Abstract
Introduction:
Obesity has become an important issue affecting both males and females. Obesity is now regarded as an independent risk factor for atherosclerosis-related diseases. Metabolic syndrome is associated with increased risk for development of cardiovascular disease. Urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine concentration has been used to express oxidation status.
Methods:
Twenty-seven obese patients with metabolic syndrome, 25 obese patients without metabolic syndrome and 31 healthy subjects were included in our study. They were subjected to full history and clinical examination; fasting blood sugar (FBS), 2 hour post prandial blood sugar (2HPP), lipid profile, urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine and carotid duplex, A/B index and tibial diameters were all assessed.
Results:
There was a statistically significant difference (p = 0.027) in diameter of the right anterior tibial artery among the studied groups, with decreased diameter of the right anterior tibial artery in obese patients with metabolic syndrome compared to those without metabolic syndrome; the ankle brachial index revealed a lower index in obese patients with metabolic syndrome compared to those without metabolic syndrome. There was a statistically insignificant difference (p = 0.668) in the 8-oxodG in the studied groups. In obese patients with metabolic syndrome there was a positive correlation between 8-oxodG and total cholesterol and LDL.
Conclusion:
Urinary 8-oxodG is correlated to total cholesterol and LDL in obese patients with metabolic syndrome; signifying its role in the mechanism of dyslipidemia in those patients. Our study highlights the importance of anterior tibial artery diameter measurement and ankle brachial index as an early marker of atherosclerosis, and how it may be an earlier marker than carotid intima-media thickness.
Keywords: metabolic syndrome, oxidative DNA, vascular affection
Introduction
Metabolic syndrome is associated with increased cardiovascular morbidity and mortality. The endothelial dysfunction is an early pathogenic event in the metabolic syndrome. Endothelial dysfunction of either the coronary, the peripheral or the cerebral vasculature is a predictor of vascular events and appears to be a marker of uncontrolled atherosclerotic risk that adds to the burden of the genetic predisposition to cardiovascular disease.1 Diabetes mellitus (DM) has been routinely described as a metabolic disorder characterized by hyperglycemia that develops as a consequence of defects in insulin secretion, insulin action or both.2
Peripheral artery disease (PAD) is defined as an atherosclerotic occlusive disease of the lower extremities. PAD is associated with increased risk of lower extremity amputation and it is also a marker for atherosclerosis in cardiovascular, cerebrovascular and renovascular beds. Patients with PAD therefore have an increased risk of MI, stroke and death.3
The alterations in vascular homeostasis due to endothelial and smooth muscle cell dysfunction are the main features of diabetic vasculopathy favoring a pro-inflammatory/thrombotic state that ultimately leads to atherosclerosis. Macro- and microvascular diabetic complications are mainly due to prolonged exposure to hyperglycemia clustering with other risk factors such as arterial hypertension and dyslipidemia, as well as genetic susceptibility.4
There is considerable evidence that hyperglycemia results in the generation of reactive oxygen species (ROS), ultimately leading to increased oxidative stress in a variety of tissues and playing an important role in diabetic complications.5
Mitochondria and nuclei are two major targets of oxidative stress, and contain a variety of DNA repair enzymes to repair oxidant-induced DNA modifications.6 Damage most likely occurs when the endogenous antioxidant network and DNA repair systems are overwhelmed.7 However, it is essential for the cell to repair the DNA damage induced by oxidants.
The ankle brachial index (ABI) serves as a measure of systemic atherosclerosis and thus is associated with both atherosclerotic risk factors and prevalent cardiovascular disease (CVD) in other vascular beds. A low ABI is associated with many cardiovascular risk factors, including traditional ones (hypertension, DM, dyslipidemia, smoking history) and several novel cardiovascular risk factors (e.g. C-reactive protein, interleukin-6, homocysteine and chronic kidney disease). The majority of studies use an ABI of 0.90 as a threshold to define PAD.8
American Heart Association/American College of Cardiology guidelines designated carotid intima-media thickness (CIMT) along with coronary artery calcium (CAC) score a class IIa recommendation for cardiovascular risk assessment in asymptomatic adults at intermediate risk of CVD.9
Several pathophysiological explanations for the metabolic syndrome have been proposed, involving insulin resistance, chronic inflammation and ectopic fat accumulation following adipose tissue saturation. However, current concepts create several paradoxes, including limited cardiovascular risk reduction with intensive glucose control in diabetics, therapies that result in weight gain and presence of some of the metabolic traits among some lipodystrophies.10
Patients and methods
This study was conducted on 83 subjects from the outpatient endocrinology clinic. They were classified into three groups (we used NCEP/ATP III criteria in our definition of metabolic syndrome): Group A – 27 obese patients with metabolic syndrome; Group B – 25 obese patients without metabolic syndrome; Group C – 31 healthy subjects serving as controls.
Local ethical committee approval was granted. Verbal consent was given by all our patients after informing them of all the procedures done in this study. Exclusion criteria were: (1) patients with known vascular disease such as vasculitis; (2) patients with renal failure.
Methodology
All patients were subjected to the following workup: (1) full clinical history; (2) thorough clinical examination, with especial emphasis on measurements of BMI and waist circumference; (3) laboratory assessment (fasting and 2 h post prandial blood glucose level), HBA1c, lipid profile (total cholesterol, LDL, HDL cholesterol and triglycerides) after fasting for 12 h, urine analysis, serum creatinine level, urinary albumin/creatinine ratio, serum SGOT, SGPT and alkaline phosphatase, urinary 8-oxodG (OHdG) by enzymatic assay; (4) imaging procedure – all patients were subjected to an imaging procedure in the form of carotid Doppler and ABI assessments.
Measurements of 8-oxodG
Urinary OHdG was measured using an ELISA kit.
Carotid duplex
A carotid duplex scan was done to measure intima-media thickness of the distal common carotid to detect any atherosclerotic plaques if present, the peak systolic velocity, end diastolic velocity and resistive index of the internal carotid artery by carotid ultrasound. B-mode (brightness) grayscale, color and spectral Doppler techniques were used to investigate the carotid arteries according to standardized protocol. The same operator interpreted all studies in a blind fashion and the same ultrasound unit (HD 5000) was used, using a linear probe (7.5 MHz) for scanning all participants.
Ankle brachial index
The ABI was calculated for all patients.
Diameters of tibial arteries (posterior and anterior tibial)
These diameters were measured.
Results
Our patients were classified to:
Group A: 27 obese patients with metabolic syndrome.
Group B: 25 obese patients without metabolic syndrome.
Group C: 31 healthy subjects (control).
We found a very high statistically significant difference (p <0.001) for weight among the studied groups, with increased values in the obese patients with metabolic syndrome compared to the obese patients without metabolic syndrome and the control group.
We found a very high statistically significant difference (p <0.001) in waist circumference among the studied groups, with increased values in the obese patients with metabolic syndrome compared to the obese patients without metabolic syndrome and the control group. We also found a very high statistically significant difference (p < 0.001) in FBS and 2HPP among the studied groups, with increased values in the obese patients with metabolic syndrome group compared to the obese patients without metabolic syndrome and the control groups (Table 1).
Table 1.
Comparison between studied groups regarding waist circumference and laboratory workup.
| Group A |
Group B |
Group C |
p-value | |
|---|---|---|---|---|
| (n = 27) | (n = 25) | (n = 31) | ||
| Waist circumference (cm) | ||||
| Range | 113.0–160.0 | 114.0–158.0 | 53.0–83.0 | <0.001 |
| Mean ± SD | 132.9 ± 10.1 | 129.3 ± 11.9 | 69.8 ± 7.6 | |
| FBS mg/dl | ||||
| Range | 110.0–364.0 | 79.0–118.0 | 84.0–122.0 | <0.001 |
| Mean ± SD | 185.4 ± 54.2 | 101.1 ± 14.7 | 100.7 ± 10.4 | |
| 2HPP mg/dl | ||||
| Range | 153.0–389.0 | 100.0–300.0 | 110.0–138.0 | <0.001 |
| Mean ± SD | 263.7 ± 51.0 | 136.5 ± 40.0 | 126.5 ± 6.4 | |
| Creat. (0.6–1.4 mg/dl) | ||||
| Range | 0.4–1.4 | 0.4–1.4 | 0.4–1.4 | |
| Mean ± SD | 0.9 ± 0.3 | 0.9 ± 0.4 | 0.9 ± 0.3 | 0.563 |
| ACR (<30 mg/g creat.) | ||||
| Range | 8.0–26.0 | 7.0–24.0 | 11.0–28.0 | 0.008 |
| Mean ± SD | 15.5 ± 5.4 | 14.1 ± 4.4 | 18.2 ± 4.3 | |
| ALT (IU/L) normal value (35 IU/L) | ||||
| Range | 13.0–34.0 | 12.0–32.0 | 15.0–40.0 | 0.103 |
| Mean ± SD | 21.0 ± 5.0 | 22.6 ± 5.2 | 24.0 ± 5.4 | |
| AST U/L (N: 35 IU/L) | ||||
| Range | 12.0–33.0 | 13.0–31.0 | 10.0–34.0 | 0.008 |
| Mean ± SD | 20.0 ± 6.9 | 21.2 ± 5.5 | 24.9 ± 6.0 | |
| 8-oxodG (0.78–50 ng/ml) | ||||
| Range | 0.1–6.3 | 0.1–7.5 | 0.1–6.3 | 0.668 |
| Mean ± SD | 1.9 ± 1.7 | 1.9 ± 2.0 | 1.5 ± 1.4 | |
| Rt. ant. tib. diameter | ||||
| Range | 0.07–0.25 | 0.10–0.30 | 0.12–0.30 | |
| Mean ± SD | 0.18 ± 0.05 | 0.16 ± 0.05 | 0.19 ± 0.05 | 0.027 |
| Rt. post. tib. diameter | ||||
| Range | 0.10–0.32 | 0.10–0.48 | 0.12–0.35 | |
| Mean ± SD | 0.19 ± 0.06 | 0.22 ± 0.08 | 0.21 ± 0.06 | 0.166 |
| Lt. ant. tib. diameter | ||||
| Range | 0.08–0.29 | 0.08–0.26 | 0.10–0.29 | |
| Mean ± SD | 0.16 ± 0.06 | 0.17 ± 0.05 | 0.18 ± 0.04 | 0.185 |
| Lt. post tib. diameter | ||||
| Range | 0.08–0.33 | 0.13–0.36 | 0.13–0.45 | 0.105 |
| Mean ± SD | 0.19 ± 0.07 | 0.22 ± 0.06 | 0.22 ± 0.07 |
The study revealed a statistically significant difference (p = 0.027) in diameter of the right anterior tibial artery among the studied groups, with decreased diameter of the right anterior tibial artery in the obese patients with metabolic syndrome compared to the obese patients without metabolic syndrome and the control group. There were no significant differences or abnormalities in diameter of the right posterior tibial artery and both left anterior and posterior tibial arteries between the studied groups (Table 1). There was a statistically insignificant difference (p = 0.668) in the urinary 8-oxodG in the studied groups (Table 1).
The CIMT was highly statistically significantly increased in both obese patients with and without metabolic syndrome more than the control group with p < 0.001 and p = 0.007, respectively. There was no statistically significant difference in CIMT between the obese patients with or without metabolic syndrome (p = 0.064) (Table 2).
Table 2.
Comparison of carotid intima-media thickness (CIMT) and ankle brachial index (ABI) among the studied groups.
| Group A |
Group B |
Group C |
p-value | |
|---|---|---|---|---|
| (n = 27) | (n = 25) | (n = 31) | ||
| CIMT mm (⩽0.9) | ||||
| Range | 0.7 ± 1.9 | 0.6 ± 1.3 | 0.064 | |
| Mean ± SD | 1.0 ± 0.2 | 0.9 ± 0.2 | ||
| 0.7 ± 1.9 | 0.5 ± 1.2 | |||
| 1.0 ± 0.2 | 0.8 ± 0.2 | <0.001 | ||
| 0.6 ± 1.3 | 0.5 ± 1.2 | |||
| 0.9 ± 0.2 | 0.8 ± 0.2 | 0.007 | ||
| ABI | 0.9 ± 1.7 | 1.0 ± 2.3 | ||
| 1.3 ± 0.2 | 1.5 ± 0.3 | 0.004 | ||
| ABI (0.9 ± 1.3) | 0.9 ± 1.7 | 1.0 ± 1.2 | ||
| Range | 1.3 ± 0.2 | 1.3 ± 0.1 | 0.639 | |
| Mean ± SD | 1.0 ± 2.3 | 1.0 ± 1.3 | ||
| 1.5 ± 0.3 | 1.3 ± 0.1 | 0.001 |
The ABI revealed a lower index in the obese patients with metabolic syndrome than in the obese patients without metabolic syndrome, with the difference being highly statistically significant (p = 0.004). However, the ABI revealed a lower index in the control than in the obese patients without metabolic syndrome, again with a statistically significant difference (p = 0.001). All our patients and control group had normal ABIs. There was a statistically insignificant difference (p = 0.639) in ABI between Group A and Group C (Table 2). There was statistically insignificant correlation of 8-oxodG with CIMT, ABI or diameter of other branches of the tibial artery in the studied groups (Table 3).
Table 3.
Correlation of 8-oxodG with carotid intima-media thickness (CIMT), ankle brachial index (ABI) and diameters of the tibial arteries in the studied groups.
| 8-oxodG ng/ml |
||||
|---|---|---|---|---|
| Group A | Group B | Group C | ||
| CIMT mm | r | −0.019 | −0.155 | −0.111 |
| p | 0.923 | 0.459 | 0.551 | |
| ABI | r | 0.133 | 0.164 | 0.266 |
| p | 0.507 | 0.433 | 0.148 | |
| Rt. ant. tib. | r | 0.298 | −0.093 | −0.423 |
| Diameter | p | 0.132 | 0.660 | 0.018 |
| Rt. post. tib. | r | −0.003 | 0.102 | −0.121 |
| Diameter | p | 0.989 | 0.626 | 0.516 |
| Lt. ant. tib. | r | 0.226 | 0.060 | 0.018 |
| Diameter | p | 0.256 | 0.776 | 0.925 |
| Lt. post tib. | r | −0.124 | 0.015 | −0.073 |
| Diameter | p | 0.537 | 0.941 | 0.697 |
We found a statistically significant correlation (p = 0.034) of CIMT with the diameter of the right anterior tibial artery in the obese patients with metabolic syndrome, and there was a statistically insignificant correlation of CIMT with ABI or diameter of left anterior and posterior tibial arteries, and right posterior tibial artery (Table 4).
Table 4.
Correlation of carotid intima-media thickness (CIMT) with ankle brachia index (ABI) and diameter of tibial arteries among the studied groups.
| CIMT mm |
||||
|---|---|---|---|---|
| Group A | Group B | Group C | ||
| ABI | r | 0.021 | 0.115 | 0.080 |
| p | 0.917 | 0.585 | 0.669 | |
| Rt. ant. tib. | r | −0.410 | −0.326 | 0.241 |
| Diameter | p | 0.034 | 0.112 | 0.191 |
| Rt. post. tib. | r | −0.039 | 0.173 | −0.172 |
| Diameter | p | 0.848 | 0.408 | 0.355 |
| Lt. ant. tib. | r | −0.340 | −0.225 | 0.193 |
| Diameter | p | 0.083 | 0.280 | 0.298 |
| Lt. post tib. | r | −0.223 | −0.103 | −0.215 |
| Diameter | p | 0.263 | 0.624 | 0.245 |
We found a very highly statistically significant correlation (p < 0.001) of CIMT with age, SBP, DBP, weight, BMI, waist circumference, FBS, 2HPP and HA1c, total cholesterol, LDL and alkaline phosphatase (Table 5).
Table 5.
Correlation of carotid intima-media thickness (CIMT) with other parameters in the studied groups.
| CIMT mm | ||
|---|---|---|
| Age | r | 0.544 |
| p | <0.001 | |
| Weight (kg) | r | 0.365 |
| p | 0.001 | |
| Height (cm) | r | −0.157 |
| p | 0.158 | |
| BMI | r | 0.369 |
| p | 0.001 | |
| Waist circumference (cm) | r | 0.353 |
| p | 0.001 | |
| SBP (mm hg) | r | 0.451 |
| p | <0.001 | |
| DBP (mm hg) | r | 0.460 |
| p | <0.001 | |
| FBS (mg/dl) | r | 0.350 |
| p | 0.001 | |
| 2HPP (mg/dl) | r | 0.331 |
| p | 0.002 | |
| HA1c (%) | r | 0.342 |
| p | 0.002 | |
| Chol. (mg/dl) | r | 0.219 |
| p | 0.047 | |
| LDL (mg/dl) | r | 0.259 |
| p | 0.018 | |
| HDL (mg/dl) | r | 0.150 |
| p | 0.175 | |
| TGA (mg/dl) | r | 0.120 |
| p | 0.281 | |
| Urea (mg/dl) | r | 0.002 |
| p | 0.985 | |
| Creat. (mg/dl) | r | −0.074 |
| p | 0.508 | |
| Albumin/creatinine ratio (mg/g cr.) | r | −0.162 |
| p | 0.142 | |
| ALT (U/L) | r | −0.116 |
| p | 0.297 | |
| AST (U/L) | r | −0.256 |
| p | 0.019 | |
| ALP (U/L) | r | −0.352 |
| p | 0.001 |
A statistically insignificant correlation of CIMT with height, HDL, triglyceride, urea, creatinine, albumin–creatinine ratio and ALT (p = 0.297) was found (Table 5).
There was a highly statistically significant difference (p < 0.001) in BMI among the studied groups, with increased BMI values in the obese patients without metabolic syndrome compared to the obese patients with metabolic syndrome and the healthy group (Figure 1).
Figure 1.
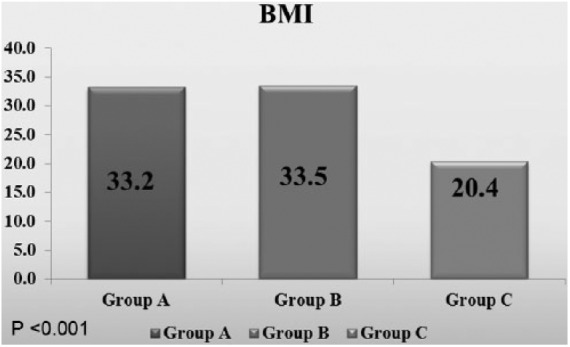
Comparison between the studied groups regarding BMI.
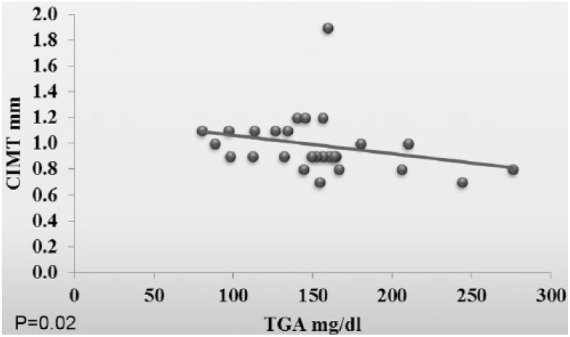
In the obese patients with metabolic syndrome, there was a positive correlation between 8-oxodG and total cholesterol and LDL (p = 0.04 and p = 0.02, respectively). Also, the CIMT was positively correlated with serum triglyceride (p = 0.02) (Figures 2–4).
Figure 2.
Correlation between the 8-oxodG and the total cholesterol for the whole groups.
Figure 3.
Correlation between the 8-oxodG and the LDL in Group A.
Figure 4.
Correlation between the carotid intima-media thickness (CIMT) and the triglycerides (TGA) in Group A.
Statistical analysis
Data was entered on the computer using Microsoft’s Excel (2010) for Windows, then transferred to the Statistical Package of Social Science program, version 21 (SPSS) to be statistically analyzed. Data were summarized using range, mean, standard deviation and median for quantitative variables or frequency, and percentage for qualitative ones. Comparison between groups was performed using the Kruskal–Wallis test followed by pairwise comparisons through the Mann–Whitney U test for quantitative variables. Spearman correlation coefficients were calculated to signify the association between different quantitative variables; p-values less than 0.05 were considered statistically significant. Graphs were used to illustrate some information.
Level of significance
For all the statistical tests, the threshold of significance is fixed at 5%. If the value is >0.05 this indicates a non-significant result; 0.05 or less indicates a significant result. In this case the p-value measures the degree of significance. The smaller the p-value obtained, the more significant is the result. If the p-value falls below or equal to 0.01, the result is considered to be highly significant [p > 0.05 is insignificant (NS); p < 0.05 is significant (S); p < 0.001 is very highly significant (VHS)].
Discussion
Obesity is characterized by increased adipose tissue mass resulting from a chronic imbalance between energy intake and expenditure. There is a defined relationship between fat mass expansion, chronic low-grade systemic inflammation and ROS generation, leading to ROS-related pathological events.11 Metabolic syndrome describes a group of clinical features that together increase the incidence of coronary artery disease, stroke and type 2 diabetes. Insulin resistance is a major risk factor for developing metabolic syndrome. A chronic state of inflammation accompanies the accumulation of serum lipids in adipose and liver tissue, frequently involved in insulin resistance. 8-oxo-2′-deoxyguanosine (8-oxo-dG) is a potent anti-inflammatory agent that inactivates both Rac1 and Rac2, which are critical to initiating the inflammatory responses in various cell types, including macrophages.12
The association between body fat and pathological effects has not been fully elucidated. Though BMI, calculated as weight in kilograms divided by height in meters squared, is the most common measure of obesity, this does not reflect body shape – it can be misleading in individuals with a high proportion of lean muscle mass. Waist circumference, a more accurate measure of the distribution of body fat, has been shown to be more strongly associated with morbidity and mortality.13
In our study we found statistically significant difference in waist circumference among the studied groups, with increased values in the obese patients with metabolic syndrome compared to the obese patients without metabolic syndrome and the control group. BMI was statistically significantly increased in the obese patients without metabolic syndrome compared to the obese patients with metabolic syndrome and the healthy group, indicating that waist circumference is an earlier predictor of atherosclerosis.
Atherosclerosis is an inflammatory process. Furthermore, the coronary plaque environment is composed of multiple cell types, including macrophages and other inflammatory cells that secrete cytokines and ROS that drive inflammation and oxidative stress.14 Levels of DNA damage base lesion, 8-oxo-7,8-dihydro-2′deoxyguanosine (8-oxodG), can be associated with increased oxidative stress as high levels of serum 8-oxodG can be detected in patients with systemic lupus erythematosus, Parkinson’s disease, end-stage renal disease and type 2 diabetes.15 This study found a statistically significant difference in the diameter of the right anterior tibial artery among the studied groups, with decreased diameter of the right anterior tibial artery in the obese patients with metabolic syndrome compared to the obese patients without metabolic syndrome and the control group. There was a statistically significant negative correlation (p = 0.018) of 8-oxodG with the diameter of the right anterior tibial artery in Group C (control). This may highlight the importance of 8-oxodG and early vascular affection as appears with decreasing artery diameter.
However, there was no statistically significant difference in CIMT between obese patients with and without metabolic syndrome, denoting the importance of anterior tibial diameter in early detection of vascular affection in our study.
The diameter of the anterior tibial artery in the first group is lower than the other group and this was statistically significant and it is also negatively correlated with the CIMT, indicating its early affection in sub-clinical atherosclerosis in obese patients with metabolic syndrome. There was also a statistically significant correlation (p = 0.034) of CIMT with the diameter of the right anterior tibial artery in the obese patients with metabolic syndrome.
The ABI which was changed early in our patients as there was highly statistically significant lower index in the obese patients with metabolic syndrome compared to the obese patients without metabolic syndrome (p = 0.004).
The ABI can be a surrogate marker for atherosclerosis, and recent studies indicate its utility as a predictor of future CVD and all-cause mortality.16 ABI thresholds of <0.9 and >1.3 are highly suspicious for PAD and high cardiovascular risk in diabetic patients.17
Atherosclerosis is the cause of a majority of cardiovascular events and is accelerated by DM and metabolic syndrome. Many risk factors are associated with metabolic syndrome and help in explanation of the increased risk of CVD in that condition.18
In our study there was a very high statistically significant correlation (p < 0.001) of CIMT with BMI, waist circumference, FBS, 2HPP and HA1c, total cholesterol and LDL, and this denotes that blood glucose level together with lipid profile, BMI and waist circumferences plays a critical role in atherosclerosis development.
However, there was statistically insignificant correlation of CIMT with height, HDL and triglycerides, which may suggest the importance of cholesterol and LDL in atherosclerosis development rather than triglycerides and HDL levels.
Higher urine albumin–creatinine ratio (ACR) is associated with CVD events, an association that is stronger than that between spot urine albumin on its own and CVD.19
We found a highly statistically significant correlation (p = 0.003) of ABI with the ACR. On the contrary, there was no statistically significant correlation of CIMT with the ACR, denoting the early effect on the ABI before the effect on the CIMT, so ACR may be used as an early predictor of atherosclerosis and nephropathy.
Conclusion
Urinary 8-oxo-7,8-dihydro-2′deoxyguanosine (8-oxodG) was correlated to total cholesterol and LDL in obese patients with metabolic syndrome, which may signify its role in dyslipidemia in those patients, and the risk for early atherosclerosis. However, it was negatively correlated with the diameter of the right anterior tibial artery in the control group, with its change in response to vascular damage occurring in the earlier phase only.
Our study highlights the importance of anterior tibial artery diameter measurement and the ABI as an early marker of atherosclerosis, and that it may be used as an even earlier marker than CIMT.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Mary Wadie Fawzy  http://orcid.org/0000-0002-7067-2115
http://orcid.org/0000-0002-7067-2115
Contributor Information
Rokayaa Abd El Aziz, Internal Medicine Cairo University, Egypt.
Mary Wadie Fawzy, Internal Medicine, Cairo University, 18th Street El Sherbiney Soliman Gohar El Dokki, Egypt.
Noha Khalil, Internal Medicine Cairo University, Egypt.
Sahar Abdel Atty, Chemical Pathology, Cairo University, Egypt.
Zainab Sabra, Internal Medicine Cairo University, Egypt.
References
- 1. Fornoni A, Raij L. Metabolic syndrome and endothelial dysfunction. Curr Hypertens Rep 2005; 7: 88–95. [DOI] [PubMed] [Google Scholar]
- 2. Standards of medical care in diabetes – 2016: summary of revisions. Diabetes Care 2016; 39(Suppl. 1): S4–S5. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care 2003; 26: 3333–3341. [DOI] [PubMed] [Google Scholar]
- 4. DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991; 14: 173–194. [DOI] [PubMed] [Google Scholar]
- 5. Chandie Shaw PK, Baboe F, van Es LA, et al. South-Asian type 2 diabetic patients have higher incidence and faster progression of renal disease compared with Dutch-European diabetic patients. Diabetes Care 2006; 29: 1383–1385. [DOI] [PubMed] [Google Scholar]
- 6. Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res 2004; 567: 1–61. [DOI] [PubMed] [Google Scholar]
- 7. Hütter E, Skovbro M, Lener B, et al. Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell 2007; 6: 245–256. [DOI] [PubMed] [Google Scholar]
- 8. Allison MA, Criqui MH, McClelland RL, et al. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol 2006; 48: 1190–1197. [DOI] [PubMed] [Google Scholar]
- 9. Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56: e50–e103. [DOI] [PubMed] [Google Scholar]
- 10. Laclaustra M, Corella D, Ordovas JM. Metabolic syndrome pathophysiology: the role of adipose tissue. Nutr Metab Cardiovasc Dis 2007; 17: 125–139. [DOI] [PubMed] [Google Scholar]
- 11. Luperini BC, Almeida DC, Porto MP, et al. Gene polymorphisms and increased DNA damage in morbidly obese women. Mutat Res 2015; 776: 111–117. [DOI] [PubMed] [Google Scholar]
- 12. Ko SH, Lee JK, Lee HJ, et al. 8-Oxo-2′-deoxyguanosine ameliorates features of metabolic syndrome in obese mice. Biochem Biophys Res Commun 2014; 443: 610–616. [DOI] [PubMed] [Google Scholar]
- 13. Seidell JC. Waist circumference and waist/hip ratio in relation to all-cause mortality, cancer and sleep apnea. Eur J Clin Nutr 2010; 64: 35–41. [DOI] [PubMed] [Google Scholar]
- 14. Shishehbor MH, Hazen SL. Inflammatory and oxidative markers in atherosclerosis: relationship to outcome. Curr Atheroscler Rep 2004; 6: 243–250. [DOI] [PubMed] [Google Scholar]
- 15. Haghdoost S, Maruyama Y, Pecoits-Filho R, et al. Elevated serum 8-oxo-dG in hemodialysis patients: a marker of systemic inflammation? Antioxid Redox Signal 2006; 8: 2169–2173. [DOI] [PubMed] [Google Scholar]
- 16. Khan TH, Farooqui FA, Niazi K. Critical review of the ankle brachial index. Curr Cardiol Rev 2008; 4: 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Potier L, Abi Khalil C, Mohammedi K, et al. Use and utility of ankle brachial index in patients with diabetes. Eur J Vasc Endovasc Surg 2011; 41: 110–116. [DOI] [PubMed] [Google Scholar]
- 18. Reilly MP, Rader DJ. The metabolic syndrome: more than the sum of its parts? Circulation 2003; 108: 1546–1551. [DOI] [PubMed] [Google Scholar]
- 19. Carter CE, Katz R, Kramer H, et al. Influence of urine creatinine concentrations on the relation of albumin–creatinine ratio with cardiovascular disease events: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 2013; 62: 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]


