Abstract
Therapy with α-radiation has issues associated with internal exposure; its clinical use has been avoided. However, phase III clinical tests of the α-emitting nuclide 223Ra on patients with cancer have been conducted, and results were reported in 2011 to 2012. Since then, research has being carried out on targeted internal therapy by introducing α-emitting nuclides directly into the cancers. For many decades, nontargeted radon therapy has been carried out and is controversial because its mechanism of action is stimulation. The low-level radiation sends powerful signals to upregulate many biological protection systems, which protect against the effects of radiogenic and nonradiogenic toxins. These vital systems prevent, repair, and remove DNA and other biomolecular damage being produced endogenously at a very high rate by the very abundant reactive oxygen species associated with aerobic metabolism. Stimulation of protection systems results in beneficial effects, including a lower risk of cancer. This article reports the results of treatments on 4 patients with cancer and reviews the clinical use of α-radiation from 223Ra and radon. It discusses the prospect of using the novel 225Ac-prostate-specific membrane antigen ligand-617 ligand as a therapeutic agent for prostate cancer. It presents a new treatment system that we developed, α-Radiorespiro-Rn, which seems to be extremely effective in treating cancer.
Keywords: α-emitting nuclides, radon, 223Ra, 225Ac-PSMA ligand, α-Radiorespiro-Rn
Introduction
Therapy with α-radiation has issues associated with internal exposure; its clinical use has been avoided. This article describes fundamental and clinical knowledge of cancer treatments using targeted 223RaCl2 and 225Ac-prostate-specific membrane antigen ligand-617 (225Ac-PSMA-617) and nontargeted 222Rn gas. Alpha rays are released from radionuclides having an atomic number of 82 or higher, and more than 400 of them exist. Those whose half-life is not too long or too short are suitable, and Table 1 lists the main α-emitters that can be clinically used.
Table 1.
Clinically Available α-Emitting Nuclides.
| Radionuclide | Half-Life | E α , average (MeV) | Decay Series | Production |
|---|---|---|---|---|
| 149Tb | 4.12 hours | 3.97 | Cyclotron | |
| 211At | 7.21 hours | 5.87 | Actinium | Cyclotron |
| 212Bi | 60.55 minutes | 6.05 | Thorium | Generator |
| 213Bi | 45.59 minutes | 5.85 | Neptunium | Generator |
| 222Rn | 3.82 days | 5.49 | Uranium | Uranium ore |
| 223Ra | 11.43 days | 5.67 | Actinium | 227Ac source |
| 225Ac | 9.92 days | 5.79 | Neptunium | 229Th source |
Targeted Internal Radiotherapy
Radiotherapy by intravenous or oral administration of a nonsealed radionuclide itself or in a medication is called internal radiotherapy. When the target tissue is cancer, it is internal radiotherapy for cancer. Radiations usable for this therapy include α−radiation, β-radiation, γ-radiation, X-rays, auger electrons, Compton electrons, internal conversion electrons, and the like. Among these, β-rays from nuclides such as 89Sr, 90Y, and 131I have been widely used in clinical treatments for many decades. Because of their long tracks in tissues (up to 12 mm), β-rays also affect the healthy tissues that surround the targeted cancer cells. Incorporating an α-emitter into cancer cells or other target cells (0.05-0.06 mm in diameter) is advantageous because α-tracks are much shorter, 0.03 to 0.1 mm. They inflict lethal damage only to cells that are very near the target cells. The energy lost by the α-ray per unit distance, its linear energy transfer (LET), is 80 keV/μm, increasing to 240 keV/μm at the end of its track. This is 400 to 1200 times the LET of the β-ray, 0.2 keV/μm. The α-ray is not scattered; it passes straight through cells, densely ionizing or exciting the nearby atoms, while losing energy and reaching maximum effectiveness just before stopping. The lethality of damage to DNA is proportional to LET, so α-rays kill cancer cells much better than β-rays. A prime target of internal therapy is the tissue of a disease that is highly sensitive to radiation, such as a cancer that has metastasized to bone marrow. Studies that exploit the superior capabilities of α-rays for cancer treatment have been reported since 1981. For example, 211At was employed to suppress cancer growth in mice bearing malignant ascites.1 Use of 212Bi-labeled antibody has been reported to delay the deaths of mice with cancerous ascites.2
Nontargeted Radiotherapy
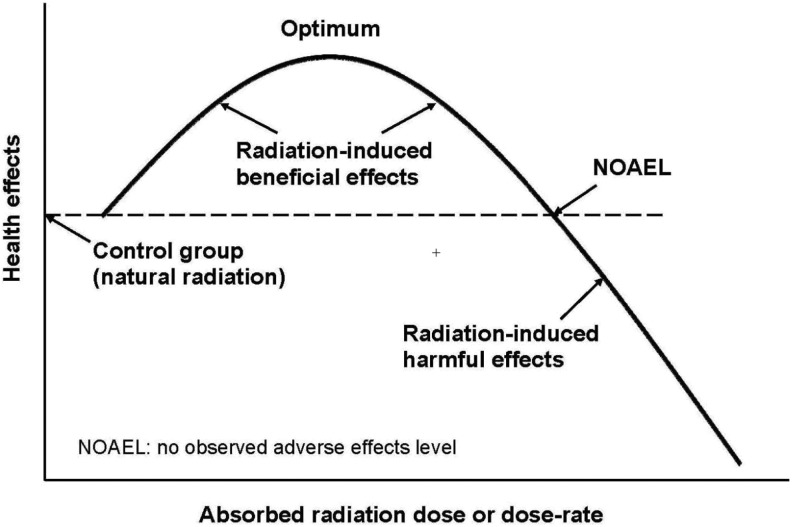
The mechanism for nontargeted radiation therapy is different from direct cell killing. Since about three quarters of human tissue is water, radiation-induced reactive oxygen species (ROS) is a very important effect. Reactive oxygen species and direct hits are a double-edged sword. They damage molecules but also send signals to stimulate or inhibit genes.3 As shown in Figure 1, the response of the patient depends on the radiation dose or the dose rate. As dose (oxidative stress) is increased, a point is reached at which protective systems begin to induce beneficial effects. As dose is raised further, an optimum response is reached at which stimulation of protection is maximal. As the dose is increased beyond the optimal point, inhibition of protection intensifies and stimulation weakens until, at the threshold point, the health effect is the same as for the unexposed patient. A dose or dose rate higher than this threshold produces net harmful effects. Therefore, the dose or dose rate administered is controlled to be in the range for high stimulation of the patient’s protection systems.
Figure 1.
Dose–response for nontargeted radiotherapy.
Reactive oxygen species are produced abundantly and constantly by the patient’s aerobic metabolism. The rate of DNA damage caused by endogenously produced ROS far exceeds the rate of DNA damage caused by low-dose hits and the ROS that they produced.4 Studies on experimental living systems and on humans have shown that low doses of radiation upregulate biological protective mechanisms, which also operate against nonradiogenic toxins and produce beneficial effects, including a lower risk of cancer.5 The degree of stimulation and inhibition depends on the individual genome. These biological effects are caused by the direct hits and by the burst of ROS that they produce. Although they damage cells, they send powerful signals also to activate many genes (>150) at the same time that they stimulate various biological protective functions originally provided to the cells. These vital protection systems prevent, repair, and remove DNA damage and other biomolecuar damage being produced endogenously at a very high rate by the abundant ROS associated with aerobic metabolism. Upregulation of protection systems by a small amount of oxidative stress results in significant beneficial effects.
A recent analysis of 2 studies on dogs that received lifelong low-dose rates of ionizing radiation, one study with γ−rays and the other with α-rays, provided evidence of increased lifespan and well-defined dose-rate thresholds for the onset of reduced longevity.6 Short-lived dogs received a greater relative benefit than the 50% mortality dogs. The study on dogs that inhaled 239PuO2 aerosols (α-emitter) demonstrated very strong signaling to the protection systems of the entire animal, by local α-particle hits in the group of dogs with the lowest initial lung burden.6 An analysis of another study on dogs that inhaled 239PuO2 aerosols demonstrated a threshold dose rate for lung cancer mortality. Two groups of dogs had lung cancer mortalities below that of the control dogs. The group with the lowest plutonium intake had no lung cancers.
Nontargeted Radon Therapy
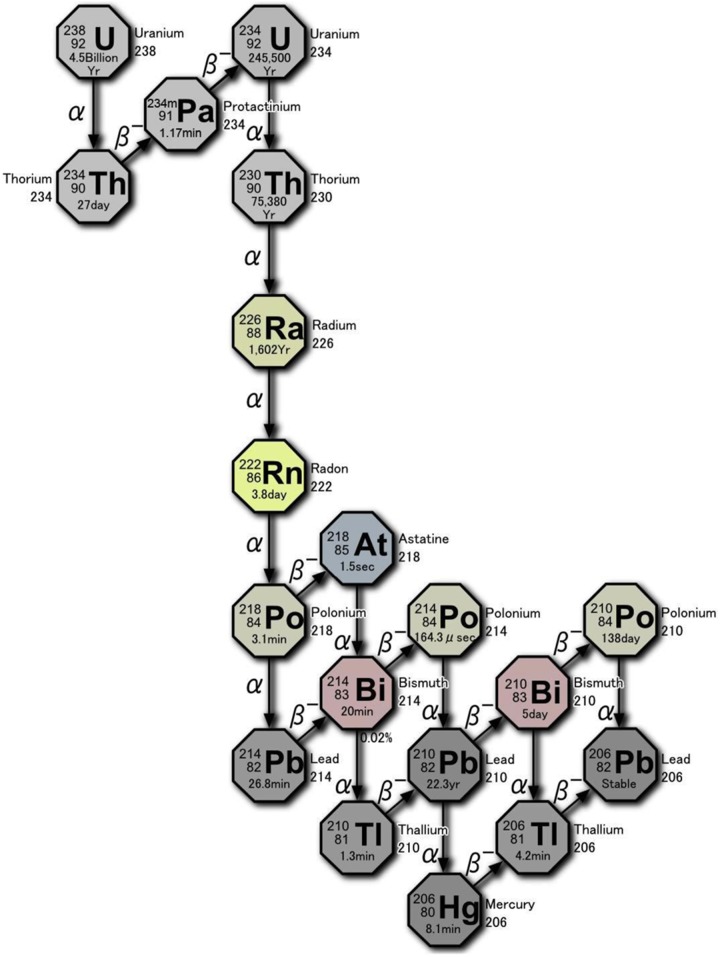
As shown in Figure 2, 222Rn gas is released from the radium present in uranium ore (pitchblende). Deposits of high-grade ore are found in countries, such as Kazakhstan, Canada, and Australia. In hot radium spring facilities, radon is absorbed mainly by inhalation. Most is exhaled, but a small amount of gas and decay products (progeny) adhere to the mucosa of the trachea and the lung surface. Some are taken up by alveolar epithelial cells and transferred into the blood together with oxygen. After 2 weeks, the gas (3.8-day half-life) almost disappears. There is no evidence of adverse health effects from these treatments and no significant long-term accumulation in any specific tissue. Radon is also absorbed into the bloodstream through the skin when the patient is immersed in warm radium bathwater. A third way is by drinking hot spring water (drinking therapy). In this case, dissolved radon is swallowed and transferred from the stomach to the blood. The subsequent kinetics in the body is the same as radon transferred from the lung to the blood. During the transit of radon, α-particles hit cells, imparting a dose of about 0.5 to 1 Gy to each. Water molecules are ionized and various ROS, mainly hydroxyl radicals, are formed. They damage biomolecules including DNA molecules.
Figure 2.
Decay chain of 238U showing the production of 222Rn and its α-emissions.
For many decades, radon has been employed to treat various diseases, such as low-back pain, high blood pressure, and cancer at radium hot springs in Misasa and in Tamagawa Onsen, Japan. Clinical trials have been carried out at the Misasa Medical Center in Okayama University Hospital, where patients are treated by inhalation of radon volatilized from radon-containing water. Patients inhale radon at a concentration of about 2000 Bq/m3 (54 pCi/L) for 40 minutes, every 2 days in a room that is maintained at 42°C and 90% relative humidity. Diseases that are treated with radon therapy are those related to ROS or oxidative stress, such as arteriosclerosis, osteoarthritis, and bronchial asthma. The effective absorbed radiation dose of each radon treatment is estimated to be 50 to 67 μSv. Nontargeted therapies with X-rays, γ−rays, and radon are performed in clinics in Japan that have an established radon room.7–11 Three patients were treated several years ago. Two had widespread bone metastasis of prostate cancer, diagnosed to be inoperable, and one had ulcerative colitis. Their diseases are now in remission, as described in a recent article about these 3 case reports.12
Radon therapy has been practiced in Central Europe and in Russia for many years.13 The pain relieving treatment of rheumatic disease by radon was reviewed in 2005.14 This report states that “to bathe in about 0.3 to 3 kBq/L of radon water for about 20 minutes for therapeutic purposes” or “to stay in caverns or galleries of about 30 to 160 kBq/m3 for about 1 hour” made it possible to obtain a statistically significant pain relieving effect. Unfortunately, the US Environmental Protection Agency (EPA) and many other organizations responsible for public safety use the linear no-threshold model to assess the risk of radiation-induced cancer. They do not consider any exposure to radon to be safe. The EPA action level for radon in homes is 4 pCi/L (150 Bq/m3). Therefore, radon therapy has not been accepted as an approved medical treatment in many countries; it remains in the category of an “alternative therapy.”
Nontargeted Effect of Radon on Melanoma in Mice
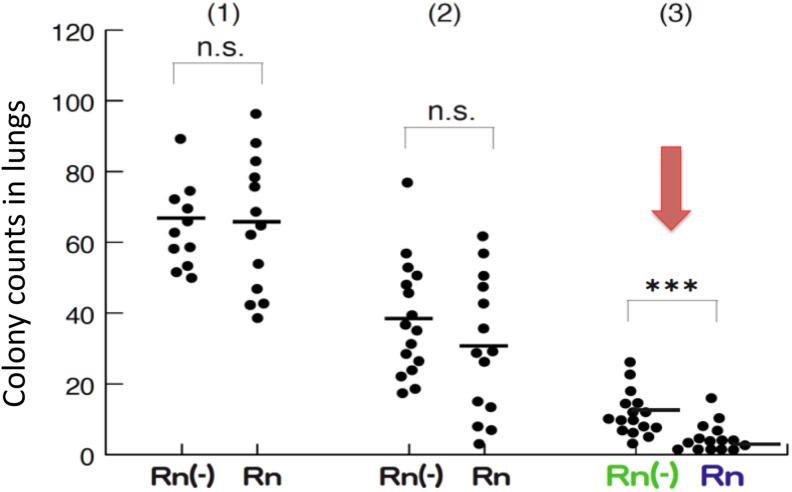
In our laboratory, skin cancer B-16 melanoma cells (2 × 105, 1 × 105, and 5 × 104) were injected into the tail vein of 3 groups of C57 black 6 (C57BL/6) mice (male, 6 weeks old). They were given radon-containing water (203 Bq/L) every day to determine the effect of radon on lung metastatic cancers.15 The colonies formed were counted 14 days later. As shown in Figure 3, metastasis was not significantly suppressed in the group with a large number of cancer cells, but significant inhibition (P < .005) was observed in the group with the smallest number of cells (5 × 104 cells). When the radon concentration in water was diluted twice, the inhibitory effect on cancer was not observed in the group in which metastasis had been suppressed. From these results, it is concluded that there is a threshold for radon concentration to suppress metastasis of the cancer to the lung, and the effect is not induced below that concentration.
Figure 3.
Effect of ingestion of 222Rn-containing water (203 Bq/L) on the formation of metastatic lung colonies after intravenous injection of B16 melanoma cells via tail vein in 3 groups of C57 black 6 (C57BL/6) mice. Radon treatment was started 2 weeks before the injection of the melanoma cells and continued for 14 days. Rn (−) is tap water; Rn is radon hot spring water (203 Bq/L); (1) melanoma cells = 2 × 105; (2) melanoma cells = 1 × 105; (3) melanoma cells = 5 × 104. ***P < .005 (n = 7 each). NS indicates not significant.
Targeted 223RaCl2 Therapy Against Bone Metastasis
Radiopharmaceuticals have been developed that can alleviate pain, but none have been able to extend survival. Bone-seeking 223Ra was studied for alleviating the pain of metastasis; however, this therapy has been observed to prolong survival time.16–18 In Europe and the United States, 223Ra has become a focus of attention. Phase I/II tests are underway in Europe and the United States on other α-emitting nuclides.
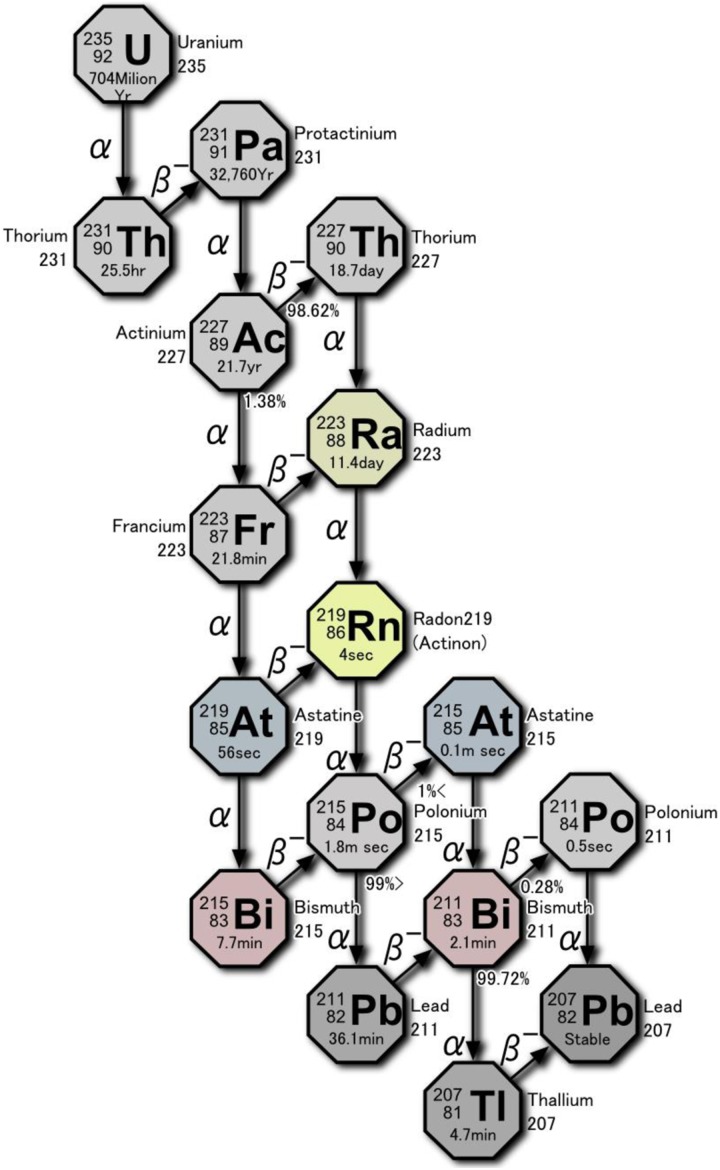
As shown in Figure 4, 227Ac transitions to 223Ra. Four α-particles are emitted as 223Ra transitions to 207Pb, producing strong cell killing action. Irradiation of 226Ra in a nuclear reactor produces 227Ra, which β decays with a half-life of 42.2 minutes to 227Ac. A reagent is added to a generator that contains 21.8-year 227Ac to “milk” 11.4-day 223Ra for use in bone cancer therapy.19 Comparing the efficacy of α-particles from 223Ra with β−rays from 89Sr, the radionuclide commonly used to treat metastatic bone tumors, it is noted that the α-particle energy is about 50 times larger than the β-ray energy, and the energy lost per micrometer of range is 400 times larger (80 keV vs 0.2 keV). 223Ra inflicts irreparable damage to the DNA of the target cell. Furthermore, the cell killing effect is active also during the S phase, since the action of α particles does not depend on cell cycle.
Figure 4.
Decay chain of 235U showing 223Ra and its α-emissions.
Studies have been conducted worldwide on the use of 223RaCl2 to inhibit bone metastases in castration-resistant prostate cancer (CRPC).20–24 Phase III clinical trial reports issued in the United States and European countries from 2011 to 2012 state that this drug has a life-prolonging effect by relieving pain and delaying the occurrence of bone-related events such as fracture. It is said to be an excellent antitumor agent with fewer side effects than β-emitting treatments. In the phase III clinical study of 223RaCl2 (Xofigo) that led to its Food and Drug Administration (FDA) approval in 2013, the mean survival time in the treated group was 14.9 months versus 11.3 in the placebo group, a 30% reduction in mortality risk. The average time to the onset of bone-related events was 15.6 months versus 9.8 months in the placebo group, a 34% reduction in risk. A drop in the alkaline phosphatase increase at the time of bone metastasis was shown. Improved quality of life was recognized. No significant difference in the incidence of adverse side effects was noted between the Xofigo group and the placebo group. The rate of treatment dropout due to adverse side effects was lower, 16% versus 21% for the placebo group.25
In March 2016, 223RaCl2 (Xofigo) was approved for clinical use in Japan for CRPC with bone metastasis. Its efficacy and safety for bone tumors, other than castration refractory prostate cancer, has not been confirmed. Further research will be needed. Because the drug price is high, about 700 000 yen (US$6300), multiple treatments would be a heavy economic burden on patients.
Targeted Treatment of 2 Patients With Metastatic Cancer Using 225Ac-PSMA-617 Ligand
Prostate cancer is very common in elderly men in many western countries.26 Prostate-specific membrane antigen is a promising target for prostate cancer, and the α-emitting PSMA ligand, 225Ac-PSMA-617, has been successfully synthesized. Studies on the use of PSMA-617 have been carried out over the past few years.27,28 We discuss here a recent case report about 2 patients who were treated successfully by 225Ac-PSMA- 617 therapy.28
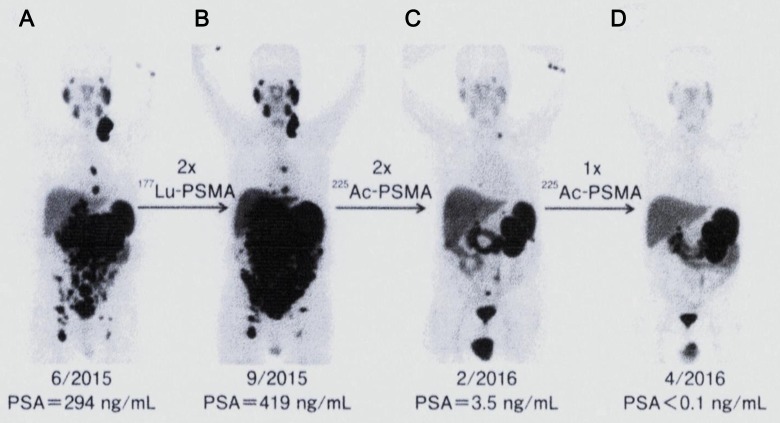
The first patient had peritoneal carcinomatosis and liver infiltration and was given an accepted treatment of β-emitting 177Lu-PSMA-617 ligand (7.4 GBq per treatment). Referring to Figure 5A and B, the initial prostate-specific antigen (PSA) value was 294 ng/mL in June 2015, but after the second treatment with 177Lu-PSMA ligand, the PSA value rose to 419 ng/mL in September 2015, and tumor progression was also seen with positron emission tomography (PET) diagnosis. Therapy with α-emitting 225Ac-PSMA-617 ligand was offered to rescue the patient. He was given 3 cycles of 6.4 MBq (100 kBq/kg body weight) at bimonthly intervals. No lesions were observed in the PET image after the second treatment, as shown in Figure 5C, and complete remission was achieved by 1 additional dose thereafter, Figure 5D. No related toxicity was observed, and the PSA value on the final day (in April 2016) was below the detection limit (<0.1 ng/mL).
Figure 5.
68Ga-PSMA-11 PET-CT scans of the first patient with prostate cancer. Initial tumor spread (A) versus tumor progression after 2 cycles of β-emitting 177Lu-PSMA-617 (B). Impressive response after second (C) and third (D) cycles of α-emitting 225Ac-PSMA-617. Reprinted with permission from Kratochwil et al28 Copyright© 2016, The Society of Nuclear Medicine and Molecular Imaging, Inc. All rights reserved. PSMA indicates prostate-specific membrane antigen; 68Ga-PSMA-11 PET-CT, positron emission tomography-computed tomography.
The second patient was also treated with 225Ac-PSMA-617 ligand (data not shown). In the PET images, diffuse red marrow invasion was observed. Treatment with β-emitting 177Lu-PSMA ligand was considered contraindicated. Therefore, 225Ac-PSMA-617 ligand (100 kBq/kg body weight) was intravenously administered to this patient 3 times at intervals of 2 months at doses of 9 to 10 MBq each. A target tumor was confirmed in a PET image scan immediately after treatment in December 2014. In the image after the third administration in July 2015, the PSMA positive lesion disappeared completely. The PSA value decreased from 3000 ng/mL or more (in December 2014) to 0.26 ng/mL (in July 2015). In addition, 6 MBq of 225Ac-PSMA-617 ligand was administered to the patient as an integrated medical care, resulting in the image becoming much clearer and the PSA value decreasing to below 0.1 ng/mL.
Due to the short range of α-particles, 225Ac-PSMA-617 needs to be taken into the cancer cell in order to destroy it. The uptake of this ligand into prostate cancer cells has been confirmed—54% and 75% of the ligand were incorporated into the cells after 1 and 3 hours, respectively.29 The cases suggest that radioligand therapy using 225Ac-PSMA-617 is an effective α-particle therapy targeting metastatic CRPC. This is important for patients who are in a clinically difficult stage, such as those showing resistance to diffuse red bone marrow infiltration and other treatments. A study should be carried out on a large cohort to confirm the effectiveness of this therapy; however, this will not happen soon because routine supply of this radionuclide has not been established.
Nontargeted Treatment of 2 Patients With Metastatic Cancer Using Radon
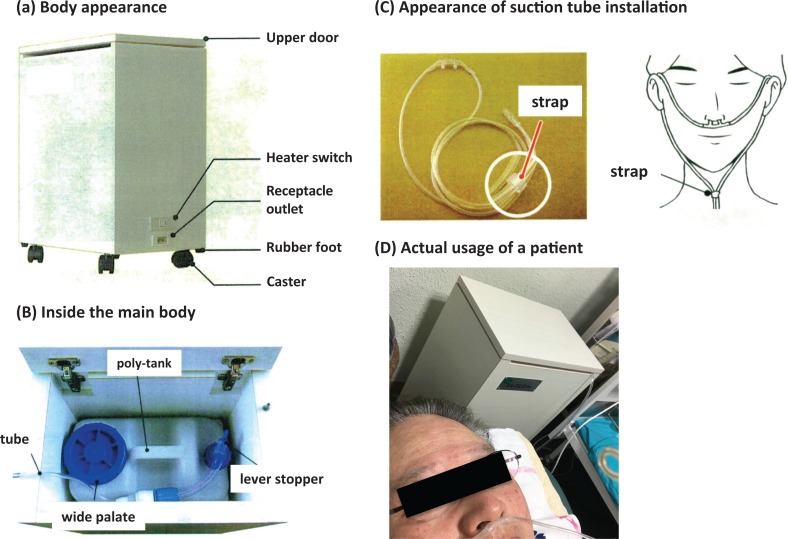
The α-Radiorespiro-Rn apparatus has been specially developed to deliver radon inhalation therapy. As shown in Figure 6A and B, it is made from simple parts and stored in a wooden cabinet, 450 mm wide, 300 mm deep, and 600 mm high. Particles of high-grade uranium ore, averaging 4 mm in diameter, are spread evenly on the bottom of a 16 L polyethylene container. About 2.5 L of distilled water is poured into this tank and maintained at a temperature of 35°C. The amount of ore is adjusted to allow the radon gas to accumulate to a concentration of about 8 MBq/m3 (216 nCi/L) in the volume above the water. As prescribed by the physician, the patient inhales radon through the suction tube into a special respirator, as shown in Figure 6C and D, for the time specified.
Figure 6.
System of α-Respiro-Rn and its actual usage. Particles of uranium ore, about 4 mm in diameter, are spread evenly on the bottom of the 16-L polyethylene tank. About 2.5 L of distilled water is poured into the tank and maintained at a temperature of 35°C. Radon gas with a concentration of about 8 MBq/m3 in air accumulates in the tank on the day before use. The patient inhales radon through the suction tube.
The first patient with breast cancer is a 42-year-old woman with metastasis to her brain. In 2013, she felt a sting on her left chest. The lump gradually enlarged to about 2 cm in diameter and was diagnosed to be breast cancer at a hospital. Only private therapy was carried out for 2 years, during which time the whole breast grew bigger and harder. Compression fracture in the lumbar vertebrae and inflammation throughout her chest were apparent in July 2016. In addition, the growth of the tumor in her brain pressed her left ocular nerve, affecting her field of vision; the image became blurred. There was bleeding from her breast, and she was taking analgesics to relieve the low-back pain.
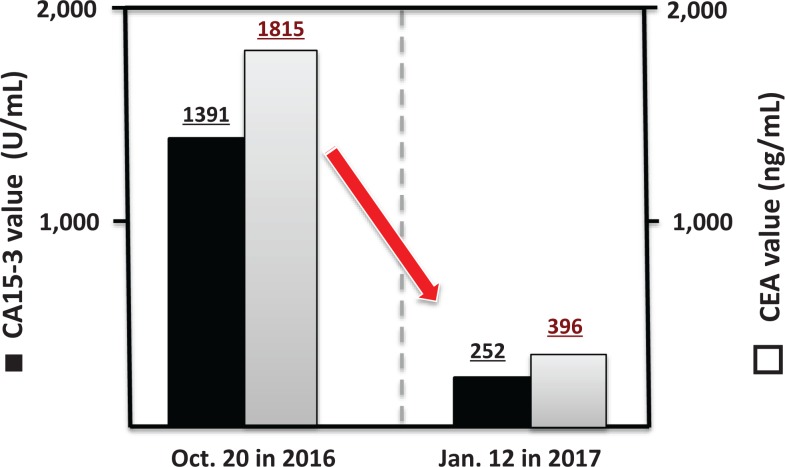
On August 22, 2016, radon inhalation treatment was started using the α-Radiorespiro-Rn apparatus. Three days per week, she inhaled 0.5 to 1.0 MBq/m3 of radon for 40 minutes, twice each day. In November 2016, the patient’s condition was observed to improve. The rash on her breast started to disappear. A dramatic recovery was recorded in February of 2017. Her left eye ball, which had been rotated to the upper right side at the start of treatment, returned to almost normal position. Her visual acuity recovered to normal vision. Furthermore, she was able to walk normally without a cane and without back pain. Figure 7A is a photo of the patient on November 13, 2016, after 2.5 months of radon treatment, and Figure 7B is a photo on April 14, 2017, after 8 months of treatment. Breast cancer marker values, cancer antigen 15-3 (CA15-3, 1391) and carcinoembryonic antigen (CEA, 1815) on October 20, 2016, fell to 252 and 396, respectively, on January 12, 2017, as shown in Figure 8.
Figure 7.

Eye of first patient with breast cancer before and after radon treatment using α-Respiro-Rn system.
Figure 8.
Breast cancer marker of first patient before and after treatment using α-Radiorespiro-Rn system. Cancer antigen 15-3 values for October 20, 2016, and January 12, 2017, are 1391 and 252, respectively. CA15-3 indicates cancer antigen 15-3; CEA, carcinoembryonic antigen; ▪, CA 15-3; □, CEA.
The hormesis room exposes the occupants to γ-radiation and radon gases from radiation sources in the walls, supplied by Lead & Company Co (Yokohama, Japan). The sources are natural monazite excavated from a mountainous area in Japan. The average γ-radiation level in the room is 11 μSv/h, and the average concentration of radon is 9800 Bq/m3.12 Temperature and humidity in the room are maintained at about 40°C and 70%, respectively.
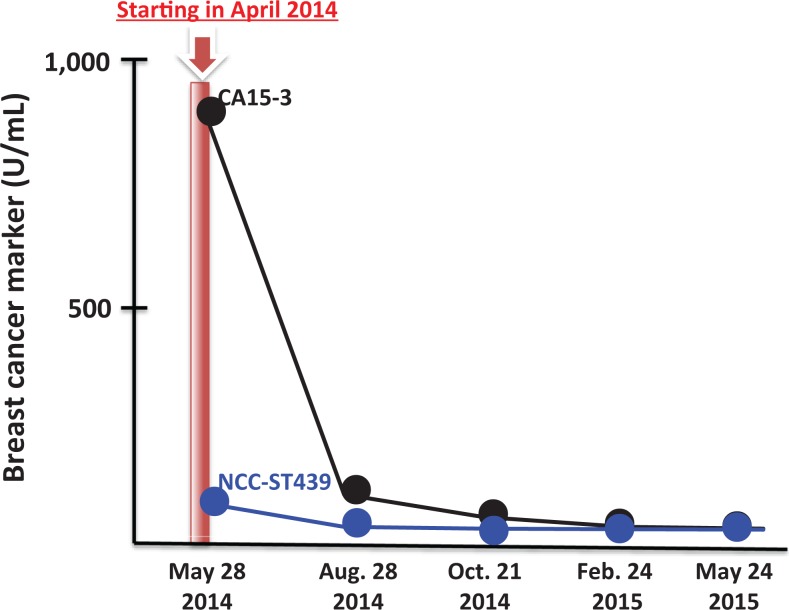
The second patient with breast cancer is a 47-year-old woman with metastasis to her bones. She was diagnosed with breast cancer 5 years ago. She refused chemotherapy, opting instead for folk remedies such as hyperthermia. Her breast cancer gradually progressed to stage IV. Her treatment began on May 28, 2014. At the start, her body weight was only 38 kg and she wore a neck brace because of bone metastasis. Twice daily, she received radon therapy in the room for 40 minutes. No improvement was observed in the first week. She lost weight during the following week, but the secretion of pus from her chest stopped. This treatment continued into the following year. As shown in Figure 9, her breast cancer markers of CA15-3 and National Cancer Center-Stomach-439 returned to their normal values in August 28, 2014, and the patient returned to work. In May 2015, the tumor tissue became scab, and in June, she was walking 7 km every 2 weeks, an indication of good physical condition and improved quality of life. Subsequently, she went to Germany for 2 weeks of company training. Her cancer markers are still at normal levels. The patient’s weight, which was 38 kg at the start of treatment, increased to 51 kg.
Figure 9.
Changes in CA 15-3 and NCC-ST-439 of second patient with breast cancer with bone metastasis after hormesis room therapy. Hormesis room treatment: 40 minutes, twice daily, May 28, 2014, to May 24, 2015. Average radiation level and radon concentration in the room: 11 μSv/h and 9800 Bq/m3. Temperature and relative humidity: 39°C to 40°C and 70%. CA15-3 indicates cancer antigen 15-3; NCC-ST-439, National Cancer Center-Stomach-439.
Conclusions
Therapy with α-radiation has been regarded as having significant concerns associated with internal exposure, and its clinical use has been avoided. However, a phase III clinical trial of targeted therapy with 223RaCl2 produced evidence of its efficacy for the treatment of metastatic bone tumors, and it was approved for clinical use by the US FDA in 2013. Since then, fundamental and applied research is underway on internal therapy with other α-emitting nuclides. The recent targeted treatment of metastatic prostate cancer by 225Ac-PSMA-617 ligand therapy is one of the most promising results.
Clinical use of nontargeted α-radiation from radon gas on 2 of our patients with advanced breast cancer brought their disease into remission. One patient received inhaled radon emanating from natural monazite ore in the walls of our hormesis treatment room. The other inhaled radon from uranium ore contained in a new treatment apparatus that we developed, the α-Radiorespiro-Rn system. Treatment with radon gas stimulated the patient’s protection systems to produce their very remarkable recoveries from advanced breast cancer. Our α-Radiorespiro-Rn system is very convenient to use and very effective in reversing the progression of their illnesses. We expect it to be potent for other types of cancer and for other illnesses that would benefit from upregulation of inherent biological protection. Further studies are recommended to optimize the treatment protocol for cancer and to identify other important applications.
This article reviewed the present and future prospects of treating cancer using α-emitting nuclides for internal radiation exposures. It examined the application of 223RaCl2 and 225Ac-PSMA ligand for targeted therapy and 222Rn gas for nontargeted therapy. Employing α-emitters for treating cancer could be a very important method for curing many types of cancer and other illnesses.
Acknowledgments
The authors would like to thank our patients (MY and YO) for granting permission to report their cases.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Bloomer WD, McLaughlin WH, Neirinckx RD, et al. Astatine-211-tellurium radiocolloid cures experimental malignant ascites. Science. 1981;212(4492):340–341. [DOI] [PubMed] [Google Scholar]
- 2. Macklis RM, Kinsey BM, Kassis AI, et al. Radio-immunotherapy with alpha-particle-emitting immunoconjugates. Science. 1988;240(4855):1024–1026. [DOI] [PubMed] [Google Scholar]
- 3. Feinendegen LE. Reactive oxygen species in cell responses to toxic agents. Hum Exp Toxicol. 2002;21(2):85–90. [DOI] [PubMed] [Google Scholar]
- 4. Pollycove M, Feinendegen LE. Radiation-induced versus endogenous DNA damage: possible effect of inducible protective responses in mitigating endogenous damage. Hum Exp Toxicol. 2003;22(6):290–306. [DOI] [PubMed] [Google Scholar]
- 5. Feinendegen LE, Pollycove M, Neumann RD. Hormesis by low dose radiation effects: low-dose cancer risk modeling must recognize up-regulation of protection In: Baum RP, ed. Therapeutic Nuclear Medicine. Berlin, Heidelberg, Germany: Springer-Verlag; 2013; 789–805. [Google Scholar]
- 6. Cuttler JM, Feinendegen LE, Socol Y. Evidence that lifelong low dose rates of ionizing radiation increase lifespan in long- and short-lived dogs. Dose Response. 2017;15(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamaoka K, Mifune T, Mitsunobu F, et al. Basic study on radon effects and thermal effects on humans in radon therapy. Physiol Chem Phys Med NMR. 2001;33(2):133–138. [PubMed] [Google Scholar]
- 8. Yamaoka K, Mitsunobu F, Kojima S, et al. The elevation of p53 protein level and SOD activity in the resident blood of the misasa radon hot spring district. J Radiat Res. 2005;46(1):21–24. [DOI] [PubMed] [Google Scholar]
- 9. Mitsunobu F, Yamaoka K, Hanamoto K, et al. Elevation of antioxidant enzymes in the clinical effects of radon therapy and thermal therapy for bronchial asthma. J Radiat Res. 2003;44(2):95–99. [DOI] [PubMed] [Google Scholar]
- 10. Cuttler JM, Sanders CL. Threshold for radon-induced lung cancer from inhaled plutonium data. Dose Response. 2015;13(4):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Etani R, Kataoka T, Kanzaki N, et al. Difference in the action mechanism of radon inhalation and radon hot spring water drinking in suppression of hyperuricemia in mice. J Radiat Res. 2016;57(3):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kojima S, Tsukimoto M, Shimura N, Koga H, Murata A, Takara T. Treatment of cancer and inflammation with low-dose ionizing radiation: three case reports. Dose Response. 2017;15(1):1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Becker K. Health effects of high radon environments in central Europe: another test for the LNT hypothesis? Nonlinearity Biol Toxicol Med. 2003;1(1):3–35. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2651614/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Falkenbach A, Kovacs J, Franke A, Jorgens K, Ammer K. Radon therapy for the treatment of rheumatic diseases—review and meta-analysis of controlled clinical trials. Rheumatol Int. 2005;25(5):205–210. [DOI] [PubMed] [Google Scholar]
- 15. Takahashi M, Kojima S. Suppression of atopic dermatitis and tumor metastasis in mice by small amounts of radon. Radiat Res. 2006;165(3):337–342. [DOI] [PubMed] [Google Scholar]
- 16. Parker CC, Pascoe S, Chodacki A, et al. Randomized, double-blind, dose-finding, multicenter, phase 2 study of radium chloride (Ra-223) in patients with bone metastases and castration-resistant prostate cancer. Eur Urol. 2013;63(2):189–197. [DOI] [PubMed] [Google Scholar]
- 17. Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15(7):738–746. [DOI] [PubMed] [Google Scholar]
- 18. Sridhar SS, Freedland SJ, Gleave ME, et al. Castration-resistant prostate cancer: from new pathophysiology to new treatment. Eur Urol. 2014;65(2):289–299. [DOI] [PubMed] [Google Scholar]
- 19. Nilsson S, Larsen RH, FossÔ SD, et al. First clinical experience with α-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res. 2005;11(12):4451–4459. [DOI] [PubMed] [Google Scholar]
- 20. Parker C, Sartor C. Radium-223 in prostate cancer. N Engl J Med. 2013;369(17):1659–1660. [DOI] [PubMed] [Google Scholar]
- 21. Heinzer H, König F, Klutmann S. Alpha emitter radium-223 dichloride: new therapy in castration-resistant prostate cancer with symptomatic bone metastases. Urologe A. 2014;53(4):519–523. [DOI] [PubMed] [Google Scholar]
- 22. Humm JL, Sartor O, Parker C, Bruland OS, Macklis R. Radium-223 in the treatment of osteoblastic metastases: a critical clinical review. Int J Radiat Oncol Biol Phys. 2015;91(5):898–906. [DOI] [PubMed] [Google Scholar]
- 23. Lewis B, Chalhoub E, Chalouhy C, Sartor O. Radium-223 in bone-metastatic prostate cancer: current data and future prospects. Oncology (Williston Park). 2015;29(7):483–488. [PubMed] [Google Scholar]
- 24. Baldaria S, Bonib G, Bortolusc R, et al. Management of metastatic castration-resistant prostate cancer: a focus on radium-223: opinions and suggestions from an expert multidisciplinary panel. Crit Rev Oncol Hematol. 2017;133(5):43–51. [DOI] [PubMed] [Google Scholar]
- 25. Parker C, Zhan L, Cislo P, et al. Effect of radium-223 dichloride (Ra-223) on hospitalisation: an analysis from the phase 3 randomised Alpharadin in Symptomatic Prostate Cancer Patients (ALSYMPCA) trial. Eur J Cancer. 2017;71:1–6. doi:10.1016/j.ejca.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 26. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 27. Benešová M, Schäfer M, Bauder-Wüst U, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med. 2015;56(6):914–920. [DOI] [PubMed] [Google Scholar]
- 28. Kratochwil C, Bruchertseifer F, Giesel FL, et al. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med. 2016;57(12):1941–1944. [DOI] [PubMed] [Google Scholar]
- 29. Song H, Hobbs RF, Vajravelu R, et al. Radioimmunotherapy of breast cancer metastases with α-particle emitter 225Ac: comparing efficacy with 213Bi and 90Y. Cancer Res. 2009;69(23):8941–8948. [DOI] [PMC free article] [PubMed] [Google Scholar]