Abstract
Carious lesions develop in tooth surfaces where there is an imbalance of the processes of acid and alkali production by supragingival biofilms. Since low pH is the main driving factor in the development of carious lesions, most efforts to identify an effective anticaries therapy have focused on targeting the acid-producing bacteria and their mechanisms of acid production. An expanding area of oral microbiology has now been devoted to explore microbial metabolic activities that help to neutralize biofilm pH and thus inhibit the caries process. Arginine metabolism via the arginine deiminase pathway (ADS) produces alkali in the form of ammonia that counteracts the effects of biofilm acidification from bacterial glycolysis. ADS also functions as an adaptive strategy used by certain bacteria to thrive in oral biofilms. Substantial evidence accumulated from laboratory and clinical observations supports the hypotheses that measurements of arginine metabolism via ADS may serve as an important caries risk assessment criterion and that providing arginine regularly to supragingival biofilms can be an effective therapy for caries intervention. This article reviews the potential of arginine-based therapies such as the use of arginine as prebiotic, ADS+ strains as probiotics, and oral care formulations containing arginine for prevention and management of dental caries.
Keywords: dental caries, bacteria, plaque, biolfim, health, antagonism
Introduction
Insights from the Human Microbiome Project reveal that microbial ecological balance in human biofilms is essential for health (Turnbaugh et al. 2007). Not surprisingly, there has been an increasing interest in therapeutic interventions that modulate the microbiome of biofilms to restore ecological balance and thus health. One way to restore biofilm homeostasis is to equilibrate the acidity and alkalinity processes to maintain a neutral biofilm pH. In the active process of caries, continuous acid production from bacterial glycolysis of dietary carbohydrates shifts the composition of supragingival biofilms toward the emergence of an acid-producing and acid-resistant microflora and loss of tooth minerals (Marsh 2006). Physiological factors that can counterbalance the acidification of biofilms include the clearance and buffering capacity of saliva and the metabolism of salivary substrates, such as urea and arginine, which generates alkali in the form of ammonia. In particular, ammonia production from arginine metabolism of oral bacteria inhibits tooth demineralization by neutralizing glycolytic acids and by favoring the growth of a desirable microflora that is compatible with dental health (Nascimento and Burne 2014).
Early in vitro studies from Kleinberg and collaborators identified the amino acid arginine as the main salivary component responsible for the pH-raising effect of saliva, even in the presence of carbohydrates (Kleinberg et al. 1979; Kanapka and Kleinberg 1983). The sources of arginine (L-arginine) can be exogenous (through the diet) or endogenous (protein turnover and de novo arginine synthesis from citrulline) in which arginine is naturally produced by the human body and secreted in saliva in free form or as salivary peptides (Morris 2006). In supragingival biofilms, arginine is metabolized mainly by the arginine deiminase pathway (ADS) of certain oral bacteria to produce citrulline, ornithine, CO2, adenosine triphosphate (ATP), and ammonia. Ammonia production from arginine metabolism results in cytoplasmic and environmental pH rises and serves as a mechanism used by oral bacteria for (1) protection against acid killing; (2) bioenergetic advantages, including the increase of ΔpH and synthese of ATP; and (3) maintaining a relatively neutral environmental pH that favors the persistence of ADS-positive (ADS+) bacteria while being competitive against caries pathogens (Burne and Marquis 2000). Therefore, ADS activity in supragingival biofilms is an important metabolic activity that affects pH homeostasis and microbial ecology and pathogenicity. This article aims to review the knowledge gained thus far from laboratory and clinical studies that support a significant role of arginine metabolism in the ecological balance of supragingival biofilms and inhibition of caries and also to report promising clinical applications of arginine in caries intervention.
Arginine Metabolism as a Caries Risk Criterion
A series of in vitro studies (Turtola and Luoma 1972; Sissons and Cutress 1988) and clinical observations (Peterson et al. 1985; Margolis et al. 1988; Van Wuyckhuyse et al. 1995) disclosed that ammonia production via ureolysis and arginolysis may prevent dental caries. Based on these previous findings, our research group posed the question of whether there is a correlation between the ammonia-producing capacity of saliva and supragingival biofilms (or dental plaque) with caries experience. Indeed, our first clinical study revealed a positive correlation between low ureolysis and arginolysis via ADS in oral samples and caries activity (Nascimento et al. 2009). More specifically, saliva and pooled plaque samples from caries-free adults presented higher ammonia production from these pathways as compared with those from caries-active adults. While in vitro studies have demonstrated the anticaries effect of urea, problems associated with supplementation of urea in humans have limited its use as an anticaries agent. Hence, our main research focus has been on arginine metabolism.
ADS activity of oral samples can be measured in the laboratory by monitoring ammonia or citrulline production from arginine using a protocol described elsewhere (Liu et al. 2008). In our second clinical study, we were able to optimize such a protocol to measure ADS activity in smaller amounts of plaque samples. This was highly significant from a clinical perspective because it allowed the study of site-specific plaque samples instead of pooled plaque. In brief, we measured the ADS activity of plaque collected from caries-free tooth surfaces (PF), enamel carious lesions (PE), and dentin carious lesions (PD) of children who were either caries free or caries active (Nascimento et al. 2013). Mixed-model analysis evaluated whether age, type of dentition, children’s caries status, and plaque caries status could be used as predictors of ADS activity. However, plaque caries status was the only factor significantly related to ADS activity (P < 0.0001), meaning that health-associated plaque (PF) predicted higher levels of ADS activity compared with carious plaque (PE or PD).
An ongoing prospective study has been investigating the relationship between arginine metabolism and caries experience over time by assessing children every 6 mo (Hanway et al. 2016). The preliminary results from the 18-mo study visit revealed that the ADS activity of caries-free children was significantly higher (P < 0.0001) and considerably more stable when compared with that of caries-active children. The ADS activity of PF samples was also significantly higher compared with those of PE (P < 0.01) and PD (P < 0.0001) samples, but only ADS activity of PD was considered stable over the period of 18 months (Fig. 1). The most rational explanation for the differences observed in ADS activity between healthy-associated and carious plaque may be related to the composition of the microbiomes inhabiting these different sites. It is also possible that the microenvironments in biofilms of caries-active individuals and caries-active tooth sites may not favor high levels of ADS expression or may contain inhibitory factors that decrease ADS expression or enzyme activity. Nevertheless, our findings to date support the hypothesis that arginine metabolism in supragingival biofilms may greatly affect the resistance or susceptibility of the host to dental caries. They also suggest that measurements of arginine metabolism by plaque bacteria can be used to differentiate the caries risk of individuals and tooth surfaces. For further validation of plaque ADS activity as a caries risk assessment criterion, future clinical trials should be designed using an integrated model that includes arginine metabolism as a caries-protective factor and other recognized pathologic risk factors as predictors of caries. Conceivably, novel chairside tests using plaque ADS activity as a caries risk assessment tool could also be developed in the future.
Figure 1.

Arginine deiminase pathway (ADS) activity of PF samples was significantly higher (*) compared with that of PE (P < 0.01) and PD (P < 0.0001) samples. Plaque ADS activity was measured at each study point by monitoring citrulline production from arginine. ADS activity was normalized to protein content and defined as nmol of citrulline generated (minute × [mg protein])–1. PD, plaque from active, dentin carious lesions; PE, plaque from active, enamel carious lesions; PF, supragingival plaque samples from caries-free tooth surfaces.
Highly Arginolytic Biofilms Are Desirable Noncariogenic Biofilms
The use of OMICS technologies in caries research is providing a broader understanding of the composition of the oral microbiome that may contribute to dental health or to the caries process (Nascimento et al. 2017). Many of these molecular biology studies, and also earlier studies based on traditional microbiology, have shown strong associations between the presence of ammonia-producing organisms and dental health (Aas et al. 2008; van Houte et al. 1994). The group of oral bacteria that are known to express the ADS include Streptococcus sanguinis, Streptococcus gordonii, Streptococcus parasanguis, Streptococcus mitis, Streptococcus oralis, Streptococcus rattus, certain Lactobacillus species, and a few spirochetes (Burne and Marquis 2000). However, some acid-producing bacteria that are associated with caries also possess the ADS, including species of Actinomyces and Bifidobacterium/Scardovia (van Ruyven et al. 2000; Aas et al. 2008; Beighton et al. 2010; Tanner et al. 2011). Moreover, the human oral microbiome database (HOMD) indicated the presence of potential “arginine deiminase” enzymes in the genome of more than 130 oral taxa, mostly not-yet-recognized ADS+ bacteria. The fact that ADS+ bacteria can be found in both health and caries underlines the many different metabolic functions of this system. Perhaps ADS+ bacteria with low constitutional arginolytic activity have maintained the ADS for bioenergetic advantages only, while those with high constitutional arginolytic activity may produce sufficient levels of ammonia to raise cytoplasmic and environmental pH that contribute to pH homeostasis of biofilms. To clarify the complex acid-base relationships of the oral microbiome, it is critical to differentiate the healthy-associated “commensals” from the bacteria that are aciduric but insufficiently alkalinogenic to moderate biofilm acidification and therefore may be considered cariogenic bacteria.
A recent study employed the Human Oral Microbe Identification using Next Generation Sequencing (HOMINGS) to explore the association between caries activity and microbial profiles of site-specific supragingival plaque samples (Richards et al. 2017). Distance-based redundancy analysis showed that the bacterial communities of PF, PE, and PD were well differentiated from each other (Fig. 2), which supports that the microbiome of healthy tooth surfaces differs substantially from that found when there is evidence of caries activity. Taxa most commonly found in PD include (in order of prevalence) Streptococcus mutans and other acidogenic/aciduric species such as Scardovia wiggsiae, Parascardovia denticolens, Veillonella parvula, and Lactobacillus salivarius. Intriguingly, PD samples in which S. mutans was not detected, or was detected at very low proportions, presented high community diversity. Taxa most commonly found in PF include (also in order of prevalence) S. sanguinis, Lautropia mirabilis, Abiotrophia defectiva, Corynebacterium durum, and Rothia aeria, and the abundance of these taxa progressively decreased from healthy-associated to carious plaque. This microbiome study strengthens the well-known association of S. mutans with caries and S. sanguinis, which is an ADS+ species, with health but also highlights that other species of lower frequency may also be involved in transition from health to caries disease. Site-specific supragingival plaque samples collected during this study are being currently analyzed to investigate whether ADS+ bacteria with low constitutional arginolytic activity are more commonly isolated from caries-active subjects and/or plaque from carious sites and whether ADS+ bacteria with high constitutional arginolytic activity are more commonly isolated from caries-free subjects and/or plaque from caries-free tooth sites.
Figure 2.

Ordination analysis: distance-based redundancy analysis of plaque bacterial communities. PD, plaque from active, dentin carious lesions; PE, plaque from active, enamel carious lesions; PF, supragingival plaque samples from caries-free tooth surfaces.
To continue to elucidate the role of bacterial arginolytic activity in the caries process, our laboratory isolated a panel of ADS+ strains from supragingival plaque and characterized these strains for their ability to metabolize arginine under conditions known to induce or repress the ADS (Huang et al. 2015). Strains of the commensals, S. sanguinis, S. gordonii, S. parasanguinis, S. intermedius, S. australis, and S. cristatus presented enormous variability in their constitutive and condition-specific arginolytic capacities. The study demonstrated that the ADS of the various clinical strains respond differently to the availability and source of carbohydrate, low pH, and oxygen. Given that acid tolerance and adaption to nutrient limitation and oxidative stress are complex phenotypes, the different regulatory patterns of these systems provides evidence that oral ADS+ strains differ markedly in the strategies they use to cope with environmental stresses in the oral environment.
From our library of clinical ADS+ strains (Huang et al. 2015), a novel strain called A12 caught our attention for expressing significantly high ADS activity under cariogenic conditions (Huang et al. 2016). High constitutional ADS activity of oral bacteria such as A12 sustains a neutral environmental pH in supragingival biofilms and can be considered as a nonspecific defense mechanism against caries pathogens by decreasing their competitive advantage at low pH. A12 was isolated from a supragingival plaque sample of a caries-free individual and was shown to have a potent inhibitory effect on the growth of S. mutans, mainly through pyruvate oxidase–dependent H2O2 production (Fig. 3). Antagonism between beneficial commensals and cariogenic bacteria is a major factor that shapes the composition and ecology of supragingival biofilms. Organisms such as A12 that can moderate plaque pH and interfere with the growth and virulence of caries pathogens may play central roles in the promotion of health-associated oral biofilm communities.
Figure 3.
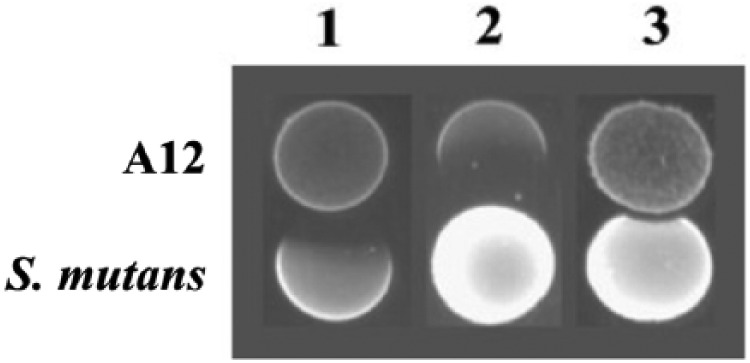
Competition assays between S. mutans UA159 and A12 on half-strength BHI agar plates. (1) A12 strain was inoculated first, followed by inoculation of S. mutans 24 h later and an additional 24 h of incubation; (2) S. mutans was inoculated first, followed by inoculation of A12 24 h later, then an additional 24 h of incubation; and (3) A12 and S. mutans were inoculated at the same time and incubated for a total of 24 h (Huang et al. 2016).
Arginine Applications in Caries Prevention: Prebiotics, Probiotics, and Oral Care Formulations
Numerous therapies have been proposed to target specific caries pathogens or indiscriminately eliminate oral biofilms (e.g., xylitol, chlorhexidine [James et al. 2010], immunization, and bacterial replacement therapy; Hillman 2002). However, the effectiveness of these methods is yet to be recognized, and safety concerns have been raised with regard to their negative impact in the ecology of the oral microbiota. Novel therapies that seek to provide arginine as a substrate for ammonia production in oral biofilms may have high cost-effective potential for at-risk populations facing challenges accessing dental care. These promising approaches may include the use of arginine as prebiotic, selected ADS+ strains as probiotic, and/or introduction of arginine in oral care formulations.
Prebiotics are defined as fermented food ingredients that can change the composition and/or activity of the resident microflora and confer benefits on host well-being and health (Roberfroid 2007). The concept of prebiotic has attracted and inspired research in many areas of nutrition and medical sciences. Several studies have shown that dietary consumption of certain food products can selectively modulate the indigenous composition of the gut microbiota (Roberfroid et al. 2010). As a natural dietary supplement, arginine has been extensively researched in medicine primarily to improve the symptoms of cardiovascular disease (Lorin et al. 2014). This is so because arginine is a precursor of nitric oxide (NO), which plays important roles in vasodilatation, bacterial challenge and cytokine stimulation, regulation of mineralized tissue function, neurotransmission, and platelet aggregation (Morris 2016).
Recent in vitro studies have shown that providing L-arginine to supragingival biofilms disrupts the process of biofilm matrix assembly and the microbial interactions that are associated with the development of cariogenic biofilms (He et al. 2016) and also confers biofilm pH homeostasis (Agnello et al. 2017). Interestingly, dietary questionnaires used in our clinical studies revealed that a group of caries-free participants presenting extremely high levels of plaque ADS activity reported high consumption of protein bars or protein shakes, which are major sources of arginine (data not published). Although arginine supplementation may have a positive impact in supragingival biofilms, NO generated from arginine metabolism may be involved in the pathogenesis of periodontitis (Parwani and Parwani 2015). Clearly, long-term randomized clinical trials are needed to determine whether dietary supplementation of arginine could be advantageous for oral health. Besides, it is critical to determine the correct supplementation level of dietary arginine before this amino acid can be used as a prebiotic approach for caries intervention.
Probiotics are defined as viable microorganisms that confer health benefits when administered in sufficient doses. There is a long history behind the use of probiotics for prevention and treatment of many medical conditions. Probiotics are an alternative to pharmaceutical management, notwithstanding the constant debates on their beneficial versus adverse effects. The increased popularity of using probiotic bacteria to improve gastrointestinal health has prompted interest in the value of this approach for oral applications. Consequently, much attention has been given lately to the role of probiotics in preventing caries, and the administration of different strains of Lactobacilli and/or Bifidobacteria have been tested to battle cariogenic bacteria (Cagetti et al. 2013). The mechanisms of action of probiotics are thought to combine local and systemic effects including adhesion, coaggregation, growth inhibition, production of organic acids and bacteriocins, and immune modulation (Devine and Marsh 2009), with the ultimate goal of displacing and perhaps replacing pathogens. These mechanisms may vary according to the specific bacterial strain or combinations of strains used, the delivery system, and the stage of the disease process in which the probiotic is administered.
Although the potential of using probiotics to manage caries appears to be high, the probiotic strains tested for oral health are, at the moment, microorganisms used mainly for gastrointestinal benefits, and they are likely not to adapt well to the unique environmental conditions and complex ecology of oral biofilms (Cagetti et al. 2013). These probiotic strains do not seem to colonize the oral cavity permanently, which may be related to the number of receptors in the dental pellicle available for colonization of these nonoral strains as compared with receptors for indigenous oral bacteria (Comelli et al. 2002). It has been proposed that naturally occurring oral strains with diminished cariogenic potential and desirable antagonistic properties on cariogenic bacteria may be proven successful as probiotic therapies for caries (Hillman et al. 2009). In this context, A12 and other arginolytic clinical strains with constitutionally high ADS-expressing phenotypes and those capable of expressing ADS under conditions known to cause caries (e.g., sugar availability and acidic environment) are being tested as probiotic strains for caries prevention.
A technology designed to deliver arginine for ammonia production by plaque bacteria (Kleinberg 1999) has been incorporated into toothpastes, mints, and chews. Over the past 10 y, clinical trials have been conducted to evaluate the anticaries efficacy of products containing this original arginine technology or other optimized arginine formulations with or without fluoride (Acevedo et al. 2008; Kraivaphan et al. 2013). A 2-y clinical study demonstrated that the 1.5% arginine fluoride-free toothpaste was more effective in inhibiting caries than the control fluoride toothpaste (Acevedo et al. 2005). In another trial, caries onset and progression were reduced in children using sugarless mints containing 1.5% arginine, 4 times/d during 12 mo (Acevedo et al. 2008). The use of a 1.5% arginine fluoride-free toothpaste for 4 wk increased ADS activity in plaque of caries-active adults and caused a shift in their plaque microbial profile to a bacterial community comparable to that of caries-free adults (Nascimento et al. 2014). A toothpaste containing 1.5% arginine, an insoluble calcium compound, and 1,450 ppm of fluoride was also shown to reduce caries increments in low- and moderate-risk children, to arrest and reverse carious lesions in children and adults, and to have superior caries benefits as compared with a regular toothpaste containing 1,450 ppm of fluoride alone (Kraivaphan et al. 2013). Thus, the mechanisms of action of arginine appear to complement those of fluoride by directly influencing biofilm pH while affecting the composition of oral biofilms. No negative side effects or potential risks have been reported following the use of these products containing arginine at 1.5%. Even though the supplementation of arginine to oral biofilms can be effective against caries, the long-term impact of these novel arginine-based technologies on oral health and etiology of other oral diseases such as periodontal disease remains to be investigated.
Conclusion
Compelling in vitro and in vivo evidence support the continued investigation of oral arginine metabolism as a promising approach for caries intervention. A key property of the bacteria that are present in higher proportions in healthy-associated supragingival biofilms is their ability to produce ammonia from arginine metabolism. Ammonia production via ADS contributes to pH homeostasis and ecological balance of biofilms, thus reducing the risk for the development of carious lesions. Measurements of ADS activity in dental plaque samples offer the opportunity for the design of novel tools for caries risk assessment. Potential arginine-based approaches for caries intervention, which can be used separately or in combination, include arginine supplementation as prebiotics, arginine incorporation in oral care products and probiotic formulations composed by clinical strains presenting high ADS-expressing phenotypes, low cariogenic potential, as well as desirable antagonistic properties on cariogenic bacteria. Yet, future long-term randomized clinical trials should be performed to further evaluate the effect of arginine supplementation in oral health.
Author Contributions
M.M. Nascimento, contributed to conception and design, drafted and critically revised the manuscript. The author gave final approval and agrees to be accountable for all aspects of the work.
Footnotes
Some of the author’s research works cited in this article have been supported by the National Institute of Dental and Craniofacial Research K23-DE023579 and also by Colgate-Palmolive.
As a potential conflict of interest, Colgate-Palmolive holds the patent of the arginine-containing toothpaste mentioned in this article. The author declares no other potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, Leys EJ, Paster BJ. 2008. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 46(4):1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo AM, Machado C, Rivera LE, Wolff M, Kleinberg I. 2005. The inhibitory effect of an arginine bicarbonate/calcium carbonate CaviStat-containing dentifrice on the development of dental caries in Venezuelan school children. J Clin Dent. 16(3):63–70. [PubMed] [Google Scholar]
- Acevedo AM, Montero M, Rojas-Sanchez F, Machado C, Rivera LE, Wolff M, Kleinberg I. 2008. Clinical evaluation of the ability of CaviStat in a mint confection to inhibit the development of dental caries in children. J Clin Dent. 19(1):1–8. [PubMed] [Google Scholar]
- Agnello M, Cen L, Tran NC, Shi W, McLean JS, He X. 2017. Arginine improves ph homeostasis via metabolism and microbiome modulation. J Dent Res. 96(8):924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beighton D, Al-Haboubi M, Mantzourani M, Gilbert SC, Clark D, Zoitopoulos L, Gallagher JE. 2010. Oral bifidobacteria: caries-associated bacteria in older adults. J Dent Res. 89(9):970–974. [DOI] [PubMed] [Google Scholar]
- Burne RA, Marquis RE. 2000. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett. 193(1):1–6. [DOI] [PubMed] [Google Scholar]
- Cagetti MG, Mastroberardino S, Milia E, Cocco F, Lingstrom P, Campus G. 2013. The use of probiotic strains in caries prevention: a systematic review. Nutrients. 5(7):2530–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comelli EM, Guggenheim B, Stingele F, Neeser JR. 2002. Selection of dairy bacterial strains as probiotics for oral health. Eur J Oral Sci. 110(3):218–224. [DOI] [PubMed] [Google Scholar]
- Devine DA, Marsh PD. 2009. Prospects for the development of probiotics and prebiotics for oral applications. J Oral Microbiol. 1. doi: 10.3402/jom.v1i0.1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanway S, Huang X, Alvarez AJ, Mugayar L, Perry S, Luce A, Hong H, Shaddoxx LM, Burne RA, Nascimento MM. 2016. New insights into the oral biofilm of children with caries. Paper presented at: 69th Annual Session of the AAPD; San Antonio, TX. [Google Scholar]
- He J, Hwang G, Liu Y, Gao L, Kilpatrick-Liverman L, Santarpia P, Zhou X, Koo H. 2016. L-arginine modifies the exopolysaccharides matrix and thwarts Streptococcus mutans outgrowth within mixed-species oral biofilms. J Bacteriol. 198(19):2651–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman JD. 2002. Genetically modified Streptococcus mutans for the prevention of dental caries. Antonie van Leeuwenhoek. 82(1–4):361–366. [PubMed] [Google Scholar]
- Hillman JD, McDonell E, Cramm T, Hillman CH, Zahradnik RT. 2009. A spontaneous lactate dehydrogenase deficient mutant of Streptococcus rattus for use as a probiotic in the prevention of dental caries. J Appl Microbiol. 107(5):1551–1558. [DOI] [PubMed] [Google Scholar]
- Huang X, Palmer SR, Ahn SJ, Richards VP, Williams ML, Nascimento MM, Burne RA. 2016. A highly arginolytic Streptococcus species that potently antagonizes Streptococcus mutans. Appl Environ Microbiol. 82(7):2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Schulte RM, Burne RA, Nascimento MM. 2015. Characterization of the arginolytic microflora provides insights into pH homeostasis in human oral biofilms. Caries Res. 49(2):165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Parnell C, Whelton H. 2010. The caries-preventive effect of chlorhexidine varnish in children and adolescents: a systematic review. Caries Res. 44(4):333–340. [DOI] [PubMed] [Google Scholar]
- Kanapka JA, Kleinberg I. 1983. Catabolism of arginine by the mixed bacteria in human salivary sediment under conditions of low and high glucose concentration. Arch Oral Biol. 28(11):1007–1015. [DOI] [PubMed] [Google Scholar]
- Kleinberg I. 1999. A new saliva-based anti-caries composition. Dent Today. 18(2):98–103. [PubMed] [Google Scholar]
- Kleinberg I, Kanapka J, Chatterjee R, Craw D, D’Angelo NK, Sandham HG. 1979. Metabolism of nitrogen by the oral mixed bacteria. In: Kleinberg I, Ellison SA, Mandel ID, editors. Saliva and dental caries. Washington (DC) and London: Information Retrieval; p. 357–377. [Google Scholar]
- Kraivaphan P1, Amornchat C, Triratana T, Mateo LR, Ellwood R, Cummins D, DeVizio W, Zhang YP. 2013. Two-year caries clinical study of the efficacy of novel dentifrices containing 1.5% arginine, an insoluble calcium compound and 1,450 ppm fluoride. Caries Res 47(6):582–590. [DOI] [PubMed] [Google Scholar]
- Liu Y, Dong Y, Chen YY, Burne RA. 2008. Environmental and growth phase regulation of the Streptococcus gordonii arginine deiminase genes. Appl Environ Microbiol. 74(16):5023-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorin J, Zeller M, Guilland JC, Cottin Y, Vergely C, Rochette L. 2014. Arginine and nitric oxide synthase: regulatory mechanisms and cardiovascular aspects. Mol Nutr Food Res. 58(1):101–116. [DOI] [PubMed] [Google Scholar]
- Margolis HC, Duckworth JH, Moreno EC. 1988. Composition of pooled resting plaque fluid from caries-free and caries-susceptible individuals. J Dent Res. 67(12):1468–1475. [DOI] [PubMed] [Google Scholar]
- Marsh PD. 2006. Dental plaque as a biofilm and a microbial community: implications for health and disease. BMC Oral Health. 6(Suppl 1):S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SM., Jr. 2006. Arginine: beyond protein. Am J Food Clin Nutr. 83(2):508S–512S. [DOI] [PubMed] [Google Scholar]
- Morris SM., Jr. 2016. Arginine metabolism revisited. J Nutr. 146(12):2579S–2586S. [DOI] [PubMed] [Google Scholar]
- Nascimento MM, Browngardt C, Xiaohui X, et al. The effect of arginine on oral biofilm communities. Mol Oral Microbiol 2014;29(1):45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento MM, Burne RA. 2014. Caries prevention by arginine metabolism in oral biofilms: translating science into clinical success. Curr Oral Health Rep. 1(1):79–85. [Google Scholar]
- Nascimento MM, Gordan VV, Garvan CW, Browngardt CM, Burne RA. 2009. Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol Immunol. 24(2):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento MM, Liu Y, Kalra R, Perry S, Adewumi A, Xu X, Primosch RE, Burne RA. 2013. Oral arginine metabolism may decrease the risk for dental caries in children. J Dent Res. 92(7):604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento MM, Zaura E, Mira A, Takahashi N, Ten Cate JM. 2017. Second era of OMICs in caries research: moving past the phase of disillusionment. J Dent Res. 96(7):733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parwani SR, Parwani RN. 2015. Nitric oxide and inflammatory periodontal disease. Gen Dent. 63(2):34–40. [PubMed] [Google Scholar]
- Peterson S, Woodhead J, Crall J. 1985. Caries resistance in children with chronic renal failure: plaque pH, salivary pH, and salivary composition. Pediatr Res. 19(8):796–799. [DOI] [PubMed] [Google Scholar]
- Richards VP, Alvarez AA, Luce AR, Perry S, Burne RA, Nascimento MM. 2017. The microbiome of site-specific dental plaque of children with different caries status. Infect Immun. 85(8). pii: e00106-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberfroid M. 2007. Prebiotics: the concept revisited. J Nutr. 137(3 Suppl 2):830S–837S. [DOI] [PubMed] [Google Scholar]
- Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, et al. 2010. Prebiotic effects: metabolic and health benefits. Br J Nutr. 104(Suppl 2):S1–63. [DOI] [PubMed] [Google Scholar]
- Sissons CH, Cutress TW. 1988. pH changes during simultaneous metabolism of urea and carbohydrate by human salivary bacteria in vitro. Arch Oral Biol. 33(8):579–587. [DOI] [PubMed] [Google Scholar]
- Tanner AC, Mathney JM, Kent RL, Chalmers NI, Hughes CV, Loo CY, Pradhan N, Kanasi E, Hwang J, Dahlan MA, et al. 2011. Cultivable anaerobic microbiota of severe early childhood caries. J Clin Microbiol. 49(4):1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. 2007. The human microbiome project. Nature. 449(7164):804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtola LO, Luoma H. 1972. Plaque pH in caries-active and inactive subjects modified by sucrose and fluoride, with and without bicarbonate-phosphate. Scand J Dent Res. 80(4):334–343. [DOI] [PubMed] [Google Scholar]
- van Houte J, Lopman J, Kent R. 1994. The predominant cultivable flora of sound and carious human root surfaces. J Dent Res. 73(11):1727–1734. [DOI] [PubMed] [Google Scholar]
- van Ruyven FO, Lingstrom P, van Houte J, Kent R. 2000. Relationship among mutans streptococci, “low-ph” bacteria, and lodophilic polysaccharide-producing bacteria in dental plaque and early enamel caries in humans. J Dent Res. 79(2):778–784. [DOI] [PubMed] [Google Scholar]
- van Wuyckhuyse BC, Perinpanayagam HE, Bevacqua D, Raubertas RF, Billings RJ, Bowen WH, Tabak LA. 1995. Association of free arginine and lysine concentrations in human parotid saliva with caries experience. J Dent Res. 74(2):686–690. [DOI] [PubMed] [Google Scholar]


