Abstract
Background
In the general population, low circulating testosterone levels are associated with higher risk of cardiovascular disease and death. While testosterone deficiency is common in dialysis patients, studies of testosterone and mortality in this population have been mixed. We hypothesized that lower testosterone levels are associated with higher mortality in male dialysis patients.
Methods
We examined a nationally representative cohort of male dialysis patients from a large US dialysis organization who underwent one or more total testosterone measurements over 1/2007–12/2011. The association between total testosterone categorized as quartiles and all-cause mortality was studied using Cox models adjusted for expanded case-mix and laboratory covariates. We also examined total testosterone as a continuous predictor of all-cause mortality using restricted cubic splines.
Results
Among 624 male dialysis patients, 51% of patients demonstrated testosterone deficiency (total testosterone <300ng/dl); median (IQR) total testosterone levels were 297 (190, 424) ng/ml. In expanded case-mix+laboratory adjusted Cox analyses, we observed a graded association between lower testosterone levels and higher mortality risk (ref: Quartile 3): adjusted HRs (95% CI) 2.32 (1.33–4.06), 1.80 (0.99–3.28), and 0.68 (0.32–1.42) for Quartiles 1, 2, and 4, respectively. In adjusted spline analyses, the lower testosterone—higher mortality risk association declined with higher testosterone levels until reaching a threshold of 400ng/dl above which risk plateaued.
Conclusion
Lower testosterone levels were independently associated with higher mortality risk in male dialysis patients. Further studies are needed to determine underlying mechanisms, and if testosterone replacement ameliorates death risk in this population.
Keywords: Testosterone, mortality, survival, dialysis
Introduction
In the general population, testosterone deficiency is a highly prevalent condition, affecting 6–12% of men over the age of 30 years and up to 30% of men greater than the age of 60 years.[1–3] Observational data have also shown that testosterone supplementation in adult males with testosterone deficiency is associated with a significant reduction in their risk for myocardial infarction, stroke, and all-cause death.[4, 5] These findings may bear particular relevance among male end-stage renal disease (ESRD) patients, who commonly manifest low testosterone levels,[6] and who suffer from disproportionately high mortality rates due to cardiovascular disease (40% of deaths).[7]
Epidemiologic data have indeed shown a disproportionately high prevalence of testosterone deficiency in ESRD patients. For example, in a study of 260 men with ESRD, it was shown that 44% of patients had testosterone deficiency and 33% had testosterone insufficiency (defined as total testosterone levels <10nmol/L [<288ng/dl] and 10–14nmol/L [288–404ng/dl], respectively), while only 23% demonstrated normal testosterone concentrations (total testosterone >14nmol/L [>404ng/dl]).[8] Other data suggest that as many as two-thirds of ESRD patients have biochemical evidence of testosterone deficiency using these criteria.[9] The etiologic factors for low testosterone levels in kidney disease may be multifactorial and include alterations in the hypothalamic-pituitary-axis and metabolic milieu (i.e., decreased gonadotropin-releasing hormone pulsation, prolactin retention with inhibition of luteinizing hormone signaling), heightened inflammation (i.e., increased C-reactive protein, interleukin-6, and fibrinogen levels), advanced age, and co-existing comorbidities.[10–12]
Given their exceedingly high cardiovascular death risk, there has been considerable interest in understanding the implications of testosterone deficiency upon mortality risk in ESRD patients. Prior studies of testosterone and mortality in European, Canadian, and Australian dialysis cohorts have shown mixed findings, likely due to heterogeneous study cohort composition and sizes, varying definitions of testosterone deficiency, inconsistent consideration of confounders, and differential follow-up periods.[8, 9, 13–16] To date, there has not been a large population-based study examining the relationship between testosterone deficiency and mortality specifically in US male dialysis patients who manifest a differential racial/ethnic composition and comorbidity burden versus international dialysis populations. Thus, to better inform the field, we sought to examine the association between serum total testosterone levels and mortality risk among a large national cohort of US dialysis patients. We additionally examined predictors of testosterone deficiency in this population.
Materials and Methods
Source Population
We conducted a historical cohort study of incident/prevalent adult male hemodialysis and peritoneal dialysis patients receiving treatment within the outpatient facilities of a large US dialysis organization over the period of January 1, 2007 to December 30, 2011 with detailed information on socio-demographics, comorbidities (ascertained by International Classification of Disease 9th Revision codes), laboratory tests, dialysis treatment characteristics, clinical events, and vital status.[17, 18] Patients were included provided that they underwent at least one or more serum total testosterone measurement(s) anytime during the study period, and were age 18 years or older at the time of study entry (date of testosterone measurement). Patients were excluded if they underwent treatment with a dialysis modality other than in-center hemodialysis or peritoneal dialysis at study entry. The study was approved by the Institutional Review Committees of the Los Angeles Biomedical Research Institute at Harbor-UCLA, University of California Irvine Medical Center, and the University of Washington.
Exposure and Outcome Ascertainment
The exposure of interest was serum total testosterone level ascertained at study entry (baseline testosterone level). In primary analyses, we examined total testosterone levels categorized as quartiles defined by the following thresholds: total testosterone ≤190, >190–296, >296–423, and >423ng/dl for Quartiles 1, 2, 3, and 4, respectively. In order to flexibly examine total testosterone as a continuous predictor of mortality, we conducted restricted cubic spline analyses with knots defined at the 33rd and 66th percentiles of observed values (corresponding to total testosterone levels of 227 and 361ng/dl, respectively) in sensitivity analyses.
The outcome of interest was all-cause mortality. At-risk time began the day after the baseline total testosterone measurement. Patients were censored for kidney transplantation, transfer to a dialysis facility operated by another provider, or at the end of the study (December 31, 2011).
Laboratory Data Collection
Serum samples for total testosterone and other laboratory tests were drawn at the indication of providers using standardized techniques within the outpatient dialysis clinics of the large dialysis organization, transported to a single central laboratory in Deland, Florida typically within 24 hours of collection, and measured using automated and standardized methods. Most blood samples were collected pre-dialysis, except for post-dialysis serum urea nitrogen to calculate urea kinetics.
Statistical Analysis
Baseline characteristics between exposure groups were compared using chi-square, analysis of variance, and Kruskal-Wallis tests according to data type. We first examined the association between relevant clinical characteristics with testosterone deficiency (defined as total testosterone <300ng/dl according to Endocrine Society guidelines[19]; reference: total testosterone ≥300ng/dl) using logistic regression. We then estimated the association between total testosterone levels and all-cause mortality using Cox proportional hazard models. Logistic and Cox regression models were analyzed using three hierarchical levels of adjustment with covariates ascertained at baseline:
Minimally adjusted model: Adjusted for calendar quarter of study entry;
Case-mix model: Adjusted for minimally adjusted model covariates, as well as age, sex, race/ethnicity, and diabetes;
Expanded case-mix+laboratory model: Adjusted for case-mix model covariates, as well as dialysis vintage, cause of ESRD, modality, dialysis access, congestive heart failure, coronary heart disease, and serum albumin level.
We a priori defined the expanded case-mix+laboratory model as our preferred model, which included core socio-demographic measures and other confounders of the association between total testosterone level and mortality. Proportional hazards assumptions were checked by graphical and formal testing.
We conducted subgroup analyses of testosterone level (dichotomized as total testosterone <300 vs. ≥300ng/dl[19]; reference: total testosterone ≥300ng/dl) and mortality across clinically relevant categories of socio-demographics, comorbidity status, and laboratory measures. There were no missing values for any of the covariates, including age, sex, race/ethnicity, diabetes, vintage, cause of ESRD, dialysis access, congestive heart failure, coronary heart disease, or serum albumin levels. Analyses and figures were generated using SAS version 9.4 (SAS Institute Inc., Cary, NC), Stata version 13.1 (Stata Corporation, College Station, TX), and SigmaPlot Version 12.5 (Systat Software, San Jose, CA).
Results
Study Cohort
Among 624 patients meeting eligibility criteria, the mean ± SD and median (IQR) baseline total testosterone values were 323±193 and 297 (190, 424) ng/dl (Supplementary Figure 1). Fifty-one percent of patients (N=317) had testosterone deficiency (defined as total testosterone <300ng/dl). When total testosterone levels were examined across strata of age (<40, 40–60, >60 years of age), there was a dose-response relationship between older age and lower mean ± SD serum testosterone levels: 371 ± 222 and 336 (224, 480) vs. 280 ± 192 and 247 (158, 359) ng/dl, respectively (Supplementary Table 1).
Compared with patients in highest testosterone quartile, those in the lowest quartile were of older age; were less likely to be African-American; had shorter dialysis vintage; and were more likely to have diabetes and congestive heart failure (Table 1).
Table 1.
Baseline characteristics among dialysis patients according to baseline total testosterone quartile.
| TOTAL TESTOSTERONE LEVEL | |||||
|---|---|---|---|---|---|
|
| |||||
| Overall | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|
| |||||
| N | 624 | 158 | 154 | 156 | 156 |
|
| |||||
| Testosterone range (ng/dl) | |||||
| Min–Max | 0–1644 | 0–190 | 191–296 | 297–423 | 425–1644 |
|
| |||||
| Age (years) | |||||
| Mean + SD | 58 ± 14 | 61 ± 14 | 60 ± 15 | 56 ± 13 | 54 ± 14 |
|
| |||||
| Race (%) | |||||
| Black | 32 | 21 | 29 | 37 | 44 |
| Non-black | 68 | 79 | 71 | 63 | 56 |
|
| |||||
| Vintage (days) | |||||
| Median (IQR) | 370 | 281 (97, 660) | 374 (160, 720) | 396 (167, 735) | 417 (168, 727) |
|
| |||||
| Vascular Access (%) | |||||
| CVC | 21 | 24 | 19 | 21 | 18 |
| AVF/AVG | 56 | 56 | 58 | 54 | 59 |
| PD Catheter | 29 | 16 | 19 | 22 | 23 |
| Unknown/Missing | 3 | 4 | 4 | 2 | 1 |
|
| |||||
| Cause of ESRD (%) | |||||
| Diabetes | 40 | 42 | 40 | 40 | 37 |
| Hypertension | 29 | 30 | 25 | 31 | 31 |
| Glomerulonephritis | 14 | 11 | 16 | 11 | 19 |
| Cystic Disease | 2.7 | 1.9 | 1.9 | 5.1 | 1.9 |
| Other | 14 | 15 | 16 | 13 | 10 |
|
| |||||
| Modality (%) | |||||
| HD | 79.6 | 83.5 | 81.2 | 77.6 | 76.3 |
| PD | 20.3 | 16.5 | 18.8 | 22.4 | 23.7 |
|
| |||||
| Diabetes (%) | 61.9 | 65.2 | 60.4 | 62.8 | 59.0 |
|
| |||||
| CHF (%) | 47.3 | 54.4 | 50.0 | 40.4 | 44.2 |
|
| |||||
| CHD (%) | 18.6 | 18.4 | 15.6 | 22.4 | 18.0 |
|
| |||||
| Serum albumin (g/dl) | |||||
| Median (IQR) | 3.9 (3.6, 4.1) | 3.9 (3.6, 4.1) | 3.9 (3.6, 4.2) | 3.9 (3.6, 4.2) | 3.9 (3.6, 4.2) |
Abbreviations: CVC, central venous catheter; AVF, arteriovenous fistula; AVG, arteriovenous grat; PD, peritoneal dialysis; HD, hemodialysis; CHF, congestive heart failure; CHD, coronary heart disease.
Clinical Characteristics Associated with Testosterone Deficiency
In minimally adjusted logistic regression analyses, patients of older age, with underlying congestive heart failure, higher body mass index, and higher serum ferritin had higher risk of having testosterone deficiency (defined as total testosterone <300ng/dl), whereas patients with higher hemoglobin levels were less likely to have testosterone deficiency (Table 2). These associations persisted with further adjustment for case-mix and expanded case-mix+laboratory covariates.
Table 2.
Clinical characteristics associated with testosterone deficiency (total testosterone <300 ng/dl) using logistic regression.
| Minimally adjusted OR (95% CI) |
Case-mix adjusted OR (95% CI) |
Expanded case-mix + laboratory adjusted OR (95% CI) |
|
|---|---|---|---|
|
| |||
| Age (Δ10 years) | 1.30 (1.15, 1.46) | 1.23 (1.09, 1.40) | 1.23 (1.08, 1.40) |
|
| |||
| Black race (ref: Non-Black) | 0.45 (0.32, 0.64) | 0.52 (0.36, 0.75) | 0.51 (0.35, 0.76) |
|
| |||
| Vintage (Δ1 month) | 1.00 (0.99, 1.02) | 1.01 (0.99, 1.02) | 1.01 (0.99, 1.03) |
|
| |||
| Vascular Access (ref: CVC) | |||
| AVF/AVG | 1.04 (0.68, 1.59) | 1.02 (0.66, 1.59) | 1.03 (0.65, 1.64) |
| PD Catheter | 0.68 (0.40, 1.13) | 0.63 (0.37, 1.07) | 0.66 (0.37, 1.16) |
| Unknown/Missing | 3.87 (0.99, 15.1) | 4.67 (1.14, 19.2) | 4.75 (1.14, 19.8) |
|
| |||
| Cause of ESRD (ref: Diabetes) | |||
| Hypertension | 0.77 (0.52, 1.14) | 0.82 (0.52, 1.30) | 0.88 (0.55, 1.41) |
| Glomerulonephritis | 0.83 (0.50, 1.38) | 0.93 (0.53, 1.62) | 1.07 (0.60, 1.91) |
| Cystic Disease | 0.54 (0.19, 1.54) | 0.59 (0.19, 1.76) | 0.69 (0.22, 2.19) |
| Other | 1.25 (0.74, 2.11) | 1.19 (0.67, 2.13) | 1.36 (0.75, 2.46) |
|
| |||
| Diabetes | 1.11 (0.79, 1.56) | 1.08 (0.76, 1.54) | 1.12 (0.73, 1.71) |
|
| |||
| CHF | 1.66 (1.19, 2.31) | 1.62 (1.15, 2.28) | 1.44 (1.00, 2.07) |
|
| |||
| CHD | 0.81 (0.53, 1.23) | 0.74 (0.48, 1.14) | 0.69 (0.44, 1.08) |
|
| |||
| Serum albumin (Δ0.5 g/dl) | 0.86 (0.71, 1.03) | 0.92 (0.76, 1.11) | 0.85 (0.69, 1.05) |
|
| |||
| BMI (Δ5 kg/m2) | 1.16 (1.01, 1.32) | 1.23 (1.07, 1.41) | 1.22 (1.05, 1.42) |
|
| |||
| Serum creatinine (mg/dl) | 0.94 (0.90, 0.99) | 1.00 (0.95, 1.06) | 1.03 (0.96, 1.09) |
|
| |||
| Calcium (mg/dl) | 1.03 (0.76, 1.41) | 0.98 (0.71, 1.35) | 1.06 (0.76, 1.48) |
|
| |||
| Phosphorus (mg/dl) | 1.00 (0.88, 1.14) | 1.09 (0.94, 1.25) | 1.07 (0.93, 1.24) |
|
| |||
| PTH (Δ25pg/ml) | 0.98 (0.96, 1.00) | 0.99 (0.98, 1.01) | 0.99 (0.97, 1.01) |
|
| |||
| Bicarbonate (Δ5meq/L) | 0.87 (0.67, 1.13) | 0.79 (0.60, 1.04) | 0.82 (0.61, 1.10) |
|
| |||
| Hemoglobin (g/dl) | 0.76 (0.65, 0.89) | 0.77 (0.65, 0.90) | 0.76 (0.64, 0.91) |
|
| |||
| Transferrin saturation (Δ5%) | 0.97 (0.90, 1.04) | 0.97 (0.90, 1.04) | 0.98 (0.91, 1.06) |
|
| |||
| Ferritin (Δ50ng/ml) | 1.03 (1.01, 1.06) | 1.03 (1.00, 1.05) | 1.02 (1.00, 1.05) |
|
| |||
| Renal urea clearance (ml/min) | 0.98 (0.88, 1.10) | 1.00 (0.88, 1.15) | 0.98 (0.85, 1.14) |
|
| |||
| spKt/V (Δ0.2) | 1.09 (0.98, 1.22) | 1.04 (0.93, 1.16) | 1.07 (0.94, 1.22) |
|
| |||
| nPCR (Δ0.2g/kg/day) | 1.11 (0.97, 1.28) | 1.09 (0.94, 1.26) | 1.15 (0.98, 1.35) |
Abbreviations: CVC, central venous catheter; AVF, arteriovenous fistula; AVG, arteriovenous graft; PD, peritoneal dialysis; ESRD, end stage renal disease; CHF, congestive heart failure; CHD, coronary heart disease; BMI, body mass index; PTH, parathyroid hormone; nPCR, normalized protein catabolic rate. Minimally adjusted analyses adjusted for calendar quarter of study entry; Case-mix adjusted analyses adjusted for minimally adjusted model covariates, as well as age, sex, race/ethnicity, and diabetes; Expanded case-mix models adjusted for case-mix model covariates, as well as dialysis vintage, cause of ESRD, dialysis access, modality, CHF, CHD, and serum albumin level.
Testosterone and All-Cause Mortality
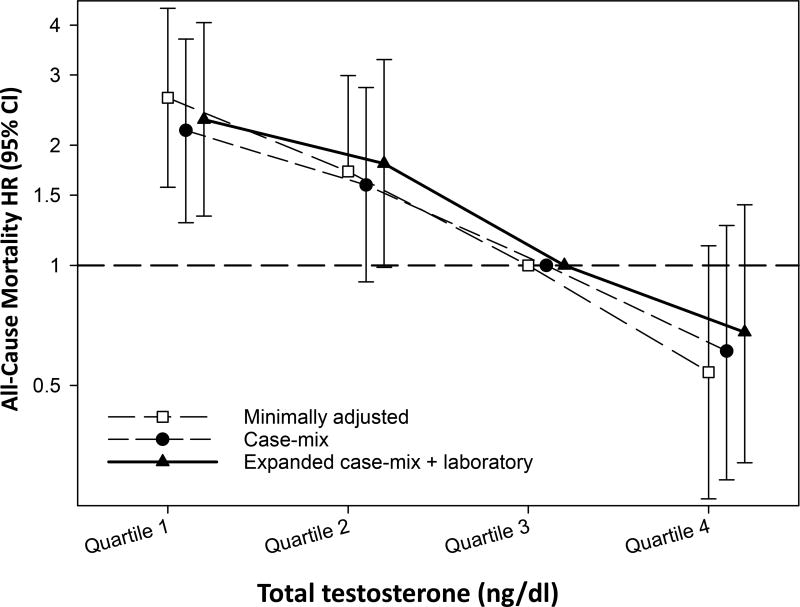
Patients contributed a total of 806 patient-years of follow up, during which time 108 all-cause deaths occurred. The median (IQR) at-risk time was 1.2 (0.5, 1.9) years. In minimally adjusted analyses, point estimates for increasingly lower testosterone quartiles were associated with numerically higher mortality risk (ref: Quartile 3): HRs (95% CI) 2.63 (1.57–4.41), 1.72 (0.99–2.99), and 0.54 (0.26–1.12) for Quartiles 1, 2, and 4, respectively (Figure 1 and Supplementary Table 2). With incremental adjustment for case-mix covariates, the association between Quartile 1 and higher mortality remained statistically significant. In both minimally adjusted and case-mix models, Quartile 2 was associated with numerically higher mortality risk but did not reach statistical significance. In expanded case-mix+laboratory adjusted analyses, point estimates for increasingly lower testosterone quartiles were associated with numerically higher mortality risk (ref: Quartile 3): adjusted HRs (aHR) (95% CI) 2.32 (1.33–4.06), 1.80 (0.99–3.28), and 0.68 (0.32–1.42) for Quartiles 1, 2, and 4, respectively.
Figure 1. Association between baseline total testosterone levels and all-cause mortality in dialysis patients in minimally adjusted, case-mix, and expanded case-mix+laboratory adjusted models.
Minimally adjusted analyses adjusted for calendar quarter of study entry; Case-mix adjusted analyses adjusted for minimally adjusted model covariates, as well as age, sex, race/ethnicity, and diabetes; Expanded case-mix models adjusted for case-mix model covariates, as well as dialysis vintage, cause of end-stage renal disease, modality, dialysis access, congestive heart failure, coronary heart disease, and serum albumin level.
In restricted cubic spline analyses of total testosterone as a continuous predictor and all-cause mortality, we observed that mortality risk declined with increasingly higher testosterone levels until reaching a threshold of 400ng/dl above which risk plateaued in case-mix and expanded case-mix+laboratory models (Supplementary Figure 2).
Subgroup Analyses
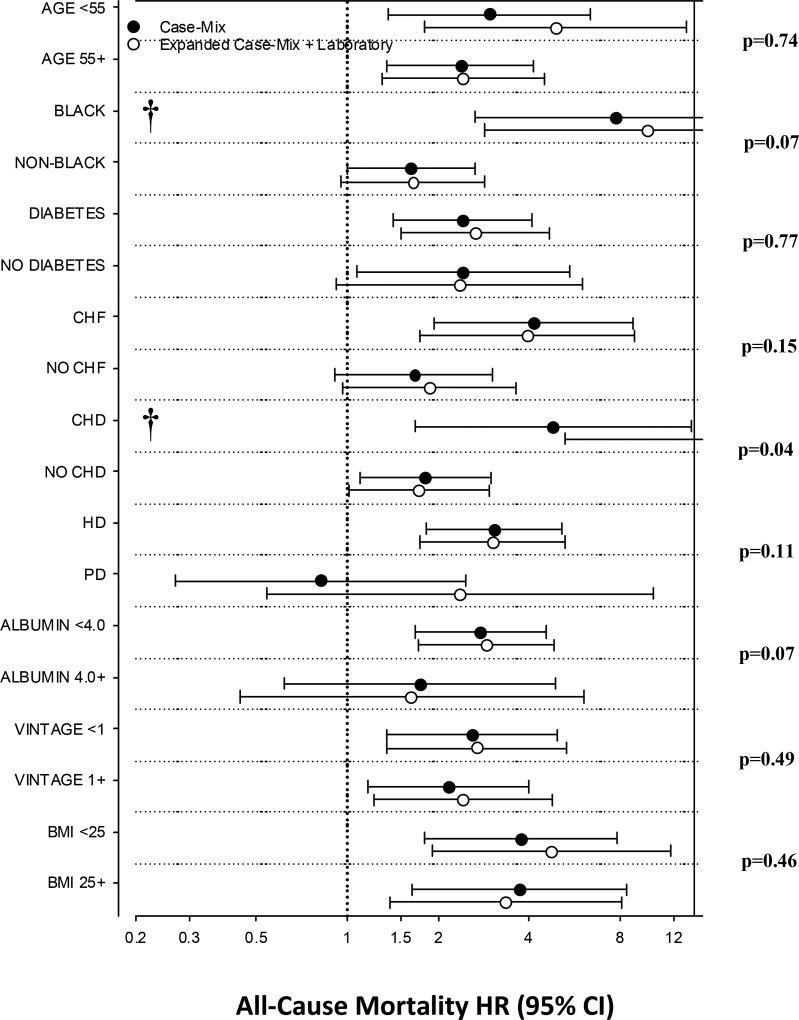
In expanded case-mix+laboratory adjusted analyses, there was effect modification on the basis of underlying coronary heart disease, such that the relationship between testosterone deficiency and mortality was stronger in those with coronary heart disease vs. those without coronary heart disease (p-interaction=0.04) (Figure 2 and Supplementary Table 3). In expanded case-mix+laboratory adjusted analyses, nominal HRs for testosterone deficiency were >1 across all subgroups; nominal associations were statistically significant in the following subgroups: age >55 years, age ≥55 years, Black race, presence of diabetes, presence of congestive heart failure, presence of coronary heart disease, absence of coronary heart disease, receipt of hemodialysis, serum albumin ≥4.0g/dl, vintage <1 year, vintage ≥1 year, body mass index <25 kg/m2, and body mass index ≥25 kg/m2.
Figure 2. Associations between baseline total testosterone level (dichotomized as total testosterone level <300ng/dl vs. ≥300ng/dl; reference: total testosterone ≥300ng/dl) and all-cause mortality.
Case-mix adjusted analyses adjusted for calendar quarter of study entry, age, sex, race/ethnicity, and diabetes; Expanded case-mix models adjusted for case-mix model covariates, as well as dialysis vintage, cause of end-stage renal disease, modality, dialysis access, congestive heart failure, coronary heart disease, and serum albumin level.
†Upper bound of 95% CI exceeds figure limits.
Discussion
In a nationally representative cohort of US dialysis patients, we observed that the prevalence of testosterone deficiency (total testosterone <300ng/dl) was substantially higher than that of the general population, but similar to that of other international dialysis cohorts.[8, 9, 13, 14, 16] In analyses of total testosterone as a categorical predictor, we found that point estimates of incrementally lower testosterone quartiles were associated with increasingly higher mortality risk independent of case-mix and laboratory characteristics. In subgroup analyses that dichotomized testosterone as <300ng/dl (i.e., testosterone deficiency[19]) vs. ≥300ng/dl, we found that testosterone deficiency was associated with higher death risk across various subgroups defined by socio-demographic, comorbidity, and nutritional status.
In international, non-US based dialysis cohorts, there have been several studies of total testosterone and mortality that have shown mixed findings.[8, 9, 13–16] In one of the seminal studies which examined a cohort of 126 Swedish hemodialysis patients by Carrero et al., those in the lowest total testosterone tertile (<233ng/dl) vs. highest tertile (>345ng/dl) had a higher mortality risk independent of socio-demographics, comorbidities, medications, and laboratory tests; however, upon adjustment for serum creatinine as a proxy of muscle mass, these associations were attenuated to the null.[14] Similarly, in a study of 420 Turkish hemodialysis patients by Gungor et al., crude analyses showed a significant association between the lowest total testosterone tertile (<6.8nmol/L or <196ng/dl) and mortality, which was attenuated to the null in adjusted analyses.[9] Yet in two subsequent studies of 260 Swedish ESRD patients by Carrero et al. and 111 Greek hemodialysis patients by Kyriazis et al., those in the lowest total testosterone tertile (<10nmol/L [288ng/dl] and <5.2nmol/L [150ng/dl], respectively) had a significantly higher mortality risk independent of socio-demographics, comorbidity status, and inflammatory markers.[8, 16] Most recently, in a study of 623 Canadian hemodialysis patients by Bello et al., the impact of low testosterone levels upon mortality was dependent on age, such that low testosterone levels (<231ng/dl) were associated with higher mortality in those of younger age (<63 years old) but not in those of older age (≥63 years old).[13]
To our knowledge, this is the largest study of testosterone and mortality in US dialysis patients conducted to date. Our primary analyses of testosterone as a categorical (i.e., quartiles) predictor suggest that levels below ~296ng/dl are associated with lower survival. In secondary analyses of testosterone as a continuous (i.e., splines) predictor, the lower testosterone—higher mortality risk declined with increasingly higher testosterone levels until reaching a threshold of 400ng/dl above which risk plateaued. These associations between lower testosterone level and higher mortality risk were independent of age, medical comorbidities, dialysis vintage, and race, thereby suggesting that testosterone plays an independent physiologic role that contributes to the survival of the ESRD population. In addition, compared to the aforementioned international studies,[8, 9, 13, 14, 16] our study population was observed to have a high prevalence of African Americans and those with underlying diabetes and cardiovascular disease, which is more reflective of the racial/ethnic diversity and high burden of medical comorbidities typically encountered in the US dialysis population.[7] Despite these case-mix differences, we observed a similar testosterone threshold associated with greater survival as observed in prior dialysis studies.[8, 13, 16]
Another novel finding of our study was the potent interaction by patient characteristics, such as underlying cardiovascular risk. For example, we observed that stronger testosterone—mortality associations were observed in those with coronary heart disease vs. those without coronary heart disease. In the general population, testosterone deficiency has been associated with multiple atherosclerotic risk factors including dyslipidemia,[20], obesity,[21] inflammation,[22] and incident diabetes.[23] Given that observational studies have shown a relationship between low testosterone and coronary heart disease risk factors such as endothelial dysfunction[24], increased arterial stiffness[16], anemia and erythropoietin-stimulating agent resistance[25] in chronic kidney disease and ESRD patients, it has been speculated that testosterone deficiency may be linked to higher death risk in these populations via cardiovascular pathways. Thus, it is plausible that dialysis patients with underlying coronary heart disease may be predisposed to cardiovascular mortality associated with testosterone deficiency, resulting from a metabolic milieu conferring heightened vulnerability to testosterone-related perturbations. We also observed a trend towards more potent testosterone—mortality associations among Black vs. non-Black patients, although interaction tests narrowly missed significance. While Black dialysis patients tend to have higher testosterone levels and greater survival,[26] these data suggest that testosterone deficiency is more potently associated with death risk in this racial/ethnic subgroup. Notably, although incrementally lower mean testosterone levels were observed with increasingly older age strata, in contrast to the Bello et al. study we did not observe a differential association between lower testosterone level and mortality across age groups, suggesting that testosterone deficiency may be equally detrimental among younger and older patients.
To date, there has been sparse study of the impact of testosterone replacement upon hard outcomes in dialysis patients. However, limited evidence suggests that correction of testosterone deficiency may improve surrogate endpoints such as body anthropometry, nutritional, and hematologic status in this population. For example, an important trial conducted by Johansen et al. examined the impact of nandrolone replacement vs. lower extremity resistive exercise upon lean body mass among 79 maintenance hemodialysis patients over a 12 week period.[27] Notably, patients randomized to nandrolone replacement were observed to have a statistically significant gain in lean body mass while those randomized to exercise alone did not. Similarly, an observational study wherein hemodialysis patients were administered nandrolone were observed to exhibit improvements in albumin, cholesterol, and lipoprotein suggestive of nutritional benefits of androgen supplementation in ESRD patients.[28] Finally, as androgen use preceded the advent of erythropoietin, prior research has shown the utility of androgen replacement in correcting anemia of renal disease. Indeed, a recent meta-analysis comparing nandrolone vs. erythropoietin in the treatment of anemia of renal disease showed no significant difference in hemoglobin level across the two groups.[29] These collective data warrant future studies that will examine whether correction of testosterone deficiency ameliorates hard outcomes such as cardiovascular disease and death in dialysis patients, as well as the optimal total testosterone target range in this population.
The strengths of our study include its examination of a large, nationally representative cohort of US dialysis patients; comprehensive availability of detailed, longitudinal patient-level comorbidity, laboratory, and dialysis-treatment characteristics; and laboratory data collected in the outpatient setting and uniformly measured at a single facility. However, several limitations of our study bear mention. First, an inherent disadvantage of a retrospective analysis using clinical data is that indications for serum testosterone measurement are not evident (i.e., patients who underwent testosterone measurement may have had higher pre-test probability of underlying disease). However, while the indications for testing in this study cohort are unknown, this requirement applied equally to patients irrespective of testosterone status and should not impair the study’s internal validity. Second, our study solely relied on total testosterone measurements and did not include analyses of free testosterone. However, it should be noted that the recommended methodologic approach for measuring free testosterone (i.e., equilibrium dialysis) is not routinely available in the clinical setting, and total testosterone is considered to be an accurate metric of testosterone secretion.[19] Third, while all serum samples for total testosterone measurement were collected pre-dialysis, due to data limitations we were unable to determine what time of day samples were drawn and thus could not account for diurnal variations in levels. Fourth, due to data limitations, we were unable to determine which patients were receiving testosterone replacement therapy and certain medications that might impact testosterone levels (e.g., beta blockers). Thus, patients were categorized according to their biochemical total testosterone status irrespective of treatment. However, it should be noted that prior studies of CKD and dialysis patients that excluded recipients of testosterone replacement have observed similar findings.[9, 16, 30] Lastly, given the inherent limitations of an observational study, we cannot exclude the possibility of residual confounding.
In conclusion, our study found that lower total testosterone levels are associated with higher death risk in dialysis patients, and that testosterone deficiency was linked with heightened mortality across multiple subgroups. At this time, future studies are needed to investigate underlying pathways by which low testosterone levels adversely impact survival. Furthermore, interventional studies are needed to determine whether correction of testosterone deficiency leads to improved outcomes, and to clarify the causal relationships between low testosterone and mortality in dialysis patients.
Supplementary Material
Acknowledgments
Funding Support:
The authors are supported by the research grants from the NIH/NIDDK including K23-DK102903 (CMR), K24-DK091419 (KKZ), R01-DK09568 (KKZ), R01-DK096920 (CPK and KKZ), U01-DK102163 (KKZ and CPK), and philanthropist grants from Mr. Harold Simmons, Mr. Louis Chang, and Dr. Joseph Lee.
Footnotes
Conflicts of Interest:
None of the authors have any disclosures to report.
Portions of these data have been presented as an oral abstract at the 2016 Annual Dialysis Conference, Seattle, WA, February 28th to March 2nd, 2016.
References
- 1.Araujo AB, Kupelian V, Page ST, Handelsman DJ, Bremner WJ, McKinlay JB. Sex steroids and all-cause and cause-specific mortality in men. Arch Intern Med. 2007;167(12):1252–60. doi: 10.1001/archinte.167.12.1252. [DOI] [PubMed] [Google Scholar]
- 2.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. The Journal of clinical endocrinology and metabolism. 2001;86(2):724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 3.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. The Journal of clinical endocrinology and metabolism. 2008;93(1):68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma R, Oni OA, Gupta K, Chen G, Sharma M, Dawn B, Sharma R, Parashara D, Savin VJ, Ambrose JA, Barua RS. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36(40):2706–15. doi: 10.1093/eurheartj/ehv346. [DOI] [PubMed] [Google Scholar]
- 5.Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, Almeida OP, Golledge J, Norman PE, Flicker L. In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. The Journal of clinical endocrinology and metabolism. 2014;99(1):E9–18. doi: 10.1210/jc.2013-3272. [DOI] [PubMed] [Google Scholar]
- 6.Kakiya R, Shoji T, Hayashi T, Tatsumi-Shimomura N, Tsujimoto Y, Tabata T, Shima H, Mori K, Fukumoto S, Tahara H, Koyama H, Emoto M, Ishimura E, Nishizawa Y, Inaba M. Decreased serum adrenal androgen dehydroepiandrosterone sulfate and mortality in hemodialysis patients. Nephrol Dial Transplant. 2012;27(10):3915–22. doi: 10.1093/ndt/gfs162. [DOI] [PubMed] [Google Scholar]
- 7.System. URD. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney DIsease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2013. [Google Scholar]
- 8.Carrero JJ, Qureshi AR, Nakashima A, Arver S, Parini P, Lindholm B, Barany P, Heimburger O, Stenvinkel P. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant. 2011;26(1):184–90. doi: 10.1093/ndt/gfq397. [DOI] [PubMed] [Google Scholar]
- 9.Gungor O, Kircelli F, Carrero JJ, Asci G, Toz H, Tatar E, Hur E, Sever MS, Arinsoy T, Ok E. Endogenous testosterone and mortality in male hemodialysis patients: is it the result of aging? Clin J Am Soc Nephrol. 2010;5(11):2018–23. doi: 10.2215/CJN.03600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrero JJ. Testosterone deficiency at the crossroads of cardiometabolic complications in CKD. Am J Kidney Dis. 2014;64(3):322–5. doi: 10.1053/j.ajkd.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. The Journal of clinical endocrinology and metabolism. 2004;89(7):3313–8. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt A, Luger A, Horl WH. Sexual hormone abnormalities in male patients with renal failure. Nephrol Dial Transplant. 2002;17(3):368–71. doi: 10.1093/ndt/17.3.368. [DOI] [PubMed] [Google Scholar]
- 13.Bello AK, Stenvinkel P, Lin M, Hemmelgarn B, Thadhani R, Klarenbach S, Chan C, Zimmerman D, Cembrowski G, Strippoli G, Carrero JJ, Tonelli M. Serum testosterone levels and clinical outcomes in male hemodialysis patients. Am J Kidney Dis. 2014;63(2):268–75. doi: 10.1053/j.ajkd.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Carrero JJ, Qureshi AR, Parini P, Arver S, Lindholm B, Barany P, Heimburger O, Stenvinkel P. Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol. 2009;20(3):613–20. doi: 10.1681/ASN.2008060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossmann M, Hoermann R, Ng Tang Fui M, Zajac JD, Ierino FL, Roberts MA. Sex steroids levels in chronic kidney disease and kidney transplant recipients: associations with disease severity and prediction of mortality. Clin Endocrinol (Oxf) 2015;82(5):767–75. doi: 10.1111/cen.12656. [DOI] [PubMed] [Google Scholar]
- 16.Kyriazis J, Tzanakis I, Stylianou K, Katsipi I, Moisiadis D, Papadaki A, Mavroeidi V, Kagia S, Karkavitsas N, Daphnis E. Low serum testosterone, arterial stiffness and mortality in male haemodialysis patients. Nephrol Dial Transplant. 2011;26(9):2971–7. doi: 10.1093/ndt/gfq847. [DOI] [PubMed] [Google Scholar]
- 17.Kuttykrishnan S, Kalantar-Zadeh K, Arah OA, Cheung AK, Brunelli S, Heagerty PJ, Katz R, Molnar MZ, Nissenson A, Ravel V, Streja E, Himmelfarb J, Mehrotra R. Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol Dial Transplant. 2015;30(7):1208–17. doi: 10.1093/ndt/gfv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhee CM, Kim S, Gillen DL, Oztan T, Wang J, Mehrotra R, Kuttykrishnan S, Nguyen DV, Brunelli SM, Kovesdy CP, Brent GA, Kalantar-Zadeh K. Association of thyroid functional disease with mortality in a national cohort of incident hemodialysis patients. The Journal of clinical endocrinology and metabolism. 2015;100(4):1386–95. doi: 10.1210/jc.2014-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2010;95(6):2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 20.Haffner SM, Mykkanen L, Valdez RA, Katz MS. Relationship of sex hormones to lipids and lipoproteins in nondiabetic men. The Journal of clinical endocrinology and metabolism. 1993;77(6):1610–5. doi: 10.1210/jcem.77.6.8263149. [DOI] [PubMed] [Google Scholar]
- 21.Ohlsson C, Barrett-Connor E, Bhasin S, Orwoll E, Labrie F, Karlsson MK, Ljunggren O, Vandenput L, Mellstrom D, Tivesten A. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. The MrOS (Osteoporotic Fractures in Men) study in Sweden. Journal of the American College of Cardiology. 2011;58(16):1674–81. doi: 10.1016/j.jacc.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan SA, Johnson-Levonas AO, Lin J, Shah AK, Meehan AG. Elevated high sensitivity C-reactive protein levels in aging men with low testosterone. The aging male : the official journal of the International Society for the Study of the Aging Male. 2010;13(2):108–12. doi: 10.3109/13685530903440424. [DOI] [PubMed] [Google Scholar]
- 23.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes care. 2004;27(5):1036–41. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 24.Yilmaz MI, Sonmez A, Qureshi AR, Saglam M, Stenvinkel P, Yaman H, Eyileten T, Caglar K, Oguz Y, Taslipinar A, Vural A, Gok M, Unal HU, Yenicesu M, Carrero JJ. Endogenous testosterone, endothelial dysfunction, and cardiovascular events in men with nondialysis chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(7):1617–25. doi: 10.2215/CJN.10681210. [DOI] [PubMed] [Google Scholar]
- 25.Carrero JJ, Barany P, Yilmaz MI, Qureshi AR, Sonmez A, Heimburger O, Ozgurtas T, Yenicesu M, Lindholm B, Stenvinkel P. Testosterone deficiency is a cause of anaemia and reduced responsiveness to erythropoiesis-stimulating agents in men with chronic kidney disease. Nephrol Dial Transplant. 2012;27(2):709–15. doi: 10.1093/ndt/gfr288. [DOI] [PubMed] [Google Scholar]
- 26.Rhee CM, Lertdumrongluk P, Streja E, Park J, Moradi H, Lau WL, Norris KC, Nissenson AR, Amin AN, Kovesdy CP, Kalantar-Zadeh K. Impact of age, race and ethnicity on dialysis patient survival and kidney transplantation disparities. Am J Nephrol. 2014;39(3):183–94. doi: 10.1159/000358497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansen KL, Mulligan K, Schambelan M. Anabolic effects of nandrolone decanoate in patients receiving dialysis: a randomized controlled trial. JAMA. 1999;281(14):1275–81. doi: 10.1001/jama.281.14.1275. [DOI] [PubMed] [Google Scholar]
- 28.Ghorbanihaghjo A, Argani H, Rohbaninoubar M, Rashtchizadeh N. Effect of Nandrolone Decanoate on serum lipoprotein (a) and its isoforms in hemodialysis patients. Lipids Health Dis. 2004;3:16. doi: 10.1186/1476-511X-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adamu B, Ma'aji SM, Erwin PJ, Tleyjeh IM. Meta-Analysis of Randomized Controlled Trials on Androgens versus Erythropoietin for Anaemia of Chronic Kidney Disease: Implications for Developing Countries. Int J Nephrol. 2012;2012:580437. doi: 10.1155/2012/580437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haring R, Nauck M, Volzke H, Endlich K, Lendeckel U, Friedrich N, Dorr M, Rettig R, Kroemer HK, Wallaschofski H. Low serum testosterone is associated with increased mortality in men with stage 3 or greater nephropathy. Am J Nephrol. 2011;33(3):209–17. doi: 10.1159/000324562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.