Abstract
The dysfunction of vascular endothelial cells (ECs) influenced by flow shear stress is crucial for vascular remodeling. However, the roles of nuclear envelope (NE) proteins in shear stress-induced EC dysfunction are still unknown. Our results indicated that, compared with normal shear stress (NSS), low shear stress (LowSS) suppressed the expression of two types of NE proteins, Nesprin2 and LaminA, and increased the proliferation and apoptosis of ECs. Targeted small interfering RNA (siRNA) and gene overexpression plasmid transfection revealed that Nesprin2 and LaminA participate in the regulation of EC proliferation and apoptosis. A protein/DNA array was further used to detect the activation of transcription factors in ECs following transfection with target siRNAs and overexpression plasmids. The regulation of AP-2 and TFIID mediated by Nesprin2 and the activation of Stat-1, Stat-3, Stat-5 and Stat-6 by LaminA were verified under shear stress. Furthermore, using Ingenuity Pathway Analysis software and real-time RT-PCR, the effects of Nesprin2 or LaminA on the downstream target genes of AP-2, TFIID, and Stat-1, Stat-3, Stat-5 and Stat-6, respectively, were investigated under LowSS. Our study has revealed that NE proteins are novel mechano-sensitive molecules in ECs. LowSS suppresses the expression of Nesprin2 and LaminA, which may subsequently modulate the activation of important transcription factors and eventually lead to EC dysfunction.
Keywords: Vascular remodelling, Shear stress, Nuclear envelope proteins, Endothelial cells, Dysfunction, Transcription factor
1. Introduction
Vascular endothelial cells (ECs) covering the inner surfaces of blood vessels are constantly exposed to fluid shear stress created by blood flow. Homeostasis of ECs is required for maintaining vascular physiological functions and protection against pathophysiological changes, such as atherosclerosis [1]. Atherosclerosis develops preferentially at branches and curvatures of arterial trees, where blood flow patterns are low or disturbed [2]. ECs respond to changes in local shear stress, which subsequently lead to the alterations in gene expression, the metabolism of biochemical substances or cellular functions, such as proliferation, apoptosis, differentiation and migration [2]. The exposure of ECs to shear stress activates multiple mechanosensors, including membrane glycocalyx [3], membrane proteins, such as integrins [4], G proteins and G protein-coupled receptors [5], receptor tyrosine kinases [6] and Ca2+ channels [7]. It has also become clear that the cytoskeleton forming the internal scaffold can sense and absorb the mechanical loading of the cell [8]. However, it remains unclear, besides mechanosensors located on membrane structures and cytoskeleton, whether nuclear skeletal elements, such as nuclear envelope (NE) proteins, are mechano-sensitive and affect EC behaviors.
Our previous proteomic analysis revealed that LaminA, which is a major protein constituent of the mammalian nuclear lamina, may participate in modulating EC proliferation and migration during shear stress application, suggesting that NE proteins may be mechano-responsive molecules that contribute to vascular remodeling [9]. The nucleus is the largest and stiffest organelle in the cell, and it is the site of transcriptional regulation and contains the genome [10]. NE proteins maintain the skeletal structure of the nucleus, but their roles in mechano-sensing and gene expression regulation are still unclear.
The NE is composed of the outer nuclear membrane (ONM), which is continuous with the endoplasmic reticulum, the inner nuclear membrane (INM), and the nuclear lamina underlying the INM [11]. Nesprin proteins at the ONM contain an N-terminal actin-binding domain in the cytoplasm and a C-terminal transmembrane domain, which interacts with the INM, in the perinuclear space [12]. The nuclear lamina, which is composed of LaminA and C (A-type lamins) and LaminB1 and B2 (B-type lamins), links with chromatin and participates in gene transcription [11,13]. LaminA, which is expressed in all differentiated cell types, participates in tissue-specific gene expression, cell signaling and maintenance of the nuclear structure. Defects in LaminA result in several diseases, such as cardiomyopathy, muscular dystrophy, lipodystrophy, and so on [14]. Because our previous work revealed that LaminA might contribute to vascular remodeling during shear stress application [9], we hypothesized that NE proteins are novel mechano-sensitive molecules that participate in the EC functions regulated by shear stress.
In the current study, two core components of NE proteins, Nesprin2 and LaminA, were studied. Using a parallel flow chamber system, the effects of Nesprin2 and LaminA on EC proliferation and apoptosis under shear stress were first investigated. Furthermore, the interrelationship between the changed expression of NE proteins and the activation of transcription factors (TFs), and their downstream target genes were demonstrated. The aim of the current study was to understand how NE proteins mediate EC proliferation and apoptosis in response to shear stress.
2. Materials and methods
2.1. Cell culture and shear stress loading
The animal care and experimental protocols conformed to the Animal Management Rules of China (Documentation 55, 2001, Ministry of Health, China), and the investigation was approved by the Animal Research Committee of Shanghai Jiao Tong University. Primary rat aortic ECs were obtained by the digestive method [15] and grown in Clonetics EGM™-2 Endothelial Cell Growth Medium-2 (Lonza, USA). ECs between passages 2 and 4 and cell populations with more than 95% purity were used in all experiments. The ECs were characterized by immunohistochemical staining for von Willebrand factor (Dako, Denmark).
A parallel-plate flow chamber system was used [9]. ECs were seeded onto the outer side of a polyethylene terephthalate (PET) membrane (Becton Dickinson Labware, NJ) at a density of 2 × 105 cells per cup. For the co-culture system, VSMCs were seeded onto the inner side of the membrane. For the mono-culture system, the inner side of the membrane was kept unseeded (Supplementary Fig. 1). Laminar flow is generated by the pressure difference between the upper and lower reservoirs. The flow path is 28 mm in width (w) and 200 μm in height (h). Shear stress (τ, dyn/cm2) intensity is calculated using the formula τ = 6 μQ/wh2, where μ is the viscosity of the medium (poise), and Q is the flow rate (ml/min). A normal laminar shear stress was applied of 15 dyn/cm2. Thus, the ECs were subjected to shear stress for 6, 12 and 24 h. Flow-loading experiments were performed at 37 °C in an incubator with 5% CO2. Normal shear stress (NSS, 15 dyn/cm2) and low shear stress (LowSS, 5 dyn/cm2) were applied to the ECs for 6, 12 and 24 h.
2.2. Western blotting analysis
For the detection of protein expression levels, EC lysates were subjected to electrophoretic separation with freshly prepared 10% SDS–PAGE gels and transferred to nitrocellulose membranes (Amersham, USA). After a blocking step with 5% of non-fat milk, the membranes were incubated with specific antibodies overnight at 4 °C. Western blots were performed using antibodies against Nesprin2 (Santa Cruz Technologies, 1:500), LaminA (Santa Cruz Technologies, 1:500), GAPDH (Proteintech Technologies, 1:1000), AP-2 (Proteintech Technologies, 1:500), TFIID (Proteintech Technologies, 1:500), Stat-1 (Cell Signaling Technologies, 1:500), phospho-Stat-1 (Cell Signaling Technologies, 1:500), Stat-3 (Cell Signaling Technologies, 1:500), phospho-Stat-3 (Cell Signaling Technologies, 1:500), Stat-5 (Cell Signaling Technologies, 1:500), phospho-Stat-5 (Cell Signaling Technologies, 1:500), Stat-6 (Cell Signaling Technologies, 1:500), and phospho-Stat-6 (Cell Signaling Technologies, 1:500). The stains were deposited by a BCIP/NBT phosphatase substrate system (KPL, USA), scanned with an image scanner (Amersham Biosciences, USA), and quantified using Quantity One software (Bio-Rad, USA).
2.3. RNA extraction and SYBR Green Real-Time PCR Assay
Total RNA was extracted using TRIZOL reagent (Invitrogen, Carlsbad, CA). Optimization reactions consisting of different annealing temperatures and different primer concentrations were performed to determine the most appropriate annealing temperature (Ta) and primer concentration. Negative controls were run with each experiment. Real-time RT-PCR was performed using a Quanti-Tect SYBR Green PCR Kit (Bio-Rad) with 1 μl of cDNA using an iCycler (Bio-Rad). Amplification of target genes and the reference gene (GAPDH) were performed in triplicate, and PCR products were verified via melt curve analysis and agarose gel electrophoresis. The results were normalized to the GAPDH expression levels, and relative gene expression was measured by the 2−ΔΔCT method.
2.4. Small interference RNA to knockdown Nesprin2 or LaminA
The double strands of small interfering RNAs (siRNAs) targeting Nesprin2 were as follows: 5′-CCAG UGUA GAAU CAAC AUAT T-3′ and 5′-UAUG UUGA UUCU ACAC UGGT T-3′. The sequences of the siRNAs targeting LaminA were as follows: 5′-CAGG UCUG AAGC CAAA GAAT T-3′ and 5′-UUCU UUGG CUUC AGAC CUGT T-3′.
For RNA interference studies, cells were seeded into 6-well cell culture plates at a density of 2 × 105 cells. After an 8-hour incubation to allow for attachment, the cells were transfected with siRNAs and Lipofectamine™ 2000 (Invitrogen) according to the manufacturer’s instructions. Briefly, 100 nmol siRNAs and 5 μl Lipofectamine™ 2000 were diluted in serum- and antibiotic-free opti-MEM (Invitrogen) at a final volume of 800 μl. After mixing for 20 min at room temperature, the siRNA/Lipofectamine™ 2000 mixture was added dropwise onto the cells and incubated at 37 °C in a humidified CO2 incubator. Non-silencing siRNA with no known homology to rat genes was synthesized as a negative control (NC).
2.5. Plasmid construction and transfection
Full-length Nesprin2 and LaminA fragments were obtained by PCR amplification from rat cDNA with the following primers: Nesprin2: 5′-ACGT AAGG ATCC GATG GCAT CTAG TCCT GAGC T-3′ and 5′-GCGG AACC GAAT TCTC ACAA GTAG CTGG CACA AG-3′; and LaminA: 5′-ACGT AAGG ATCC GATG GAGA CCCC GTCT CAGC-3′ and 5′-GCGG AACC GAAT TCTC AGCG GCGG CTGC CAC-3′. Then, the fragments were subcloned into a pcDNA3.1(+) vector (Invitrogen, USA) with BamHI and EcoRI restriction sites (New England BioLabs, USA). Both of the plasmid constructs were verified by DNA sequencing.
For plasmid transfection, optimization reactions using different transfection reagents were performed to determine the most efficient protocol. Using a Basic Nucleofector Kit for Primary Mammalian Endothelial Cells (Amaxa, Germany), 3 μg of Nesprin2 or LaminA plasmid was transfected into 5 × 105 ECs with the program A-33 of Nucleofector II (Amaxa, Germany).
2.6. Cell proliferation assay
Cell proliferation was analyzed using an in situ BrdU kit (Roche Diagnostics, Germany) by fluorescence microscopy. Generally, ECs were labeled with 10 μM BrdU for 3 h under different conditions. After labeling, the cells were incubated with an anti-BrdU-FLUOS antibody for 1 h at 37 °C in a humidified atmosphere in the dark and evaluated by laser-scanning confocal microscopy (Olympus FV1000, Japan) using wavelengths of 488 nm for excitation and 515 nm for detection. The nuclei of all cells were visualized using PI (Invitrogen, USA).
The cell cycle was assessed by flow cytometric analysis with FACSCalibur (BD, USA), and the proportions of cells at the G0/G1, S, and G2/M phases were analyzed with ModFit software. The proliferation index (S + G2/M)/(G0/G1 + S + G2/M) was then calculated.
2.7. Cell apoptosis assay
Cell apoptosis was detected with an annexinV-FITC apoptosis detection kit (Roche Diagnostics, Germany) according to the manufacturer’s instructions. Briefly, cells were collected, washed twice with PBS, gently resuspended in annexinV binding buffer, incubated with annexinV-FITC/PI in the dark for 10 min, and analyzed by flow cytometry using FACSCalibur (Becton, Dickinson and Company, USA). In the scatter diagrams of the FACS assay, the x-axis represents annexinV-FITC fluorescence, and the y-axis depicts PI fluorescence. The upper right quadrant indicates the necrotic cells, the lower right indicates the apoptotic cells, and the lower left shows the living cells.
2.8. Transcription factor array analysis
Nuclear proteins were prepared from ECs transfected with siRNA or an overexpression plasmid by using a nuclear extraction kit (Panomics, USA) following the manufacturer’s instructions. TranSignal Protein/DNA Arrays I (Panomics, USA), which identify sequence-specific DNA-binding property of 54 transcription factors (TFs), were used according to the manufacturer’s directions. In general, nuclear extracts from ECs were incubated with TranSignal probe mix (Panomics) containing 54 biotin-labeled double-stranded DNA oligonucleotides for 30 min at 15 °C. The biotin-labeled oligonucleotides that were specifically bound to the TFs were eluted and hybridized to a TranSignal array membrane containing oligonucleotides. The blots were then washed and incubated with horseradish peroxidase (HRP)-conjugated streptavidin according to the manufacturer’s instructions. Hybridization signals on the array were detected using standard chemiluminescence procedures with Hyperfilm ECL (2–10 min). Relative spot intensities were determined using ScanAlyze software to obtain numerical data for comparisons. All changes that were more than 2-fold or less than 0.5-fold were considered significant [16].
2.9. Ingenuity Pathway Analysis
Transcriptional regulatory networks of TFs with significant P values (P < 0.05) were generated by the Ingenuity Pathway Analysis (IPA) server (http://www.ingenuity.com/products/ipa), which integrated more than 5 million manually curated biological findings and 1.5 million interaction relationships derived from the Ingenuity Pathways Knowledge Database Literature [17].
2.10. Transmission electron microscope (TEM) analysis
ECs were transfected with Nesprin2 or LaminA target siRNA under static conditions. Sample preparation was followed as previously described [18]. Ultrathin sections were prepared with an Ultratome (UC6-FC6, Leica, Germany), counterstained with uranyl acetate and lead citrate, and observed under an electron microscope (Tecnai G2 Spirit, FEI, USA).
2.11. Statistical analysis
All results were expressed as the mean ± SD from at least five independent experiments for each condition. The statistical significance of the results was evaluated with one-way ANOVA. P values of lower than 0.05 were considered statistically significant.
3. Results
3.1. Low shear stress repressed expression of Nesprin2 and LaminA and accelerated proliferation and apoptosis of mono-cultured ECs
Compared with NSS, LowSS significantly decreased the expression of Nesprin2 and LaminA at 6 h, 12 h, and 24 h (Fig. 1A). These results indicated that the expression of NE proteins in the ECs was affected by shear stress.
Fig. 1.
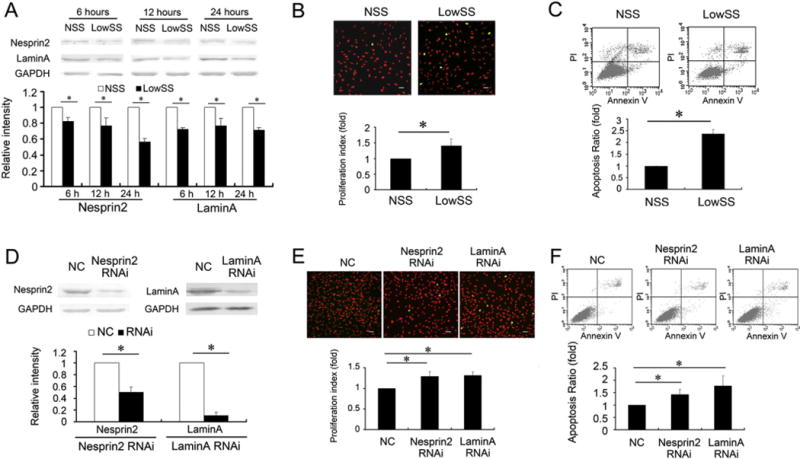
Shear stress regulated the expression of Nesprin2 and LaminA, which modulated EC proliferation and apoptosis. (A) The expression levels of Nesprin2 and LaminA in ECs were downregulated following LowSS applications for 6 h, 12 h and 24 h. (B) LowSS applied for 12 h significantly increased the proliferation of ECs compared with the NSS group. (upper) The images depict typical BrdU immunofluorescence results, in which the ECs are dyed with propidium iodide (red), and the BrdU-positive cells are shown in green. Bar = 40 μm. (lower) The histogram shows the proliferation index as calculated by (S + G2/M)/(G0/G1 + S + G2/M) and verified by flow cytometric analysis. (C) LowSS remarkably increased the apoptosis of ECs, which was detected by annexinV-FITC and flow cytometric analysis. (D) Nesprin2 or LaminA RNA interference (RNAi) remarkably suppressed the expression of Nesprin2 and LaminA, respectively, under static conditions. (E) Suppression of the expression of Nesprin2 and LaminA in the ECs increased cell proliferation. (upper) The images depict typical BrdU immunofluorescence results. Bar = 40 μm. (lower) The histogram shows the proliferation index, which was determined by flow cytometric analysis. (F) Suppression of the expression of Nesprin2 and LaminA in the ECs increased cell apoptosis. Non-silencing siRNA with no known homology to rat genes was synthesized as a negative control (NC). The values represent the mean ± SD. * P < 0.05 vs. the NSS group or NC (n = 5).
Compared with NSS, LowSS applied for 12 h significantly increased the proliferation of the ECs, which was detected by BrdU immunofluorescence, and the proliferation index was assessed by flow cytometry (Fig. 1B). Apoptosis in the LowSS group was also increased compared with the NSS group (Fig. 1C). These results implied that LowSS induced the dysfunction of the ECs. The accompanying variation in NE protein expression and EC dysfunction after shear stress application suggested that NE proteins might participate in the modulation of shear stress to promote EC homeostasis.
3.2. Repressed expression of NE proteins induced proliferation and apoptosis of ECs
Because NE protein expression was decreased by the application of LowSS, the roles of the repressed NE proteins on EC proliferation and apoptosis were further analyzed using their respective specific siRNAs. Under the static condition, transfection of the Nesprin2-targeted siRNA specifically decreased the expression of Nesprin2. In addition, the silencing of LaminA also resulted in a significant decrease in LaminA expression (Fig. 1D).
Both Nesprin2 and LaminA “silencing” increased proliferation and apoptosis significantly in the ECs under the static condition (Fig. 1E and F). Considering the effects of shear stress on NE protein expression and EC functions, our results suggested that LowSS repressed the expression of NE proteins, which might have contributed to the regulation of EC proliferation and apoptosis.
3.3. Overexpression of Nesprin2 and LaminA reversed the effects of LowSS
To gain further insight into the role of NE proteins in LowSS-induced EC proliferation and apoptosis, Nesprin2 and LaminA overexpression plasmids were constructed. In comparison to the pcDNA3.1 (+) vector control, the expression levels of Nesprin2 and LaminA were increased by more than 3-fold in the ECs transfected with the Nesprin2 and LaminA overexpression plasmids, respectively (Fig. 2A).
Fig. 2.
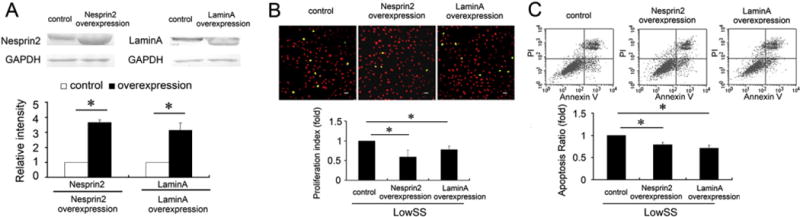
Overexpressed Nesprin2 and LaminA reversed the effects of LowSS on the proliferation and apoptosis of ECs. (A) Overexpression of Nesprin2 or LaminA increased their expression by more than three-fold in the ECs under the static condition. (B) Overexpression of Nesprin2 or LaminA repressed the proliferation of ECs under LowSS. (upper) The images depict typical BrdU immunofluorescence results. Bar = 40 μm. (lower) The histogram shows the proliferation index, as verified by flow cytometric analysis. (C) Overexpression of Nesprin2 or LaminA decreased the apoptosis of ECs under LowSS. ECs transfected with a blank pcDNA3.1 (+) vector plasmid were used as a control. The values represent the mean ± SD. * P < 0.05 vs. the control (n = 5).
After transfection of the ECs with the Nesprin2 or LaminA overexpression plasmids, these cells were subjected to LowSS. Compared with the pcDNA3.1 (+) control, both Nesprin2 and LaminA overexpression significantly repressed EC proliferation and apoptosis (Fig. 2B, C). LowSS downregulated the expression of Nesprin2 and LaminA, and also accelerated EC proliferation and apoptosis. However, these effects could be reversed by the overexpression of Nesprin2 or LaminA. These results suggested that Nesprin-2 and Lamin A had crucial roles in maintaining the normal homeostasis of the ECs.
3.4. Varied expression of Nesprin2 and LaminA regulated the activation of TFs in ECs
It has been reported that NE proteins have roles in regulating DNA synthesis, chromatin organization and gene transcription [14,19]. Therefore, protein/DNA arrays were used to identify the TFs that are involved in the EC dysfunction modulated by NE protein expression. ECs transfected with Nesprin2- or LaminA-targeted siRNA and a Nesprin2 or LaminA overexpression plasmid were used for a protein/DNA interaction array to analyze the activities of 54 different TFs (Panomics Inc., USA).
Relative spot intensities were recorded, and changes of more than 2-fold or of less than 0.5-fold compared with the control were classified by cluster analysis (Fig. 3A and D). There were 3 TFs, AP-2, TFIID and HSE, the activation of which was increased by more than 2-fold in the ECs transfected with Nesprin2-targeted siRNA and decreased less than 0.5-fold in the ECs overexpressing Nesprin2 (Fig. 3B and E, Supplementary Table 1). In the ECs transfected with the LaminA-targeted siRNA and overexpression plasmid, there were 10 TFs the activation of which was decreased by less than 0.5-fold by repressed expression of LaminA and the expression of which was increased by more than 2-fold by overexpression (Fig. 3C and F, Supplementary Table 1).
Fig. 3.
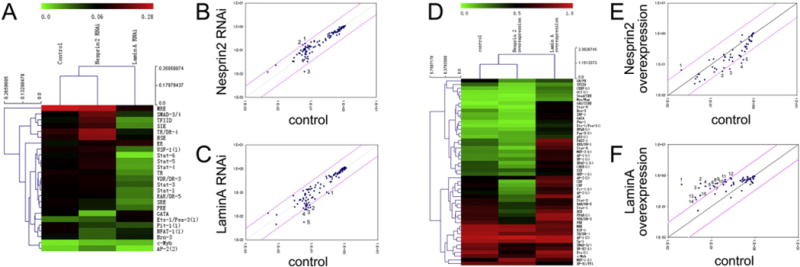
The effects of Nesprin2 and LaminA on the activation of transcription factors (TFs). (A) Clustering analysis based on the protein/DNA interaction array showed the TFs that were altered by more than 2-fold or less than 0.5-fold by Nesprin2 or LaminA RNA interference (RNAi), respectively. A color bar is shown at the top of the figure. Pseudocolors indicate the relative activities of the different TFs. (B) After the suppression of the expression of Nesprin2 by RNAi, the activation of SMAD-3/4 (1) and TFIID (2), which are shown as spots above the top-left of the diagonal pink line, was increased more than 3-fold, and the activation of GATA (3), which is depicted as the spot below the lower-right of the diagonal pink line, was decreased less than one-third-fold. The standard values of the 54 TFs are displayed on the scatter plots. (C) After the suppression of the expression of Lamin A by RNAi, the activation of Stat-5 (1), TR (2), SRE (3), Stat-4 (4), and Stat-6 (5), which are shown as spots below the lower-right of the diagonal pink line, were decreased by less than one-third-fold. (D) Clustering analysis based on the protein/DNA interaction array showed that the TFs changed by more than 2-fold or less than 0.5-fold by Nesprin2 or LaminA overexpression, respectively. The color bar is shown at the top of the figure. Pseudocolors indicate the relative activities of the different TFs. (E) In the Nesprin-2-overexpressed ECs, the activation of GAS/ISRE (1), which is shown as the spot above the top-left of the diagonal pink line, was increased by more than 3-fold, and the activation of Brn-3 (2), TFIID (3), CDP (4), and CBF (5), which are shown as spots below the lower-right of the diagonal pink line, was decreased by less than one-third-fold. (F) In the Lamin A-overexpressed ECs, the activation of GAS/ISRE (1), Stat-5 (2), Ets-1/Pea-3 (3), Pbx-1 (4), p53 (5), NFκB (6), Pax-5 (7), IRF-1 (8), GATA (9), NF-1 (10), Stat-6 (11), FAST-1 (12), Smad/SBE (13), Myc/Max (14), and OCT (15), which are shown as spots above the top-left of the diagonal pink line, was increased more than 3-fold.
To corroborate the results of the protein/DNA interaction array, the effects of Nesprin2 on the expression of AP-2 and TFIID and the effects of LaminA on the phosphorylation of Stat family members, including Stat-1, Stat-3, Stat-5 and Stat-6, were then verified. The expression levels of AP-2 and TFIID were found to be remarkably promoted by the silencing of Nesprin2, and the phosphorylation of Stat-1, Stat-3, Stat-5 and Stat-6 was significantly decreased by Lamin A-targeted siRNA transfection (Fig. 4) under the static condition. These findings suggested that Nesprin2 and LaminA could modulate the activities of TFs that might participate in the regulation of EC proliferation and apoptosis.
Fig. 4.
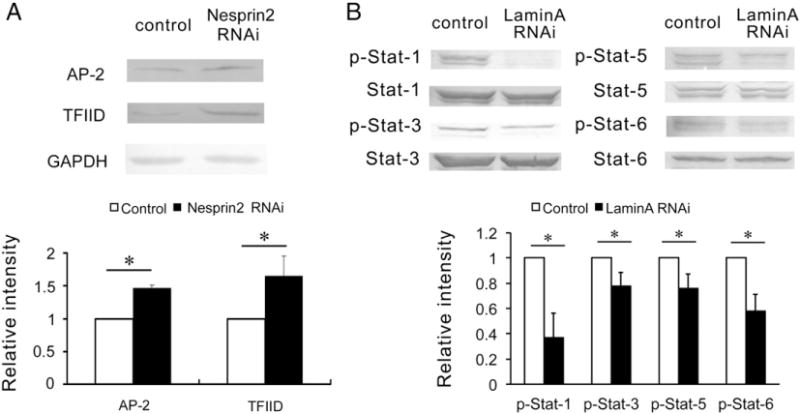
The effects of Nesprin2 or LaminA RNA interference (RNAi) on the activation of target transcription factors under static conditions. (A) Nesprin2-targeted RNA interference (RNAi) markedly increased the expression of AP-2 and TFIID under static conditions. (B) LaminA-targeted RNA interference (RNAi) significantly decreased the phosphorylation of Stat-1, Stat-3, Stat-5 and Stat-6 under static conditions. The values represent the mean ± SD. * P < 0.05 vs. the control (n = 5).
3.5. Low shear stress induced expression of AP-2 and TFIID and repressed phosphorylation of Stat-1, Stat-3, Stat-5 and Stat-6
To further clarify the variations in the TFs under shear stress, ECs were then subjected to NSS and LowSS for 12 h. As shown in Fig. 5, compared to the NSS group, LowSS significantly increased the expression of AP-2 and TFIID, whereas the phosphorylation levels of Stat-1, Stat-3, Stat-5 and Stat-6 were suppressed. These results revealed that LowSS downregulated the expression of NE proteins and then modulated the activities of their corresponding TFs.
Fig. 5.
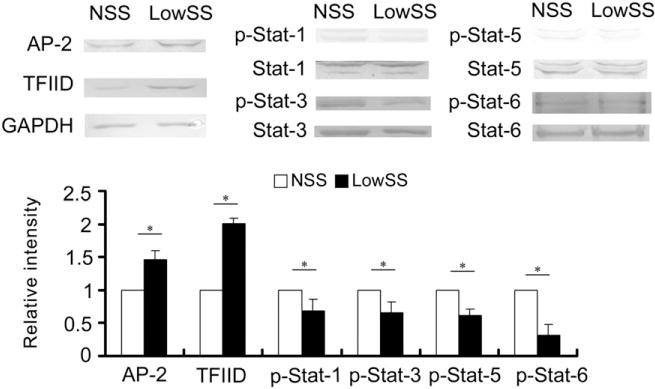
LowSS increased the expression of AP-2 and TFIID and decreased the phosphorylation of Stat-1, Stat-3, Stat-5 and Stat-5 in the ECs compared with NSS. The values represent the mean ± SD. * P < 0.05 vs. the NSS group (n = 5).
3.6. Overexpression of Nesprin2 or LaminA reversed the effects of low shear stress on the activation of TFs
To better understand the roles of Nesprin2 and LaminA in LowSS-induced EC proliferation and apoptosis, ECs were transfected with a Nesprin2 or LaminA overexpression plasmid and then subjected to LowSS. Compared with the pcDNA3.1 (+) control, the expression levels of AP-2 and TFIID were significantly downregulated by Nesprin2 overexpression, and the phosphorylation levels of Stat-1, Stat-3, Stat-5 and Stat-6 were elevated by LaminA overexpression (Fig. 6A).
Fig. 6.
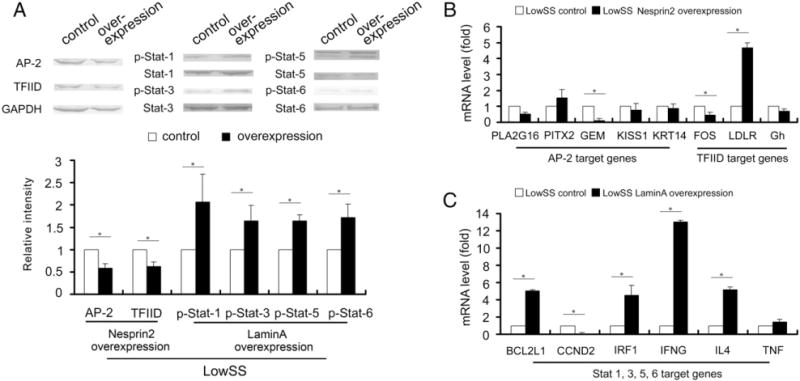
The effects of Nesprin2 or LaminA overexpression on transcription factors and target genes under LowSS. (A) The expression levels of AP-2 and TFIID were significantly decreased by Nesprin2 overexpression, while the phosphorylation levels of Stat-1, Stat-3, Stat-5 and Stat-6 were significantly increased by LaminA overexpression. (B) The mRNA levels of GEM and FOS were significantly decreased, while LDLR was increased by Nesprin2 overexpression under LowSS. (C) The mRNA levels of BCL2L1, IRF1, IFNG and IL4 were significantly increased, while CCND2 was decreased by LaminA overexpression under LowSS. The values represent the mean ± SD. * P < 0.05 vs. the control (n = 5).
3.7. Potential networks based on TFs modulated by NEs
To elucidate the mechanisms underlying Nesprin2- and LaminA-regulated EC proliferation and apoptosis, IPA software was used to investigate the downstream target gene of the TFs revealed above. As shown in Supplementary Fig. 2, potential networks of AP-2, TFIID, Stat-1, Stat-3, Stat-5 and Stat-6 were revealed. Genes in these networks that were important for cellular proliferation and apoptosis (Supplementary Table 2) were then analyzed by real-time PCR.
Nesprin2 overexpression significantly repressed the mRNA level of GEM, which is the target gene of AP-2, and the mRNA level of FOS, which is the target gene of TFIID, and increased the mRNA level of TFIID, which is the target gene of LDLR (Fig. 6B), under LowSS. The overexpression of LaminA upregulated the mRNA levels of BCL2L1, IRF1, IFNG and IL4 but decreased the mRNA level of CCND2. All of these molecules are common downstream targets of Stat-1, Stat-3, Stat-5 and Stat-6 (Fig. 6C).
4. Discussion
ECs, by virtue of their unique location in the vessel wall, respond rapidly and sensitively to mechanical stimuli caused by blood flow and are major regulators of cell structure and function. Disturbed and low shear stress upregulate the proatherosclerotic genes and proteins that promote the development of atherosclerosis [20]. However, the molecular mechanisms of the mechanosensing and mechanotransduction of ECs are still unclear. Our previous proteomic study suggested that LaminA, which is a type of NE protein, might participate in vascular remodeling resulting from LowSS [9]. Hence, in the current study, we demonstrated the effects of NE proteins on EC functions modulated by shear stress.
NE proteins, which form the skeletal structure of the nucleus, have important roles in maintaining nuclear integrity and in regulating gene expression and cell functions [19]. Nesprins are a family of spectrin repeat-containing proteins [21] that localize to the outer part of the NE and bind with actin in the cytoplasm [11,12]. It has been suggested that Nesprins contribute to the structural integrity of the NE by maintaining the precise separation of the two nuclear membranes [12] and even nuclear positioning and migration [22]. LaminA is one of the most common A-type lamins encoded by the LMNA gene, and it is present in almost all differentiated cells [23]. It has been reported that mutations in the LMNA gene lead to the development of several diseases, such as muscle dystrophy [24], Hutchinson–Gilford progeria syndrome [25] and cardiomyopathy [26]. LaminA and Nesprin2 are the core components of the linker of nucleoskeleton and cytoskeleton (LINC) complex. The LINC complex connects the nucleoskeleton and cytoskeleton and has a role in the transfer of mechanically induced signals along the NE into the nucleus, and its components are thought to function in maintaining nuclear and cellular organization as well as signal transduction. A recent study revealed that mutations in LMNA may modulate the LaminA– Nesprin2 interaction and cause alterations in the LINC complex [27]. Additionally, it has been reported that the localization and function of Nesprin2 depend on LaminA [28]. This information suggests that Nesprin2 might have a close relationship with LaminA. Hence, in addition to LaminA, we investigated Nesprin2 as a potential mechano-sensitive molecule in the current study.
Our current study revealed that NE proteins, including Nesprin2 and LaminA, were sensitive to mechanical stimuli and were closely involved in the functioning of ECs. To determine the effects of differentially expressed Nesprin2 and LaminA on EC functions, their expression was upregulated by transfecting an overexpression plasmid and knocked down by target siRNA, respectively. Consistent with the results of the LowSS application, the repressed expression of NE proteins increased both the proliferation and apoptosis of ECs, suggesting that the lack of Nesprin-2 and Lamin A caused by LowSS contributed to EC dysfunction.
ECs are exposed to shear stress on the lumen side and are also neighbors to vascular smooth muscle cells (VSMCs) on the opposite side in vivo. Studies have proven that cross-talk and paracrine effects between ECs and VSMCs may influence the response of ECs to hemodynamic forces and may affect their function [9,29,30]. We also investigated the role of VSMCs in the expression of the Nesprin2 and LaminA proteins and EC functions. The current results revealed that VSMCs had no significant effect on NE protein expression in co-cultured ECs in both the direct contact and the indirect contact models in static or under shear stress (Supplementary Figs. 3, 4), suggesting that these proteins in ECs are specific mechano-sensitive molecules that are not affected by neighboring VSMCs.
In addition to the well-established role of NEs in maintaining the mechanical stability of the nucleus, the current data suggest that Nesprin2 and LaminA may act as scaffolds to regulate the activities of TFs. TFIID has been found to be activated by approximately 3-fold following transfection with Nesprin-2-targeted siRNA and to be repressed by less than 0.3-fold following transfection with an overexpression plasmid in ECs. TFIID is an RNA polymerase II general TF composed of a TATA-box binding protein (TBP) and a group of 13–14 conserved TBP-associated factors (TAFs) [31]. Previous studies have shown that TFIID mediates the proliferation and differentiation of keratinocytes [32] and has important effects on cell survival [33]. Combined with our results, the increased activity of TFIID caused by repressed Nesprin2 might be involved in the proliferation and apoptosis of ECs induced by LowSS. In addition, our results suggested that AP-2 was activated after the expression of Nesprin2 was repressed. The AP-2 transcription factor has a crucial role in the progression of several human tumors, and its expression has been shown to contribute to functional changes in several gene products that are involved in cellular proliferation and differentiation [34].
The activities of Stat-1, Stat-3, Stat-5 and Stat-6 were all significantly decreased in the ECs transfected with LaminA-targeted siRNA and were increased in the ECs transfected with the LaminA overexpression plasmid. Stats are a seven-member family, and they initiate the transcriptional activation of different genes and are also the downstream regulators of the JAK/STAT signaling pathway [35,36]. According to their specific functions, Stats are divided into two groups. One group includes Stat-2, Stat-4 and Stat-6, and the other group includes Stat-1, Stat-3 and Stat-5. Activated Stat-5 translocates into the nucleus, binds to specific DNA response elements, and induces a cascade of cellular responses, including the upregulation of anti-apoptotic genes and inhibition of cell proliferation [37]. The activation of Stats revealed in our study suggests that the repressed expression of LaminA might induce the proliferation and apoptosis of ECs via the modulation of Stat activation during LowSS.
Further results involving cell proliferation- and apoptosis-associated genes suggest that AP-2 and TFIID, which are regulated by Nesprin2, exert their effects by altering the expression of GEM, FOS and LDLR. GEM is a member of the RGK family of GTP-binding proteins within the Ras superfamily, and it possesses a ras-like core and terminal extensions and acts as a potent inhibitor of voltage-dependent (VDCC) Ca2+ channels and regulators of cytoskeletal remodeling [38,39]. It has been reported that overexpression of GEM promotes EC growth and cytoskeletal reorganization [40]. This result is consistent with our observation that a decreased level of GEM, which is a target of AP-2, repressed EC proliferation and apoptosis in the Nesprin2 overexpression group under LowSS. The latest research has suggested that ubiquitinated Stat-3 has a key role in anti-apoptosis and cellular proliferation by up-regulating the BCL2L1 gene [41], which is consistent with our finding that Stat-1, 3, 5, and 6 contribute to EC proliferation and apoptosis by cooperation with BCL2L1. In addition, IFNG has been reported to exert potent antiproliferative and tumoricidal effects in tumor cells [42]. Moreover, CCND2 has been shown to induce DNA synthesis and the proliferation of cardiomyocytes [43]. Based on our results and the Ingenuity® Knowledge Base, a network of the relationships between the target genes and EC functions was revealed. Supplementary Fig. 5 illustrates the effects of these molecules on the inhibition of EC proliferation and apoptosis. In addition, Hay et al. have shown that nuclear transcription factor NF-κB can be activated by shear stress in human endothelial cells [44], which is in support of our idea that shear stress induces gene transactivation by certain transcription factors. Taken together, the altered expression levels of target genes, such as GEM, FOS, BCL2L1, IFNG and CCND2, are affected by the transcription factors modulated by Nesprin2 or LaminA and participate in the EC dysfunction induced by LowSS.
Transmission electron microscopy (TEM) revealed that exposure to both Nesprin2 and LaminA siRNAs resulted in the degradation of the NE phospholipid bilayer (Supplementary Fig. 6). It has been suggested that Nesprin2 and LaminA have essential roles in maintaining NE stability and a normal nuclear structure. Brosig et al. [45] have proved that dominant-negative Nesprin and SUN protein constructs enhance the transcriptional activities of NFκB C2C12 cells, suggesting that the induction of nuclear deformation contributes to conformational changes in chromatin structure and organization and then modulates transcription factor binding or transcriptional processes. The elucidation of the roles of Nesprin2 and LaminA in the maintenance of the nuclear structure might help to explain their effects on transcription factors and endothelial functions.
5. Conclusion
The current study has revealed that the NE proteins Nesprin2 and LaminA are novel mechano-sensitive molecules in ECs. We found that LowSS suppressed the expression of Nesprin2 and LaminA, which might have subsequently modulated the activation of different TFs and regulated their respective genes, ultimately affecting the proliferation and apoptosis of ECs (Fig. 7). These results provide evidence that shear stress regulates EC functions through an NE protein-mediated mechanism and shed light on the role of nuclear skeletal proteins in mechanotransduction.
Fig. 7.
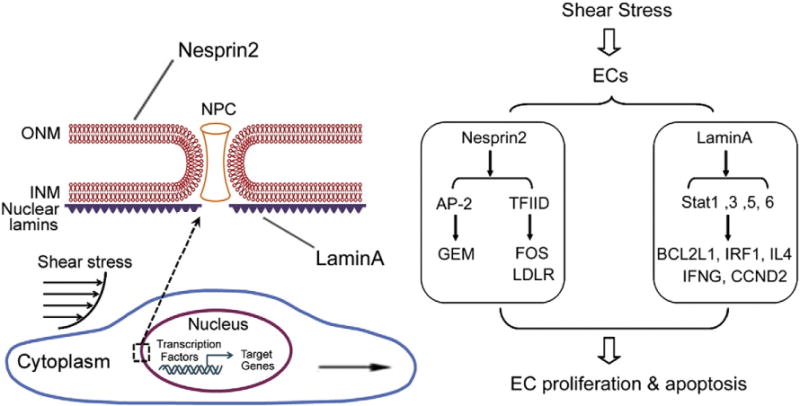
The schematic diagram of the possible signaling pathways involved in the effects of Nesprin2 and LaminA on the proliferation and apoptosis of ECs in response to shear stress. LowSS repressed the expression of Nesprin2 and LaminA, which affected the activation of the transcription factors AP-2, TFIID and Stat-1, Stat-3, Stat-5, Stat-6, and then regulated the mRNA levels of their downstream target genes, ultimately inducing the proliferation and apoptosis of the ECs.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China, nos. 11222223, 11232010, 11428207, 10972140 and 11002091.
Abbreviations
- AP-2
activator protein-2
- ECs
endothelialcells
- HRP
horseradish peroxidase
- IFNG
interferon-gamma
- INM
inner nuclear membrane
- IPA
ingenuity pathway analysis
- LINC
linker of nucleoskeleton and cytoskeleton
- LowSS
low shear stress
- NC
negative control
- NE
nuclear envelope
- NSS
normal shear stress
- ONM
outer nuclear membrane
- PET
polyethylene terephthalate
- siRNA
small interfering RNAs
- TAFs
TBP-associatedfactors
- TBP
TATA-box binding protein
- TEM
transmission electron microscope
- TF
transcription factors
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbamcr.2015.02.013.
Footnotes
Conflict of interest
None.
Transparency document
The Transparency document associated with this article can be found, in the online version.
References
- 1.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–H1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol. 2014;34:2191–2198. doi: 10.1161/ATVBAHA.114.303422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotrasduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci U S A. 2003;100:7988–7995. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Chen BP, Lu M, Zhu Y, Stemerman MB, Chien S, Shyy JY. Shear stress activation of SREBP1 in endothelial cells is mediated by integrins. Arterioscler Thromb Vasc Biol. 2002;22:76–81. doi: 10.1161/hq0102.101822. [DOI] [PubMed] [Google Scholar]
- 5.Traub O, Ishida T, Ishida M, Tupper JC, Berk BC. Shear stress-mediated extracellular signal-regulated kinase activation is regulated by sodium in endothelial cells. Potential role for a voltage-dependent sodium channel. J Biol Chem. 1999;274:20144–20150. doi: 10.1074/jbc.274.29.20144. [DOI] [PubMed] [Google Scholar]
- 6.Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JY. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999;274:18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, Fukuda T, Sato T, Sekine K, Kato S, Isshiki M, Fujita T, Kobayashi M, Kawamura K, Masuda H, Kamiya A, Ando J. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med. 2006;12:133–137. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- 8.Houben F, Ramaekers FC, Snoeckx LH, Broers JL. Role of nuclear lamina–cytoskeleton interactions in the maintenance of cellular strength. Biochim Biophys Acta. 2007;1773:675–686. doi: 10.1016/j.bbamcr.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Qi YX, Jiang J, Jiang XH, Wang XD, Ji SY, Han Y, Long DK, Shen BR, Yan ZQ, Chien S, Jiang ZL. PDGF-BB and TGF-β1 on cross-talk between endothelial and smooth muscle cells in vascular remodeling induced by low shear stress. Proc Natl Acad Sci U S A. 2011;108:1908–1913. doi: 10.1073/pnas.1019219108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caille N, Thoumine O, Tardy Y, Meister JJ. Contribution of the nucleus to the mechanical properties of endothelial cells. J Biomech. 2002;35:177–187. doi: 10.1016/s0021-9290(01)00201-9. [DOI] [PubMed] [Google Scholar]
- 11.Kris ND, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ Res. 2008;102:1307–1318. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holaska JM. Emerin and the nuclear lamina in muscle and cardiac disease. Circ Res. 2008;103:16–23. doi: 10.1161/CIRCRESAHA.108.172197. [DOI] [PubMed] [Google Scholar]
- 14.Isermann P, Lammerding J. Nuclear mechanics and mechanotransduction in health and disease. Curr Biol. 2013;23:R1113–R1121. doi: 10.1016/j.cub.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwan HY, Leung PC, Huang Y, Yao X. Depletion of intracellular Ca2+ stores sensitizes the flow-induced Ca2+ influx in rat endothelial cells. Circ Res. 2003;92:286–292. doi: 10.1161/01.res.0000054625.24468.08. [DOI] [PubMed] [Google Scholar]
- 16.Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 17.Zeng Q, Zhang P, Wu Z, Xue P, Lu D, Ye Z, Zhang X, Huang Z, Feng J, Song L, Yang D, Jiang T, Yan X. Quantitative proteomics reveals ER-α involvement in CD146-induced epithelial–mesenchymal transition in breast cancer cells. J Proteome. 2014:00155–00159. doi: 10.1016/j.jprot.2014.03.033. (pii: S1874–3919) [DOI] [PubMed] [Google Scholar]
- 18.Liu YY, Wong-Riley MT, Liu JP, Jia Y, Liu HL, Jiao XY, Ju G. GABAergic and glycinergic synapses onto neurokinin-1 receptor-immunoreactive neurons in the pre-Bötzinger complex of rats: light and electron microscopic studies. Eur J Neurosci. 2002;16:1058–1066. doi: 10.1046/j.1460-9568.2002.02163.x. [DOI] [PubMed] [Google Scholar]
- 19.Andrés V, González JM. Role of A-type lamins in signaling, transcription, and chromatin organization. J Cell Biol. 2009;187:945–957. doi: 10.1083/jcb.200904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cecchi E, Giglioli C, Valente S, Lazzeri C, Gensini GF, Abbate R, Mannini L. Role of hemodynamic shear stress in cardiovascular disease. Atherosclerosis. 2011;214:249–256. doi: 10.1016/j.atherosclerosis.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Skepper JN, Yang F, Davies JD, Hegyi L, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J Cell Sci. 2001;114:4485–4498. doi: 10.1242/jcs.114.24.4485. [DOI] [PubMed] [Google Scholar]
- 22.Yu J, Lei K, Zhou M, Craft CM, Xu G, Xu T, Zhuang Y, Xu R, Han M. KASH protein Syne-2/Nesprin-2 and SUN proteins SUN1/2 mediate nuclear migration during mammalian retinal development. Hum Mol Genet. 2011;20:1061–1073. doi: 10.1093/hmg/ddq549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutchison CJ, Worman HJ. A-type lamins: guardians of the soma? Nat Cell Biol. 2004;6:1062–1067. doi: 10.1038/ncb1104-1062. [DOI] [PubMed] [Google Scholar]
- 24.Lammerding J, Lee RT. Torn apart: membrane rupture in muscular dystrophies and associated cardiomyopathies. J Clin Invest. 2007;117:1749–1752. doi: 10.1172/JCI32686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez JM, Pla D, Perez-Sala D, Andres V. A-type lamins and Hutchinson–Gilford progeria syndrome: pathogenesis and therapy. Front Biosci. 2011;3:1133–1146. doi: 10.2741/216. [DOI] [PubMed] [Google Scholar]
- 26.Muchir A, Wu W, Worman HJ. Mitogen-activated protein kinase inhibitor regulation of heart function and fibrosis in cardiomyopathy caused by lamin A/C gene mutation. Trends Cardiovasc Med. 2010;20:217–221. doi: 10.1016/j.tcm.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, Munck M, Swaminathan K, Kapinos LE, Noegel AA, Neumann S. Mutations in LMNA modulate the lamin A–Nesprin-2 interaction and cause LINC complex alterations. PLoS One. 2013;8:e71850. doi: 10.1371/journal.pone.0071850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Libotte T, Zaim H, Abraham S, Padmakumar VC, Schneider M, Lu W, Munck M, Hutchison C, Wehnert M, Fahrenkrog B, Sauder U, Aebi U, Noegel AA, Karakesisoglou I. Lamin A/C-dependent localization of Nesprin-2, a giant scaffolder at the nuclear envelope. Mol Biol Cell. 2005;16:3411–3424. doi: 10.1091/mbc.E04-11-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu JJ, Chen LJ, Lee CI, Lee PL, Lee DY, Tsai MC, Lin CW, Usami S, Chien S. Mechanisms of induction of endothelial cell E-selectin expression by smooth muscle cells and its inhibition by shear stress. Blood. 2007;110:519–528. doi: 10.1182/blood-2006-08-040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang HQ, Huang LX, Qu MJ, Yan ZQ, Liu B, Shen BR, Jiang ZL. Shear stress protects against endothelial regulation of vascular smooth muscle cell migration in a coculture system. Endothelium. 2006;13:171–180. doi: 10.1080/10623320600760282. [DOI] [PubMed] [Google Scholar]
- 31.Davidson I, Kobi D, Fadloun A, Mengus G. New insights into TAFs as regulators of cell cycle and signaling pathways. Cell Cycle. 2005;4:1486–1490. doi: 10.4161/cc.4.11.2120. [DOI] [PubMed] [Google Scholar]
- 32.Fadloun A, Kobi D, Pointud JC, Indra AK, Teletin M, Bole-Feysot C, Testoni B, Mantovani R, Metzger D, Mengus G, Davidson I. The TFIID subunit TAF4 regulates keratinocyte proliferation and has cell-autonomous and non-cell-autonomous tumour suppressor activity in mouse epidermis. Development. 2007;134:2947–2958. doi: 10.1242/dev.005041. [DOI] [PubMed] [Google Scholar]
- 33.Mohan WS, Scheer E, Jr, Wendling O, Metzger D, Tora L. TAF10 (TAF(II) 30) is necessary for TFIID stability and early embryogenesis in mice. Mol Cell Biol. 2003;23:4307–4318. doi: 10.1128/MCB.23.12.4307-4318.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heimberger AB, McGary EC, Suki D, Ruiz M, Wang H, Fuller GN, Bar-Eli M. Loss of the AP-2alpha transcription factor is associated with the grade of human gliomas. Clin Cancer Res. 2005;11:267–272. [PubMed] [Google Scholar]
- 35.Darnell JE, Kerr IM, Jr, Stark GR. Jak–STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 36.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 37.Ji YQ, Zhang YQ, Li MQ, Du MR, Wei WW, Li DJ. EPO improves the proliferation and inhibits apoptosis of trophoblast and decidual stromal cells through activating STAT-5 and inactivating p38 signal in human early pregnancy. Int J Clin Exp Pathol. 2011;4:765–774. [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly K. The RGK family: a regulatory tail of small GTP-binding proteins. Trends Cell Biol. 2005;15:640–643. doi: 10.1016/j.tcb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Correll RN, Pang C, Niedowicz DM, Finlin BS, Andres DA. The RGK family of GTP-binding proteins: regulators of voltage-dependent calcium channels and cytoskeleton remodeling. Cell Signal. 2008;20:292–300. doi: 10.1016/j.cellsig.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan JY, Fieles WE, White AM, Egerton MM, Silberstein Ges DS. A human GTPase of the Rad/Gem/Kir family, promotes endothelial cell sprouting and cytoskeleton reorganization. J Cell Biol. 2000;149:1107–1116. doi: 10.1083/jcb.149.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ray S, Zhao Y, Jamaluddin M, Edeh CB, Lee C, Brasier AR. Inducible STAT3 NH2 terminal mono-ubiquitination promotes BRD4 complex formation to regulate apoptosis. Cell Signal. 2014;26:1445–1455. doi: 10.1016/j.cellsig.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labeur M, Refojo D, Wölfel B, Stalla J, Vargas V, Theodoropoulou M, Buchfelder M, Paez-Pereda M, Arzt E, Stalla GK. Interferon-gamma inhibits cellular proliferation and ACTH production in corticotroph tumor cells through a novel janus kinases-signal transducer and activator of transcription 1/nuclear factor-kappa B inhibitory signaling pathway. J Endocrinol. 2008;199:177–189. doi: 10.1677/JOE-08-0011. [DOI] [PubMed] [Google Scholar]
- 43.Busk PK, Hinrichsen R, Bartkova J, Hansen AH, Christoffersen TE, Bartek J, Haunsø S. Cyclin D2 induces proliferation of cardiac myocytes and represses hypertrophy. Exp Cell Res. 2005;304:149–161. doi: 10.1016/j.yexcr.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 44.Hay DC, Beers C, Cameron V, Thomson L, Flitney FW, Hay RT. Activation of NF-kappaB nuclear transcription factor by flow in human endothelial cells. Biochim Biophys Acta. 2003;1642:33–44. doi: 10.1016/s0167-4889(03)00084-3. [DOI] [PubMed] [Google Scholar]
- 45.Brosig M, Ferralli J, Gelman L, Chiquet M, Chiquet-Ehrismann R. Interfering with the connection between the nucleus and the cytoskeleton affects nuclear rotation mechanotransduction and myogenesis. Int J Biochem Cell Biol. 2010;42:1717–1728. doi: 10.1016/j.biocel.2010.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


