Abstract
BACKGROUND
Chronic angina is more common in diabetes mellitus (DM) patients with poor glucose control. Ranolazine both treats chronic angina and improves glucose control.
OBJECTIVES
This study sought to examine ranolazine’s antianginal effect in relation to glucose control.
METHODS
We performed a secondary analysis of RIVER-PCI, a clinical trial in which 2,604 patients with chronic angina and incomplete revascularization following percutaneous coronary intervention (PCI) were randomized to ranolazine versus placebo. Mixed-effects models were used to compare the effects of ranolazine versus placebo on hemoglobin A1c (HbA1c) at 6 and 12 months of follow-up. Interaction between baseline HbA1c and ranolazine’s effect on Seattle Angina Questionnaire (SAQ) angina frequency at 6 and 12 months was tested.
RESULTS
Overall, 961 (36.9%) had DM at baseline. Compared with placebo, ranolazine significantly decreased HbA1c by 0.42±0.08% (adjusted mean difference ± standard error) and 0.44±0.08% from baseline to 6 and 12 months, respectively, in DM patients, and by 0.19±0.02% and 0.20±0.02% at 6 and 12 months, respectively, in non-DM patients. Compared with placebo, ranolazine significantly reduced SAQ angina frequency at 6 months among DM patients, but not at 12 months. The reductions in angina frequency were numerically greater among patients with baseline HbA1c ≥7.5% than those with HbA1c <7.5% (interaction p=0.07).
CONCLUSIONS
In patients with DM and chronic angina with incomplete revascularization after PCI, ranolazine’s effect on glucose control and angina at 6 months was proportionate to baseline HbA1C, but the effect on angina dissipated by 12 months.
Keywords: coronary artery disease, chronic angina, diabetes mellitus
More than 20% of patients with diabetes mellitus (DM) have coronary artery disease (CAD), and in patients with DM between 65 and 74 years of age, that proportion increases to 45% (1). Despite aggressive use of traditional secondary prevention medications, nearly 50% of adults with both DM and CAD have chronic angina, and those with poorer glucose control are more likely to have severe angina (2).
Ranolazine is an oral antianginal agent that acts to inhibit the late sodium ion current, and through that action, reduces calcium overload in the myocytes (3). Clinically, ranolazine has been shown to reduce angina frequency, particularly among patients with more frequent angina or DM (4–6). Unexpectedly, ranolazine has also been observed to reduce hemoglobin A1c (HbA1c) in patients with and without DM (7). The hypothesized mechanism of ranolazine’s effect on HbA1c is through inhibition of sodium channels in pancreatic alpha cells (analogous to the myocardial action), but in this case, resulting in reduced glucagon release (8). Since patients with DM are particularly responsive to ranolazine’s antianginal properties, interactions between ranolazine’s effect on glucose and angina control are of particular interest (5, 6).
The Ranolazine in Patients with Incomplete Revascularization after Percutaneous Coronary Intervention (RIVER-PCI) randomized trial examined the utility of ranolazine in patients with a history of chronic angina who had incomplete revascularization following percutaneous coronary intervention (PCI) (9). Compared to placebo, ranolazine did not reduce the rate of the trial’s primary endpoint, ischemia-driven revascularization or rehospitalization, nor did it improve measures of quality of life or angina frequency. As part of the trial, glycometabolic parameters were prospectively collected from participants at baseline, 6 months, and 12 months to further understand the relationship between glucose control and antianginal efficacy. The results of this pre-specified substudy are reported herein.
METHODS
RIVER-PCI was a multicenter, randomized, double-blind, placebo-controlled trial conducted in 245 centers in 15 countries; the design and primary results have been published, as have the effects on angina burden and quality of life (6, 9, 10). Briefly, patients with a history of chronic angina who had undergone PCI with resultant incomplete revascularization were randomized to receive ranolazine 1000 mg twice daily or placebo. Chronic angina was defined as ≥2 episodes of typical angina, with episodes occurring on ≥2 separate days between 30 days and 1 year prior to PCI. Qualifying PCI could be due either to acute coronary syndrome or stable angina, and patients could have additional angina within 30 days of their PCI. Incomplete revascularization was defined as the presence of at least one lesion with ≥50% diameter stenosis in a coronary artery ≥2.0 mm in diameter, in either a PCI-treated or non-treated vessel. In patients with prior coronary artery bypass graft surgery, incomplete revascularization was defined as at least one ≥50% diameter stenosis in a non-bypassed coronary artery ≥2.0 mm in diameter, or at least one ≥50% diameter stenosis in a bypass graft supplying an otherwise non-revascularized territory. The primary endpoint of RIVER-PCI was the cumulative rate of ischemia-driven hospitalization or revascularization.
PATIENT POPULATION AND STUDY PROCEDURES
RIVER-PCI randomized 2,651 patients, stratified by acute coronary syndrome versus non-acute coronary syndrome, and DM versus no DM. Among those randomized, 2,604 patients who had a qualifying PCI and received at least one dose of study drug were included in the full efficacy analysis. For analyses of the effect of ranolazine on glycometabolic parameters, we included all patients in the full analysis set; 1,317 patients were randomized to receive ranolazine, and 1,287 to placebo. Analyses of angina frequency were performed on patients with DM who participated in the quality of life substudy; this population included 864 patients, of whom 432 were randomized to ranolazine and 422 to placebo. For all analyses, treatment group assignment was based on the intention-to-treat principle.
Patients were classified as having DM if they presented with a medical history of type 1 or type 2 DM as indicated on the study’s case report form, were taking a DM medication at the time of trial enrollment, or had a baseline HbA1c ≥6.5%. If patients met none of these criteria, then they were included in the group without DM. Patients without a prior diagnosis of DM, or DM medications at the time of trial enrollment who were missing baseline HbA1c, were included in the group without DM.
Due to the potential for pharmacokinetic interactions between ranolazine and simvastatin, lovastatin, or metformin, patients in both the ranolazine and placebo group were not allowed to take >1000 mg metformin, >20 mg simvastatin, or >40 mg lovastatin daily; other statins could be used at any dose, and there were no restrictions on the use of any other DM medications. Treatment for secondary prevention of vascular events was left to the discretion of treating physicians; the protocol did not specify goals for lipid or glucose management.
Patients had study visits at baseline, as well as at 1, 6, and 12 months after randomization. At the baseline, 6-month, and 12-month visits, fasting blood samples were collected and processed at a central laboratory; HbA1c, blood glucose, and lipid profiles were measured at these time points. HbA1c was not measured at the 1-month visit, since it is a marker of glucose control over the prior 6–8 weeks, and 1-month follow-up is not long enough to see the full effect that a medication might have on this parameter. At baseline, 57 patients (2.2%) had missing HbA1c data; 450 (17.3%) and 564 (21.7%) patients had missing HbA1c data at 6 and 12 months, respectively. Angina was assessed using the Seattle Angina Questionnaire (SAQ) at baseline and at 1, 6, and 12 months. The SAQ angina frequency score is determined from two questions about angina frequency and nitroglycerin usage; scores range from 0–100, with 100 representing no angina and 0 representing very frequent angina (11).
STATISTICAL ANALYSIS
Baseline characteristics for patients with and without DM were reported by treatment group, with categorical variables reported as number (percent) and continuous variables reported as median (25th, 75th percentile). Since randomization was stratified by DM status, no formal statistical comparisons were performed.
For HbA1c, descriptive statistics were generated for observed values, as well as the change from baseline at 6 and 12 months in all patients, and separately in patients with and without DM. Repeated measures mixed models with unstructured covariance matrices were used to compare least squares mean change in HbA1c from baseline between treatment groups at 6 and 12 months. The model included age, sex, race, baseline HbA1c, treatment group, visit, and treatment-by-visit interaction.
We determined the proportion of patients with new-onset DM, defined as HbA1c ≥6.5% or a reported adverse event indicating type 2 DM, at month 6 and month 12 among patients without DM at baseline and who had not died or had not discontinued from the study prior to the respective study month. Among patients with and without DM who had HbA1c measured at baseline and month 6 or month 12, we determined the proportion with worsening glucose control, defined as an increase in HbA1c ≥1%. To test the association between randomized treatment strategy and the incidence of new-onset DM or worsening glucose control, a logistic regression analysis, adjusting for age, sex, race, and randomized treatment, was performed for each endpoint at month 6 and month 12.
To explore the effect of ranolazine on angina frequency in patients with DM only, as well as the effect of baseline HbA1c on this effect, we generated descriptive statistics for SAQ angina frequency score at baseline, month 1, month 6, and month 12, as well as change from baseline data at each time point. To place these results into clinical context, we also determined the percentage of patients with no angina (SAQ angina frequency score = 100), monthly angina (61–99), weekly angina (31–60), and daily angina (0–30) in the ranolazine and placebo arms at baseline, 6 months, and 12 months. Using a repeated measures mixed model with an unstructured covariance matrix, we tested the association between treatment group and least squares mean change from baseline SAQ angina frequency score at month 1, month 6, and month 12. Terms for age, sex, race, baseline angina, treatment group, visit, and treatment-by-visit interaction were included in the model. We repeated this analysis for baseline glucose control subgroups with HbA1c ≥ and <6.5%, 7.0%, 7.5%, and 8.0%. Tests for interaction between SAQ angina frequency and HbA1c subgroup were performed. To explore the effect of sex on ranolazine’s effect on angina frequency, we repeated these analyses, testing for interaction between SAQ angina frequency and sex.
The investigators had full access to all of the data. Faculty and staff statisticians at the Duke Clinical Research Institute performed all analyses using SAS version 9.4 (Cary, NC, USA).
RESULTS
Among 2,604 patients included in the full efficacy analysis, 961 (36.9%) had DM; 87.4% had a history of type 2 DM prior to trial enrollment, 3.4% had a history of type 1 DM, and the remainder were diagnosed at enrollment with HbA1c value ≥6.5%. For patients with and without DM, baseline characteristics were similar for patients randomized to ranolazine or placebo (Table 1). Overall, discontinuation of treatment by month 12 occurred in 25.1% of patients, and was more common among those randomized to ranolazine versus placebo at 6-month (21.0% vs. 14.6%) and 12-month (28.0% vs. 22.1%) follow-up. Among patients with DM, discontinuation of treatment occurred in 30.6%, and was more common among those randomized to ranolazine versus placebo at 6 months (26.6% vs. 17.9%) and 12 months (34.6% vs. 26.5%); among patients randomized to ranolazine, the rate of treatment discontinuation at 12 months was greater for patients with baseline HbA1c ≥7.5 than <7.5% (40.2 vs. 30.9%).
Table 1.
Baseline Characteristics by Randomized Treatment and DM Status*
| All patients | DM | No DM | ||||
|---|---|---|---|---|---|---|
| Ranolazine (n=1317) | Placebo (n=1287) | Ranolazine (n=482) | Placebo (n=479) | Ranolazine (n=835) | Placebo (n=808) | |
| Age (years) | 63 (56, 71) | 63 (56, 71) | 65 (59, 72) | 64 (58, 72) | 62 (55, 71) | 62 (56, 70) |
| Age ≥75 years | 206 (15.6%) | 192 (14.9%) | 72 (14.9%) | 81 (16.9%) | 134 (16.0%) | 111 (13.7%) |
| Female | 274 (20.8%) | 257 (20.0%) | 122 (25.3%) | 127 (26.5%) | 152 (18.2%) | 130 (16.1%) |
| Caucasian race | 1199 (91.0%) | 1187 (92.2%) | 422 (87.6%) | 422 (88.1%) | 777 (93.1%) | 765 (94.7%) |
| BMI, kg/m2 | 29 (26, 32) | 29 (26, 32) | 31 (27, 34) | 30 (28, 34) | 28 (25, 31) | 28 (25, 31) |
| Weight, kg | 85 (75, 97) | 85 (75, 96) | 90 (78, 103) | 87 (78, 101) | 82 (74, 94) | 84 (75, 94) |
| Waist circumference, cm | 104 (96, 112) | 104 (96, 112) | 107 (100, 117) | 109 (99, 116) | 102 (94, 109) | 102 (94, 110) |
| History of DM | 443 (33.6%) | 430 (33.4%) | 443 (91.9%) | 430 (89.8%) | -- | -- |
| Type 1 | 20 (1.5%) | 13 (1.0%) | 20 (4.1%) | 13 (2.7%) | -- | -- |
| Type 2 | 423 (32.1%) | 417 (32.4%) | 423 (87.8%) | 417 (87.1%) | -- | -- |
| Diabetes medication | ||||||
| Insulin | 160 (12.1%) | 141 (11.0%) | 160 (33.2%) | 141 (29.4%) | -- | -- |
| Metformin | 238 (18.1%) | 217 (16.9%) | 238 (49.4%) | 217 (45.3%) | -- | -- |
| Other agents | 157 (11.9%) | 175 (13.6%) | 157 (32.6%) | 175 (36.5%) | -- | -- |
| HbA1c ≥ 7.5% | 194 (15.0%) | 172 (13.7%) | 194 (40.8%) | 172 (37.1%) | -- | -- |
| HbA1c < 7.5% | 1102 (85.0%) | 1079 (86.3%) | 282 (59.2%) | 291 (62.9%) | 820 (100.0%) | 788 (100.0%) |
| Hypertension | 1121 (85.1%) | 1130 (87.8%) | 447 (92.7%) | 458 (95.6%) | 674 (80.7%) | 672 (83.2%) |
| Hyperlipidemia | 1145 (86.9%) | 1096 (85.2%) | 436 (90.5%) | 414 (86.4%) | 709 (84.9%) | 682 (84.4%) |
| Lipid lowering agents | ||||||
| Statin | 1236 (93.8%) | 1188 (92.3%) | 451 (93.6%) | 443 (92.5%) | 785 (94.0%) | 745 (92.2%) |
| Non-statin lipid-lowering agent | 194 (14.7%) | 185 (14.4%) | 87 (18.0%) | 93 (19.4%) | 107 (12.8%) | 92 (11.4%) |
| Smoking, current | 277 (21.0%) | 264 (20.5%) | 75 (15.6%) | 88 (18.4%) | 202 (24.2%) | 176 (21.8%) |
| Chronic kidney disease | 106 (8.0%) | 109 (8.5%) | 67 (13.9%) | 69 (14.4%) | 39 (4.7%) | 40 (5.0%) |
| Peripheral arterial disease | 159 (12.1%) | 151 (11.7%) | 77 (16.0%) | 79 (16.5%) | 82 (9.8%) | 72 (8.9%) |
| Prior myocardial infarction | 614 (46.6%) | 603 (46.9%) | 218 (45.2%) | 209 (43.6%) | 396 (47.4%) | 394 (48.8%) |
| History of revascularization (any) | 679 (51.6%) | 612 (47.6%) | 256 (53.1%) | 259 (54.1%) | 423 (50.7%) | 353 (43.7%) |
| Prior PCI other than index PCI | 593 (45.0%) | 527 (40.9%) | 225 (46.7%) | 221 (46.1%) | 368 (44.1%) | 306 (37.9%) |
| Prior CABG | 209 (15.9%) | 196 (15.2%) | 92 (19.1%) | 95 (19.8%) | 117 (14.0%) | 101 (12.5%) |
| Prior CHF | 229 (17.4%) | 236 (18.3%) | 90 (18.7%) | 91 (19.0%) | 139 (16.6%) | 145 (17.9%) |
| LVEF, median | 55 (48, 60) | 55 (50, 60) | 55 (47, 60) | 55 (45, 60) | 55 (49, 60) | 55 (50, 62) |
| Reason for index PCI | ||||||
| ACS | 433 (32.9%) | 455 (35.4%) | 125 (25.9%) | 151 (31.5%) | 308 (36.9%) | 304 (37.6%) |
| Non-ACS | 884 (67.1%) | 832 (64.6%) | 357 (74.1%) | 328 (68.5%) | 527 (63.1%) | 504 (62.4%) |
| Baseline angina frequency† | ||||||
| No angina (SAQ AF=100) | 178 (15.0%) | 200 (17.1%) | 56 (13.1%) | 72 (17.2%) | 122 (16.1%) | 128 (17.1%) |
| Angina monthly (SAQ AF=61–99) | 485 (40.8%) | 520 (44.5%) | 174 (40.6%) | 174 (41.5%) | 311 (40.9%) | 346 (46.1%) |
| Angina weekly (SAQ AF=31–60) | 396 (33.3%) | 349 (29.9%) | 143 (33.3%) | 134 (32.0%) | 253 (33.3%) | 215 (28.7%) |
| Angina daily (SAQ AF=0–30) | 130 (10.9%) | 100 (8.6%) | 56 (13.1%) | 39 (9.3%) | 74 (9.7%) | 61 (8.1%) |
| Angina daily or weekly (SAQ AF ≤60) | 526 (44.2%) | 449 (38.4%) | 199 (46.4%) | 173 (41.3%) | 327 (43.0%) | 276 (36.8%) |
Continuous data presented as median (25th, 75th percentile); categorical variables presented as number (percent).
Data for patients included in the quality of life substudy
ACS = acute coronary syndrome; AF = atrial fibrillation; BMI = body mass index; CABG = coronary artery bypass grafting; CHF = congestive heart failure; DM = diabetes mellitus; HbA1c = hemoglobin A1c; LVEF = left ventricular ejection fraction; PCI = percutaneous coronary intervention; SAQ = Seattle Angina Questionnaire
Compared to patients without DM, patients with DM were older, heavier, and more likely to be female. They had a higher prevalence of hypertension, hyperlipidemia, chronic kidney disease, and peripheral arterial disease. Patients with DM were less likely to have undergone index PCI for an acute coronary syndrome indication. Angina frequency at the time of enrollment was similar in patients with and without DM.
EFFECT OF RANOLAZINE ON GLYCOMETABOLIC PARAMETERS
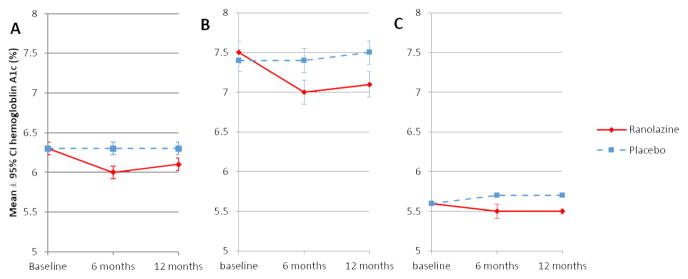
Overall, HbA1c remained stable in placebo-treated patients from baseline to 6 and 12 months (mean ± standard deviation 6.3 ± 1.3% at all 3 time periods), but on average decreased in ranolazine-treated patients (6.3 ± 1.4% at baseline, 6.0 ± 1.2% at 6 months, 6.1 ± 1.2% at 12 months) (Figure 1). A statistically significant reduction in HbA1c among ranolazine-treated patients was present in those with and without DM. The least mean squares difference in HbA1c (± standard error) for patients randomized to ranolazine compared to those randomized to placebo was −0.28 ± 0.03 at 6 months and −0.29 ± 0.03% at 12 months. The least squares mean reduction in HbA1c for ranolazine compared to placebo was greater in patients with DM (0.42 ± 0.08% and 0.44 ± 0.08% decrease at 6 and 12 months, respectively) when compared with patients without DM (0.19 ± 0.02% and 0.20 ± 0.02% decrease at 6 and 12 months, respectively, p<0.001 for interaction at both time points).
Figure 1. Effect of Ranolazine on HbA1c in Patients With and Without DM.
Displayed is the effect of ranolazine on HbA1c in patients with and without DM in: A) all patients; B) patients with DM at baseline; and C) patients without DM at baseline.
*p<0.001; error bars represent standard error of the mean
DM = diabetes mellitus; HbA1c = hemoglobin A1c
Among patients without DM at baseline, patients treated with ranolazine compared to placebo had a significantly lower incidence of new DM diagnoses at 6-month follow-up, but not at 12 months (Table 2). Patients randomized to ranolazine were less likely than placebo patients to have an increase in HbA1c ≥1% at 6-month follow up (30.2% vs. 52.9%; odds ratio [OR] 0.39, 95% confidence interval [CI] 0.33–0.46; p<0.001) and at 12-month follow-up (29.1% vs. 51.8%; OR 0.38, 95% CI 0.31–0.46; p<0.001) (Table 2), which was similar in patients with and without DM (interaction p=0.220 at 6 months, and interaction p=0.248 at 12 months).
Table 2.
Outcomes Related to Glucose Control at 6 and 12 Months
| 6 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|
| Endpoint | Ranolazine | Placebo | OR (95% CI) | p-value | Ranolazine | Placebo | OR (95% CI) | p-value |
| New onset DM (non-diabetic patients at baseline) | 14/798 (1.8%) | 27/776 (3.5%) | 0.49 (0.25–0.93) | 0.031 | 18/796 (2.3%) | 29/752 (3.9%) | 0.59 (0.32–1.06) | 0.079 |
| Worsening glucose control* | ||||||||
| All patients | 327/1083 (30.2%) | 567/1071 (52.9%) | 0.39 (0.33–0.47) | <0.001 | 295/1013 (29.1%) | 532/1027 (51.8%) | 0.38 (0.31–0.46) | <0.001 |
| Baseline DM | 114/380 (30.0%) | 187/381 (49.1%) | 0.45 (0.34–0.61) | <0.001 | 112/354 (31.6%) | 189/364 (51.9%) | 0.44 (0.32–0.60) | <0.001 |
| No DM | 213/703 (30.3%) | 380/690 (55.1%) | 0.36 (0.29–0.45) | <0.001 | 183/659 (27.8%) | 343/663 (51.7%) | 0.35 (0.28–0.44) | <0.001 |
Worsening glucose control was defined as an increase in HbA1c from baseline by ≥1%
CI = confidence interval; DM = diabetes mellitus; OR = odds ratio
EFFECT OF RANOLAZINE ON ANGINA FREQUENCY IN PATIENTS WITH DM
Patients with DM had a substantial reduction in angina frequency, regardless of randomized treatment (Supplemental Table 1). At 6 months, 192 (53.3%) patients randomized to ranolazine had resolution of angina, compared with 179 (49.6%) randomized to placebo (Supplemental Table 2). From baseline to 1 month, ranolazine-treated DM patients had an increase (improvement) in SAQ angina frequency score of 18.9 as compared to 16.8 for placebo-treated patients (least squares mean difference 2.15; p=0.11). SAQ angina frequency score adjusted for baseline score indicated improved angina frequency scores in patients randomized to ranolazine compared with those randomized to placebo at 6 months (88.3 vs. 85.4; least squares mean difference 2.86; p=0.033), but this difference did not persist at 12 months (least squares mean difference 1.77; 88.2 vs. 86.6; p=0.18). Outcomes among patients without DM have been previously reported, and showed no significant difference in SAQ angina frequency between patients randomized to ranolazine or placebo at any time point (6).
Among patients with DM and with worse blood glucose control at baseline (HbA1c ≥7.5%), randomization to ranolazine reduced angina; improvement was associated with less angina and better SAQ angina frequency scores at month 1 (87.9 vs. 83.5 for ranolazine vs. placebo; p=0.036) and month 6 (89.7 vs. 83.9, p=0.008), but not month 12 (86.0 vs. 85.1, p=0.68). The least squares mean difference ± standard error in SAQ angina frequency score between ranolazine- and placebo-treated patients was 4.40 ± 2.08 at 1 month, 5.80 ± 2.17 at 6 months, and 0.98 ± 2.39 at 12 months in patients with baseline HbA1c ≥7.5% (Figure 2). By contrast, in patients with HbA1c <7.5% at baseline, SAQ angina frequency score was not significantly different for patients randomized to ranolazine and placebo at all follow-up intervals. Interaction between HbA1c subgroup and treatment assignment revealed a trend toward greater effect on angina among the patients with worse glucose control at baseline (p=0.074), but this effect dissipated by 12 months. When we analyzed treatment by subgroup interactions for different HbA1c cut-offs (≥ and <6.5%, 7.0%, 7.5%, and 8.0%) at 6 months, the adjusted mean improvement in SAQ angina frequency from baseline was numerically greatest in patients with worse baseline blood glucose control; however, interaction testing between HbA1c subgroup and treatment assignment was not significant (Supplemental Figure 1). Similar to the results for patients with HbA1c ≥ and <7.5%, this benefit was not observed at 12 months.
Figure 2. SAQ Angina Frequency between Treatment Groups by HbA1c Subgroup.
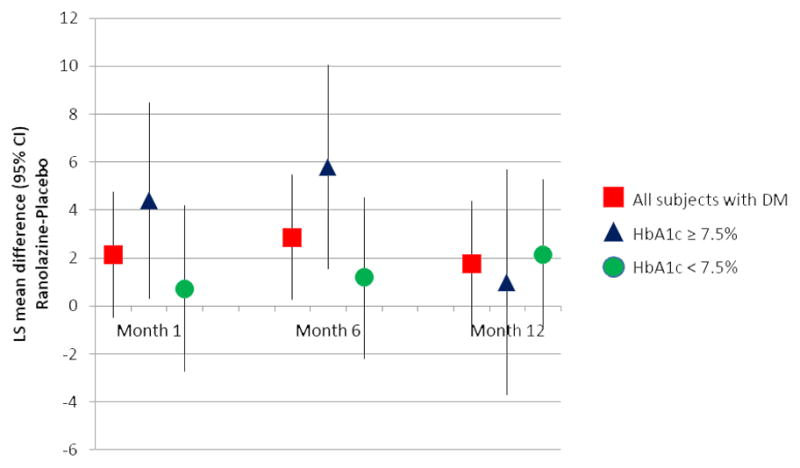
Adjusted mean difference of SAQ angina frequency between treatment groups by HbA1c subgroup at month 1, month 6, and month 12
* = p<0.05
Treatment by subgroup interaction p=0.20 at 1 month, 0.074 at 6 months, and 0.98 at 12 months
CI = confidence interval; HbA1c = hemoglobin A1c; LS = least squares; SAQ = Seattle Angina Questionnaire
No difference in ranolazine’s effect on SAQ angina frequency was noted by sex (p for interaction = 0.91 at 1 month, 0.98 at 6 months, and 0.85 at 12 months). Subgroup analysis within regions (North America, Israel and Western Europe, Russia and Eastern Europe) demonstrated findings consistent with the overall results.
DISCUSSION
In this pre-specified secondary analysis from the RIVER-PCI trial, treatment with ranolazine reduced HbA1c among patients with and without DM, with a greater absolute treatment effect in patients with DM. Ranolazine also reduced the incidence of worsening glucose control and new DM among patients without DM at baseline. This study confirms the glycometabolic effects of ranolazine, now observed across several randomized clinical trials (12–15), including RIVER-PCI. In addition to glycometabolic effects, ranolazine had a significant effect on angina frequency in patients with DM at 6 months as measured by the SAQ angina frequency score, with numerically greater efficacy at higher levels of baseline HbA1c, providing additional insight into the antianginal effects at 6 months seen in the main trial results; however, ranolazine’s effect on SAQ angina frequency dissipated at 12 months and was not significant. These findings suggest a particular benefit of ranolazine for chronic angina among patients with DM and poor glucose control.
Ranolazine has been evaluated in nine clinical trials, including six in patients with CAD (4, 12–19). Six of these trials (including RIVER-PCI) have reported the effects of ranolazine on HbA1c (Figure 3). Across these trials, the average absolute reduction in HbA1c with ranolazine versus placebo was approximately 0.45%, with a range from 0.11% to 0.70%. All trials found that ranolazine significantly reduced HbA1c, except for one study that enrolled only metformin-treated patients and reduced the dose of metformin in the ranolazine arm, but not the placebo arm. Several potential mechanisms underlying ranolazine’s effect on glucose control have been examined. Ranolazine preserves pancreatic β-cell mass in streptozocin-treated mice by unclear molecular mechanisms (20), reduces glucagon secretion via inhibition of sodium channels (8), and diminishes fatty acid oxygenation in the liver, shifting the liver’s energy source from fatty acids to glucose (21). Ranolazine also increases steady-state metformin concentrations in the serum (22), and some of ranolazine’s effect on HbA1c seen in our study may be mediated by potentiation of metformin’s effect, though this mechanism would not explain ranolazine’s effect on HbA1c in patients without DM. The results of the present analysis, combined with the emerging mechanistic data, reinforce the results of prior trials of ranolazine’s glucose-lowering efficacy in patients with CAD.
Figure 3. Effect of Ranolazine on HbA1c in Patients with Diabetes Mellitus in Published Clinical Trials.
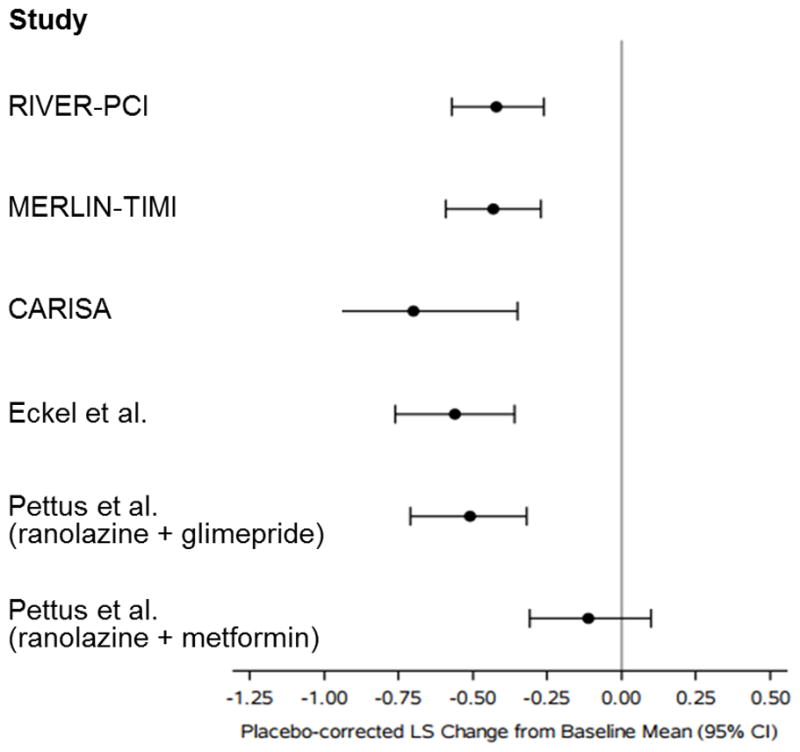
RIVER-PCI and CARISA enrolled patients with stable angina, and MERLIN-TIMI 36 enrolled patients with acute coronary syndromes; the remaining studies enrolled patients with diabetes mellitus and no CAD. Data displayed for CARISA is for 1000 mg twice daily dose of ranolazine. For the displayed data, follow-up duration ranged from 3–6 months.
CAD = coronary artery disease; CARISA = Combination Assessment of Ranolazine in Stable Angina; HbA1c = hemoglobin A1c; MERLIN-TIMI 36 = Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST-Elevation Acute Coronary Syndromes-Thrombolysis in Myocardial Infarction; RIVER-PCI = Ranolazine in Patients with Incomplete Revascularization after Percutaneous Coronary Intervention
In addition to confirming the effect of ranolazine on glycometabolic parameters, we also confirm its particular antianginal efficacy in patients with DM and poor glucose control. In Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST-Elevation Acute Coronary Syndromes-Thrombolysis in Myocardial Infarction 36 (MERLIN TIMI-36) trial, ranolazine reduced recurrent ischemia at 12 months by 25% in patients with DM, compared to 13% among all participants in the trial (13). In the Type 2 Diabetes Evaluation in Patients with Chronic Stable Angina (TERISA) trial, which only enrolled patients with type 2 DM, ranolazine significantly reduced patients’ number of weekly angina episodes over 8 weeks of follow-up (17), with greater efficacy in patients with higher baseline HbA1c (5). In our study, patients with HbA1c ≥7.5% had a significant reduction in angina burden, which was not seen in patients with HbA1c <7.5%; however, this benefit persisted only through 6 months, and was not present at 12 months. The improvements in mean SAQ angina frequency score at 6 months for all patients with DM (~3) and those with baseline HbA1c ≥7.5% (~6) are modest, but similar to those seen in clinical trials of PCI versus medical therapy for patients with obstructive CAD (23). The large proportion of patients that were asymptomatic or had minimal angina following index PCI also reduced the potential impact of ranolazine on angina frequency.
The exact reason for lack of a benefit at 12 months is unclear, but ranolazine was discontinued more often than placebo over time, diminishing its effect at longer duration of follow-up in analyses performed using the intention-to-treat principle. Moreover, fewer patients completed SAQ angina frequency questionnaires over time (sample size decreased from 848 patients at baseline to 656 patients), reducing statistical power to detect a significant difference. Also, if ranolazine’s effect on angina and glucose are proportionate, as might be suggested by these data, the metabolic effects of ranolazine were greatest at 6 months, and are unlikely to change more with longer follow-up. Finally, angina is not a static condition, and angina in most patients resolves over time with or without changes in treatments (6), making differences most likely to be observed sooner after the angina population is identified. To surmise, dissipation of ranolazine’s reduction in angina frequency by 12 months raises concerns about the durability of its antianginal effect.
While the specific mechanisms explaining ranolazine’s particular efficacy in patients with DM are unclear, several mechanisms can be considered. Ranolazine reduces angina frequency more effectively in patients with a greater baseline angina burden (6), and patients with worse blood glucose control have more severe CAD, impaired endothelial cell function, increased inflammation, and reduced collateralization (24–27). Cardiac myocytes from diabetic mice have increased sodium influx due to a reduction in phosphoinositide 3-kinase signaling (28), perhaps making them more susceptible to ranolazine’s action as a sodium channel inhibitor (3). Ranolazine also increases serum concentrations of metoprolol in extensive cytochrome 2D6 metabolizers (29), which may potentiate its effect, though this would not explain differential efficacy in patients with worse baseline blood glucose control.
STUDY LIMITATIONS
Our study had several limitations. First, we evaluated the effect of ranolazine on multiple separate glycometabolic parameters, and also evaluated the effect of ranolazine in a subgroup of patients from a clinical trial with a neutral primary outcome, which raises concerns about the interpretation of any single p-value. Nonetheless, the analysis of changes in HbA1c with ranolazine was pre-specified, is consistent with previous reports, and is biologically plausible, lending credence to these conclusions. Second, although our study of ranolazine’s glycometabolic effects was prospectively designed, it was ancillary to the main objectives of RIVER-PCI, and some patients did not have HbA1c measured at baseline and follow-up. Nevertheless, the number of patients without HbA1c measurements was small and unlikely to qualitatively affect our conclusions. Moreover, glycometabolic data was collected from >75% of patients at all follow-up visits, analyses were performed at a central laboratory, reliable baseline HbA1c testing allowed patients without previously-diagnosed DM to be included in the DM group for all analyses, and patients were all followed for 12 months. Finally, more than one in four patients in the ranolazine arm discontinued treatment by 12 months; treatment discontinuation obscures study drug effects as analyzed with intention to treat. As a result, our results may underestimate the effect of ranolazine on glucose control and angina in patients with DM who are adherent to therapy at longer follow-up intervals.
CONCLUSIONS
Ranolazine significantly lowered HbA1c and lessened the new onset of DM in RIVER-PCI patients, including those with and without DM. Moreover, ranolazine was numerically more effective at reducing angina frequency at 6 months (but not 12 months) in diabetics with HbA1c ≥7.5% and incomplete revascularization, suggesting a possible synergy between the drug’s effect on angina and glucose control.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge
Ranolazine is an antianginal agent with additional effects on glucose metabolism, lowering HbA1c and reducing the incidence of new onset diabetes mellitus (DM).
Competency in Clinical Care
Ranolazine may be more effective at reducing angina in patients with worse baseline glucose control, suggesting a possible synergistic effect in angina and glucose control; therefore, ranolazine may be particularly effective for patients with poorly controlled DM also experiencing frequent chronic angina.
Translational Outlook
Future studies are needed to confirm the interaction between ranolazine’s effects on angina and glucose control, and elucidate potential mechanisms.
Acknowledgments
The authors thank Erin Campbell, MS, for expert editorial assistance. Ms. Campbell did not receive payment for her assistance, apart from her employment at the institution where this study was conducted.
Sources of funding: The RIVER-PCI Trial was sponsored by Gilead Sciences, Inc., and is co-funded by The Menarini Group.
ABBREVIATIONS
- CAD
coronary artery disease
- DM
diabetes mellitus
- HbA1c
hemoglobin A1c
- MERLIN TIMI-36
Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST-Elevation Acute Coronary Syndromes-Thrombolysis in Myocardial Infarction 36
- PCI
percutaneous coronary intervention
- RIVER-PCI
Ranolazine in Patients with Incomplete Revascularization after Percutaneous Coronary Intervention
- SAQ
Seattle Angina Questionnaire
- TERISA
Type 2 Diabetes Evaluation in Patients with Chronic Stable Angina
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and its Burden in the United States, 2014. Centers for Disease Control and Prevention web site; [Accessed November 18, 2016]. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Updated May 15, 2015. [Google Scholar]
- 2.Hui G, Koch B, Calara F, Wong ND. Angina in coronary artery disease patients with and without diabetes: US National Health and Nutrition Examination Survey 2001-2010. Clin Cardiol. 2016;39:30–6. doi: 10.1002/clc.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaitman BR. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation. 2006;113:2462–72. doi: 10.1161/CIRCULATIONAHA.105.597500. [DOI] [PubMed] [Google Scholar]
- 4.Chaitman BR, Pepine CJ, Parker JO, et al. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291:309–16. doi: 10.1001/jama.291.3.309. [DOI] [PubMed] [Google Scholar]
- 5.Arnold SV, McGuire DK, Spertus JA, et al. Effectiveness of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina according to baseline hemoglobin A1c. Am Heart J. 2014;168:457–65. e2. doi: 10.1016/j.ahj.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Alexander KP, Weisz G, Prather K, et al. Effects of ranolazine on angina and quality of life after percutaneous coronary intervention with incomplete revascularization: results From the Ranolazine for Incomplete Vessel Revascularization (RIVER-PCI) Trial. Circulation. 2016;133:39–47. doi: 10.1161/CIRCULATIONAHA.115.019768. [DOI] [PubMed] [Google Scholar]
- 7.Greiner L, Hurren K, Brenner M. Ranolazine and its effects on hemoglobin A1C. Ann Pharmacother. 2016;50:410–5. doi: 10.1177/1060028016631757. [DOI] [PubMed] [Google Scholar]
- 8.Dhalla AK, Yang M, Ning Y, et al. Blockade of Na+ channels in pancreatic alpha-cells has antidiabetic effects. Diabetes. 2014;63:3545–56. doi: 10.2337/db13-1562. [DOI] [PubMed] [Google Scholar]
- 9.Weisz G, Genereux P, Iñiguez A, et al. Ranolazine in patients with incomplete revascularisation after percutaneous coronary intervention (RIVER-PCI): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387:136–45. doi: 10.1016/S0140-6736(15)00459-6. [DOI] [PubMed] [Google Scholar]
- 10.Weisz G, Farzaneh-Far R, Ben-Yehuda O, et al. Use of ranolazine in patients with incomplete revascularization after percutaneous coronary intervention: design and rationale of the Ranolazine for Incomplete Vessel Revascularization Post-Percutaneous Coronary Intervention (RIVER-PCI) trial. Am Heart J. 2013;166:953–9. e3. doi: 10.1016/j.ahj.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Arnold SV, Kosiborod M, Li Y, et al. Comparison of the Seattle Angina Questionnaire with daily angina diary in the TERISA clinical trial. Circ Cardiovasc Qual Outcomes. 2014;7:844–50. doi: 10.1161/CIRCOUTCOMES.113.000752. [DOI] [PubMed] [Google Scholar]
- 12.Eckel RH, Henry RR, Yue P, et al. Effect of ranolazine monotherapy on glycemic control in subjects with type 2 diabetes. Diabetes Care. 2015;38:1189–96. doi: 10.2337/dc14-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrow DA, Scirica BM, Chaitman BR, et al. Evaluation of the glycometabolic effects of ranolazine in patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation. 2009;119:2032–9. doi: 10.1161/CIRCULATIONAHA.107.763912. [DOI] [PubMed] [Google Scholar]
- 14.Pettus J, McNabb B, Eckel RH, et al. Effect of ranolazine on glycaemic control in patients with type 2 diabetes treated with either glimepiride or metformin. Diabetes Obes Metab. 2016;18:463–74. doi: 10.1111/dom.12629. [DOI] [PubMed] [Google Scholar]
- 15.Timmis AD, Chaitman BR, Crager M. Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur Heart J. 2006;27:42–8. doi: 10.1093/eurheartj/ehi495. [DOI] [PubMed] [Google Scholar]
- 16.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007;297:1775–83. doi: 10.1001/jama.297.16.1775. [DOI] [PubMed] [Google Scholar]
- 17.Kosiborod M, Arnold SV, Spertus JA, et al. Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina: results from the TERISA randomized clinical trial (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina) J Am Coll Cardiol. 2013;61:2038–45. doi: 10.1016/j.jacc.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Chaitman BR, Skettino SL, Parker JO, et al. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol. 2004;43:1375–82. doi: 10.1016/j.jacc.2003.11.045. [DOI] [PubMed] [Google Scholar]
- 19.Stone PH, Gratsiansky NA, Blokhin A, Huang I-Z, Meng L ERICA Investigators. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol. 2006;48:566–75. doi: 10.1016/j.jacc.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 20.Ning Y, Zhen W, Fu Z, et al. Ranolazine increases beta-cell survival and improves glucose homeostasis in low-dose streptozotocin-induced diabetes in mice. J Pharmacol Exp Ther. 2011;337:50–8. doi: 10.1124/jpet.110.176396. [DOI] [PubMed] [Google Scholar]
- 21.Mito MS, Constantin J, de Castro CV, Yamamoto NS, Bracht A. Effects of ranolazine on fatty acid transformation in the isolated perfused rat liver. Mol Cell Biochem. 2010;345:35–44. doi: 10.1007/s11010-010-0557-8. [DOI] [PubMed] [Google Scholar]
- 22.Zack J, Berg A, Juan A, et al. Pharmacokinetic drug-drug interaction study of ranolazine and metformin in subjects with type 2 diabetes mellitus. Clin Pharmacol Drug Dev. 2015;121:121–9. doi: 10.1002/cpdd.174. [DOI] [PubMed] [Google Scholar]
- 23.Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359:677–687. doi: 10.1056/NEJMoa072771. [DOI] [PubMed] [Google Scholar]
- 24.Moura FA, Figueiredo VN, Teles BS, et al. Glycosylated hemoglobin is associated with decreased endothelial function, high inflammatory response, and adverse clinical outcome in non-diabetic STEMI patients. Atherosclerosis. 2015;243:124–30. doi: 10.1016/j.atherosclerosis.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Shen Y, Lu L, Ding FH, et al. Association of increased serum glycated albumin levels with low coronary collateralization in type 2 diabetic patients with stable angina and chronic total occlusion. Cardiovasc Diabetol. 2013;12:165. doi: 10.1186/1475-2840-12-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravipati G, Aronow WS, Ahn C, Sujata K, Saulle LN, Weiss MB. Association of hemoglobin A(1c) level with the severity of coronary artery disease in patients with diabetes mellitus. Am J Cardiol. 2006;97:968–9. doi: 10.1016/j.amjcard.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 27.Hong LF, Li XL, Guo YL, et al. Glycosylated hemoglobin A1c as a marker predicting the severity of coronary artery disease and early outcome in patients with stable angina. Lipids Health Dis. 2014;13:89. doi: 10.1186/1476-511X-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Z, Jiang YP, Wu CY, et al. Increased persistent sodium current due to decreased PI3K signaling contributes to QT prolongation in the diabetic heart. Diabetes. 2013;62:4257–65. doi: 10.2337/db13-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranexa [package insert] Foster City, CA: Gilead Sciences, Inc; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.