Abstract
Currently, researchers are using neural stem cell transplantation to promote regeneration after peripheral nerve injury, as neural stem cells play an important role in peripheral nerve injury repair. This article reviews recent research progress of the role of neural stem cells in the repair of peripheral nerve injury. Neural stem cells can not only differentiate into neurons, astrocytes and oligodendrocytes, but can also differentiate into Schwann-like cells, which promote neurite outgrowth around the injury. Transplanted neural stem cells can differentiate into motor neurons that innervate muscles and promote the recovery of neurological function. To promote the repair of peripheral nerve injury, neural stem cells secrete various neurotrophic factors, including brain-derived neurotrophic factor, fibroblast growth factor, nerve growth factor, insulin-like growth factor and hepatocyte growth factor. In addition, neural stem cells also promote regeneration of the axonal myelin sheath, angiogenesis, and immune regulation. It can be concluded that neural stem cells promote the repair of peripheral nerve injury through a variety of ways.
Keywords: nerve regeneration, neural stem cells, peripheral nerve, regeneration, Schwann-like cells, neurons, neurotrophic factors, neuroprotection, axons, angiogenesis, immune regulation, neural regeneration
Introduction
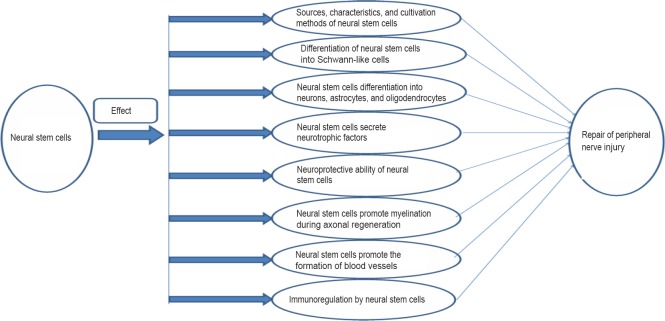
A large proportion of peripheral nerve injuries result in imperfect or no functional recovery, particularly after a complete disruption (Evans, 2001). Peripheral nerve injuries are mainly caused by trauma, birth injury, bone dysplasia, elevated levels of lead in the blood, or alcoholism, and are associated with some degree of disruption or complete disturbance of sensory and/or functional abilities in the areas innervated by the affected nerve. Approximately 13–23 out of 100,000 people suffer peripheral nerve damage each year (Taylor et al., 2008; Asplund et al., 2009), which is important because these injuries can be devastating and life-altering. Recently, the proportion of peripheral nerve injuries among trauma patients increased to approximately 2.8% and more than 50% of these patients failed to recover normal motor and sensory functions following treatment (Scholz et al., 2011; Grinsell and Keating, 2014). Although peripheral nerve axons possess an intrinsic ability to regenerate and form functional connections with their targets, it is difficult to achieve full functional recovery following proximal nerve injury or damage causing crucial nerve gaps (Sachanandani et al., 2014). This is likely due to the death of a large number of neurons in the proximal dorsal root ganglion and anterior horn of the spinal cord (Scheib and Hoke, 2013). Furthermore, after injury, Wallerian degeneration occurs in the distal axon, the required time for axonal regeneration is too long, and there are mismatched results in terms of target organ dysfunction (Zhao et al., 2014). In 1951, Sunderland classified peripheral nerve injuries into Grades I–V (Sunderland, 1951) (Table 1) such that the degree of axonal fracture is described by the first three levels and levels IV and V require surgery to repair the damage (Sullivan et al., 2016). Autologous nerve grafting is often used and has long been considered the gold standard for the treatment of large segmental peripheral nerve defects. However, this therapeutic technique can lead to a series of problems, including nerve dysfunction, scarring, and the requirement of two surgical procedures to obtain tissue prior to its implantation (Jiang et al., 2016). As the development of techniques for peripheral nerve regeneration continues, a large number of studies have shown that stem cells play neuroprotective and regenerative roles in the repair of peripheral nerves (Salibian et al., 2013; Grochmal and Midha, 2014; Fairbairn et al., 2015; Zack-Williams et al., 2015); however, the mechanisms that underlie and support this repair remain unclear. Thus, the present article reviews the mechanisms underlying the actions of neural stem cells (NSCs) during regeneration following peripheral nerve damage (Figure 1).
Table 1.
Peripheral nerve injury grading system (Sunderland, 1951)
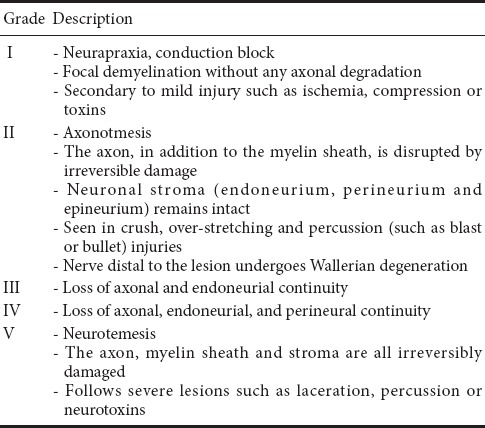
Figure 1.
Neural stem cell repair of peripheral nerve damage.
Sources, Characteristics, and Cultivation Methods of NSCs
NSCs isolated from the adult mouse striatum have the ability to divide, proliferate, and undergo multi-lineage differentiation in vitro; thus, their existence and capabilities dispute the traditional theory that neural tissues cannot regenerate (Reynolds and Weiss, 1992). McKay (1997) summarized the concept of NSCs by describing their ability to differentiate into neurons, astrocytes, and oligodendrocytes and demonstrating that they can be renewed to a degree that provides a large number of brain cells. NSCs are widely distributed in the cerebral cortex, hippocampus, striatum, olfactory bulb, lateral ventricle ependymal zone/subventricular zone, and spinal marrow of embryonic, fetal, and adult central nervous systems (Kornack and Rakic, 1999; Gritti et al., 2002; Alvarez-Buylla and Lim, 2004; Liste et al., 2004; Lu et al., 2005; Selkoe, 2005; Wen et al., 2005; Wong et al., 2005; Harrower et al., 2006). Several studies have shown that mesenchymal stem cells (Koshizuka et al., 2004), human gingiva-derived mesenchymal stem cells (Zhang et al., 2016), induced pluripotent stem cells (Wernig et al., 2008), and the precursor cells of oligodendrocytes (Kondo and Raff, 2000) can be induced to differentiate into NSCs. Furthermore, human NSCs can be obtained from H9 (WA09) human embryonic stem cells (Moore et al., 2017) and neurospheres can be derived from human adipose-derived stromal stem cells (Jahanbazi Jahan-Abad et al., 2017) and, at 10–10.5 weeks of gestational age, from the interbrain and telencephalon (Villa et al., 2000). Stem cells obtained from CD34+ cells in human adult peripheral blood differentiate into adherent NSCs (Wang et al., 2013). Additionally, these authors established a unique in vitro autologous body model of neuroinflammatory disease with the potential for assessing individual pathophysiologies in personalized medical cases (Wang et al., 2013). Other scholars have developed models using human NSCs obtained from the fetal cerebral cortex at 14 weeks of gestation. These human NSCs were cultured using two- and three-dimensional methods (Ghourichaee et al., 2017). Human NSCs differentiate and possess the therapeutic potential to promote locomotor recovery in spinal cord-injured mice (Cummings et al., 2005). Accordingly, many studies have shown that human NSCs can repair central nervous system injuries (Goh et al., 2003; Trounson et al., 2015). Thus, there is great potential for these cells in the repair of peripheral nerve injuries. NSCs can be classified according to their differentiation potential and cell-type generation as follows: (1) neural tube epithelial cells, (2) neuroblasts, and (3) neural progenitor cells. NSCs can also be classified according to their location: (1) neural crest stem cells, and (2) central NSCs. Furthermore, Parker et al. (2015) summarized NSC characteristics as follows: (1) NSC multi-differentiation potential can produce three main cell types in the central nervous system (neurons, astrocytes, and oligodendrocytes) in a number of regions, (2) NSCs can be generated following nerve damage, (3) NSCs can be produced by serial transplantation, and (4) these cells are self-renewing.
Differentiation of NSCs into Schwann-Like Cells
The regeneration of damaged peripheral nerves occurs during a multiplex course in which Schwann cells play a crucial role (Ren et al., 2012). NSCs have been used to repair peripheral nerve injury by initially differentiating them into Schwann-like cells that exhibit biological characteristics similar to their in vivo counterparts. Tong et al. (2010) found that hippocampal NSCs differentiate into Schwann-like cells with similar morphological, phenotypic, and functional characteristics and that differentiated NSCs enhance neurite outgrowth when co-cultured with NG108-15 cells. In that study, the ability of NSCs to differentiate into stem cells highlights their potential use in a wide range of nerve injuries and diseases. Murakami et al. (2003) reported that NSCs derived from the hippocampi of fetal rats differentiate into Schwann-like supportive cells positive for anti-S100 and anti-p75 antibodies. Additionally, when transplanted into areas with peripheral nerve defects, some of these cells differentiated into Schwann-like Sertoli cells that aid and promote axonal regeneration. The implantation of NSCs into the nervous system in mice resulted in formation of a peripheral myelin sheath, similar to Schwann-like cells that exhibit specific M2/M6 markers and glial/Schwann cells (Blakemore, 2005). These findings support the idea that transplanted mouse embryonic stem cell-derived neural progenitor cells may differentiate into Schwann-like cells following severe sciatic nerve transection injury (Cui et al., 2008). Zhang et al. (2016) reported for the first time that gingiva-derived mesenchymal stem cells can be directly induced into pluripotent and extensive neural progenitor-like cells after direct transplantation into the area of sciatic nerve compression injury in rats. These cells differentiated into neuronal cells and stem cells and exhibited potential treatment effects in the damaged nerve and distal injured nerve via the promotion of axonal regeneration. Lee et al. (2017) reported that interleukin 12 p80 activates the differentiation of mouse NSCs that are phosphorylated by signal transducer and activator of transcription 3, which increases the diameter of regenerating nerves and enhances functional recovery following sciatic nerve damage in the mouse (Lee et al., 2017). The findings of Gu et al. (2014) indicate that dorsal root ganglion-derived NSCs exhibit self-renewal and multi-directional differentiation abilities and that basic fibroblast growth factor effectively induces the differentiation of dorsal root ganglion-derived NSCs into Schwann-like cells with biological characteristics that are the same as primary stem cells. These authors also proposed that the basic fibroblast growth factor-induced differentiation of dorsal root ganglion-derived NSCs into stem cells might be mediated, in conjunction with fibroblast growth factor receptor 1, by modulation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway (Gu et al., 2014). Although a large number of studies have shown that NSCs can be induced to differentiate into stem cells and promote axonal regeneration, the differentiation of stem cells predominantly occurs through other stem cells, and the dorsal root ganglion cells, after their induction into NSCs, in turn differentiated into Schwann-like cells. Only a few research studies have supported the direct differentiation of NSCs into stem cells; therefore, further research is warranted. Moreover, future studies will be required to determine whether the differentiation of NSCs into Schwann-like cells generates a sufficient number of functional cells to support nerve regeneration.
Differentiation of NSCs into Neurons, Astrocytes and Oligodendrocytes
NSCs differentiate into neurons, astrocytes, and oligodendrocytes upon receipt of appropriate signals (Guo and Dong, 2009). For example, transplanted NSCs differentiate into motor neurons and functionally innervate muscles following peripheral nerve injury (Thomas et al., 2000; MacDonald et al., 2003; Miles et al., 2004; Li et al., 2005). However, the relationship between regenerated axons and transplanted NSCs remains to be elucidated. Hu et al. (2005) demonstrated that NSCs transduced with neurogenin 2 differentiated into cells that were positive for the neuronal marker TUJ1 and were more efficient at differentiating into neurons when transplanted. NSCs can survive for at least four weeks after being transplanted into the mature inner ear. NSCs also migrate to other important functional structures, such as auditory nerve bundles, the organ of Corti, and spiral-shaped ganglia (Hu et al., 2005). Spinal cord-derived neural progenitor cells cultured in vitro and transplanted into the distal transected sciatic nerve can divide into motor neurons that express markers for choline acetyltransferase, insulin-1, regenerating gene-2, and voltage-sensitive calcium (Ca2+) channels (MacDonald et al., 2003). These authors also showed that the axons of these cells reached the muscle and contributed to formation of cholinergic terminals. Nitric oxide regulates the growth and guidance of axons, synaptic plasticity, the proliferation of the anterior mass of neurons, and the survival of neurons (Madhusoodanan and Murad, 2007). Tao et al. (2010) first demonstrated the manner in which nitric oxide/cyclic guanosine monophosphate signals promote the development of neural precursors from human embryonic stem cells and enhance precursors involved in peripheral nerve regeneration and the function of neurons. A previous study showed that in peripheral nerves NSCs differentiate into neurons and that transplanted fetal NSCs that differentiate into neurons play a role in peripheral nerve regeneration and functional recovery of the neuromuscular unit following muscle denervation, which may be useful for the treatment of peripheral nerve injuries (Gu et al., 2010a). Gao et al. (2005) reported the potential of human fetal NSCs to differentiate into motoneurons in newborns using an animal model of damage to the sciatic nerve axis. They reported for the first time that human NSC-derived motoneurons provided sciatic nerve axons to form neuromuscular connections with the muscles around the target. Furthermore, this novel cholinergic nerve dominance was associated with local improvements in motor function, because NSCs can differentiate into neurons, astrocytes, and oligodendrocytes (McKay, 1997), which play predominant roles in central nervous system repair (Fu et al., 2013; Grade et al., 2013; Liu et al., 2015). Using novel methods, significant progress has been made in terms of understanding the characteristics of endogenous stem cells and their development in the adult spinal cord. Endogenous stem cells can continuously differentiate into neurons (Weiss et al., 1996; Horner et al., 2000; Shihabuddin, 2008). Following the activation of endogenous stem cells by neurotrophic factors in an in vivo microenvironment, the stem cells are induced to become nerve-like cells and participate in the repair of nerve injuries. Faigle and Song (2013) reported that key signaling pathways that regulate the different stages of adult neurogenesis enable the proliferation and lineage differentiation of NSCs as well as the migration and integration of immature neurons in the adult brain. The proliferation of NSCs is associated with a very high level of endogenous reactive oxygen species and can regulate neurogenesis via the phosphoinositide 3-kinase/Akt pathway (Le Belle et al., 2011). Laterza et al. (2017) indicated that infiltrating monocytes, which are a specific component of the early inflammatory response following stroke in the subventricular zone and adjacent striatum, compromise the short-term neurogenic response of endogenous neural stem/progenitor cells. Thus, endogenous stem cells interact with the neurogenic process and are primarily evident in the central nervous system. However, few studies have investigated these processes in the peripheral nerve and, thus, this area has great research potential. However, in studies of peripheral nerve injury, these cells play relatively small roles, especially oligodendrocytes and astrocytes. Taken together, these findings suggest that these three NSC-differentiated cell types should be a focus of future studies investigating the regeneration of injured peripheral nerves.
NSCs Secrete Neurotrophic Factors
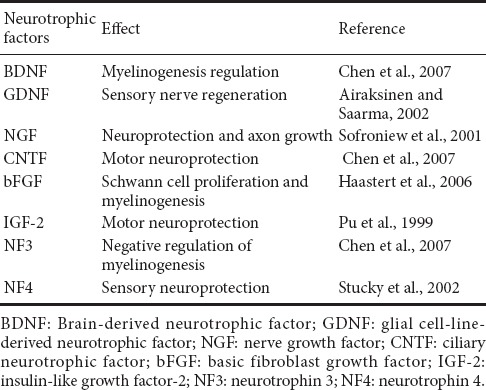
NSCs produce a number of neurotrophic factors (Llado et al., 2004; Xu et al., 2012) that can improve peripheral regeneration in vivo (Guenard et al., 1992; Mosahebi et al., 2001). In both in vitro and in vivo models, NSCs secrete brain-derived neurotrophic factor, basic fibroblast growth factor, nerve growth factor, hepatocyte growth factor, ciliary neurotrophic factor, glial cell-line-derived neurotrophic factor, insulin-like growth factor, neurotrophin 3, and neurotrophin 4, as well as a series of neurotrophic factor proteins (Mirsky et al., 2002; Llado et al., 2004; Kornblum, 2007; Dong and Yi, 2010; Xu et al., 2012; Flachsbarth et al., 2014). These proteins play unique roles in the peripheral nervous system because the expression of neurotrophic factors promotes regeneration, improves the level of axonal myelination, and enhances the recovery of sensory and motor functions (Table 2). In recent years, studies on neurotrophic factors secreted by NSCs have been limited; therefore, the mechanisms associated with the secretion of these factors warrant further exploration.
Table 2.
Effect of neurotrophic factors in the peripheral nervous system
Neuroprotective Ability of NSCs
NSCs operate through the actions of a series of secreted factors to improve the survival rates of motor and sensory neurons located in injured peripheral nerves. To date, few studies have investigated the mechanisms underlying this neuroprotection, but Llado et al. (2004) reported that NSCs produce neurotrophic factors. In particular, glial cell-line-derived neurotrophic factor effectively protects ventral horn neurons and promotes nerve growth; NSCs protect spinal motor neurons against glutamate-induced neurotoxicity, and NSCs ameliorate facial motor neuron apoptosis both in vivo and in vitro following facial nerve injury (Llado et al., 2004). Taken together, these findings explain, at least in part, the protective mechanisms of NSCs following peripheral nerve injury.
NSCs Promoted Myelination during Axonal Regeneration
Myelination of axons is important for the repair of peripheral nerve damage. NSC-derived glial cell-line-derived neurotrophic factor enhances axonal area and axonal numbers following facial nerve injury (Shi et al., 2009). Gu et al. (2010b) transplanted F344 rat nerve segments and F344 rat NSCs into host green fluorescent protein transgenic F344 rats and found that NSCs attenuated denervation-induced muscle atrophy and promoted peripheral nerve regeneration. Furthermore, the regenerated host axons formed synapses with the transplanted and differentiated NSCs. However, further investigation will be required to determine the longevity and functionality of these synapses. Cui et al. (2008) successfully induced murine embryonic stem cells prepared from the wild-type D3 embryonic stem cell line using the 4–/4+ protocol (4 days of leukemia inhibitory factor withdrawal followed by 4 days in retinoic acid) into neural progenitor cells. These authors also explored the possibility that the transplantation of embryonic stem/neural progenitor cells into severely injured peripheral nerves would promote the myelination of regenerated axons. However, the embryonic stem/neural progenitor cells were not able to achieve the axonal numbers necessary for a normal myelin sheath (Cui et al., 2008). Lee et al. (2015) reported that the transplantation of NSCs into mice expressing vascular endothelial growth factor (SV-VEGF-NSCs) resulted in optimal fiber conduction and the regeneration of myelin in a sciatic nerve injury model. Guo and Dong (2009) repaired 10 mm rabbit facial nerve defects with NSCs. The transplanted NSC group showed large numbers of regenerative axons with partial myelination, nerve fibers with uniform diameter, and smooth myelin sheathes. Franchi et al. (2012) found that NSCs promoted the regeneration of small myelinated axon fibers and some thinly myelinated fibers during chronic constriction of sciatic nerve.
NSCs Promoted the Formation of Blood Vessels
Angiogenesis is an important component of peripheral nerve regeneration. Zochodne and Nguyen (1997) found that new microvasculature was prominent in adventitial connective tissue as well as between the anterior fascicular tract and the cell layer at the site of neuroma formation following peripheral nerve injury in Sprague-Dawley rats. These authors proposed that the microvessels likely shared nutrition signals with other proliferative cell elements in the injured peripheral nerve trunk. A previous study showed that SV-VEGF-NSCs secrete high levels of VEGF, and the use of a combined strategy of stem cells and therapeutic genes resulted in a synergistic effect (Oh et al., 2012). Lee et al. (2015) found that transplanted NSCs express higher levels of VEGF and there was a significant increase in the number of blood vessels in the SV-VEGF-NSC group. These findings suggest that VEGF was secreted from SV-VEGF-NSCs transplanted into injured sciatic nerves and subsequently improved angiogenesis (Lee et al., 2015). In addition to their environmental support functions, NSCs may differentiate into epithelial cells. Estradiol management and the transplantation of NSCs increased endovascular blood infusion and significantly increased the recruitment of NSCs to new blood vessels following peripheral nerve injury (Sekiguchi et al., 2013). Taken together, these findings indicate that NSCs secrete VEGF and differentiate into vascular epithelial cells to promote angiogenesis in injured peripheral nerves.
Immunoregulation by NSCs
Recent advances in the field of tissue engineering have allowed for the testing of the immunomodulatory properties of NSCs in several nervous system diseases (Bacigaluppi et al., 2009). Reports on the immunomodulatory effects of NSCs on regeneration following peripheral nerve injury are relatively rare, but this may become a popular field of research in the future. Human neural progenitor cells and a variety of adhesion molecules, such as α2, α6, and β1 integrin, CD44, and numerous chemokine receptors, are associated with neuroinflammation (Hall et al., 2006; Rampon et al., 2008). Furthermore, in vitro analyses have shown that porcine, rat, and mouse neural stem/progenitor cells inhibit the proliferation of activated T cells (Bonnamain et al., 2011).
Conclusions and Expectations
This article describes the characteristics of NSCs, their differentiation into Schwann like-cells and neurons, their expression of neurotrophic factors involved in neuronal protection and angiogenesis, and their roles in the repair and regeneration of damaged peripheral nerves, including myelination and immunoregulation. NSCs may play a role in the repair of peripheral nerve injury. The regeneration of injured peripheral nerves involves a multiplex process in which Schwann cells play an important role; however, it remains unclear whether NSCs directly differentiate into Schwann-like cells and whether this differentiation generates a sufficient number of functional repair cells. Additionally, neurons, astrocytes, and oligodendrocytes develop following NSC differentiation and should represent a primary focus of research in studies of the regeneration of injured peripheral nerves. These questions must be addressed in future research. With current techniques, it is challenging to transplant NSCs into humans. Observations of neural-immune interactions following NSC transplantation and a number of clinical trials aimed at developing applications for NSCs to repair central nervous system damage have illustrated the challenges associated with applying the findings of animal models of disease to the use of NSC derivatives in humans (Giusto et al., 2014; Trounson et al., 2015). For example, three of four patients with the early onset of severe Pelizaeus-Merzbacher disease who received transplantation of neural progenitor cells exhibited slight improvements in neurological function (Gupta et al., 2012). However, further progress remains limited, because it is difficult to recruit young patients with an advanced disease phenotype prior to disease presentation. A few experimental studies have investigated regeneration following peripheral nerve injury, but one researcher has suggested that there is a lack of scientific data to sufficiently support any clinical benefits (Dimmeler et al., 2014). The present article offers a focus for future research regarding the roles of NSCs in peripheral nerve repair/regeneration. Although a better understanding of the biological characteristics of NSCs has been achieved, the mechanisms by which NSCs elicit the repair of peripheral nerves require further investigation. There are many studies concerning use of NSCs in injury of the central nervous system, but NSCs may also have great potential for the repair of peripheral nerve injury. If NSCs can be applied to clinical practice, they will be of great benefit to human beings.
Footnotes
Funding: This study was supported by the Major State Basic Research and Development Program of China (973 Program), No. 2014CB542201; the National Key Research and Development Program of China, No. 2016YFC1101601, 2017YFA0104702; the Natural Science Foundation of Beijing of China, No. 7172202; a grant from the 13th Five-Year Plan Period of People's Liberation Army of China, No. BWS13C029-5; a grant from the Science and Technology Project of Beijing of China, No. Z161100005016059.
Conflicts of interest: None declared.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Benson, Wui-Man Lau, The Hong Kong Polytechnic University, China.
Copyedited by Turnley A, Haase R, Wang J, Li CH, Qiu Y, Song LP, Zhao M
References
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Asplund M, Nilsson M, Jacobsson A, von Holst H. Incidence of traumatic peripheral nerve injuries and amputations in Sweden between 1998 and 2006. Neuroepidemiology. 2009;32:217–228. doi: 10.1159/000197900. [DOI] [PubMed] [Google Scholar]
- Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, Brambilla E, West MJ, Comi G, Martino G, Hermann DM. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- Blakemore WF. The case for a central nervous system (CNS) origin for the Schwann cells that remyelinate CNS axons following concurrent loss of oligodendrocytes and astrocytes. Neuropathol Appl Neurobiol. 2005;31:1–10. doi: 10.1111/j.1365-2990.2005.00637.x. [DOI] [PubMed] [Google Scholar]
- Bonnamain V, Neveu I, Naveilhan P. In vitro analyses of the immunosuppressive properties of neural stem/progenitor cells using anti-CD3/CD28-activated T cells. Methods Mol Biol. 2011;677:233–243. doi: 10.1007/978-1-60761-869-0_17. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- Cui L, Jiang J, Wei L, Zhou X, Fraser JL, Snider BJ, Yu SP. Transplantation of embryonic stem cells improves nerve repair and functional recovery after severe sciatic nerve axotomy in rats. Stem Cells. 2008;26:1356–1365. doi: 10.1634/stemcells.2007-0333. [DOI] [PubMed] [Google Scholar]
- Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Ding S, Rando TA, Trounson A. Translational strategies and challenges in regenerative medicine. Nat Med. 2014;20:814–821. doi: 10.1038/nm.3627. [DOI] [PubMed] [Google Scholar]
- Dong MM, Yi TH. Stem cell and peripheral nerve injury and repair. Facial Plast Surg. 2010;26:421–427. doi: 10.1055/s-0030-1265023. [DOI] [PubMed] [Google Scholar]
- Faigle R, Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim Biophys Acta. 2013;1830:2435–2448. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn NG, Meppelink AM, Ng-Glazier J, Randolph MA, Winograd JM. Augmenting peripheral nerve regeneration using stem cells: a review of current opinion. World J Stem Cells. 2015;7:11–26. doi: 10.4252/wjsc.v7.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachsbarth K, Kruszewski K, Jung G, Jankowiak W, Riecken K, Wagenfeld L, Richard G, Fehse B, Bartsch U. Neural stem cell-based intraocular administration of ciliary neurotrophic factor attenuates the loss of axotomized ganglion cells in adult mice. Invest Ophthalmol Vis Sci. 2014;55:7029–7039. doi: 10.1167/iovs.14-15266. [DOI] [PubMed] [Google Scholar]
- Franchi S, Valsecchi AE, Borsani E, Procacci P, Ferrari D, Zalfa C, Sartori P, Rodella LF, Vescovi A, Maione S, Rossi F, Sacerdote P, Colleoni M, Panerai AE. Intravenous neural stem cells abolish nociceptive hypersensitivity and trigger nerve regeneration in experimental neuropathy. Pain. 2012;153:850–861. doi: 10.1016/j.pain.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Fu ZY, Shi JG, Liu N, Jia LS, Yuan W, Wang Y. Differentiation of neonatal dorsal root ganglion-derived neural stem cells into oligodendrocytes after intrathecal transplantation into a cauda equina lesion model. Genet Mol Res. 2013;12:6092–6102. doi: 10.4238/2013.December.2.7. [DOI] [PubMed] [Google Scholar]
- Gao J, Coggeshall RE, Tarasenko YI, Wu P. Human neural stem cell-derived cholinergic neurons innervate muscle in motoneuron deficient adult rats. Neuroscience. 2005;131:257–262. doi: 10.1016/j.neuroscience.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Ghourichaee SS, Powell EM, Leach JB. Enhancement of human neural stem cell self-renewal in 3D hypoxic culture. Biotechnol Bioeng. 2017;114:1096–1106. doi: 10.1002/bit.26224. [DOI] [PubMed] [Google Scholar]
- Giusto E, Donega M, Cossetti C, Pluchino S. Neuro-immune interactions of neural stem cell transplants: from animal disease models to human trials. Exp Neurol. 2014;260:19–32. doi: 10.1016/j.expneurol.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh EL, Ma D, Ming GL, Song H. Adult neural stem cells and repair of the adult central nervous system. J Hematother Stem Cell Res. 2003;12:671–679. doi: 10.1089/15258160360732696. [DOI] [PubMed] [Google Scholar]
- Grade S, Bernardino L, Malva JO. Oligodendrogenesis from neural stem cells: perspectives for remyelinating strategies. Int J Dev Neurosci. 2013;31:692–700. doi: 10.1016/j.ijdevneu.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014;2014:698256. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti A, Bonfanti L, Doetsch F, Caille I, Alvarez-Buylla A, Lim DA, Galli R, Verdugo JM, Herrera DG, Vescovi AL. Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents. J Neurosci. 2002;22:437–445. doi: 10.1523/JNEUROSCI.22-02-00437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grochmal J, Midha R. Recent advances in stem cell-mediated peripheral nerve repair. Cells Tissues Organs. 2014;200:13–22. doi: 10.1159/000369450. [DOI] [PubMed] [Google Scholar]
- Gu S, Shen Y, Xu W, Xu L, Li X, Zhou G, Gu Y, Xu J. Application of fetal neural stem cells transplantation in delaying denervated muscle atrophy in rats with peripheral nerve injury. Microsurgery. 2010a;30:266–274. doi: 10.1002/micr.20722. [DOI] [PubMed] [Google Scholar]
- Gu SH, Xu WD, Xu L, Li XK, Ochiya T, Wang Y, Li JF, Gu YD, Xu JG. Regenerated host axons form synapses with neurons derived from neural stem cells transplanted into peripheral nerves. J Int Med Res. 2010b;38:1721–1729. doi: 10.1177/147323001003800517. [DOI] [PubMed] [Google Scholar]
- Gu Y, Xue C, Zhu J, Sun H, Ding F, Cao Z, Gu X. Basic fibroblast growth factor (bFGF) facilitates differentiation of adult dorsal root ganglia-derived neural stem cells toward Schwann cells by binding to FGFR-1 through MAPK/ERK activation. J Mol Neurosci. 2014;52:538–551. doi: 10.1007/s12031-013-0109-2. [DOI] [PubMed] [Google Scholar]
- Guenard V, Kleitman N, Morrissey TK, Bunge RP, Aebischer P. Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J Neurosci. 1992;12:3310–3320. doi: 10.1523/JNEUROSCI.12-09-03310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo BF, Dong MM. Application of neural stem cells in tissue-engineered artificial nerve. Otolaryngol Head Neck Surg. 2009;140:159–164. doi: 10.1016/j.otohns.2008.10.039. [DOI] [PubMed] [Google Scholar]
- Gupta N, Henry RG, Strober J, Kang SM, Lim DA, Bucci M, Caverzasi E, Gaetano L, Mandelli ML, Ryan T, Perry R, Farrell J, Jeremy RJ, Ulman M, Huhn SL, Barkovich AJ, Rowitch DH. Neural stem cell engraftment and myelination in the human brain. Sci Transl Med. 2012;4:155ra137. doi: 10.1126/scitranslmed.3004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haastert K, Lipokatic E, Fischer M, Timmer M, Grothe C. Differentially promoted peripheral nerve regeneration by grafted Schwann cells over-expressing different FGF-2 isoforms. Neurobiol Dis. 2006;21:138–153. doi: 10.1016/j.nbd.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Hall PE, Lathia JD, Miller NG, Caldwell MA, ffrench-Constant C. Integrins are markers of human neural stem cells. Stem Cells. 2006;24:2078–2084. doi: 10.1634/stemcells.2005-0595. [DOI] [PubMed] [Google Scholar]
- Harrower TP, Tyers P, Hooks Y, Barker RA. Long-term survival and integration of porcine expanded neural precursor cell grafts in a rat model of Parkinson's disease. Exp Neurol. 2006;197:56–69. doi: 10.1016/j.expneurol.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Andang M, Ni D, Ulfendahl M. Neural cograft stimulates the survival and differentiation of embryonic stem cells in the adult mammalian auditory system. Brain Res. 2005;1051:137–144. doi: 10.1016/j.brainres.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Jahanbazi Jahan-Abad A, Morteza-Zadeh P, Sahab Negah S, Gorji A. Curcumin attenuates harmful effects of arsenic on neural stem/progenitor cells. Avicenna J Phytomed. 2017;7:376–388. [PMC free article] [PubMed] [Google Scholar]
- Jiang CQ, Hu J, Xiang JP, Zhu JK, Liu XL, Luo P. Tissue-engineered rhesus monkey nerve grafts for the repair of long ulnar nerve defects: similar outcomes to autologous nerve grafts. Neural Regen Res. 2016;11:1845–1850. doi: 10.4103/1673-5374.194757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum HI. Introduction to neural stem cells. Stroke. 2007;38:810–816. doi: 10.1161/01.STR.0000255757.12198.0f. [DOI] [PubMed] [Google Scholar]
- Koshizuka S, Okada S, Okawa A, Koda M, Murasawa M, Hashimoto M, Kamada T, Yoshinaga K, Murakami M, Moriya H, Yamazaki M. Transplanted hematopoietic stem cells from bone marrow differentiate into neural lineage cells and promote functional recovery after spinal cord injury in mice. J Neuropathol Exp Neurol. 2004;63:64–72. doi: 10.1093/jnen/63.1.64. [DOI] [PubMed] [Google Scholar]
- Laterza C, Wattananit S, Uoshima N, Ge R, Pekny R, Tornero D, Monni E, Lindvall O, Kokaia Z. Monocyte depletion early after stroke promotes neurogenesis from endogenous neural stem cells in adult brain. Exp Neurol. 2017;297:129–137. doi: 10.1016/j.expneurol.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Chen JH, Hsu TY, Chang LH, Chang H, Chi YH, Chiu IM. Neural stem cells promote nerve regeneration through IL12-induced Schwann cell differentiation. Mol Cell Neurosci. 2017;79:1–11. doi: 10.1016/j.mcn.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Lee HL, Oh J, Yun Y, Lee HY, You Y, Che L, Lee M, Kim KN, Ha Y. Vascular endothelial growth factor-expressing neural stem cell for the treatment of neuropathic pain. Neuroreport. 2015;26:399–404. doi: 10.1097/WNR.0000000000000359. [DOI] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Liste I, Garcia-Garcia E, Martinez-Serrano A. The generation of dopaminergic neurons by human neural stem cells is enhanced by Bcl-XL, both in vitro and in vivo. J Neurosci. 2004;24:10786–10795. doi: 10.1523/JNEUROSCI.3208-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tan B, Wang L, Long Z, Li Y, Liao W, Wu Y. Endogenous neural stem cells in central canal of adult rats acquired limited ability to differentiate into neurons following mild spinal cord injury. Int J Clin Exp Pathol. 2015;8:3835–3842. [PMC free article] [PubMed] [Google Scholar]
- Llado J, Haenggeli C, Maragakis NJ, Snyder EY, Rothstein JD. Neural stem cells protect against glutamate-induced excitotoxicity and promote survival of injured motor neurons through the secretion of neurotrophic factors. Mol Cell Neurosci. 2004;27:322–331. doi: 10.1016/j.mcn.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Lu L, Zhao C, Liu Y, Sun X, Duan C, Ji M, Zhao H, Xu Q, Yang H. Therapeutic benefit of TH-engineered mesenchymal stem cells for Parkinson's disease. Brain Res Brain Res Protoc. 2005;15:46–51. doi: 10.1016/j.brainresprot.2005.03.002. [DOI] [PubMed] [Google Scholar]
- MacDonald SC, Fleetwood IG, Hochman S, Dodd JG, Cheng GK, Jordan LM, Brownstone RM. Functional motor neurons differentiating from mouse multipotent spinal cord precursor cells in culture and after transplantation into transected sciatic nerve. J Neurosurg. 2003;98:1094–1103. doi: 10.3171/jns.2003.98.5.1094. [DOI] [PubMed] [Google Scholar]
- Madhusoodanan KS, Murad F. NO-cGMP signaling and regenerative medicine involving stem cells. Neurochem Res. 2007;32:681–694. doi: 10.1007/s11064-006-9167-y. [DOI] [PubMed] [Google Scholar]
- McKay R. Stem cells in the central nervous system. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- Miles GB, Yohn DC, Wichterle H, Jessell TM, Rafuse VF, Brownstone RM. Functional properties of motoneurons derived from mouse embryonic stem cells. J Neurosci. 2004;24:7848–7858. doi: 10.1523/JNEUROSCI.1972-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky R, Jessen KR, Brennan A, Parkinson D, Dong Z, Meier C, Parmantier E, Lawson D. Schwann cells as regulators of nerve development. J Physiol Paris. 2002;96:17–24. doi: 10.1016/s0928-4257(01)00076-6. [DOI] [PubMed] [Google Scholar]
- Moore L, Skop NB, Rothbard DE, Corrubia LR, Levison SW. Tethered growth factors on biocompatible scaffolds improve stemness of cultured rat and human neural stem cells and growth of oligodendrocyte progenitors. Methods. 2017 doi: 10.1016/j.ymeth.2017.08.015. doi: 10.1016/j.ymeth.2017.08.015. [DOI] [PubMed] [Google Scholar]
- Mosahebi A, Woodward B, Wiberg M, Martin R, Terenghi G. Retroviral labeling of Schwann cells: in vitro characterization and in vivo transplantation to improve peripheral nerve regeneration. Glia. 2001;34:8–17. doi: 10.1002/glia.1035. [DOI] [PubMed] [Google Scholar]
- Murakami T, Fujimoto Y, Yasunaga Y, Ishida O, Tanaka N, Ikuta Y, Ochi M. Transplanted neuronal progenitor cells in a peripheral nerve gap promote nerve repair. Brain Res. 2003;974:17–24. doi: 10.1016/s0006-8993(03)02539-3. [DOI] [PubMed] [Google Scholar]
- Oh JS, An SS, Gwak SJ, Pennant WA, Kim KN, Yoon DH, Ha Y. Hypoxia-specific VEGF-expressing neural stem cells in spinal cord injury model. Neuroreport. 2012;23:174–178. doi: 10.1097/WNR.0b013e32834f4f3a. [DOI] [PubMed] [Google Scholar]
- Parker MA, Anderson JK, Corliss DA, Abraria VE, Sidman RL, Park KI, Teng YD, Cotanche DA, Snyder EY. Expression profile of an operationally-defined neural stem cell clone. Exp Neurol. 2005;194:320–332. doi: 10.1016/j.expneurol.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Pu SF, Zhuang HX, Marsh DJ, Ishii DN. Insulin-like growth factor-II increases and IGF is required for postnatal rat spinal motoneuron survival following sciatic nerve axotomy. J Neurosci Res. 1999;55:9–16. doi: 10.1002/(SICI)1097-4547(19990101)55:1<9::AID-JNR2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Rampon C, Weiss N, Deboux C, Chaverot N, Miller F, Buchet D, Tricoire-Leignel H, Cazaubon S, Baron-Van Evercooren A, Couraud PO. Molecular mechanism of systemic delivery of neural precursor cells to the brain: assembly of brain endothelial apical cups and control of transmigration by CD44. Stem Cells. 2008;26:1673–1682. doi: 10.1634/stemcells.2008-0122. [DOI] [PubMed] [Google Scholar]
- Ren Z, Wang Y, Peng J, Zhao Q, Lu S. Role of stem cells in the regeneration and repair of peripheral nerves. Rev Neurosci. 2012;23:135–143. doi: 10.1515/revneuro-2011-0069. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Sachanandani NF, Pothula A, Tung TH. Nerve gaps. Plast Reconstr Surg. 2014;133:313–319. doi: 10.1097/01.prs.0000436856.55398.0f. [DOI] [PubMed] [Google Scholar]
- Salibian AA, Widgerow AD, Abrouk M, Evans GR. Stem cells in plastic surgery: a review of current clinical and translational applications. Arch Plast Surg. 2013;40:666–675. doi: 10.5999/aps.2013.40.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheib J, Hoke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013;9:668–676. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- Scholz T, Sumarto A, Krichevsky A, Evans GR. Neuronal differentiation of human adipose tissue-derived stem cells for peripheral nerve regeneration in vivo. Arch Surg. 2011;146:666–674. doi: 10.1001/archsurg.2011.148. [DOI] [PubMed] [Google Scholar]
- Sekiguchi H, Ii M, Jujo K, Thorne T, Ito A, Klyachko E, Hamada H, Kessler JA, Tabata Y, Kawana M, Asahi M, Hagiwara N, Losordo DW. Estradiol promotes neural stem cell differentiation into endothelial lineage and angiogenesis in injured peripheral nerve. Angiogenesis. 2013;16:45–58. doi: 10.1007/s10456-012-9298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Defining molecular targets to prevent Alzheimer disease. Arch Neurol. 2005;62:192–195. doi: 10.1001/archneur.62.2.192. [DOI] [PubMed] [Google Scholar]
- Shi Y, Zhou L, Tian J, Wang Y. Transplantation of neural stem cells overexpressing glia-derived neurotrophic factor promotes facial nerve regeneration. Acta Otolaryngol. 2009;129:906–914. doi: 10.1080/00016480802468153. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS. Adult rodent spinal cord-derived neural stem cells: isolation and characterization. Methods Mol Biol. 2008;438:55–66. doi: 10.1007/978-1-59745-133-8_6. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Shin JB, Lewin GR. Neurotrophin-4: a survival factor for adult sensory neurons. Curr Biol. 2002;12:1401–1404. doi: 10.1016/s0960-9822(02)01072-2. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Dailey T, Duncan K, Abel N, Borlongan CV. Peripheral nerve injury: stem cell therapy and peripheral nerve transfer. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491–516. doi: 10.1093/brain/74.4.491. [DOI] [PubMed] [Google Scholar]
- Tao Li J, Somasundaram C, Bian K, Xiong W, Mahmooduddin F, Nath RK, Murad F. Nitric oxide signaling and neural stem cell differentiation in peripheral nerve regeneration. Eplasty. 2010;10:e42. [PMC free article] [PubMed] [Google Scholar]
- Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008;87:381–385. doi: 10.1097/PHM.0b013e31815e6370. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Erb DE, Grumbles RM, Bunge RP. Embryonic cord transplants in peripheral nerve restore skeletal muscle function. J Neurophysiol. 2000;84:591–595. doi: 10.1152/jn.2000.84.1.591. [DOI] [PubMed] [Google Scholar]
- Tong L, Ji L, Wang Z, Tong X, Zhang L, Sun X. Differentiation of neural stem cells into Schwann-like cells in vitro. Biochem Biophys Res Commun. 2010;401:592–597. doi: 10.1016/j.bbrc.2010.09.107. [DOI] [PubMed] [Google Scholar]
- Trounson A, Kolaja K, Petersen T, Weber K, McVean M, Funk KA. Stem Cell Research. Int J Toxicol. 2015;34:349–351. doi: 10.1177/1091581815581423. [DOI] [PubMed] [Google Scholar]
- Wang T, Choi E, Monaco MC, Campanac E, Medynets M, Do T, Rao P, Johnson KR, Elkahloun AG, Von Geldern G, Johnson T, Subramaniam S, Hoffman DA, Major E, Nath A. Derivation of neural stem cells from human adult peripheral CD34+ cells for an autologous model of neuroinflammation. PLoS One. 2013;8:e81720. doi: 10.1371/journal.pone.0081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen T, Li H, Song H, Chen F, Zhao C, Lu W, Bao K, Jin Y. Down-regulation of specific gene expression by double-strand RNA induces neural stem cell differentiation in vitro. Mol Cell Biochem. 2005;275:215–221. doi: 10.1007/s11010-005-2049-9. [DOI] [PubMed] [Google Scholar]
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AM, Hodges H, Horsburgh K. Neural stem cell grafts reduce the extent of neuronal damage in a mouse model of global ischaemia. Brain Res. 2005;1063:140–150. doi: 10.1016/j.brainres.2005.09.049. [DOI] [PubMed] [Google Scholar]
- Xu L, Zhou S, Feng GY, Zhang LP, Zhao DM, Sun Y, Liu Q, Huang F. Neural stem cells enhance nerve regeneration after sciatic nerve injury in rats. Mol Neurobiol. 2012;46:265–274. doi: 10.1007/s12035-012-8292-7. [DOI] [PubMed] [Google Scholar]
- Zack-Williams SD, Butler PE, Kalaskar DM. Current progress in use of adipose derived stem cells in peripheral nerve regeneration. World J Stem Cells. 2015;7:51–64. doi: 10.4252/wjsc.v7.i1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Nguyen P, Xu Q, Park W, Lee S, Furuhashi A, Le AD. Neural Progenitor-like cells induced from human gingiva-derived mesenchymal stem cells regulate myelination of schwann cells in rat sciatic nerve regeneration. Stem Cells Transl Med. 2016 doi: 10.5966/sctm.2016-0177. DOI: 10.5966/sctm.2016-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Wang Y, Peng J, Ren Z, Zhang L, Guo Q, Xu W, Lu S. Improvement in nerve regeneration through a decellularized nerve graft by supplementation with bone marrow stromal cells in fibrin. Cell Transplant. 2014;23:97–110. doi: 10.3727/096368912X658845. [DOI] [PubMed] [Google Scholar]
- Zochodne DW, Nguyen C. Angiogenesis at the site of neuroma formation in transected peripheral nerve. J Anat. 1997;191:23–30. doi: 10.1046/j.1469-7580.1997.19110023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]