Abstract
Our recent work highlights unique requirements for the induction of Th17 cells at the oral/gingival mucosal barrier. Unlike other barrier sites, such as the skin and gastrointestinal tract, we found that Th17 cells can develop at the gingiva independently of commensal microbiota colonization. Instead, we identified that damage, which occurs physiologically due to mastication, promotes induction of Th17 cells and tones homeostatic immunity at the gingiva.
Keywords: oral immunity, oral barrier, barrier immunity, periodontal immunity, IL-17, mastication
The T-helper subset 17 (Th17) and its signature cytokine interleukin (IL)–17 (Amatya et al. 2017) are considered vital mediators of immunity at barrier surfaces such as the gastrointestinal (GI) tract and skin (Belkaid and Harrison 2017). Indeed, Th17 cells are enriched in these environments, where they participate in immune surveillance and maintenance of barrier integrity (Stockinger and Omenetti 2017; Veldhoen 2017). As such, residence of Th17 cells at barrier sites has been shown to ensure protective immunity against both bacterial and fungal challenge (Stockinger and Omenetti 2017; Veldhoen 2017). However, dysregulated Th17 cell responses have, over the past decade, been continuously associated with auto-inflammatory pathologies (Gaffen et al. 2014; Stockinger and Omenetti 2017). In fact, pathogenic Th17 subsets have been identified and characterized in disease lesions and shown to drive pathogenic responses (Lee et al. 2012; Burkett et al. 2015; Stockinger and Omenetti 2017). With Th17 cells being key contributors to effective barrier homeostasis, as well as autoinflammatory pathology, the factors that control their development, function, and plasticity have been well explored. However, much of this work has focused on Th17 cells resident in the GI tract and skin, and by contrast, the factors controlling Th17 cell biology at oral mucosal barriers remain less well explored. This is surprising given the critical role of Th17 cells in oral immunity. Indeed, defects in Th17 cells and/or their signature cytokines result in significant susceptibility to oral fungal infections (Conti et al. 2009; Lionakis et al. 2014; Abusleme and Moutsopoulos 2016). Moreover, amplified/uncontrolled Th17 responses are documented in the oral chronic inflammatory disease periodontitis (Moutsopoulos et al. 2014; Zenobia and Hajishengallis 2015; Dutzan et al. 2016; Moutsopoulos et al. 2017). Yet, although pathogenic subsets of Th17 cells have not been defined in periodontitis, the Th17 signature cytokine IL-17 has been shown to play a pathogenic role in a variety of periodontitis models. In fact, IL-17 inhibition has been successful in rescuing periodontitis phenotypes in models of age-related and diabetes-associated periodontal bone loss, as well as inflammatory bone loss in the setting of tissue neutropenia (Eskan et al. 2012; Moutsopoulos et al. 2014; Xiao et al. 2017). Thus, it is clear that appropriate IL-17 and Th17 cell function is vital to maintain immune homeostasis at the oral barrier, yet little is known regarding their developmental requirements at this site. Here we discuss our recent work (Dutzan et al. 2017), in which we elucidated the factors controlling the induction and regulation of Th17 cells in the gingiva, outlining that novel, tissue-specific cues train immune function at this oral barrier (Fig.).
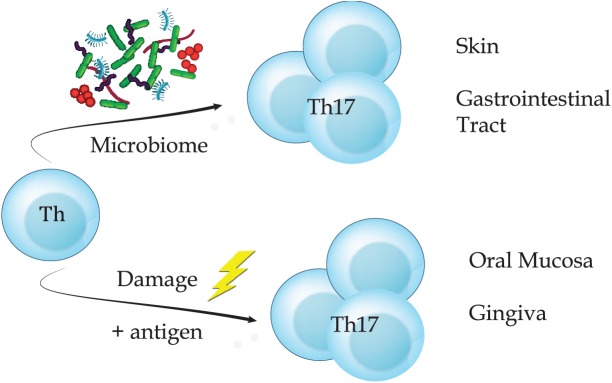
Figure.
Ongoing damage of mastication promotes accumulation of Th17 cells in the gingiva. Accumulation of tissue Th17 is dependent on microbial signals in the skin and gastrointestinal tract. However, at the oral/gingival barrier, Th17 arise under physiologic conditions independent of microbiome. At the gingiva, ongoing damage from mastication is a local, tissue-specific cue, which, in the presence of cognate antigen, will induce oral Th17 cells.
To place our findings at the oral barrier in the context of the larger field of mucosal immunity, we contrast Th17 development in the oral mucosa to findings from the GI tract and skin. At these sites, Th17 cell development has been exquisitely tied to colonization by commensal bacteria (Ivanov et al. 2009; Hooper et al. 2012; Naik et al. 2012) with Th17 cells lacking in germ-free animals. In the GI tract, specific bacterial species have been identified that support acquisition of the Th17 cell fate, most well characterized being segmented filamentous bacteria (SFB) (Ivanov et al. 2009). Moreover, GI Th17 cells have been shown to be commensal specific (Goto et al. 2014; Yang et al. 2014), further reinforcing the vital role of microbes in ensuring Th17 cell residence at this site. Importantly, in both the GI tract and skin, the downstream mediators that drive Th17 development in response to commensal colonization have also been defined. It is well established that the cytokines transforming growth factor β (TGFβ), IL-1β, IL-23, and IL-6 play crucial roles in Th17 cell development (Stockinger and Omenetti 2017). Exploring the roles of these cytokines in generation of skin and GI tract resident Th17 cells has demonstrated that IL-1β signaling is a common mediator of tissue Th17 cell specification (Naik et al. 2012; Shaw et al. 2012). In both barrier environments, IL-1β is produced in response to commensal colonization (Naik et al. 2012; Shaw et al. 2012). Yet production of this cytokine can only be stimulated by certain bacterial species (Seo et al. 2015), further highlighting the commensal-specific nature of this response. Importantly, in the absence of either commensal colonization or IL-1 signals, gut and skin resident Th17 cells fail to develop. Our understanding of Th17 cell specification in the GI tract does not end here; the antigen-presenting cells that drive development of Th17 cells in this environment have also been defined. CD103+CD11b+ IFR4-dependent dendritic cells (DCs) have been shown to be important drivers of Th17 cell development (Persson et al. 2013). Alongside this, CCR2-dependent cells, likely monocyte-derived DCs, have also been reported to be required for Th17 cell induction upon colonization by SFB (Panea et al. 2015).
With this detailed mechanistic insight in mind, we set out to establish the factors shaping Th17 cell immunity at the gingiva. Our first surprise was that in health, the gingiva of young mice was home to very few Th17 cells, contrasting other barrier sites. However, when mice were aged to 24 wk of age, this resident population of Th17 cells was significantly increased in the gingiva but not at other barriers. An increase in Th17 cells with age was also evident in healthy human gingiva. IL-17–producing cells in gingiva were increased in individuals aged 40 to 50 y compared to adults younger than 25 y, even in the absence of oral inflammation. Importantly, and perhaps most surprisingly, this increase in Th17 cells with age was not dependent upon commensal bacteria. Not only was the oral microbiome comparable in young compared to older mice, but the Th17 cell population increased with age in germ-free mice, meaning both germ-free and conventionally housed animals had a similar comparable resident population of Th17 cells. Thus, we outlined that in the gingiva, Th17 cell development occurs via commensal-independent mechanisms, starkly contrasting other barrier sites.
These data demonstrated that Th17 cell specification at the gingiva occurred via mechanisms distinct to those controlling this process at other barrier sites. Highlighting this further was the demonstration that gingival Th17 cell generation was IL-6 dependent and not dependent upon IL-1β signals, which are critical in other barrier microenvironments. In elucidating the upstream driver of IL-6 production in the gingival environment, we considered the local, tissue-specific stimuli encountered at this barrier. We reasoned that during mastication, the gingiva experiences a considerable amount of tissue damage. We hypothesized that this physiological mechanical damage could be training immune function in the gingiva. It is well recognized that tissue injury is a trigger of immune responses, initiated in response to orchestrated signals from damaged or stressed cells (Kono and Rock 2008). Thus, the concept that such signals, which would arise continuously in the oral environment, could contribute to educating T-cell responses in the gingiva formed an attractive proposition.
To address whether local gingival damage could be a stimulus promoting Th17 cell development, we examined Th17 cell generation following alterations in the levels of gingival damage. First, we directly damaged the gingiva of young mice, which resulted in a significant expansion of barrier resident Th17 cells. This damage-induced expansion of Th17 cells was dependent on IL-6 and on the presence of cognate antigen. Second, we placed weanling mice on either a softened or hardened diet and examined gingival Th17 cells at 24 wk of age. Compared to mice aged on a normal diet, those aged on the softened diet had a reduced population of Th17 cells, whereas those aged on a hard diet exhibited increased proportions of Th17 cells. Combined, these data clearly demonstrate that physiological mechanical damage promotes Th17 cell development in the gingiva (Fig.).
Further exploring this novel pathway of Th17 specification in the gingiva, we outlined that in response to gingival damage, epithelial cells produced IL-6, which was subsequently vital for Th17 cell generation at this site. A core component of cellular response to damage and/or stress is the production of proinflammatory mediators, including IL-6 (Kobayashi et al. 2003; Fukuno et al. 2011). Our data demonstrated that epithelial cells function as local, gingival resident sentinels that produce IL-6 in response to mechanical damage and therefore coordinated Th17 specification at this site.
These data suggested that gingiva mechanical damage was the major driver promoting accumulation of Th17 cells. As the gingiva is an environment experiencing constant physiological mechanical damage from mastication, it was important to understand the contribution of damage-induced Th17 cells to local immunity. We demonstrated that damage stimulated induction of IL17-dependent defenses, revealing its role as an ongoing signal that tones protective immunity at this site. Thus, we identify physiological mechanical damage as a key educator of immune function in the gingiva. These data do not preclude a role for the local oral microbiome in shaping gingival immunity but highlight a novel stimulus capable of training immune function at this unique site. A damage-induced pathway of Th17 cell generation could well be active at other sites during pathological settings when levels of damage are elevated and approach those experienced in the gingiva. That tissue damage is often associated with expansions of Th17 cells adds value to this proposition. Indeed, we demonstrated that repeated damage to the skin could promote increases in local Th17 cells, revealing the activity of this pathway even at a site where, during steady state, Th17 cells are dominantly educated by commensals. However, the levels of damage that occur physiologically at the oral barrier are substantial and continuous compared to other sites, with constant occlusal forces, epithelial abrasion, and mechanical stresses arising with every chew of food. That this local immune stimulus could be a dominant mechanism educating immune functions at this site is therefore not surprising.
Yet, as with all immune signals, we show that exaggeration of damage over time contributes to local immunopathology and periodontal bone loss. In this regard, mice develop periodontal bone loss with age, the severity of which was altered by changing the hardness of the diet consumed and therefore the level of damage experienced. In line with a role for mechanical damage in periodontal bone loss, germ-free mice were shown, both in our study and in that of others, to develop bone loss with age (Baer and Newton 1960; Taubman et al. 1981; Hajishengallis et al. 2011). However, bone loss in germ-free animals was less exaggerated than in control mice, indicating that the cooperative functions of the microbiome and damage exaggerate local inflammatory pathology. Consistent with this notion, T cell accumulation and functions have been shown to be regulated both by microbial and damage signals. Specifically, microbial signals have been shown to be critical in the development and maintenance of regulatory T cells (Tregs) (Tanoue et al. 2016), yet in the context of tissue injury, accumulation and functionality of Tregs are influenced by damage signals, including cytokines and alarmins (Burzyn et al. 2013; Arpaia et al. 2015). Similarly, functionality of innate lymphoid cells can be driven by microbial as well as damage-induced signals, such as IL-33 (Monticelli et al. 2015; Thaiss et al. 2016). Thus, our data outline damage not only as a novel pathway of Th17 generation in the gingiva but also as a mechanism that could contribute to dysregulated Th17 responses in settings of pathology.
Our findings elucidate unique immunological triggers operating in the oral barrier yet raise further questions about the training of homeostatic immunity at this site. Is damage a unique signal for the induction of Th17 immunity, or does it trigger additional mechanisms of immune responsiveness promoting barrier fitness? Indeed, continued damage would likely promote a plethora of immune functions. Are there unique functional capabilities exhibited by gingival-resident, damage-induced Th17 cells compared to Th17 cells at other barriers? As a product of their novel developmental program, gingival Th17 cells may well exhibit unique functions specifically supporting defense and/or integrity of the gingival barrier. How stable are these damage-induced Th17 cell populations? Functional plasticity and reduced stability are well-described phenotypes of Th17 cells (Hirota et al. 2013; Morrison et al. 2013; Muranski and Restifo 2013; Gagliani et al. 2015); given the pathogenic potential of these cells within the gingival environment, it will be important to establish the long-term stability of this cellular fates. Importantly, given the amplification of Th17 cells in oral inflammatory disease, what are the critical signals participating in the dysregulation of Th17 immunity at the oral barrier? Elucidation of unique requirements for the induction of immunity at the oral barrier and delineation of functionality of tissue resident populations at this interface is critical to support our understanding of immunity and inflammation at this unique mucosal site.
Author Contributions
J.E. Konkel, N.M. Moutsopoulos, contributed to conception, design, and data interpretation, drafted and critically revised the manuscript. Both authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This work was funded by the Intramural Program of the National Institute of Dental and Craniofacial Research (to N.M.), by the BBSRC (BB/M025977/1 to J.E.K), and by the Manchester Collaborative Centre for Inflammation Research (to J.E.K).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Abusleme L, Moutsopoulos NM. 2016. IL-17: overview and role in oral immunity and microbiome. Oral Dis. 23(7):854–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatya N, Garg AV, Gaffen SL. 2017. IL-17 signaling: the yin and the yang. Trends Immunol. 38(5):310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, Rudensky AY. 2015. A distinct function of regulatory T cells in tissue protection. Cell. 162(5):1078–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer PN, Newton WL. 1960. Studies on peridontal disease in the mouse. 3. The germ-free mouse and its conventional control. Oral Surg Oral Med Oral Pathol. 13:1134–1144. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Harrison OJ. 2017. Homeostatic immunity and the microbiota. Immunity. 46(4):562–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett PR, Meyer zu, Horste G, Kuchroo VK. 2015. Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity. J Clin Invest. 125(6):2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, et al. 2013. A special population of regulatory T cells potentiates muscle repair. Cell. 155(6):1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, et al. 2009. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 206(2):299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzan N, Abusleme L, Bridgeman H, Greenwell-Wild T, Zangerle-Murray T, Fife ME, Bouladoux N, Linley H, Brenchley L, Wemyss K, et al. 2017. On-going mechanical damage from mastication drives homeostatic Th17 cell responses at the oral barrier. Immunity. 46(1):133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzan N, Konkel JE, Greenwell-Wild T, Moutsopoulos NM. 2016. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 9(5):1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, et al. 2012. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 13(5):465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuno N, Matsui H, Kanda Y, Suzuki O, Matsumoto K, Sasaki K, Kobayashi T, Tamura S. 2011. TGF-β-activated kinase 1 mediates mechanical stress-induced IL-6 expression in osteoblasts. Biochem Biophys Res Commun. 408(2):202–207. [DOI] [PubMed] [Google Scholar]
- Gaffen SL, Jain R, Garg AV, Cua DJ. 2014. The IL-23–IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 14(9):585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limon P, Paiva RS, Ching T, et al. 2015. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 523(7559):221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, Laufer TM, Ignatowicz L, Ivanov II. 2014. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 40(4):594–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, et al. 2011. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 10(5):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Turner JE, Villa M, Duarte JH, Demengeot J, Steinmetz OM, Stockinger B. 2013. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell–dependent IgA responses. Nat Immunol. 14(4):372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. 2012. Interactions between the microbiota and the immune system. Science. 336(6086):1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 139(3):485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Nagino M, Komatsu S, Naruse K, Nimura Y, Nakanishi M, Sokabe M. 2003. Stretch-induced IL-6 secretion from endothelial cells requires NF-kappaB activation. Biochem Biophys Res Commun. 308(2):306–312. [DOI] [PubMed] [Google Scholar]
- Kono H, Rock KL. 2008. How dying cells alert the immune system to danger. Nat Rev Immunol. 8(4):279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, et al. 2012. Induction and molecular signature of pathogenic Th17 cells. Nat Immunol. 13(10):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis MS, Netea MG, Holland SM. 2014. Mendelian genetics of human susceptibility to fungal infection. Cold Spring Harb Perspect Med. 4(6). pii: a019638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DM, Artis D. 2015. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci USA. 112(34):10762–10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison PJ, Bending D, Fouser LA, Wright JF, Stockinger B, Cooke A, Kullberg MC. 2013. Th17-cell plasticity in Helicobacter hepaticus–induced intestinal inflammation. Mucosal Immunol. 6(6):1143–1156. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos NM, Konkel J, Sarmadi M, Eskan MA, Wild T, Dutzan N, Abusleme L, Zenobia C, Hosur KB, Abe T, et al. 2014. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci Transl Med. 6(229):229ra240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos NM, Zerbe CS, Wild T, Dutzan N, Brenchley L, DiPasquale G, Uzel G, Axelrod KC, Lisco A, Notarangelo LD, et al. 2017. Interleukin-12 and interleukin-23 blockade in leukocyte adhesion deficiency type 1. N Engl J Med. 376(12):1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P, Restifo NP. 2013. Essentials of Th17 cell commitment and plasticity. Blood. 121(13):2402–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, et al. 2012. Compartmentalized control of skin immunity by resident commensals. Science. 337(6098):1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panea C, Farkas AM, Goto Y, Abdollahi-Roodsaz S, Lee C, Koscso B, Gowda K, Hohl TM, Bogunovic M, Ivanov II. 2015. Intestinal monocyte-derived macrophages control commensal-specific Th17 responses. Cell Rep. 12(8):1314–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hagerbrand K, Marsal J, Gudjonsson S, Hakansson U, Reizis B, Kotarsky K, et al. 2013. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 38(5):958–969. [DOI] [PubMed] [Google Scholar]
- Seo SU, Kamada N, Muñoz-Planillo R, Kim YG, Kim D, Koizumi Y, Hasegawa M, Himpsl SD, Browne HP, Lawley TD, et al. 2015. Distinct commensals induce interleukin-1β via NLRP3 inflammasome in inflammatory monocytes to promote intestinal inflammation in response to injury. Immunity. 42(4):744–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw MH, Kamada N, Kim YG, Nunez G. 2012. Microbiota-induced IL-1β, but not IL-6, is critical for the development of steady-state Th17 cells in the intestine. J Exp Med. 209(2):251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Omenetti S. 2017. The dichotomous nature of T helper 17 cells. Nat Rev Immunol. 17(9):535–544. [DOI] [PubMed] [Google Scholar]
- Tanoue T, Atarashi K, Honda K. 2016. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol. 16(5):295–309. [DOI] [PubMed] [Google Scholar]
- Taubman MA, Buckelew JM, Ebersole JL, Smith DJ. 1981. Periodontal bone loss and immune response to ovalbumin in germfree rats fed antigen-free diet with ovalbumin. Infect Immun. 32(1):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss CA, Zmora N, Levy M, Elinav E. 2016. The microbiome and innate immunity. Nature. 535(7610):65–74. [DOI] [PubMed] [Google Scholar]
- Veldhoen M. 2017. Interleukin 17 is a chief orchestrator of immunity. Nat Immunol. 18(6):612–621. [DOI] [PubMed] [Google Scholar]
- Xiao E, Mattos M, Vieira GHA, Chen S, Correa JD, Wu Y, Albiero ML, Bittinger K, Graves DT. 2017. Diabetes enhances IL-17 expression and alters the oral microbiome to increase its pathogenicity. Cell Host Microbe. 22(1):120–128e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, et al. 2014. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 510(7503):152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenobia C, Hajishengallis G. 2015. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol 2000. 69(1):142–159. [DOI] [PMC free article] [PubMed] [Google Scholar]