneurol.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Rochkind S, Shainberg A. Muscle response to complete peripheral nerve injury: changes of acetylcholine receptor and creatine kinase activity over time. J Reconstr Microsurg. 2017;33:352–357. doi: 10.1055/s-0037-1598619. [DOI] [PubMed] [Google Scholar]
- Schmitte R, Tipold A, Stein VM, Schenk H, Flieshardt C, Grothe C, Haastert K. Genetically modified canine Schwann cells-in vitro and in vivo evaluation of their suitability for peripheral nerve tissue engineering. J Neurosci Methods. 2010;186:202–208. doi: 10.1016/j.jneumeth.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Shin RH, Vathana T, Giessler GA, Friedrich PF, Bishop AT, Shin AY. Isometric tetanic force measurement method of the tibialis anterior in the rat. Microsurgery. 2008;28:452–457. doi: 10.1002/micr.20520. [DOI] [PubMed] [Google Scholar]
- Trehan SK, Model Z, Lee SK. Nerve repair and nerve grafting. Hand Clin. 2016;32:119–125. doi: 10.1016/j.hcl.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Xu C, Kou Y, Zhang P, Han N, Yin X, Deng J, Chen B, Jiang B. Electrical stimulation promotes regeneration of defective peripheral nerves after delayed repair intervals lasting under one month. PLoS One. 2014;9:e105045. doi: 10.1371/journal.pone.0105045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Yao Z, Lin T, Zhu Q, Qi J, Gu L, Fang J, Zhou X, Liu X. The role of precisely matching fascicles in the quick recovery of nerve function in long peripheral nerve defects. Neuroreport. 2017;28:1008–1015. doi: 10.1097/WNR.0000000000000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, He X, Zhao F, Zhang D, Fu Z, Jiang B. Bridging small-gap peripheral nerve defects using biodegradable chitin conduits with cultured schwann and bone marrow stromal cells in rats. J Reconstr Microsurg. 2005;21:565–571. doi: 10.1055/s-2005-922437. [DOI] [PubMed] [Google Scholar]
- Zhang P, Han N, Wang T, Xue F, Kou Y, Wang Y, Yin X, Lu L, Tian G, Gong X, Chen S, Dang Y, Peng J, Jiang B. Biodegradable conduit small gap tubulization for peripheral nerve mutilation: a substitute for traditional epineurial neurorrhaphy. Int J Med Sci. 2013a;10:171–175. doi: 10.7150/ijms.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Kou Y, Yin X, Wang Y, Zhang P, Jiang B. The effect of a small gap sleeve suture at the distal anastomosis of a nerve graft on promoting nerve regeneration and functional recovery. Artif Cells Nanomed Biotechnol. 2013b;41:282–288. doi: 10.3109/21691401.2012.742097. [DOI] [PubMed] [Google Scholar]