Abstract
Preclinical evidence suggests angiotensin blockade therapy (ABT) decreases late radiation toxicities. This study aims to investigate the association between ABT and symptomatic radiation necrosis (SRN) following stereotactic radiosurgery (SRS). Resected brain metastases (rBM) and arteriovenous malformation (AVM) patients treated with SRS from 2002 to 2015 were identified. Patients in the ABT cohort were on therapy during SRS and at 1-month follow up. Kaplan Meier method and cumulative incidence model were used to analyze overall survival (OS) and intracranial outcomes. 228 consecutive patients were treated with SRS: 111 with rBM and 117 with AVM. Overall, 51 (22.4%) patients were in the ABT group: 32 (28.8%) in the rBM and 19 (16.2%) in AVM cohorts. Baseline characteristics were similar, except for higher Graded Prognostic Analysis (3–4) in the rBM (ABT: 25.0% vs. non-ABT: 49.0%, p = 0.033) and median age in the AVM (ABT: 51.4 vs. non-ABT: 35.4, p < 0.001) cohorts. In both populations, OS and intracranial efficacy (rBM—local control; AVM—obliteration rates) were statistically similar between the cohorts. ABT was associated with lower 1-year SRN rates in both populations: rBM, 3.1 versus 25.3% (p = 0.003); AVM, 6.7 vs. 14.6% (p = 0.063). On multivariate analysis, ABT was a significant predictive factor for rBM (HR: 0.17; 95% CI 0.03–0.88, p = 0.035), but did not reach statistical significance for AVM (HR: 0.36; 95% CI 0.09–1.52, p = 0.165). ABT use appears to be associated with a reduced risk of SRN following SRS, without detriment to OS or intracranial efficacy. A prospective trial to validate these findings is warranted.
Keywords: Radiation necrosis, Angiotensin converting enzyme inhibitor, Angiotensin receptor blocker, Brain metastases, Arteriovenous malformation
Introduction
Stereotactic radiosurgery (SRS) is a technique that delivers higher focal doses of radiation, while minimizing dose to healthy tissue [1, 2]. With increasing lesion size, however, the volume and dose of radiation to the surrounding normal brain escalates. Several months to years after SRS treatment, the irradiated adjacent brain tissue may elicit an inflammatory response, characterized by the development of necrotic tissue, edema and neurologic symptoms secondary to mass effect [3]. This late adverse event, known as radiation necrosis (RN), is one of the main dose-limiting toxicities associated with SRS and prevents further dose escalation for large lesions that have lower rates of local control [4, 5].
For the majority of patients, treatment with steroids (dexamethasone) is effective in curtailing the symptoms of RN; however, steroid use has been associated with significant side effects including increased risk of opportunistic infection, worsening hyperglycemia, gastritis, Cushingnoid appearance, central obesity and even psychosis [6]. These limitations call for the identification of other, safer agents to help prevent or treat symptomatic radiation necrosis.
The renin angiotensin system (RAS) is a cascade of enzymes and peptides well known in the regulation of blood pressure. Angiotensin converting enzyme (ACE) converts angiotensin I to angiotensin II; angiotensin II then binds to and activates the angiotensin receptors, signaling a downstream cascade of vascular changes [7]. Emerging evidence suggests that angiotensin receptors are associated with various physiological processes including angiogenesis, oxidative stress, inflammation and cognition [8, 9]. Moreover, recent studies have shown the ability of angiotensin receptor activation to regulate vascular endothelial growth factor (VEGF) and transforming growth factor receptor beta (TGFB) expression, which are central to the pathogenesis of radiation toxicities [10, 11]. Given the potential effect of angiotensin on vascular inflammation, fibrosis, and remodeling, the aim of this study is to investigate whether angiotensin system blockade is associated with reduced rates of symptomatic radiation necrosis (SRN).
Materials and methods
Patient selection
After obtaining Institutional Review Board approval, we retrospectively reviewed records of patients with resected brain metastases (rBM) treated with post-operative SRS from 2005 to 2015 as well as patients with arteriovenous malformation (AVM) treated with SRS from 2002 to 2015. Inclusion criteria for the cohorts was as follows: patients were assigned to the angiotensin blockade therapy (ABT) group if they were on either an ACE inhibitor (ACEi) or angiotensin receptor blocker (ARB) at least 1-month before and after SRS. Patients who did not receive either an ACEi or ARB at any time, ACEi/ARB less than 1 month before SRS, or ACEi/ARB more than 1 month after SRS were assigned to the non-ABT cohort. Exclusion criteria included receipt of prior whole brain radiation therapy, rBM from radiosensitive malignancies (e.g. small cell, germ cell, and lymphoma), and AVM patients with prior SRS or staged SRS (i.e. treatment delivered approximately within 6 months apart).
All patient charts were reviewed for the following baseline characteristics at the time of initial SRS: age, sex, and Eastern Cooperative Group Oncology performance status (ECOG PS). For rBM patients, presence of active systemic disease, and Graded Performance Assessment (GPA) score was recorded. For AVM patients, Spetlzer-Martin grade, Virginia Radiosurgery AVM Score [12], prior non-SRS treatments (i.e. embolization and or surgery), and prior rupture were recorded. For all patients, radiation treatment parameters were recorded, including the gross tumor (GTV) volume, planning target volume (PTV) margin, total dose, number of fractions and dose per fraction.
Radiation treatment
SRS was performed using a linear accelerator with 6 MV photon energies as previously described [13, 14]. For the post-operative SRS management of rBM, patients underwent high-resolution treatment planning magnetic resonance imaging (MRI) scan with and without contrast immediately before or following CT simulation. The T1 post-contrast MRI sequence encompassing the resection cavity as well as any enhancing tumor defined lesion constituted the GTV. No margin was utilized to create a clinical target volume. The GTV was expanded by 1–2.5 mm to generate the PTV based on treating physician preference and determined by resection cavity characteristics. Patients with large rBM cavities (typically > 40 mm in diameter) were treated with fractionated radiosurgery over 3–5 fractions using a frameless radiosurgery technique.
Similarly, patients with AVMs initially underwent placement of an imaging-compatible stereotactic head frame after administration of a local anesthetic supplemented by intravenous sedation. High-resolution axial plane MR imaging coupled with biplane stereotactic angiography was performed for dose planning. The GTV consisted of the entire AVM nidus volume, defined as the shunt between the afferent arteries and the draining veins. For AVM, no margin was added when expanding from the GTV to the PTV.
Follow-up
Follow-up of rBM patients consisted of history, clinical examination and brain MRI at 1 month after initial SRS, and then at 3 months intervals thereafter unless clinically indicated at an earlier time point. Local recurrence (LR) was defined as the presence of new progressive nodular enhancement within the prior 80% iso-dose line of the prior SRS treatment. Radiographic radiation necrosis (RN) was defined as development of a contrast-enhancing mass within prior SRS fields [15]; if there was a question of the nodular enhancement representing LR versus RN, cases were discussed at a multi-disciplinary tumor board to develop a consensus. Additional functional imaging was also obtained (e.g. MR perfusion, MR spectroscopy, or brain positron emission tomography [PET]) to further aid evaluation. For patients who were symptomatic, steroids were initially used. In patients with continued symptoms, hyperbaric oxygen and/or bevacizumab were also considered, while surgery was reserved for refractory patients and/or where the diagnosis remained unclear. Patients who underwent salvage surgical resection and found on pathology to have any residual disease were deemed to have a LR; patients with only necrosis (and no residual disease) were considered to have RN.
Patients with AVMs were also followed with a history, physical examination, and brain MRI at 6 weeks post-treatment, then every 6 months for 3 years, then annually. If MRI findings were suggestive of complete AVM obliteration, cerebral angiography was performed for confirmation. Complete AVM obliteration established on angiography was defined as the disappearance of the AVM shunt lying between feeding arteries and draining veins (the nidus) and the absence of early venous drainage. At any time when a new neurological symptom or sign developed, the patient underwent CT and/or MR for further evaluation.
Statistical analysis
The ABT and non-ABT groups were compared across categorical covariates using chi-squared tests or Fisher’s Exact tests, where appropriate, and were compared across continuous variables using ANOVA. For overall survival (OS), death from any cause was defined as the event, and patients were censored at time of last follow-up. OS for all patients, and radiographic obliteration rates & SRN for AVM patients, were estimated by the Kaplan–Meier product-limit method; the log-rank test was used to assess for differences between patients treated with ABT and non-ABT cohort. A univariate analysis (UVA) and multivariate analysis (MVA) was performed using the Cox proportional hazards model.
For rBM patients, LR, RN, and SRN were estimated using cumulative incidence methodology, with death without the event considered a competing risk. For patients with rBM who had additional intact lesions, these intact lesions were also included in the lesion-level (i.e. LR and RN) statistical analysis. For these intracranial outcomes, patients were censored at time of last brain imaging. Cumulative incidence curves for each non-survival outcome were compared between groups with death as a competing risk using Gray’s test for equality across groups [16]. Univariate and MVA regression analyses using the semiparametric proportional hazards model in the presence of competing risks were performed, as proposed by Fine and Gray [16]. All potentially prognostic covariates which were statistically significant in the univariate analysis were entered into the MVA model. All statistical tests were 2-sided, with p-values < 0.05 considered statistically significant. Statistical analysis was carried out using SAS version 9.4.0 statistical software (SAS Institute Inc., Cary, NC).
Results
Baseline clinical and dosimetric characteristics
Resected brain metastases
One hundred and eleven patients with rBM were identified. Thirty-two (28.8%) patients were found to be on ABT at the time of post-operative SRS and again at follow up 1-month later. These 111 patients had 156 lesions: 115 were resection cavities and 41 were intact lesions; 45 (28.8%) of the lesions were in the ABT group. Table 1 shows the rBM cohorts had similar baseline patient characteristics except that the ABT group had a lower percentage of patients with GPA 3.0–4.0 (25.0 vs. 49.4%, p = 0.033). No differences were seen in lesion-level characteristics between the two cohorts (Table 1). Median imaging follow-up for the rBM patients was statistically similar for the ABT and non-ABT cohorts, 8.7 and 13.9 months, respectively. Median single fraction dose for rBM was 18.0 Gy (range 15–21.0 Gy); for patients undergoing hypofractionated radiosurgery, the most common regimen used was 30 Gy in five fractions.
Table 1.
Baseline BM patient and lesions characteristics between ABT and non-ABT cohorts
| Covariate | ABT (32 BM patients or 45 lesions) | No ABT (79 BM patients or 111 lesions) | P-value |
|---|---|---|---|
| Sex | |||
| Male | 15 (46.9%) | 31 (39.2%) | 0.460 |
| Female | 17 (53.1%) | 48 (60.8%) | |
| Active systemic disease | |||
| Yes | 17 (54.8%) | 35 (44.3%) | 0.319 |
| No | 14 (45.2%) | 44 (55.7%) | |
| Primary histology | |||
| Lung | 13 (40.6%) | 36 (32.9%) | 0.132 |
| Breast | 4 (12.5%) | 15 (19.0%) | |
| Melanoma | 5 (15.6%) | 18 (22.8%) | |
| RCC, GI, or other | 10 (31.3%) | 10 (12.7%) | |
| Age | |||
| ≤65 | 20 (62.5%) | 62 (78.5%) | 0.083 |
| >65 | 12 (37.5%) | 17 (21.5%) | |
| Number of BM | |||
| 1 | 20 (62.5%) | 60 (75.9%) | 0.153 |
| >1 | 12 (37.5%) | 19 (24.1%) | |
| Resected lesion | |||
| Yes | 34 (75.6%) | 81 (73.0%) | 0.740 |
| No | 11 (24.4%) | 30 (27.0%) | |
| Location of BM | |||
| Frontal/parietal/temporal | 32 (71.1%) | 64 (57.7%) | 0.118 |
| Occipital/cerebellum/brainstem | 13 (28.9%) | 47 (42.3%) | |
| Extracranial metastases | |||
| Yes | 10 (22.3%) | 21 (26.6%) | 0.552 |
| No | 21 (67.7%) | 58 (73.4%) | |
| ECOG | |||
| 0 | 5 (15.6%) | 25 (31.6%) | 0.175 |
| 1 | 18 (58.1%) | 40 (50.6%) | |
| 2+ | 9 (28.1%) | 14 (17.7%) | |
| GPA class | |||
| 0–1.0 | 1 (3.1%) | 1 (1.3%) | 0.033 |
| 1.5–2.5 | 23 (71.9%) | 39 (49.4%) | |
| 3.0–4.0 | 8 (25.0%) | 39 (49.4%) | |
| Number of fractions | |||
| 1 | 31 (68.9%) | 89 (80.2%) | 0.129 |
| >1 | 14 (31.1%) | 22 (19.8%) | |
| Prescribed dose, Gy | |||
| Mean | 21.3 | 19.9 | 0.118 |
| Median | 20.0 | 18.0 | |
| CTV volume (cc) | |||
| ≤ 4 | 13 (31.0%) | 31 (27.9%) | 0.808 |
| 4–14 | 17 (40.5%) | 36 (32.4%) | |
| > 14 | 12 (28.6%) | 34 (30.6%) | |
| PTV margin (mm) | |||
| 0–1 | 13 (28.9%) | 42 (37.8%) | 0.437 |
| 1.5 | 13 (28.9%) | 23 (20.7%) | |
| 2–2.5 | 19 (42.2%) | 46 (41.4%) | |
| Conformality index | |||
| Mean | 1.56 | 1.52 | 0.502 |
| Median | 1.50 | 1.47 | |
| Prescription IDL (%) | |||
| ≤80 | 19 (44.2%) | 44 (40.0%) | 0.636 |
| >80 | 24 (55.8%) | 66 (60.0%) |
Bold p values denotes statistical significant, p < 0.05
ABT angiotensin blockade therapy, RCC renal cell carcinoma, GI gastrointestinal, BM brain metastases, ECOG eastern cooperative oncology group, RPA recursive partitioning analysis, GPA graded prognostic assessment, Gy gray, CTV clinical target volume, PTV planning target volume, IDL isodose line
AVM
Nineteen (16.2%) of the 117 patients undergoing SRS ablation for AVM met criteria for the ABT group. The patients had statistically similar baseline and dosimetric characteristics (Table 2) except for 1 factor, age: the ABT cohort was older than non-ABT cohort (51.5 vs. 38.4 years, p < 0.001). Median imaging follow-up for the AVM patients was statistically similar for the ABT and non-ABT cohorts, 24.8 and 20.9 months, respectively.
Table 2.
Baseline AVM patient and lesion characteristics between ABT and non-ABT cohorts
| Covariate | ABT (19 Patients/AVM) |
No ABT (98 Patients/AVM) |
P-value |
|---|---|---|---|
| Sex | |||
| Male | 8 (42.1%) | 40 (40.8%) | 0.917 |
| Female | 11 (57.9%) | 58 (59.2%) | |
| Age | |||
| Mean | 51.5 | 38.4 | < 0.001 |
| Median | 51.9 | 38.4 | |
| Spetlzer-Martin grade | |||
| 1–2 | 6 (31.6%) | 36 (36.7%) | 0.474 |
| 3 | 11 (57.9%) | 43 (43.9%) | |
| 4–5 | 2 (10.5%) | 19 (19.4%) | |
| Virginia radiosurgery AVM score | |||
| 0–1 | 7 (36.8%) | 33 (33.7%) | 0.436 |
| 2–3 | 10 (52.6%) | 56 (57.1%) | |
| 4 | 2 (10.5%) | 9 (9.2%) | |
| Ruptured AVM lesion | |||
| Yes | 11 (57.9%) | 42 (42.9%) | 0.228 |
| No | 8 (42.1%) | 56 (57.1%) | |
| Prior non-SRS treatment | |||
| Yes | 2 (10.5%) | 11 (11.2%) | 1 |
| No | 17 (89.5%) | 87 (88.8%) | |
| Prescribed dose, Gy | |||
| Mean | 17.7 | 17.6 | 0.817 |
| Median | 17.5 | 17.5 | |
| GTV volume (cc) | |||
| ≤4 | 13 (68.4%) | 59 (60.2%) | 0.752 |
| 4–14 | 6 (31.6%) | 33 (33.7%) | |
| >14 | 0 (0.0%) | 5 (5.1%) | |
| Prescription IDL (%) | |||
| ≤80 | 12 (63.2%) | 69 (70.4%) | 0.531 |
| >80 | 7 (36.8%) | 29 (29.6%) | |
Bold p values denotes statistical significant, p < 0.05
ABT angiotensin blockade therapy, AVM arteriovenous malformation, SRS stereotactic radiosurgery, Gy gray, GTV gross target volume, IDL isodose line
Overall survival
No OS difference was seen between ABT and non-ABT cohorts in either population (Fig. 1a, b). For rBM patients, median survival for the ABT cohort was 11.6 months and for the non-ABT cohort was 15.3 months. For AVM patients, median survival was not reached for both cohorts. ABT was not a significant factor on UVA or MVA in both populations.
Fig. 1.
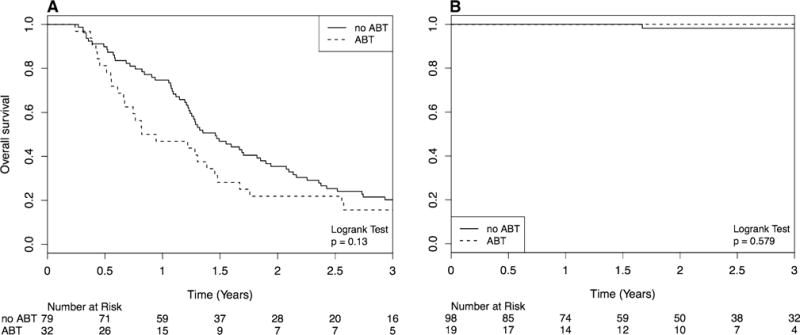
Kaplan–Meir Curve comparing OS in angiotensin blockade therapy (ABT) and stereotactic radiosurgery (SRS) vs. SRS alone for patients with resected brain metastases (a) and AVM (b)
Local intracranial efficacy
Resected brain metastases
There was no difference in the cumulative incidence of LR (Fig. 2a) for the ABT and non-ABT cohorts at 1 year (15.6 vs. 9.0%, p = 0.31). Median time to LR was 8.1 vs. 7.1 months between the two cohorts. ABT was not a significant predictor of LR on UVA or MVA. Multiple predictors for LR were identified on UVA including ECOG status, GPA, presence of extra-cranial metastases, tumor location, and GTV volume > 14; however, only GTV volume > 14 cc remained significant on MVA (Hazard Ratio [HR]: 5.22; 95% Confidence Interval [CI] 1.08–25.37, p = 0.012).
Fig. 2.
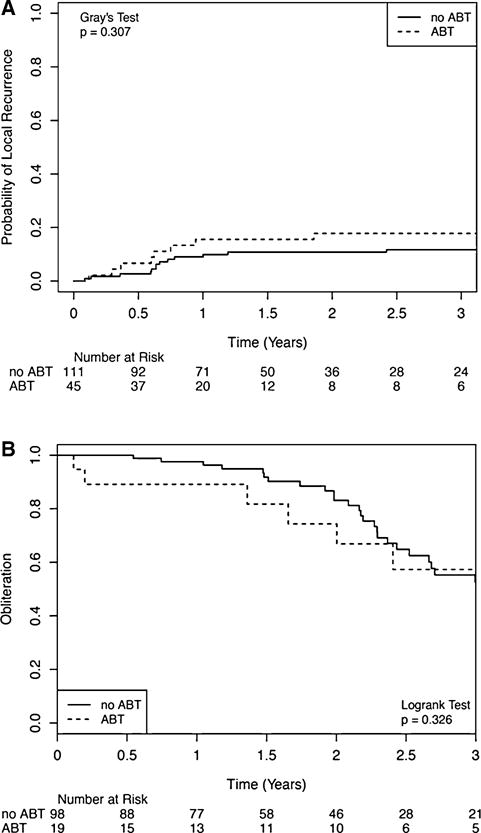
Comparison of intracranial efficacy for patients treated with angiotensin blockade therapy (ABT) and stereotactic radiosurgery (SRS) to SRS alone. Competing risk model to evaluate local control is utilized for resected brain metastases patients (a); Kaplan Meier model to compare obliteration rates for AVM patients (b) is utilized
AVM
Figure 2b demonstrates that the probability of obliteration was similar between the ABT and non-ABT cohorts: 1-year – 10.8 versus 2.4%; 2-year – 25.7 versus 16.9%. ABT was not a significant predictor for obliteration on both UVA and MVA. Ruptured AVM lesion and GTV ≤ 4 were significant on UVA, but neither was significant on MVA.
Radiation necrosis
Resected brain metastases
In the entire post-operative SRS cohort, 46 patients (41.4%) with 49 (33.5%) lesions developed radiographic evidence of RN. 1-year risk of RN was lower in the ABT cohort, 11.1 versus 21.6% (p = 0.067). Significant predictors for RN on UVA included lung histology, active systemic disease, presence of extra-cranial metastases, > 1 BM, resected lesion, prescription IDL > 80, PTV margin, GTV volume, and conformality index. ABT use showed a trend towards significance on UVA (p = 0.053). On MVA, ABT use (HR: 0.45; 95% CI 0.21–0.95, p = 0.036) was a statistically significant predictor for lower risk of RN, while larger GTV volume (HR: 4.29; 95% CI 1.13–16.19, p = 0.032) predicted for higher risk of RN.
Of the 46 patients, 29 (70.7%) were symptomatic: 2 (6.9%) in the ABT cohort and 27 (93.1%) in the non-ABT cohort. 20 (69.0%) of the symptomatic patients were treated with steroids only; 9 (31.0%) patients—all in the non-ABT cohort—developed SRN refractory to medical management, with 5 treated with surgical intervention, 3 treated with bevacizumab, and 1 treated with hyperbaric oxygen. One-year rates of SRN were significantly lower in the ABT cohort, 3.1 versus 25.3% (p = 0.003) (Fig. 3a). UVA showed ABT (HR: 0.15; 95% CI 0.04–0.64, p = 0.10) to be significant for SRN. Lung cancer histology and active systemic disease were also significant. On MVA analyses, ABT predicted for lower risk of SRN (HR: 0.17; 95% CI 0.03–0.88, p = 0.035), while the other factors no longer remained significant. Table 3 illustrates the factors identified on MVA for SRN for the rBM cohort.
Fig. 3.
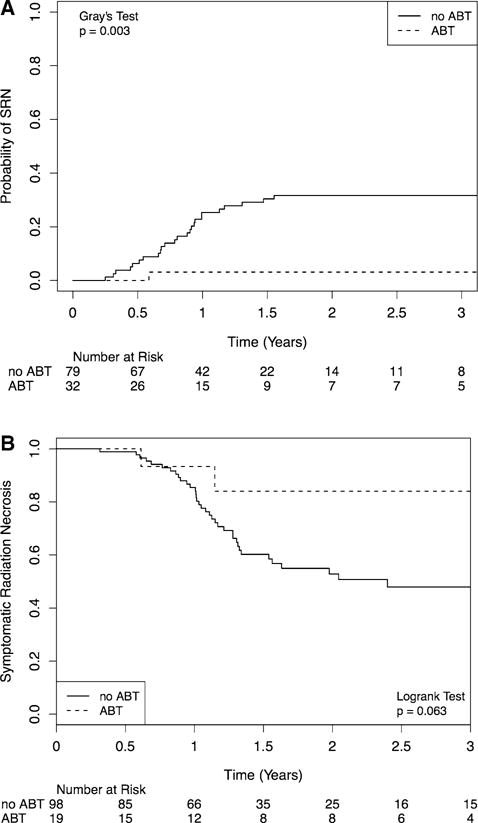
Comparison of symptomatic radiation necrosis for patients treated with angiotensin blockade therapy (ABT) and stereotactic radiosurgery (SRS) to SRS alone. Competing risk model is utilized for resected brain metastases patients (a) and Kaplan Meier model for AVM patients (b)
Table 3.
Multivariate analysis for radiation necrosis and symptomatic radiation necrosis in patients with brain metastases
| Radiographic RN
|
Symptomatic RN
|
|||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| ABT | ||||
| Yes | 0.45 (0.21–0.95) | 0.036 | 0.17 (0.03–0.88) | 0.035 |
| No | – | – | – | |
| Primary histology | ||||
| Lung | 2.22 (0.79–6.23) | 0.128 | 8.46 (0.92–77.93) | 0.060 |
| Breast | 0.68 (0.19–2.48) | 0.558 | 1.92 (0.18–20.37) | 0.590 |
| Melanoma | 0.92 (0.30–2.88) | 0.891 | 2.87 (0.31–26.32) | 0.351 |
| RCC/GI/other | – | – | – | – |
| Extracranial metastases | ||||
| Yes | 0.62 (0.28–1.34) | 0.221 | 1.14 (0.25–5.17) | 0.864 |
| No | – | – | – | – |
| Prescription IDL | ||||
| >80 | 1.78 (0.85–3.72) | 0.125 | ||
| ≤80 | – | – | ||
| CTV volume | ||||
| ≤4 | – | – | ||
| 4–14 | 2.47 (0.61–10.03) | 0.207 | ||
| >14 | 4.29 (1.13–16.19) | 0.032 | ||
| Resected lesion | ||||
| Yes | 1.92 (0.30–12.37) | 0.492 | ||
| No | – | – | ||
| ECOG performance status | ||||
| 0 | 0.49 (0.16–1.45) | 0.195 | ||
| 1 | 0.99 (0.40–2.45) | 0.987 | ||
| 2+ | – | – | ||
| Active systemic disease | ||||
| Yes | 0.42 (0.07–2.54) | 0.345 | ||
| No | – | – | ||
| GPA | ||||
| 0–2.5 | 0.51 (0.14–1.84) | 0.307 | ||
| 3.0–4.0 | – | – | ||
| Number of BM | ||||
| >1 | 1.36 (0.45–4.07) | 0.585 | ||
| 1 | – | – | ||
| Gender | ||||
| Male | 2.30 (0.96–5.52) | 0.062 | ||
| Female | – | – | ||
Bold p values denotes statistical significant, p < 0.05
RN radiation necrosis, HR hazard ratio, CI confidence interval, ABT angiotensin blockade therapy, RCC renal cell carcinoma, GI gastrointestinal, IDL isodose line, CTV clinical target volume, ECOG eastern cooperative oncology group, GPA graded prognostic assessment, BM brain metastases
AVM
44 (37.6%) patients in the AVM group developed radiographic RN. 1-year risk of RN was lower in the ABT cohort, 6.7% versus 14.6% (p = 0.063). Significant predictors for RN on UVA included age, Speltzer-Martin grade, and GTV volume. ABT use showed a trend towards significance on UVA (p = 0.063). No factors were found to be significant on MVA.
Of the 44 patients with RN, 38 (86.4%) developed symptoms. All of these patients were treated with steroids and surgical intervention was not needed. Bevacizumab was utilized in 4 patients, while no patients were prescribed hyperbaric oxygen. There was a trend towards lower rates of SRN in the ABT cohort at 1 year, 6.7 versus 14.6% (p = 0.063) (Fig. 3b). Age > 40, Spetlzer-Martin Grade, and GTV volume were significant on UVA. ABT showed a trend towards significance (HR: 0.28; 95% CI 0.07–1.17, p = 0.063). On MVA, GTV volume (HR: 1.07; 95% CI 1.01–1.14, p = 0.029) remained significant for a higher probability of SRN, while ABT use did not reach statistical significance (HR: 0.36; 95% CI 0.09–1.52, p = 0.165).
Discussion
Historically, patients with rBM have been treated with whole brain radiation therapy (WBRT) [17, 18]; however, due to results showing worse neurocognitive decline and quality of life following WBRT, clinicians have begun to turns towards post-operative SRS [13, 19, 20]. Postoperative SRS is not without its limitations, with a main dose-limiting toxicity being SRN. As patients with brain metastases begin to live longer, partly due to improvements in systemic therapy including targeted agents [21] and immunotherapy [2], they are at increased risk for developing late adverse events. For AVM patients, two recent prospective trials demonstrated that treatment for unruptured AVMs decrease OS [22, 23] at early followup; SRS is now commonly eschewed given the inferior survival and risk of SRN. Methods to decrease the risk of SRN and improve the therapeutic ratio for SRS are clearly needed for these two populations.
In this study, we demonstrate that being on ABT during and at least 1 month after post-operative SRS is associated with a lower risk of RN and SRN for rBM. Because the diagnosis of SRN is challenging and can only be truly confirmed with pathology, we attempted to validate our findings in a model without this limitation. AVM is a benign intracranial process; radiographic changes after SRS are not due to progression of a malignant disease and can be attributed to an adverse radiation event [3]. In our population of AVM patients treated with SRS, we found that ABT treatment was associated with a trend towards lower incidence of SRN. Given that ABT treatment was not associated with a detriment in intracranial efficacy or OS in both populations, we believe these results are encouraging.
Consistent with our findings, there is a growing body of clinical literature that suggests ABT is associated with lower risks of late radiation effects. Recently, the NRG Oncology Radiation Therapy Oncology Group (RTOG) 0123 reported on the toxicity outcomes after randomizing stage II and III non-small cell lung cancer patients to the ACE inhibitor captopril or observation [24]. This study showed a lower rate of grade 2 radiation pneumonitis (14% vs. 23%), but was not statistically significant as a result of being underpowered due to low accrual rates. Taken this study in context with the other published, albeit retrospective studies [25–28], the correlation that ABT decreases the incidence of late radiation toxicities within multiple organ systems [24–27] (gastrointestinal, lung, heart and kidney) and with different radiation regimens [28, 29] (standard [1.8–2 Gy] and high-dose, SBRT fractionation [> 5 Gy]) provides support that angiotensin may be part of a central targetable pathway critical in the development of late radiation effect.
Support for this hypothesis also comes from pre-clinical studies demonstrating that oxidative damage, from both cardiac etiologies [30] and radiation therapy [31], dysregulates the ACE/angiotensin pathway, leading to elevated levels of TGFB and VEGF, molecules essential to the pathogenesis of fibrosis and poor organ function; ABT is able to modulate these pathways and lead to decrease late side effects in vivo [32]. Overall, these pre-clinical and clinical studies provide multiple levels of support to our findings that ABT is associated with lower risk of SRN after intracranial SRS.
Inherent limitations of our study include recall and selection bias inherent due to the retrospective design of this analysis. Our study is limited by the lack of information on dose of ABT. To help partially account for variations in duration of ABT, we only included patients in the ABT cohort who were receiving ABT during and 1 month after SRS. Another key limitation is that for rBM patients, the differential diagnosis for RN includes LR; only surgical resection and histologic analysis of the specimen can provide a definitive diagnosis. With the majority of symptomatic rBM patients not undergoing surgery, it is possible patients diagnosed with SRN may in fact have local progression. To help address this, we also investigated our hypothesis in patients with AVM, a population where this bias does not exist. Finally, while ABT treatment predicted for lower risk of SRN in the rBM cohort (p = 0.035), it did not reach statistical significance in the AVM cohort (p = 0.165). This is likely in part due to less power: 28.8% of rBM were on ABT while only 16.2% of AVM patients were on ABT.
In conclusion, incidental ABT use concurrent with SRS is associated with a statistically significant decreased risk of SRN in patients with rBM and demonstrates a trend of decreased risk in AVM. In addition, the use of ABT during and 1 month after SRS did not negatively impact OS or intracranial efficacy. These retrospective findings warrant further investigation in a prospective, randomized fashion. In the interim, we recommend discussing the risks and benefits of ABT (both ACEi and ARB)—FDA approved, extremely economical, and well-tolerated drugs—with patients who are at high risk for SRN and have a concomitant history of HTN in order to possibly add or switch ABT to his or her medication regimen.
Acknowledgments
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with ethical standards
Conflict of interest: The author(s) declare that they have no conflict of interests.
References
- 1.Patel KR, Burri SH, Asher AL, et al. Comparing preoperative with postoperative stereotactic radiosurgery for resectable brain metastases: a multi-institutional analysis. Neurosurgery. 2016;79:279–285. doi: 10.1227/NEU.0000000000001096. [DOI] [PubMed] [Google Scholar]
- 2.Patel KR, Lawson DH, Kudchadkar RR, et al. Two heads better than one? Ipilimumab immunotherapy and radiation therapy for melanoma brain metastases. Neuro Oncol. 2015;17:1312–1321. doi: 10.1093/neuonc/nov093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao ST, Ahluwalia MS, Barnett GH, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013;87:449–457. doi: 10.1016/j.ijrobp.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Ebner D, Rava P, Gorovets D, et al. Stereotactic radiosurgery for large brain metastases. J Clin Neurosci. 2015;22:1650–1654. doi: 10.1016/j.jocn.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Prabhu R, Shu HK, Hadjipanayis C, et al. Current dosing paradigm for stereotactic radiosurgery alone after surgical resection of brain metastases needs to be optimized for improved local control. Int J Radiat Oncol Biol Phys. 2012;83:e61–6. doi: 10.1016/j.ijrobp.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Stockham AL, Ahluwalia M, Reddy CA, et al. Results of a questionnaire regarding practice patterns for the diagnosis and treatment of intracranial radiation necrosis after SRS. J Neurooncol. 2013;115:469–475. doi: 10.1007/s11060-013-1248-6. [DOI] [PubMed] [Google Scholar]
- 7.Re RN. Mechanisms of disease: local renin-angiotensin-aldosterone systems and the pathogenesis and treatment of cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2004;1:42–47. doi: 10.1038/ncpcardio0012. [DOI] [PubMed] [Google Scholar]
- 8.Escobar E, Rodriguez-Reyna TS, Arrieta O, et al. Angiotensin II, cell proliferation and angiogenesis regulator: biologic and therapeutic implications in cancer. Curr Vasc Pharmacol. 2004;2:385–399. doi: 10.2174/1570161043385556. [DOI] [PubMed] [Google Scholar]
- 9.Zhuang S, Li J, Wang X, et al. Renin-angiotensin system-targeting antihypertensive drugs and risk of vascular cognitive impairment: A meta-analysis. Neurosci Lett. 2016;615:1–8. doi: 10.1016/j.neulet.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Nordal RA, Nagy A, Pintilie M, et al. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res. 2004;10:3342–3353. doi: 10.1158/1078-0432.CCR-03-0426. [DOI] [PubMed] [Google Scholar]
- 11.Vujaskovic Z, Marks LB, Anscher MS. The physical parameters and molecular events associated with radiation-induced lung toxicity. Semin Radiat Oncol. 2000;10:296–307. doi: 10.1053/srao.2000.9424. [DOI] [PubMed] [Google Scholar]
- 12.Starke RM, Yen CP, Ding D, et al. A practical grading scale for predicting outcome after radiosurgery for arteriovenous malformations: analysis of 1012 treated patients. J Neurosurg. 2013;119:981–987. doi: 10.3171/2013.5.JNS1311. [DOI] [PubMed] [Google Scholar]
- 13.Patel KR, Prabhu RS, Kandula S, et al. Intracranial control and radiographic changes with adjuvant radiation therapy for resected brain metastases: whole brain radiotherapy versus stereotactic radiosurgery alone. J Neurooncol. 2014;120:657–663. doi: 10.1007/s11060-014-1601-4. [DOI] [PubMed] [Google Scholar]
- 14.Eaton BR, LaRiviere MJ, Kim S, et al. Hypofractionated radiosurgery has a better safety profile than single fraction radiosurgery for large resected brain metastases. J Neurooncol. 2015;123:103–111. doi: 10.1007/s11060-015-1767-4. [DOI] [PubMed] [Google Scholar]
- 15.Patel KR, Chowdhary M, Switchenko JM, et al. BRAF inhibitor and stereotactic radiosurgery is associated with an increased risk of radiation necrosis. Melanoma Res. 2016;26:387–394. doi: 10.1097/CMR.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Amer Statistical Assoc. 1999;94:496–509. [Google Scholar]
- 17.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 19.Brennan C, Yang TJ, Hilden P, et al. A phase 2 trial of stereotactic radiosurgery boost after surgical resection for brain metastases. Int J Radiat Oncol Biol Phys. 2014;88:130–136. doi: 10.1016/j.ijrobp.2013.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown PD, Ballman KV, Cerhan J, et al. N107C/CEC.3: a phase iii trial of post-operative stereotactic radiosurgery (SRS) compared with whole brain radiotherapy (WBRT) for resected metastatic brain disease. Int J Radiat Oncol Biol Phys. 2016;96:937. [Google Scholar]
- 21.Chowdhary M, Patel KR, Danish HH, et al. BRAF inhibitors and radiotherapy for melanoma brain metastases: potential advantages and disadvantages of combination therapy. Onco Targets Ther. 2016;9:7149–7159. doi: 10.2147/OTT.S119428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Shahi Salman R, White PM, Counsell CE, et al. Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA. 2014;311:1661–1669. doi: 10.1001/jama.2014.3200. [DOI] [PubMed] [Google Scholar]
- 23.Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383:614–621. doi: 10.1016/S0140-6736(13)62302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Small W, Jr, James JL, Moore TD, et al. Utility of the ACE Inhibitor captopril in mitigating radiation-associated pulmonary toxicity in lung cancer: results from NRG Oncology RTOG 0123. Am J Clin Oncol. 2016 doi: 10.1097/COC.0000000000000289. https://doi.org/10.1097/COC.0000000000000289. [DOI] [PMC free article] [PubMed]
- 25.Kharofa J, Cohen EP, Tomic R, et al. Decreased risk of radiation pneumonitis with incidental concurrent use of angiotensin-converting enzyme inhibitors and thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:238–243. doi: 10.1016/j.ijrobp.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Liao Z, Zhuang Y, et al. Do angiotensin-converting enzyme inhibitors reduce the risk of symptomatic radiation pneumonitis in patients with non-small cell lung cancer after definitive radiation therapy? Analysis of a single-institution database. Int J Radiat Oncol Biol Phys. 2013;87:1071–1077. doi: 10.1016/j.ijrobp.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alite F, Balasubramanian N, Adams W, et al. Decreased risk of radiation pneumonitis with coincident concurrent use of angiotensin-converting enzyme inhibitors in patients receiving lung stereotactic body radiation therapy. Am J Clin Oncol. 2016 doi: 10.1097/COC.0000000000000324. https://doi.org/10.1097/COC.0000000000000324. [DOI] [PubMed]
- 28.Alashkham A, Paterson C, Rauchhaus P, et al. Can angiotensin-converting enzyme inhibitors reduce the incidence, severity, and duration of radiation proctitis? Int J Radiat Oncol Biol Phys. 2016;94:93–101. doi: 10.1016/j.ijrobp.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Harder EM, Park HS, Nath SK, et al. Angiotensin-converting enzyme inhibitors decrease the risk of radiation pneumonitis after stereotactic body radiation therapy. Pract Radiat Oncol. 2015;5:e643–e649. doi: 10.1016/j.prro.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Leask A. Getting to the heart of the matter: new insights into cardiac fibrosis. Circ Res. 2015;116:1269–1276. doi: 10.1161/CIRCRESAHA.116.305381. [DOI] [PubMed] [Google Scholar]
- 31.Okwan-Duodu D, Landry J, Shen XZ, et al. Angiotensin-converting enzyme and the tumor microenvironment: mechanisms beyond angiogenesis. Am J Physiol Regul Integr Comp Physiol. 2013;305:R205–15. doi: 10.1152/ajpregu.00544.2012. [DOI] [PubMed] [Google Scholar]
- 32.Medhora M, Gao F, Jacobs ER, et al. Radiation damage to the lung: mitigation by angiotensin-converting enzyme (ACE) inhibitors. Respirology. 2012;17:66–71. doi: 10.1111/j.1440-1843.2011.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


