Abstract
Background
Professional guidelines have reduced the recommended minimum number to an average of 50 PCI procedures performed annually by each operator. Operator volume patterns and associated outcomes since this change are unknown.
Objectives
To describe PCI operator procedure volumes; characteristics of low-, intermediate-, and high-volume operators; and the relationship between operator volume and clinical outcomes in a large, contemporary, nationwide sample
Methods
Using data from the nationally representative NCDR CathPCI registry collected between July 1, 2009 and March 31, 2015, we examined operator annual PCI volume. We divided operators into low- (< 50 PCIs/year), intermediate- (50–100 PCIs/year), and high-volume (> 100 PCIs/year) groups, and determined the adjusted association between annual PCI volume and in-hospital outcomes, including mortality.
Results
The median number of annual procedures performed per operator was 59 (25th, 75th percentiles: 21, 106); 44% of operators performed < 50 PCI procedures/year. Low-volume operators more frequently performed emergency and primary PCI procedures and practiced at hospitals with lower annual PCI volumes. Unadjusted in-hospital mortality was 1.86% for low-volume operators, 1.73% for intermediate-volume operators, and 1.53% for high-volume operators. The adjusted risk of in-hospital mortality was higher for PCI procedures performed by low- and intermediate-volume operators compared with those performed by high-volume operators (adjusted OR 1.16, 95% CI 1.12–1.21 for low vs. high; adjusted OR 1.05, 95% CI 1.02–1.09 for intermediate vs. high volume) as was the risk for new dialysis post PCI. No volume relationship was seen for post-PCI bleeding.
Conclusions
Many PCI operators in the U.S. are performing fewer than the recommended number of PCI procedures annually. Though absolute risk differences are small and may be partially explained by unmeasured differences in case mix between operators, there remains an inverse relationship between PCI operator volume and in-hospital mortality that persisted in risk-adjusted analyses.
Keywords: Percutaneous coronary intervention, operator volume, volume-outcome relationship
INTRODUCTION
For a variety of reasons, percutaneous coronary intervention (PCI) volumes have declined over the past decade, and many operators have seen a corresponding decline in number of procedures performed.(1–3) The 2013 American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Intervention (ACC/AHA/SCAI) clinical competency statement reduced the recommended minimum number of PCI procedures performed annually by each operator from 75 to 50, averaged over 2 years.(4,5) Contemporary, nationwide patterns of operator volumes have not been described, and little is known about the characteristics of procedures performed by low-volume operators. Furthermore, while prior studies have examined the volume-outcome relationship (6–10), none have been nationally representative using clinical data, or conducted after the change in the recommendations. Importantly, the operator volume recommendations were based on expert opinion that the increasing safety of PCI minimizes differences in outcomes across operators regardless of the number of procedures they perform, rather than on objective data.
Using data from the nationally representative National Cardiovascular Data Registry (NCDR)® CathPCI Registry®, which collects detailed information on > 90% of PCI procedures performed in the United States, we aimed to 1) assess median operator volumes of PCI procedures; 2) evaluate potential differences in patient and procedural characteristics for high-, intermediate-, and low-volume operators; and 3) determine the relationship between operator volumes and patient outcomes in a large, contemporary sample.
METHODS
Patient population
The NCDR CathPCI registry, jointly administered by the ACC and SCAI, has been previously described.(11) It collects data from consecutive patients undergoing PCI at > 1500 hospitals in the United States (~90% of PCI centers), recording information on patient and hospital characteristics, including patient presentation, lesion and procedural details, peri-procedural and discharge medications, and in-hospital outcomes.(12) Variables collected are determined and defined by physician work groups; data collection forms and dictionaries are available at www.ncdr.com. Data collected is subject to the NCDR’s comprehensive data quality program, which includes data quality report specifications for capture and transmission, as well as auditing (13).
For this study, we included all PCI procedures entered into CathPCI using version 4 of the data collection form (July 1, 2009 through March 31, 2015); version 4 of the CathPCI data collection form was the first to include National Provider Identification (NPI) number, which allows for unique identification of the operator for each PCI. We excluded any procedure missing operator’s NPI number, which was < 1% of all PCIs in the database.
Definitions and outcomes
All study definitions were derived from the CathPCI data dictionary. The primary outcome for this analysis was in-hospital mortality, as recorded on the CathPCI data collection form. Secondary outcomes included bleeding events within 72 hours of PCI (hemoglobin drop ≥ 3 g/dl, transfusion of whole blood or packed red blood cells, or procedural intervention/surgery at the bleeding site), new need for dialysis, PCI success rate, and PCI procedure appropriateness. PCI success was defined as successful dilation of all lesions attempted. Appropriateness was based on the 2012 Appropriate Use Criteria for Coronary Revascularization, and was determined using a validated algorithm.(14–16)
The total number of PCI procedures performed or attempted for each operator was counted using each operator’s unique NPI number, and each operator’s average annual volume was calculated by dividing the operator’s total number of PCI procedures by the number of days the operator was active during the study period (date of last PCI procedure – date of first PCI procedure) and multiplying by 365. Since the NPI number is a unique ID that carries across hospitals, operator volumes could be counted without regard to where procedures were performed.
As the AHA/ACC/SCAI clinical competence statement recommends that operators perform and average of ≥ 50 PCIs/year to maintain competence, operators performing < 50 PCIs/year were defined as low-volume operators.(4) Operators performing 50–100 and > 100 PCIs/year were defined as intermediate- and high-volume operators, respectively. For a sensitivity analysis, extreme high- and low-volume operators were defined as those performing > 413 PCIs (97.5th percentile of the volume distribution) and < 26 PCIs (2.5th percentile of the distribution) annually, respectively.
Since the AHA/ACC/SCAI clinical competency statement defines low-volume operators as those performing < 50 PCIs annually averaged over a two-year period, we also examined operator volume trends using this specific definition. We divided the study period into 16 overlapping eight-quarter (two-year) intervals. For each interval, we counted the number of PCIs performed by each operator, then divided by two to calculate annual volume averaged over two years.
Statistical analysis
The distribution of operator volumes was plotted as a histogram and descriptive statistics were calculated. Median annual operator volumes for states and Dartmouth Atlas of Healthcare hospital referral regions were calculated and plotted on U.S. maps.(17) We report the percentage of low-, intermediate-, and high-volume operators (out of all operators performing at least one PCI during the interval) for each eight-quarter interval.
Patient, procedural, and hospital characteristics are presented for high-, intermediate-, and low-volume operators, with categorical variables presented as frequencies (percentages) and continuous variables presented as medians (25th, 75th percentiles). Pearson χ2 tests and Kruskal-Wallis tests were used for comparing categorical and continuous variables, respectively. A p-value threshold of < 0.05 was used to define statistical significance. We performed a sensitivity analysis, comparing extreme high- and extreme-low volume operators.
To determine the relationship between operator volume and in-hospital mortality, we created multivariable logistic regression models using the generalized estimating equation method with an exchangeable working correlation structure to account for within-operator and within-center clustering. By accounting for within-center clustering, this method accounts for the effect of hospital PCI volume. Covariates for these models were all variables included in the CathPCI in-hospital mortality risk score – age, cardiogenic shock, prior heart failure, peripheral vascular disease, chronic lung disease, eGFR (calculated using the Modification of Diet in Renal Disease equation), New York Heart Association class, and presentation with ST segment elevation myocardial infarction (STEMI) (versus no STEMI) – as well as year of PCI.(18) The first model treated operator volume as a continuous variable, and we report the risk-adjusted odds ratio (OR) with associated 95% confidence interval (CI) for a 50 unit annual decrease in PCI volume. The second model calculated unadjusted and adjusted ORs with 95% CIs for low- and intermediate-volume operators with high-volume operators as the reference. Regression models were created for the overall dataset, and separately for the STEMI subgroup, the primary PCI subgroup (defined as those patients undergoing PCI for STEMI within 12 hours of symptom onset) the unstable angina/non-STEMI (UA/NSTEMI) subgroup, and the stable angina subgroup. To examine the effect that potentially modifiable PCI process measures have on the volume-outcome relationship, we calculated the ORs for mortality with 95% CIs for low- and intermediate-volume operators with high-volume operators as the reference after adjusting for all variables included in the CathPCI in-hospital mortality risk score, year of PCI, radial access, drug-eluting stent use, glycoprotein IIb/IIIa inhibitor use, heparin use, and bivalirudin use. As a sensitivity analysis, we calculated adjusted and unadjusted ORs for mortality for extreme low-volume operators with extreme high-volume operators as the reference. We repeated each of these analyses for the secondary outcomes of bleeding complications at 72 hours using a validated predictive model for bleeding,(19) and new requirement for dialysis using a validated predictive model for post-PCI renal failure.(20) For all analyses, an OR < 1 indicates that lower PCI operator volume is associated with lower odds of the outcome compared with higher operator volume, and an OR > 1 indicates that lower PCI operator volume is associated with higher odds of the outcome compared with higher operator volume.
To assess the interaction between operator PCI volume, hospital PCI volume, and post-PCI mortality, we computed adjusted and unadjusted ORs for PCIs performed by low-, intermediate-, and high-volume operators at low- (< 400 PCIs/year), intermediate- (400–800 PCIs/year), and high-volume (> 800 PCIs/year) hospitals. For this analysis, PCIs performed by high-volume operators at high-volume hospitals were the reference; covariates for the adjusted model were the same as those included in the analysis of the association between operator volume and mortality.
All statistical analyses were performed by the Duke Clinical Research Institute using SAS version 9.3. The Duke University Medical Center Institutional Review Board granted a waiver of informed consent and authorization for this study, as data are collected for CathPCI without individual patient identifiers.
RESULTS
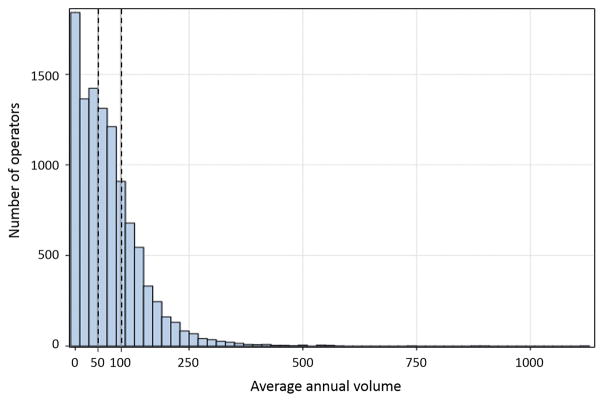
From July 1, 2009 to March 31, 2015, 10,496 operators performed 3,747,866 PCIs at 1584 sites (Online Figure 1); median annual operator volume was 59 PCIs (25th, 75th percentiles: 21, 106). We classified 4628 operators (44%) that performed < 50 PCIs/year as low-volume operators, 3001 (29%) that performed 50–100 PCIs/year as intermediate-volume operators, and 2867 (27%) that performed > 100 PCIs/year as high-volume operators (Figure 1).
Figure 1. Distribution of annual operator volumes.
Operator volumes ranged from 1 to 1121 PCIs per year; the median operator performed 59 PCIs/year. Dashed lines separate operators into low- (< 50 PCIs/year, 4628 operators, 44%), intermediate- (50–100 PCIs/year, 3001 operators, 29%), and high- (> 100 PCIs/year, 2867 operators, 27%) volume operators.
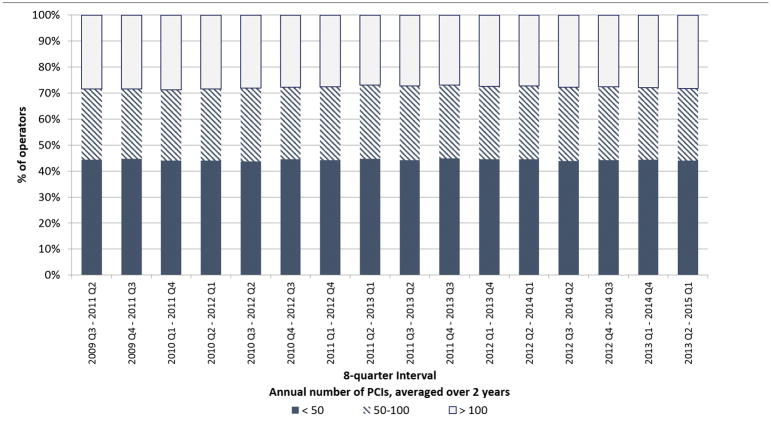
When we examined operator volume using the AHA/ACC/SCAI’s definition of total number of PCIs averaged over a two-year period, findings were similar. For each eight-quarter interval during the study period, the percentage of operators that performed < 50 PCIs, 50–100 PCIs, and > 100 PCIs averaged over two years remained stable (Figure 2). Between 43.7 and 44.8% of operators were defined as low-volume operators per the consensus statement recommendation for each eight-quarter interval during the study period. Quarterly operator volumes also remained stable: In the third quarter of 2009, the median operator volume was 17 PCIs; in the first quarter of 2015, the median volume was 18 PCIs (Online Figure 2).
Figure 2. Changes in annual operator volume over the study period.
Stacked bar graphs depict the percentage of operators performing < 50, 50–100, and > 100 PCIs annually averaged over the 16 overlapping eight-quarter (two-years) intervals that comprised the study period. Between 43.7 and 44.8% of operators performed < 50 PCIs/year in each interval.
Geographic variability in operator volumes
There was regional variability in operator volumes. Throughout the study period, operators in the western part of the U.S. had the lowest annual volumes, followed by operators from the South, Midwest, and North. At the state level, median annual operator volumes ranged from a low of 33 in Nevada to a high of 142 in Rhode Island; the total number of operators ranged from 9 in Alaska to 1197 in California (Supplemental Figure S3). There was also variability by hospital referral region (Figure 3).
Figure 3. Median annual operator volumes by hospital referral region.
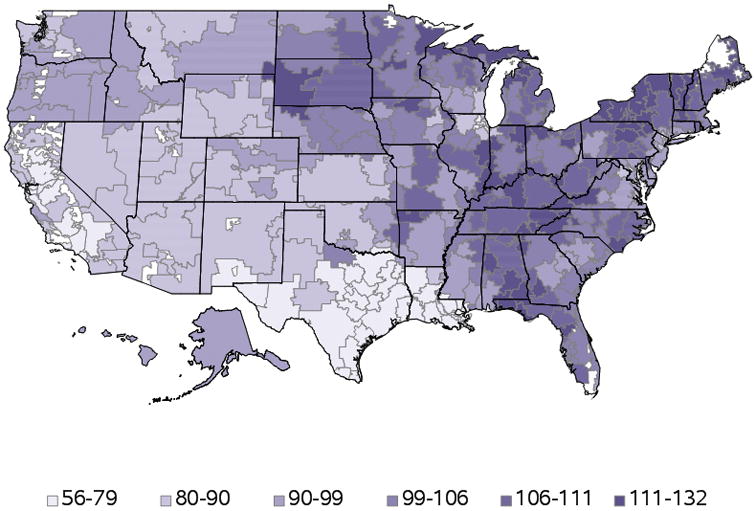
Annual operator volumes varied by Dartmouth Health Atlas hospital referral region. In general, regions in the western and southwestern U.S. had the lowest median operator volumes (depicted in lighter purple); northeastern and midwestern regions had higher median operator volumes.
Patient, procedure, and hospital characteristics for high-, intermediate-, and low-volume operators
All differences between low-, intermediate-, and high-volume operators were nominally statistically significant. Hospital characteristics differed significantly for high-, intermediate-, and low-volume operators; high-volume operators were more likely to practice at an urban or teaching hospital, and practiced at larger hospitals with higher median annual hospital PCI volumes (781 vs. 509 vs. 455 PCIs/year for high- vs. intermediate- vs. low-volume operators; p < 0.0001) (Table 1).
Table 1.
Hospital and patient characteristics by operator volume
| Overall (n = 3,747,866 PCIs; 10,496 operators) | Low (< 50 PCIs/year) (n = 371,861 PCIs; 4628 operators) | Intermediate (50–100 PCIs/year) (n = 1,037,092 PCIs; 3001 operators) | High (> 100 PCIs/year) (n = 2,338,913 PCIs; 2867 operators) | P value | |
|---|---|---|---|---|---|
| Hospital characteristics | |||||
| Hospital region | < 0.0001 | ||||
| Northeast | 578,510 (15.4%) | 40,013 (10.8%) | 140,906 (13.6% | 397,591 (17.0%) | |
| Midwest | 1,025,453 (27.4%) | 64,606 (17.4%) | 270,491 (26.1%) | 690,356 (29.5%) | |
| South | 1,553,974 (41.5%) | 163,019 (43.8%) | 419,239 (40.4%) | 971,716 (41.6%) | |
| West | 589,835 (15.7%) | 104,129 (28.0%) | 206,456 (19.9%) | 279,250 (11.9%) | |
| Urban location | 2,126,157 (56.7%) | 199,828 (53.7%) | 540,177 (52.1%) | 1,386,152 (59.3%) | < 0.0001 |
| No. of beds | 400 (269, 573) | 371 (249, 552) | 372 (250, 546) | 411 (282, 589) | < 0.0001 |
| Annual PCI volume | 661 (405, 1063) | 455 (258, 797) | 509 (313, 832) | 781 (485, 1222) | < 0.0001 |
| Surgical back-up available | 3,285,903 (87.7%) | 317,536 (85.4%) | 887,880 (85.6%) | 2,080,487 (89.0%) | < 0.0001 |
| Teaching hospital* | 1,820,676 (48.6%) | 141,786 (38.1%) | 451,974 (43.6%) | 1,226,916 (52.5%) | < 0.0001 |
| Private/community hospital | 3,256,013 (86.9%) | 327,412 (88.1%) | 900,836 (86.9%) | 2,027,765 (86.7%) | < 0.0001 |
| Patient characteristics | |||||
| Age | 65 (56,74) | 65 (56, 73) | 65 (56, 73) | 65 (56,74) | < 0.0001 |
| Male | 2,550,824 (68.1%) | 255,605 (68.7%) | 710,319 (68.5%) | 1,584,900 (67.8%) | < 0.0001 |
| White | 3,272,652 (87.3%) | 310,597 (83.5%) | 895,424 (86.3%) | 2,066,631 (88.4%) | < 0.0001 |
| Admit source | < 0.0001 | ||||
| Emergency dept | 1,469,424 (39.2%) | 166,071 (44.7%) | 449,216 (43.3%) | 854,137 (36.5%) | |
| Transfer in | 695,207 (18.6%) | 51,781 (13.9%) | 170,064 (16.4%) | 473,362 (20.2%) | |
| Other | 1,579,392 (42.1%) | 153,589 (41.3%) | 416,638 (40.2%) | 1,009,165 (43.2%) | |
| BMI | 29 (26, 33) | 29 (26, 33) | 29 (26, 33) | 29 (26, 33) | < 0.0001 |
| Prior MI | 1,129,986 (30.2%) | 100,359 (27.0%) | 300,225 (29.0%) | 729,402 (31.2%) | < 0.0001 |
| Prior CHF | 470,986 (12.6%) | 39,961 (10.8%) | 121,837 (11.8%) | 309,188 (13.2%) | < 0.0001 |
| HF within 2 weeks | 404,903 (10.8%) | 36,595 (9.8%) | 111,760 (10.8%) | 256,548 (11.0%) | < 0.0001 |
| LV systolic dysfunction | 411,881 (11.0%) | 35,846 (9.6%) | 110,050 (10.6%) | 265,985 (11.4%) | < 0.0001 |
| Cardiogenic shock within 24 hours | 111,941 (3.0%) | 12,850 (3.5%) | 34,806 (3.4%) | 64,285 (2.8%) | < 0.0001 |
| Cardiac arrest within 24 hours | 79,979 (2.1%) | 9,380 (2.5%) | 24,938 (2.4%) | 45,661 (2.0%) | < 0.0001 |
| Diabetes | 1,398,487 (37.3%) | 135,088 (36.3%) | 379,181 (36.6%) | 884,218 (37.8%) | < 0.0001 |
| Cerebrovascular disease | 469,625 (12.5%) | 40,645 (10.9%) | 123,214 (11.9%) | 305,766 (13.1%) | < 0.0001 |
| Peripheral vascular disease | 464,691 (12.4%) | 38,619 (10.4%) | 121,123 (11.7%) | 304,949 (13.0%) | < 0.0001 |
| Hypertension | 3,078,534 (82.1%) | 299,656 (80.6%) | 840,832 (81.1%) | 1,938,046 (82.9%) | < 0.0001 |
| Current/recent smoker | 1,023,367 (27.3%) | 97,825 (26.3%) | 285,815 (27.6%) | 639,727 (27.4%) | < 0.0001 |
| Dyslipidemia | 2,946,961 (78.6%) | 280,712 (75.5%) | 798,604 (77.0%) | 1,867,645 (79.9%) | < 0.0001 |
| Prior PCI | 1,517,079 (40.5%) | 135,487 (36.4%) | 396,251 (38.2%) | 985,341 (42.1%) | < 0.0001 |
| Prior CABG | 683,993 (18.3%) | 59,328 (16.0%) | 176,312 (17.0%) | 448,353 (19.2%) | < 0.0001 |
| Admission symptoms | < 0.0001 | ||||
| No symptoms | 263,404 (7.0%) | 27,015 (7.3%) | 72,061 (7.0%) | 164,328 (7.0%) | |
| Atypical chest pain | 87,934 (2.4%) | 8,629 (2.3%) | 23,745 (2.3%) | 55,560 (2.4%) | |
| Stable angina | 557,868 (14.9%) | 57,820 (15.6%) | 148,830 (14.4%) | 351,218 (15%) | |
| Unstable angina | 1,439,897 (38.4%) | 128,290 (34.5%) | 377,111 (36.4%) | 934,496 (40.0%) | |
| NSTEMI | 770,313 (20.6%) | 73,077 (19.7%) | 218,222 (21.0%) | 479,014 (20.5%) | |
| STEMI | 627,501 (16.7%) | 76,900 (20.7%) | 196,868 (19.0%) | 353,733 (15.1%) | |
| Appropriate PCI | 3,037,924 (81.1%) | 297,483 (80.0%) | 845,716 (81.6%) | 1,894,725 (81.0%) | < 0.0001 |
| PCI status | < 0.0001 | ||||
| Elective | 1,499,915 (40.0%) | 149,119 (40.1%) | 397,661 (38.3%) | 953,135 (40.8%) | |
| Urgent | 1,545,413 (41.2%) | 136,865 (36.8%) | 419,364 (40.4%) | 989,184 (42.3%) | |
| Emergency | 686,402 (18.3%) | 83,956 (22.6%) | 214,908 (20.7%) | 387,538 (16.6%) | |
| Salvage | 14,761 (0.4%) | 1776 (0.5%) | 4705 (0.5%) | 8,280 (0.4%) |
Continuous data presented as median (25th percentile, 75th percentile); categorical data presented as count (percent), except where otherwise noted.
has a residency or fellowship program; PCI, percutaneous coronary intervention; BMI, body mass index, MI, myocardial infarction; CHF, congestive heart failure; HF, heart failure; LV, left ventricular; CABG; coronary artery bypass grafting; NSTEMI, non-ST segment elevation MI; STEMI, ST segment elevation MI.
There were also significant differences in patient characteristics for PCIs performed by low-, intermediate-, and high-volume operators. Patients undergoing PCI by low-volume operators were significantly less likely to have cardiovascular comorbidities that patients undergoing PCI by intermediate- or high-volume operators (Table 1). By contrast, PCIs performed by low-volume operators were more often in patients with STEMI than those performed by intermediate- or high-volume operators (20.7% vs. 19.0% vs. 15.1%; p < 0.0001) and were more often emergency PCIs performed due to concern that ongoing ischemia or infarction could lead to death (22.6% vs. 20.7% vs. 16.6%; p < 0.001). According to the ACC/AHA/SCAI appropriateness criteria for PCI,(16) between 80.0 and 81.6% of PCIs performed by operators at all strata were considered appropriate.
High-volume operators, as compared with intermediate- and low-volume operators, more often attempted PCI on chronic total occlusions, and more often attempted two or more lesions in one lab visit (29% vs. vs. 25.2% vs. 22.5% for high-, intermediate-, and low-volume operators; p < 0.0001) (Table 2). High-volume operators also more often used radial access than intermediate- or low-volume operators (17.3% vs. 13.0% vs. 7.6%; p < 0.0001) and less often used bivalirudin and glycoprotein IIb/IIIa inhibitors. For all operators, procedure success was > 92.5%, though high-volume operators were successful significantly more often than intermediate- and low-volume operators (94.2% vs. 93.3% vs. 92.6%; p < 0.0001).
Table 2.
PCI characteristics by operator volume
| Overall (n = 3,747,866 PCIs; 10,496 operators) | Low (< 50 PCIs/year) (n = 371,861 PCIs; 4628 operators) | Intermediate (50–100 PCIs/year) (n = 1,037,092 PCIs; 3001 operators) | High (> 100 PCIs/year) (n = 2,338,913 PCIs; 2867 operators) | P value | |
|---|---|---|---|---|---|
| Lesion segment | < 0.0001 | ||||
| Left main | 70,090 (1.9%) | 4532 (1.2%) | 16,187 (1.6%) | 49,371 (2.1%) | |
| Proximal LAD | 571,396 (15.3%) | 61,971 (16.7%) | 161,407 (15.6%) | 348.018 (14.9%) | |
| pRCA/mLAD/pLCx | 1,274,902 (34.0%) | 127,830 (34.4%) | 354,833 (34.2%) | 792,239 (33.9%) | |
| Other | 1,817,196 (48.5%) | 175,825 (47.3%) | 500,460 (48.3%) | 1,140,911 (48.8%) | |
| Vein graft lesion | 217,461 (5.8%) | 20,193 (5.4%) | 58,594 (5.7%) | 138,674 (5.9%) | < 0.0001 |
| Chronic total occlusion | 109,152 (16.1%) | 10,847 (14.0%) | 28,556 (14.0%) | 69,749 (17.5%) | < 0.0001 |
| Bifurcation lesion | 425,147 (11.3%) | 38,418 (10.3%) | 116,355 (11.2%) | 270,374 (11.6%) | < 0.0001 |
| Lesion length | 16 (12, 24) | 15 (12, 22) | 16 (12, 23) | 16 (12, 24) | < 0.0001 |
| No. lesions attempted in lab visit | < 0.0001 | ||||
| 1 | 2,723,589 (72.7%) | 288,132 (77.5%) | 776,634 (74.9%) | 1,658.823 (70.9%) | |
| 2 | 806,242 (21.5%) | 68,936 (18.5%) | 210,064 (20.3%) | 527,242 (22.5%) | |
| ≥ 3 | 218,035 (5.8%) | 14,793 (4.0%) | 50,394 (4.9%) | 152,848 (6.5%) | |
| Radial access | 567,640 (15.2%) | 28.160 (7.6%) | 135,104 (13.0%) | 404,376 (17.3%) | < 0.0001 |
| Unfractionated heparin | 2,018,063 (53.9%) | 182,344 (49.1%) | 552,397 (53.3%) | 1,283,322 (54.9%) | < 0.0001 |
| Bivalirudin | 2,193,047 (58.5%) | 226,004 (60.8%) | 616,715 (59.5%) | 1,350,328 (57.8%) | < 0.0001 |
| Glycoprotein IIb/IIIa inhibitor | 893,655 (23.9%) | 101,085 (27.2%) | 282,809 (27.3%) | 509,761 (21.8%) | < 0.0001 |
| Drug eluting stent | 2,755,004 (73.5%) | 266,382 (71.6%) | 740,889 (71.4%) | 1,747,733 (74.7%) | < 0.0001 |
| Fluoroscopy time (minutes) | 11.7 (7.5, 18.6) | 12.3 (8.0, 19.4) | 12.3 (8.0, 19.3) | 11.3 (7.2, 18.1) | < 0.0001 |
| Contrast volume (mL) | 179 (130, 235) | 185 (140, 245) | 180 (140, 240) | 175 (125, 230) | < 0.0001 |
| Successful PCI | 3,500,956 (93.8%) | 342,741 (92.6%) | 962,923 (93.3%) | 2,195,292 (94.2%) | < 0.0001 |
Continuous data presented as median (25th percentile, 75th percentile); categorical data presented as count (percent). LAD, left anterior descending coronary artery; pRCA, proximal right coronary artery; mLAD, mid left anterior descending coronary artery; pLCA, proximal left circumflex artery. Successful PCI defined as dilation of all lesions attempted.
The sensitivity analysis comparing extreme low- with extreme high-volume operators showed results consistent with the main findings: Overall, compared with extreme low-volume operators, extreme high-volume operators more frequently performed elective PCI on patients with more medical comorbidities, more often performed complex PCI, and more frequently used radial access (Online Table 1). Differences in procedure characteristics between extreme high- and extreme-low volume operators were numerically greater than those between high- and low-volume operators.
Association between operator volumes and outcomes
Overall, 59,400 patients (1.6%) died while hospitalized. Operator volume, when assessed as a continuous variable, was linearly and inversely associated with in-hospital mortality: For every 50 case decrease in annual PCI volume, there was a corresponding 4% increase in the risk-adjusted odds of in-hospital mortality (OR 1.04; 95% CI 1.03–1.05). The relationship between operator volume and in-hospital mortality was significant in patients presenting with STEMI (OR 1.03; 95% CI 1.02–1.04), UA/NSTEMI (OR 1.04; 95% CI 1.03–1.05), and stable angina (OR 1.06; 95% CI 1.02–1.11). Risk-adjusted mortality for the subset of STEMI patients undergoing primary PCI was the same as for the full STEMI population.
This relationship was also apparent when examining operator volume as a categorical variable. Unadjusted in-hospital mortality was 1.86% for low-volume operators, 1.73% for intermediate-volume operators, and 1.48% for high-volume operators. After risk adjustment, in-hospital mortality was significantly higher for intermediate- and low-volume operators compared with high-volume operators (OR 1.16, 95% CI 1.12–1.21 for low vs. high; OR 1.05, 95% CI 1.02–1.09 for intermediate vs. high) (Table 3). For low-volume operators, risk-adjusted in-hospital mortality was greater than that of high-volume operators for all presentation subgroups; however, for intermediate-volume operators, risk-adjusted mortality was not significantly greater than that of high-volume operators for the STEMI and stable angina subgroups. When we further adjusted for PCI process measures, the relationship between operator volume and mortality was attenuated; in-hospital mortality remained higher for low-volume operators compared with high-volume operators (OR 1.12, 95% CI 1.08–1.19) but not for intermediate-volume operators (OR 1.02, 95% CI 0.99–1.05).
Table 3.
In-hospital mortality by PCI operator volume
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| All patients | ||
| Low vs. high volume | 1.23 (1.19–1.28) | 1.16 (1.12–1.21) |
| Intermediate vs. high volume | 1.14 (1.11–1.18) | 1.05 (1.02–1.09) |
| STEMI only | ||
| Low vs. high volume | 1.07 (1.03–1.11) | 1.13 (1.08–1.19) |
| Intermediate vs. high volume | 1.01 (0.98–1.04) | 1.03 (0.99–1.07) |
| NSTEMI/UA only | ||
| Low vs. high volume | 1.11 (1.05–1.18) | 1.20 (1.13–1.28) |
| Intermediate vs. high volume | 1.06 (1.02–1.11) | 1.07 (1.02–1.11) |
| Stable angina only | ||
| Low vs. high volume | 1.14 (0.91–1.44) | 1.31 (1.04–1.65) |
| Intermediate vs. high volume | 1.12 (0.94–1.33) | 1.15 (0.97–1.37) |
OR, odds ratio; STEMI, ST segment elevation myocardial infarction; NSTEMI, non-ST segment elevation myocardial infarction; UA, unstable angina. Low volume operators had an annual PCI volume < 50; intermediate had an annual PCI volume 50–100, and high volume operators had an annual PCI volume > 100. Covariates for the adjusted model included all variables included in the CathPCI mortality risk score, which includes age, cardiogenic shock, prior heart failure (HF), peripheral vascular disease, chronic lung disease, eGFR, New York Heart Association class, and presentation with STEMI (versus no STEMI).
The sensitivity analysis comparing in-hospital mortality for extreme low-volume operators with extreme high-volume operators showed results consistent with the comparison between low- and high-volume operators, with slightly greater ORs (Online Table 2).
There was an inverse and linear relationship between operator volume and risk-adjusted new requirement for dialysis (OR 1.02, 95% CI 1.01–1.03), but no significant association between operator volume and risk-adjusted post-procedure bleeding (OR 0.99; 95% CI 0.97–1.01). When operator volume was analyzed as a categorical variable, low-volume operators had a higher risk-adjusted rate of new requirement for dialysis compared with high-volume operators, but intermediate-volume operators did not (Online Table 3). Neither low- nor intermediate-volume operators had a higher rate of procedural bleeding compared with high-volume operators. However, in patients with UA/NSTEMI and stable angina, low-volume operators had higher rates of both new requirement for dialysis and procedural bleeding.
Association between hospital volume and mortality by operator volume
For low-, intermediate-, and high-volume operators, adjusted and unadjusted in-hospital mortality was highest at low-volume hospitals and lowest at high-volume hospitals (Online Table 4). Compared with high-volume operators performing PCI at high-volume hospitals, risk-adjusted in-hospital mortality was 28% higher for low-volume operators performing PCI at low-volume hospitals (OR 1.28, 95% CI 1.21–1.35), 18% higher for low-volume operators performing PCI at intermediate-volume hospitals (OR 1.18, 95% CI 1.10–1.25), and 12% higher for low-volume operators performing PCI at high-volume hospitals (OR 1.12, 95% CI 1.04–1.21). Among intermediate-volume operators, only those performing PCI at low- and intermediate-volume hospitals had significantly greater risk-adjusted mortality than high-volume operators performing PCI at high-volume hospitals.
DISCUSSION
In this nationally representative study, we found that a large proportion of PCI operators were performing less than an average of 50 PCIs annually, the number of procedures recommended by the 2013 ACC/AHA/SCAI clinical competency statement. Compared with high- (> 100 PCIs annually) and intermediate-volume (50–100 PCIs annually) operators, low-volume (< 50 PCIs annually) operators performed a greater number of PCIs in an emergency setting, but performed fewer anatomically complex PCIs, treated patients with fewer comorbidities, and less frequently attempted multiple lesions in a single lab visit. Procedure success and appropriateness rates were high, and similar for high-, intermediate-, and low-volume operators. While the absolute differences were small, there was an inverse linear association between PCI operator volume and adjusted in-hospital mortality across patient presentations, including emergent procedures for STEMI and elective procedures for stable angina. Among low-volume operators, hospital PCI volume influenced in-hospital mortality; operators practicing at low-volume hospitals had higher mortality than those practicing at high-volume hospitals. While these patterns also existed for post-PCI acute kidney injury, we could not find a volume relationship for post-PCI bleeding.
Low-Volume Operators
Despite the ACC/AHA/SCAI’s reduction of the minimum recommended number of annual PCIs to maintain competency to 50 in 2013, many operators do not meet this minimum requirement. In some states, largely concentrated in the less-densely populated western part of the country, the median operator volume is less than 50 PCIs/year. Using clinical and procedural details collected by the CathPCI registry, we found that low-volume operators less often use radial access, and use more contrast dye and minutes of fluoroscopy. Importantly, they also commonly practice at lower volume hospitals, treat patients with fewer comorbidities but more acute presentations, and attempt less complex lesions than higher-volume operators. Taken together, it appears that these low-volume operators serve an important role by maintaining access to primary PCI for STEMI and performing a disproportionate number of emergency cases. However, the modest increase in the risk of in-hospital mortality, even among STEMI patients, treated by low-volume operators suggests that robust quality improvement processes are necessary to maintain access to primary PCI and improve outcomes.
Volume-Outcome Relationship
Seminal studies, published in the late 1990s, demonstrated lower rates of death and cardiovascular events, including need for coronary artery bypass grafting surgery, among patients undergoing PCI performed at high-volume centers and by high-volume operators.(6,7,21,22) However, the relationship between operator volume and mortality has not been consistently reported in the era of widespread use of coronary stenting. The two most contemporary manuscripts analyzing the operator volume-outcome provided important, though conflicting data on this relationship. Moscucci et al., reporting data from PCIs performed in Michigan in 2002, divided operators into quintiles on the basis of annual volume, and found higher rates of major adverse cardiac events (but not death) in patients treated by operators in the bottom two quintiles.(8) Though this study was conducted in a quality-controlled, audited database, it was not nationally representative. Badheka et al., using data from the Nationwide Inpatient Sample from 2005–2009, demonstrated higher risk-adjusted mortality among patients undergoing PCI performed by low- (< 16 PCIs/year) compared with high-volume operators (> 100 PCIs/year), though overall mortality was low.(10) However, this study was limited by its use of administrative rather than clinical data, and the method used to identify operators may have been less reliable than using NPI number.(23) In addition, data from both studies pre-date the publication of the Appropriate Use Criteria and the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial, which may have changed operator volumes (16,24).
By contrast, the CathPCI registry collects detailed patient and procedure data on all PCI procedures performed in ~90% of U.S. catheterization labs,(12) and our analysis includes PCIs, regardless of inpatient or outpatient status, with the ability to account for instances in which an individual operator performs PCIs at multiple different hospitals. In that context, our study provides the most comprehensive contemporary examination of operator volumes to date, and adds several important insights.
We demonstrate there is persistence of the volume-outcome relationship, but as PCI has grown safer with widespread use of coronary stenting and contemporary antithrombotic therapy, the absolute differences in outcomes have attenuated.(25) The inverse of the mortality data, which may be thought of as the number of PCIs that would need to be shifted from lower to higher volume operators to prevent one death, were correspondingly large – 263 for low- versus high-volume operators and 769 for intermediate- versus high-volume operators. Moreover, post-PCI in-hospital mortality increases linearly with decreasing operator volume, without an inflection point to suggest a minimum annual number of PCIs. Despite the recommendations of the consensus competency statement, any volume threshold appears arbitrarily determined, and caution should be exercised when applying specific operator volume recommendations to individual operators. Rather than just firm annual volume recommendations, a focus on improving process and outcomes performance measures for PCI across all operators, regardless of volume, may be more appropriate.(26) After adjusting for process measures like radial access and antithrombotic choice, differences in mortality between high-, intermediate-, and low-volume operators were attenuated, suggesting that high-volume operators implement specific strategies that may improve outcomes. In addition, some low-volume operators may practice at high-volume centers (although we found that the converse was more likely) and these operators may be able to achieve excellent outcomes because of a “safety net” provided by the structure and processes at that facility.(27,28) We found that PCIs performed by low-volume operators practicing at low-volume hospitals were associated with higher mortality than those performed by low-volume operators practicing at intermediate- or high-volume hospitals. As hospitals consolidate and create large health systems, emphasis should be placed on sharing of best practices, consistent protocols, and formalizing processes for transferring the most complex PCIs to high-volume operators.
Another important aspect of our study is the reporting of the volume-outcome relationship by patient presentation. Though there was a significant relationship between operator volume and mortality regardless of how patients presented for PCI, we found that the relationship between operator volume and mortality is weakest in STEMI patients and strongest in patients with stable angina. Rates of new requirement for dialysis and procedural bleeding, potential procedure-related mediators of mortality in patients undergoing PCI, were statistically greater for low-volume operators than high-volume operators only in patients with UA/NSTEMI and stable angina. These findings suggest that operator volume may play a larger role in lower risk patients, while patient factors may overwhelm the effect of operator volumes on outcomes in patients with STEMI.
Limitations
Even though the CathPCI Registry collects data from ~ 90% of U.S. hospitals, some operators perform PCIs at hospitals that do not participate in CathPCI, and their procedures will be undercounted. Most hospitals that do not participate in CathPCI are Veterans Administration (VA) hospitals. Between 2005 and 2014, 801 operators performed diagnostic coronary angiography in the VA system;(29) even assuming that all of these operators performed PCIs at both CathPCI and VA hospitals and thus had their volumes undercounted in our study, at least 36% of operators would still be performing fewer than the minimum recommended standard. In addition, this study is a retrospective analysis, and is therefore subject to unmeasured confounding and other biases inherent to post hoc analysis; it is possible, for example, that high-volume operators perform more PCIs because they are known to be more skilled and receive more referrals, and that increasing low-volume operators’ PCI volumes would not improve outcomes. Moreover, CathPCI does not include variables that could be used to approximate overall experience, such as number of years in practice, total lifetime volume, or board certification, factors that may influence both operator competency and the relationship between annual operator volume and outcomes.(4) However, CathPCI collects comprehensive data about comorbidities and procedure characteristics, and we adjusted for measured confounders, including hospital PCI volume. Our analysis of more than 3 million PCIs performed by more than 10,000 operators in a national database with strict quality controls provides the best possible description of PCI operator volume patterns, and the best evidence to date of the contemporary relationship between volume and outcomes.
CONCLUSION
Many PCI operators perform less than an average 50 PCI procedures annually, the minimum number recommended by the 2013 ACC/AHA/SCAI clinical competency statement. Though absolute risk differences are small, operator volumes are linearly and inversely associated with in-hospital mortality, even in risk-adjusted analyses. Low-volume operators perform a disproportionate number of emergency PCIs and primary PCIs for STEMI, suggesting that they may perform an important role in maintaining access to PCI.
Supplementary Material
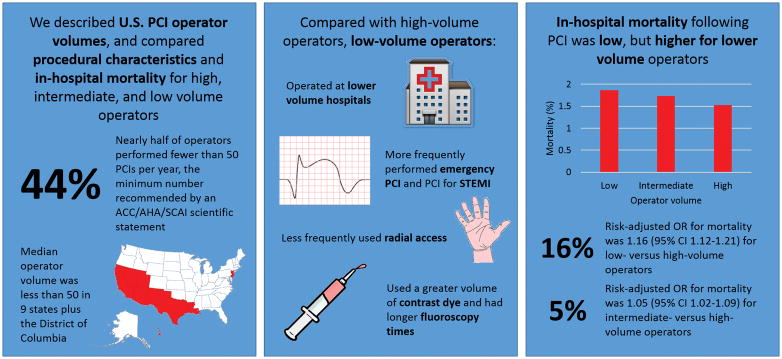
Central Illustration. Procedure Characteristics and In-Hospital Outcomes by Operator Volume.
Nearly half of all operators were low-volume operators (performed < 50 PCIs/year). Compared with intermediate- (50–100 PCIs/year) and high- (> 100 PCIs/year) volume operators, low-volume operators worked at lower volume hospitals, performed more emergency PCIs and primary PCIs for STEMI, less frequently used radial access, used more radiographic contrast dye, and more fluoroscopy minutes. Though in-hospital mortality was low (1.6% overall), it was higher for low- and intermediate-volume operators than for high-volume operators.
PERSPECTIVES.
Competency in Patient Care and Procedural Skills
A large percentage of PCI operators are performing fewer than 50 PCIs annually, the number recommended by professional societies. Despite increases in PCI safety, an inverse association between operator volume and mortality continues to exist for patients undergoing PCI, though absolute differences in mortality by operator volume are small.
Translational Outlook Implications
Further studies should focus on identifying measurable PCI process and outcome measures for professional societies to use to determine clinical competency.
ABBREVIATIONS
- ACC
American College of Cardiology
- AHA
American Heart Association
- NCDR
National Cardiovascular Data Registry
- NPI
National Provider Identification
- NSTEMI
Non-ST segment elevation myocardial infarction
- PCI
Percutaneous Coronary Intervention
- SCAI
Society for Cardiovascular Angiography and Intervention
- STEMI
ST segment elevation myocardial infarction
- UA
Unstable angina
- VA
Veterans Administration
Footnotes
Disclosures: None
References
- 1.Howard DH, Shen YC. Trends in PCI volume after negative results from the COURAGE trial. Health services research. 2014;49:153–170. doi: 10.1111/1475-6773.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maroney J, Khan S, Powell W, Klein LW. Current operator volumes of invasive coronary procedures in medicare patients: Implications for future manpower needs in the catheterization laboratory. Catheterization and Cardiovascular Interventions. 2013;81:34–39. doi: 10.1002/ccd.24366. [DOI] [PubMed] [Google Scholar]
- 3.Kim LK, Feldman DN, Swaminathan RV, et al. Rate of percutaneous coronary intervention for the management of acute coronary syndromes and stable coronary artery disease in the United States (2007 to 2011) Am J Cardiol. 2014;114:1003–1010. doi: 10.1016/j.amjcard.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Harold JG, Bass TA, Bashore TM, et al. ACCF/AHA/SCAI 2013 update of the clinical competence statement on coronary artery interventional procedures. J Am Coll Cardiol. 2013;62:357–396. doi: 10.1016/j.jacc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. J Am Coll Cardiol. 2011;58:e44–e122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Hannan EL, Racz M, Ryan TJ, et al. Coronary angioplasty volume-outcome relationships for hospitals and cardiologists. JAMA. 1997;277:892–898. [PubMed] [Google Scholar]
- 7.McGrath PD, Wennberg DE, Malenka DJ, et al. Operator volume and outcomes in 12,988 percutaneous coronary interventions fn1. J Am Coll Cardiol. 1998;31:570–576. doi: 10.1016/s0735-1097(97)00541-x. [DOI] [PubMed] [Google Scholar]
- 8.Moscucci M, Share D, Smith D, et al. Relationship between operator volume and adverse outcome in contemporary percutaneous coronary intervention practice: an analysis of a quality-controlled multicenter percutaneous coronary intervention clinical database. J Am Coll Cardiol. 2005;46:625–632. doi: 10.1016/j.jacc.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 9.Minges KE, Wang Y, Dodson JA, et al. Physician Annual Volume and In-Hospital Mortality Following Percutaneous Coronary Intervention. Circulation. 2011;124:A16550. [Google Scholar]
- 10.Badheka AO, Patel NJ, Grover P, et al. Impact of annual operator and institutional volume on percutaneous coronary intervention outcomes: a 5-year United States experience (2005–2009) Circulation. 2014;114:009281. doi: 10.1161/CIRCULATIONAHA.114.009281. CIRCULATIONAHA. [DOI] [PubMed] [Google Scholar]
- 11.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology-National Cardiovascular Data Registry™(ACC-NCDR™): building a national clinical data repository. Journal of the American College of Cardiology. 2001;37:2240–2245. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 12.Moussa I, Hermann A, Messenger JC, et al. The NCDR CathPCI Registry: a US national perspective on care and outcomes for percutaneous coronary intervention. Heart. 2013 doi: 10.1136/heartjnl-2012-303379. heartjnl-2012-303379. [DOI] [PubMed] [Google Scholar]
- 13.Messenger JC, Ho KK, Young CH, et al. The National Cardiovascular Data Registry (NCDR) data quality brief: the NCDR data quality program in 2012. J Am Coll Cardiol. 2012;60:1484–1488. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Desai NR, Bradley SM, Parzynski CS, et al. Appropriate use criteria for coronary revascularization and trends in utilization, patient selection, and appropriateness of percutaneous coronary intervention. JAMA. 2015;314:2045–2053. doi: 10.1001/jama.2015.13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan PS, Patel MR, Klein LW, et al. Appropriateness of percutaneous coronary intervention. JAMA. 2011;306:53–61. doi: 10.1001/jama.2011.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update. J Am Coll Cardiol. 2012;59:857–881. doi: 10.1016/j.jacc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Wennberg JE. The Dartmouth Atlas of Health Care in the United States (incl. Diskette) American Hospital Association; 1996. [PubMed] [Google Scholar]
- 18.Peterson ED, Dai D, DeLong ER, et al. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55:1923–1932. doi: 10.1016/j.jacc.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao SV, McCoy LA, Spertus JA, et al. An updated bleeding model to predict the risk of post-procedure bleeding among patients undergoing percutaneous coronary intervention: a report using an expanded bleeding definition from the National Cardiovascular Data Registry CathPCI Registry. JACC: Cardiovascular Interventions. 2013;6:897–904. doi: 10.1016/j.jcin.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Tsai TT, Patel UD, Chang TI, et al. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the National Cardiovascular Data Registry Cath-PCI Registry. Journal of the American Heart Association. 2014;3:e001380. doi: 10.1161/JAHA.114.001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kastrati A, Neumann F-J, Schömig A. Operator volume and outcome of patients undergoing coronary stent placement. J Am Coll Cardiol. 1998;32:970–976. doi: 10.1016/s0735-1097(98)00334-9. [DOI] [PubMed] [Google Scholar]
- 22.Jollis JG, Peterson ED, DeLong ER, et al. The relation between the volume of coronary angioplasty procedures at hospitals treating Medicare beneficiaries and short-term mortality. N Engl J Med. 1994;331:1625–1629. doi: 10.1056/NEJM199412153312406. [DOI] [PubMed] [Google Scholar]
- 23.Khera R, Cram P, Girotra S. Letter by Khera et al. Regarding Article,“Impact of Annual Operator and Institutional Volume on Percutaneous Coronary Intervention Outcomes: A 5-Year United States Experience (2005–2009)”. Circulation. 2015;132:e35–e35. doi: 10.1161/CIRCULATIONAHA.114.013342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 25.Marso SP, Amin AP, House JA, et al. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. JAMA. 2010;303:2156–2164. doi: 10.1001/jama.2010.708. [DOI] [PubMed] [Google Scholar]
- 26.Krumholz HM, Anderson JL, Bachelder BL, et al. ACC/AHA 2008 performance measures for adults with ST-elevation and non–ST-elevation myocardial infarction. J Am Coll Cardiol. 2008;52:2046–2099. doi: 10.1016/j.jacc.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Hannan EL, Wu C, Walford G, et al. Volume-outcome relationships for percutaneous coronary interventions in the stent era. Circulation. 2005;112:1171–1179. doi: 10.1161/CIRCULATIONAHA.104.528455. [DOI] [PubMed] [Google Scholar]
- 28.Kansagra SM, Curtis LH, Anstrom KJ, Schulman KA. Trends in operator and hospital procedure volume and outcomes for percutaneous transluminal coronary angioplasty, 1996 to 2001. Am J Cardiol. 2007;99:339–343. doi: 10.1016/j.amjcard.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 29.Maddox TM, Plomondon ME, Petrich M, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program) Am J Cardiol. 2014;114:1750–1757. doi: 10.1016/j.amjcard.2014.08.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.