Abstract
A variety of tissue engineering techniques utilizing different cells and biomaterials are currently being explored to construct urinary bladder walls de novo, but so far no approach is clearly superior. The aim of this study was to determine whether mesenchymal stem cells (MSCs) isolated from different sources, (bone marrow [BM-MSCs] and adipose tissue [ADSCs]), differ in their potential to regenerate smooth muscles in tissue-engineered urinary bladders and to determine an optimal number of MSCs for urinary bladder smooth muscle regeneration. Forty-eight rats underwent hemicystectomy and bladder augmentation with approximately 0.8 cm2 graft. In the first and second groups, urinary bladders were reconstructed with small intestinal submucosa (SIS) seeded with 10 × 106 or 4 × 106 ADSCs/cm2, respectively. In the third and fourth groups, urinary bladders were augmented with SIS seeded with 10 × 106 or 4 × 106 BM-MSCs/cm2, respectively. In the fifth group, urinary bladders were augmented with SIS without cells. The sixth group (control) was left intact. Smooth muscle regeneration was evaluated by real-time polymerase chain reaction (RT-PCR) and histological examinations. Histologically, there were no significant differences between urinary bladders augmented with ADSCs and BM-MSCs, but there was a marked increase in smooth muscle formation in bladders augmented with grafts seeded with MSCs in higher density (10 × 106/cm2) compared to lower density (4 × 106/cm2). Molecular analysis revealed that bladders reconstructed with ADSC-seeded grafts expressed higher levels of smooth muscle myosin heavy chain, caldesmon, and vinculin. Bladders augmented with unseeded SIS were fibrotic and devoid of smooth muscles. ADSCs and BM-MSCs have comparable smooth muscle regenerative potential, but the number of MSCs used for graft preparation significantly affects the smooth muscle content in tissue-engineered urinary bladders.
Keywords: tissue engineering, urinary bladder, smooth muscle regeneration, bone
Introduction
Development of a new method of urinary diversion following radical cystectomy is one of the major challenges in modern urology. Construction of urinary bladders de novo using tissue engineering techniques is a very promising option that may become available in the near future.1–3 Two major approaches utilized by tissue engineering involve acellular and cellular scaffolds. Cell-based strategies allow for better smooth muscle regeneration in tissue-engineered bladders compared to acellular ones.4–8 The question is: what is the best source and number of cells for urinary bladder regeneration? An ideal source of cells should be easily accessible, abundant in cells, and available from adult patients. Furthermore, it should be free from metastatic cancer cells and safe for oncological patients. Autologous urinary bladder smooth muscle and urothelial cells are not the best option because most of the patients requiring construction of urinary bladders de novo are cancer patients. Therefore, other cell sources are required. Both bone marrow and adipose tissue are rich sources of mesenchymal stem cells (MSCs). MSCs are defined as plastic adherent when maintained in standard culture conditions, express CD29, CD44, CD90, CD49a-f, CD51, CD73 (SH3), CD105 (SH2), CD106, CD166, and Stro-1 and not expressing CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA-DR surface markers, and are able to differentiate into osteoblasts, adipocytes, and chondroblasts in vitro.9 MSCs secrete numerous cytokines (among them interleukins and growth factors) that play an important role in the regulation of hematopoiesis, angiogenesis, and immune responses.10 Moreover, MSCs can migrate and home to tissues and organs in response to injury to enhance regeneration.11 Therefore, MSCs are a very attractive choice for numerous clinical applications.
Detrusor regeneration is one of the most important factors determining proper function of reconstructed urinary bladders.12,13 Several in vitro and in vivo studies revealed that adipose-derived stem cells (ADSCs) and bone marrow-derived mesenchymal stem cells (BM-MSCs) are able to differentiate into urinary bladder smooth muscle cells (SMCs).5,14–17 Zhang et al. compared the potential of BM-MSC- and SMC-seeded small intestinal submucosa (SIS) in urinary bladder regeneration and found that both types of cells induce solid smooth muscle bundle formation throughout the graft.18 Sharma et al. indicated that BM-MSCs stimulate smooth muscle regeneration in tissue-engineered bladders 1.75 times stronger than SMCs. They confirmed that undifferentiated stem cells have higher regenerative potential than mature cells. A possible explanation for this phenomenon is that terminally differentiated SMCs lost their ability to replicate over time, while BM-MSCs continued proliferation within the graft.6 Up to now, there has not been a study comparing the efficiency of BM-MSCs and ADSCs in urinary bladder smooth muscle regeneration. The optimal cell number required for urinary bladder detrusor regeneration has not been established yet. The number of MSCs used for graft preparation for urinary bladder reconstruction varies between studies. Different MSC numbers were used to prepare 1 cm2 graft for rat urinary bladder reconstruction: 1.5 × 104(6), 1 × 105(19), 1 × 106(5,20,21), or 6 × 106(12).
In this study, we compared the potential of 2 MSC populations: BM-MSCs and ADSCs for urinary bladder smooth muscle regeneration. Additionally, we investigated the importance of the number of MSCs for detrusor regeneration.
Materials and Methods
This study was carried out in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.22 The protocol was approved by the Nicolaus Copernicus University Ethics Committee (no. 4/2012).
MSC Isolation and Culture
Twelve syngeneic (8 wk old, male) Wistar rats were euthanized with an overdose of ketamine (75 mg/kg; Biowet, Poland). Adipose tissue was harvested from the retroperitoneal space and processed according to the method described previously by Safford et al.23 For this purpose, adipose tissue was cut into small pieces and digested in collagenase type I solution (1 mg/mL; Sigma, Germany) for 30 min at 37 °C. Enzyme digestion was stopped by addition of Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 (PAA, Austria) supplemented with 10% fetal bovine serum (FBS Gold; PAA) and antibiotics: amphotericin B (5 μg/mL; PAA) and penicillin/streptomycin (100 U/100 μg/mL; PAA). Next the cell suspension was filtered through a 100-μm filter (Becton Dickinson, USA) and centrifuged at 350g for 5 min. The cells were counted using the trypan blue exclusion test and seeded in a 25-cm2 cell culture flask at a density of 15 × 103 cells/cm2. BM-MSCs were isolated from femur and tibial bones according to the method described previously by Lennon and Caplan.24 Briefly, the epiphyses were cut and the bone marrow was flushed out with DMEM/Ham’s F-12 supplemented with 10% FBS and antibiotics. The cell suspension was centrifuged at 350g for 5 min. The cells (flushed from 1 femur and 1 tibia) were seeded in a 25-cm2 cell culture flask.
ADSCs and BM-MSCs were cultured in a medium consisting of DMEM/Ham’s F-12 supplemented with 10% FBS, fibroblast growth factor (FGF) (10 ng/mL; Sigma-Aldrich, Germany), penicillin/streptomycin (100 U/100 μg/mL), and amphotericin B (5 μg/mL) (PAA) at 37 °C, 5% CO2 in air, and 95% humidity, until the third passage.
Phenotypic Analysis of MSCs by Flow Cytometry
ADSCs and BM-MSCs at the third passage were detached with trypsin/ethylenediaminetetraacetic acid (EDTA) solution (0.05%/ 0.5 mM), counted using the trypan blue exclusion test, washed, and resuspended with phosphate-buffered saline (PBS). Approximately 0.5 × 106 cells were incubated with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies against CD11b, CD29, CD31, CD34, CD44, CD45, and CD90 (BD, USA; Santa Cruz Biotechnology, USA) for 30 min. FITC- or PE-conjugated IgG1, IgG2A, IgM, and IgA (BD) was used as an isotype control. Data were analyzed by collecting 3 × 104 events on a FACSCanto (BD Biosciences, USA).
Analysis of MSC Multipotency
To verify multipotency of ADSCs and BM-MSCs, the cells were differentiated in vitro into adipogenic, osteogenic, and chondrogenic lineages. The differentiation was induced by culture in appropriate differentiation media, according to the manufacturer’s instructions (Invitrogen, USA). Adipogenesis was measured by the accumulation of neutral lipids in fat vacuoles and stained with Oil Red O (Sigma-Aldrich). Osteogenesis was confirmed using Alizarin Red staining (Millipore, USA). Chondrogenic differentiation was evaluated by anticollagen type II immunocytochemical staining (anticollagen type II clone 6B3, dilution 1:100; Millipore and EnVision+/HRP antimouse; Dako, Denmark). Stained samples were analyzed using light microscopy by 2 independent pathologists.
PKH-26 Labeling of MSCs
ADSCs and BM-MSCs from the third passage were labeled with PKH-26 fluorescent tracking dye according to the manufacturer’s instructions (Sigma-Aldrich). Cell labeling was confirmed under a fluorescence microscope (Nikon, Japan).
Graft Preparation
PKH-26-labeled ADSCs or BM-MSCs were seeded on 0.8 cm2 of SIS (Surgisis; Biodesign, USA) mounted on cell insert (Scaffdex, Finland) in low (4 × 106 cells/cm2) or high (10 × 106 cells/cm2) density and cultured for 7 d. Growth of ADSCs and BM-MSCs on SIS was assessed by scanning electron microscopy. For this purpose, the specimens were fixed in 2% paraformaldehyde (PFA) and 2.5% glutaraldehyde in phosphate buffer for 2 h, postfixed in 1% OsO4, and dehydrated with graded series of ethyl alcohol followed by acetone. Next, the specimens were critically dried and coated with gold particles before observation using a scanning electron microscope (JEOL JSM-6390LV, Japan).
Urinary Bladder Augmentation
Forty-eight syngeneic male Wistar rats weighing between 250 and 300 g were randomly divided into 6 equal groups. Under general anesthesia with sodium pentobarbital (15 mg/kg intraperitoneally; Biowet) and lidocaine (20 mg/kg intramuscularly; Polfa, Poland), 40 rats underwent hemicystectomy and bladder augmentation with an approximately 1 cm2 of graft. In the first and second groups, urinary bladders were reconstructed with SIS seeded with 10 × 106 ADSCs or 4 × 106, respectively. In the third and fourth groups, urinary bladders were augmented with SIS seeded with 10 × 106 or 4 × 106 BM-MSCs, respectively. In the fifth group, urinary bladders were augmented with SIS without cells. The sixth group (control) was left intact. The anastomosis line was marked by 8.0 monofilament nonabsorbable marker sutures to identify the graft borders. All rats were euthanized with an overdose of ketamine (75 mg/kg) after 3-mo follow-up.
Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR)
The reconstructed walls of urinary bladders (marked by nonabsorbable marker sutures) were isolated, frozen immediately after harvesting, and stored at −80 °C until further analysis. The samples were homogenized on the MagNA Lyser instrument using the MagNA Lyser green beads (Roche Diagnostics GmbH, Germany). Total RNA was extracted from the homogenized samples using a High Pure RNA Tissue Kit (Roche Diagnostics GmbH). The RNA concentration was determined from absorbance at 260 nm on the Nanodrop (Thermo Scientific, USA). The integrity of the RNA was analyzed using the RNA 6000 Nano Kit on Agilent 2100 Bioanalyzer (Agilent, USA). Complementary DNA (cDNA) was synthesized from 100 ng of total RNA using the Transcriptor High Fidelity cDNA Synthesis Kit (Roche Diagnostics GmbH). Gene expression related to urinary bladder smooth muscle regeneration was determined by a real-time quantitative RT-PCR using sequence-specific probes. Ten replicates (5 biological replicates × 2 technical replicates) for each experimental group were performed. The primer and probe sequences (Table 1) were designed using publicly available rat gene sequences (NCBI) via the Roche Universal Probe Library design software (ProbeFinder, v.2.45). LightCycler 480 Probe Master Mix (Roche Diagnostics GmbH) was used for RT-PCR in accordance with the manufacturer’s protocol. The LightCycler 480 cycling parameters were 95 °C denaturation for 10 min followed by 50 cycles of 95 °C for 10 s, 60 °C for 30 s (with a single fluorescence acquisition), and 72 °C for 1 s followed by a 40 °C cooling period for 30 s. The Roche LightCycler 480 software was used to perform quantification analysis of gene expression using the advanced relative standard curve second derivative maximum analysis method, a nonlinear regression line method.
Table 1.
Primers and Probes for Quantitative Real-Time PCR.
| Gene | Primer Sequence 5′-3′ | Probea | Amplicon Length (nt) | Efficiency | |
|---|---|---|---|---|---|
| Forward | Reverse | ||||
| Caldesmon 1 | gctggagcaatataccaatgc | tgatattgcggacaccttcc | 89 | 104 | 1.98 |
| Calponin 1 | ggcctgtctgctgaggtaaa | accccttcgatccactctc | 128 | 86 | 1.94 |
| Desmin | agaggctcaaggccaagc | caattctgcgctctaggtca | 106 | 126 | 1.97 |
| Smoothelin | ccacagagccctctgatacc | acagacagggagcgttgg | 22 | 100 | 2.00 |
| Smooth muscle myosin heavy chain 11 | gcacaagaagaagaagctgga | ttgagcatgcctgtgacact | 128 | 138 | 2.12 |
| Transgelin 1 | ggtggctcaattcttgaagg | ctctgcactgctgccatatc | 73 | 105 | 1.96 |
| Vinculin | caaagcagagtattgcgaagaa | catcacataactcagcaatcttcc | 41 | 132 | 2.05 |
| Vimentin | cgagaaaaattgcaggagga | acgtgccagagaagcattgt | 69 | 96 | 1.94 |
| Hypoxanthine | ggtccattcctatgactgtagatttt | caatcaagacgttctttccagtt | 22 | 126 | 1.95 |
| Actin ß | cccgcgagtacaaccttct | cgtcatccatggcgaact | 17 | 72 | 1.98 |
Abbreviation: PCR, polymerase chain reaction.
aProbe number, Universal Probe Library, Roche Diagnostics.
In Vivo Tracking of Implanted Stem Cells
The excised urinary bladders were frozen on dry ice. Eight-micrometer-thick frozen tissue sections were prepared. The sections were stained with 4′,6-diamidino-2′-phenylindole (DAPI) dihydrochloride (Sigma-Aldrich), mounted and observed under a confocal laser scanning microscope (Nikon, Japan). The percentage of cells expressing PKH-26 in tissue-engineered bladder wall was assessed using ImageJ software.
Histological and Immunohistochemical Staining
Urinary bladders were fixed in 10% neutral-buffered formalin, dehydrated through a graded ethanol series, and embedded in paraffin. Embedded tissues were sectioned at a thickness of 8 µm. The tissue samples were processed routinely for standard staining with hematoxylin and eosin (H&E). To confirm the smooth muscle regeneration, an immunohistochemical staining with anti-smooth muscle α-myosin heavy chain (α-SMM) antibody was used. Briefly, the tissue sections were incubated with primary antibody against α-SMM (dilution 1:400; Abcam, Great Britain). After washing, the sections were overlaid with peroxidase-conjugated antimouse secondary antibody (EnVision+/HRP antimouse; Dako). Twelve tissue sections for each experimental group (3 urinary bladders × 4 tissue section each) were examined. Stained samples were analyzed using light microscopy by 2 independent pathologists. Additionally, the samples were evaluated for smooth muscle content using the ImageJ software according to the method described previously.7 Analysis was repeated for 5 areas from each specimen.
Statistical Analysis
The statistical differences between the groups were calculated by analysis of variance (ANOVA) followed by NIR or Tamhane post hoc multiple comparison tests (IBM SPSS Statistics; Predictive Solutions, Poland). Statistically significant differences were defined as having P < 0.05.
Results
MSC Characterization
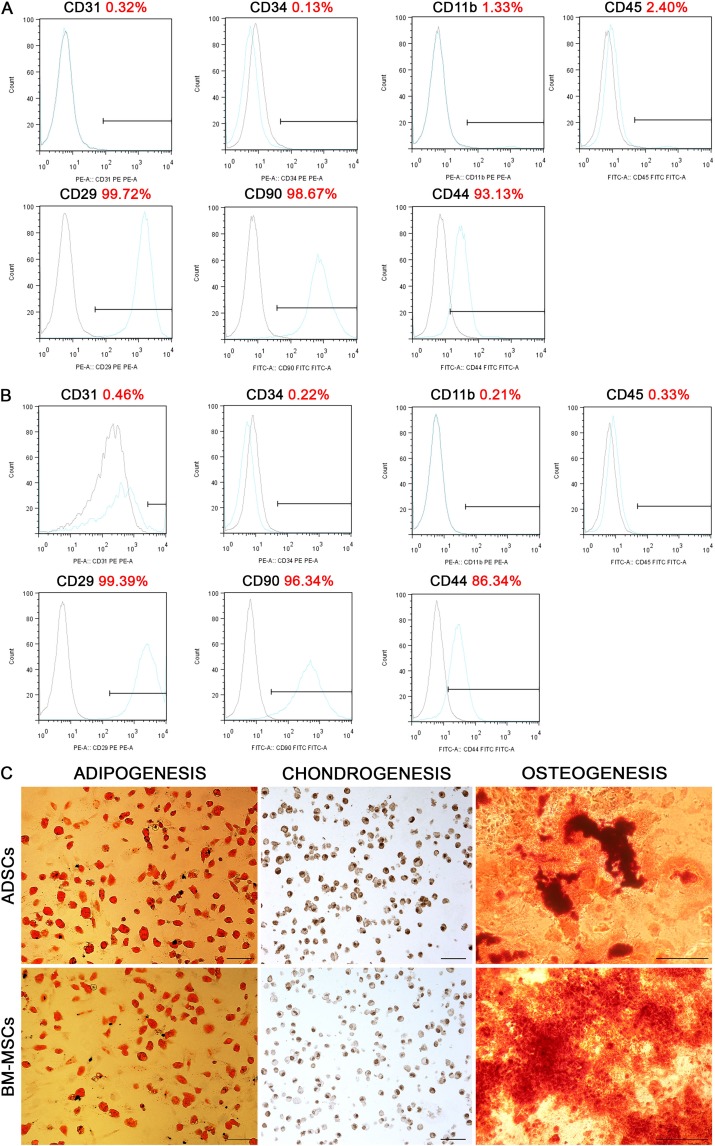
Isolated BM-MSCs and ADSCs exhibited typical fibroblast-like morphology. Flow cytometry confirmed their mesenchymal phenotype. BM-MSCs were positive for CD29 (99.83 ± 0.09), CD44 (90.63 ± 2.18), and CD90 (99.35 ± 0.59) markers and negative for typical endothelial and hematopoietic markers CD11b (0.74 ± 0.51), CD31 (0.20 ± 0.10), CD34 (0.12 ± 0.01), and CD45 (1.03 ± 1.20; Fig. 1A). Similarly, ADSCs were positive for CD29 (98.01 ± 1.53), CD44 (92.82 ± 5.62), CD90 (95.89 ± 1.44) markers and negative for CD11b (0.33 ± 0.23), CD31 (0.38 ± 0.20), CD34 (0.27 ± 0.06), and CD45 (0.27 ± 0.07) markers (Fig. 1B). They were also able to differentiate into adipocytes, osteoblasts, and chondrocytes after cultivation in respective media (Fig. 1C).
Fig. 1.
Flow cytometry analysis for the expression of cell surface antigens: CD11b, CD29, CD31, CD34, CD44, CD45, and CD90 in bone marrow-derived mesenchymal stem cells (BM-MSCs; A) and adipose-derived stem cells (ADSCs; B). The black histograms show staining with isotype controls, and the blue histograms represent staining with the specified surface marker antibody. Results from one representative BM-MSCs and ADSCs culture are shown. Differentiation potential of BM-MSCs and ADSCs (C): a positive Oil Red O staining after adipogenic induction, anticollagen type II staining after chondrogenic induction, Alizarin red staining after osteogenic induction. Scale bar 100 µm, 500 µm.
Analysis of MSC Growth on SIS
MSCs seeded on SIS at low density (4 × 106 cells/cm2) have normal morphology, but only single cells have flattened shape and elongated cellular processes (Fig. 2A, B). MSCs seeded on SIS at high density (10 × 106 cells/cm2) formed a dense layer that adhered well to the SIS surface (Fig. 2C, D).
Fig. 2.
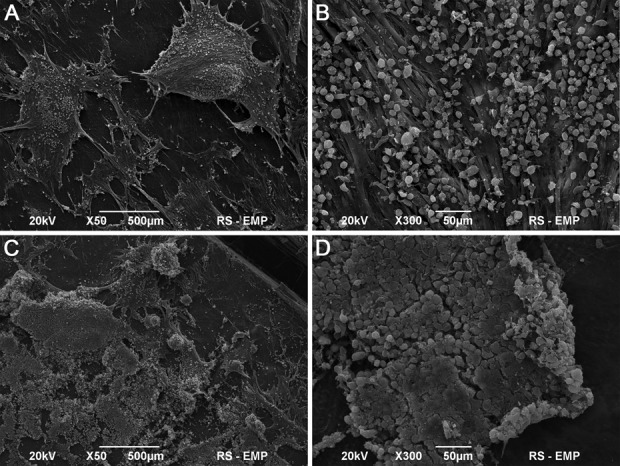
Small intestinal submucosa (SIS) seeded with mesenchymal stem cells (MSCs) in low (A, B) and high density (C, D). Scanning electron microscopy, Scale bar 500 μm, 50 μm.
Macroscopic Analysis of Reconstructed Urinary Bladders
All animals survived the 3-mo follow-up. Marked graft shrinkage was observed in urinary bladders augmented with unseeded SIS (fifth group; Fig. 3E). BM-MSCs and ADSCs decreased or even prevented graft shrinkage (first to fourth groups; Fig. 3A-D).
Fig. 3.
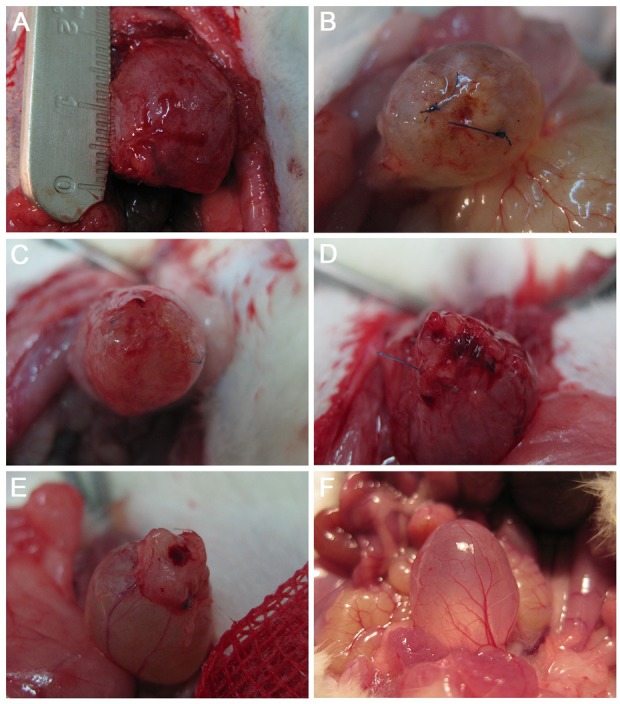
Macroscopic analysis of reconstructed urinary bladder wall 3 mo postoperatively. Urinary bladders augmented with small intestinal submucosa (SIS) seeded with 10 × 106 adipose-derived stem cells (ADSCs; first group; A), 4 × 106 ADSCs (second group; B), 10 × 106 bone marrow-derived mesenchymal stem cells (BM-MSCs; third group; C), 4 × 106 BM-MSCs (fourth group; D), SIS without cells (fifth group; E), and control (F).
Tracking of Implanted Stem Cells
Immunofluorescent imaging revealed the presence of implanted BM-MSCs and ADSCs in augmented bladders 3 mo postoperatively (Fig. 4). In bladders reconstructed with SIS seeded with MSCs at low density, only single PKH-26-labeled cells (10%-13%) were visible (Fig. 4A, B). Whereas when the bladders were grafted with SIS seeded with MSCs at high density, a plurality of PKH-26-labeled cells (33%-37%) were found in reconstructed area (Fig. 4C, D). There were no significant differences between the type of cells used (BM-MSCs vs. ADSCs) and the number of tracked cells in augmented bladders 3 mo after the implantation (first vs. third and second vs. fourth P > 0.05).
Fig. 4.
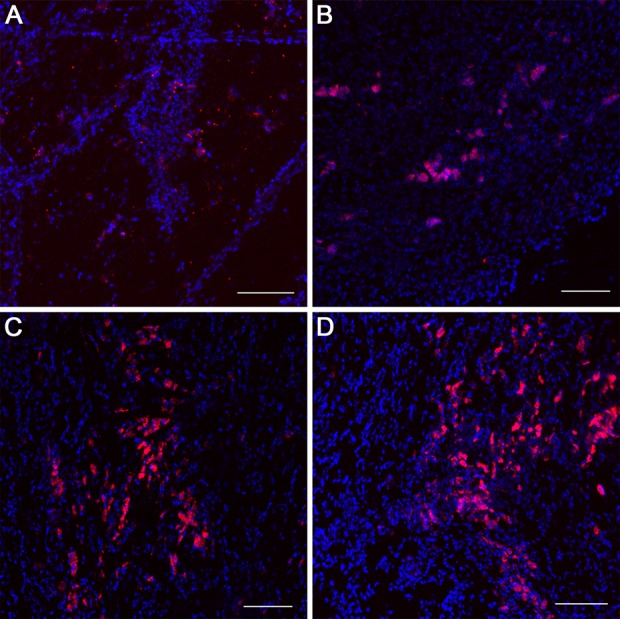
PKH-26-labeled bone marrow-derived mesenchymal stem cells (BM-MSCs) and adipose-derived stem cells (ADSCs) tracking in reconstructed urinary bladders 3 mo postoperatively. Urinary bladders augmented with small intestinal submucosa (SIS) seeded with 4 × 106 ADSCs (second group; A), 4 × 106 BM-MSCs (fourth group; B), 10 × 106 ADSCs (first group; C), and 10 × 106 BM-MSCs (third group). Confocal laser scanning microscope, Scale bar 200 µm.
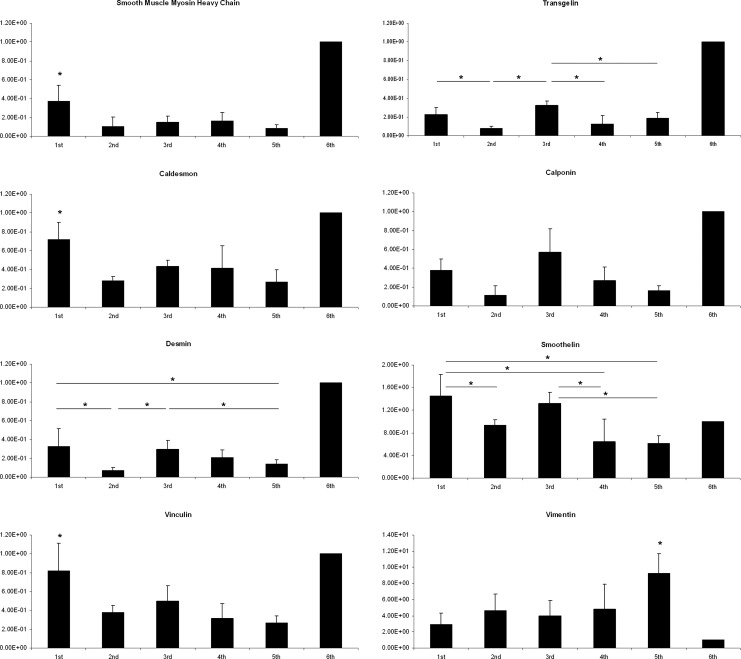
Molecular Analysis of Smooth Muscle Regeneration
The expression of smooth muscle myosin, caldesmon, and vinculin was significantly higher in urinary bladders augmented with SIS seeded with 10 × 106 ADSCs compared to SIS seeded with 10 × 106 BM-MSCs (P < 0.05), 4 × 106 ADSCs (P < 0.01), and 4 × 106 BM-MSCs (P < 0.05) and unseeded SIS (P < 0.01; Fig. 5). The source of MSCs had no effect on the expression of desmin, transgelin, and smoothelin in tissue-engineered urinary bladders. However, expression of these markers differed significantly between high and low cell density groups (P < 0.05). Interestingly, there were no statistically significant differences in the expression of smooth muscle markers: myosin, caldesmon, desmin, vinculin, transgelin, calponin, and smoothelin between urinary bladders augmented with unseeded SIS and SIS seeded with 4 × 106 ADSCs or 4 × 106 BM-MSCs (P > 0.05). Calponin expression did not differ significantly among the groups (P > 0.05); however, there was a trend toward increased calponin expression in high cell density groups that did not reach statistical significance. Vimentin expression was the highest in bladders augmented with unseeded SIS and the lowest in bladders reconstructed with SIS seeded with 10 × 106 ADSCs (Fig. 5).
Fig. 5.
The relative smooth muscle markers expression level in tissue-engineered (first to fifth groups) and control urinary bladders (sixth group). Stars indicate statistically significant differences between groups (P < 0.05).
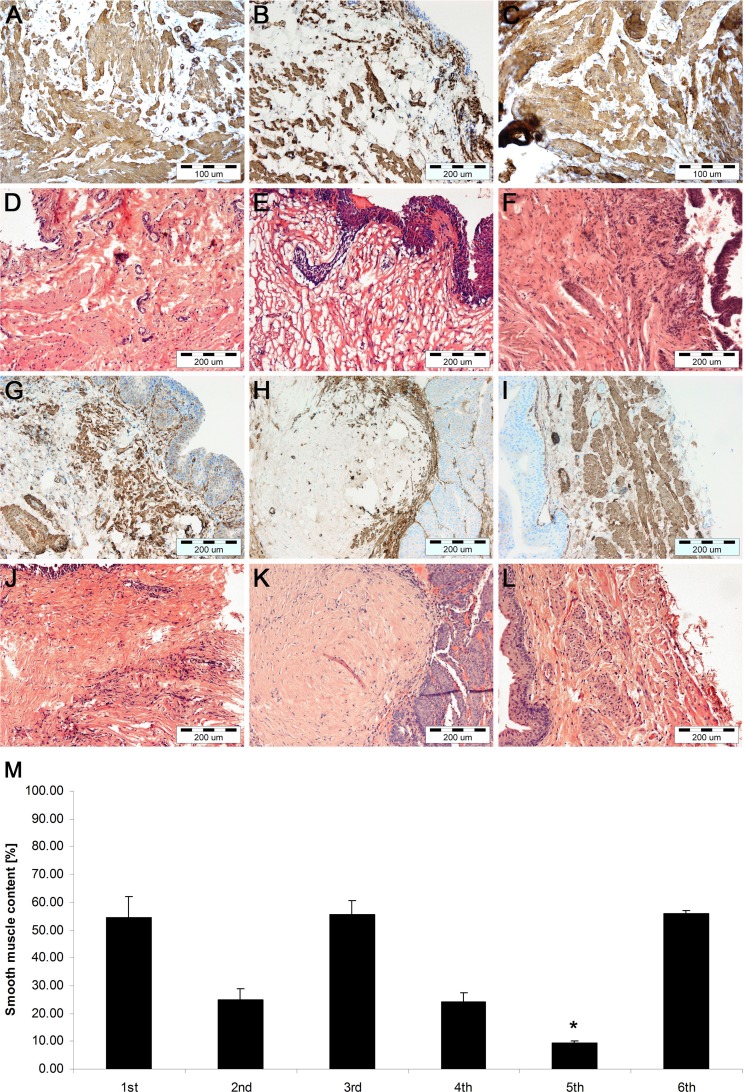
Histological and Immunohistochemical Examination
Histological and immunohistochemical staining revealed the bladder wall consisting of urothelium and smooth muscle in both ADSC (first and second) and BM-MSC (third and fourth) groups (Fig. 6A–G, J). Bladders augmented with unseeded SIS (fifth group) exhibited a thin layer of urothelium, fibrotic tissue, and generally lack of muscle (Fig. 6H, K). There were no significant differences between urinary bladders augmented with ADSCs and BM-MSCs. However, there was a marked increase in smooth muscle formation in bladders augmented with grafts seeded with MSCs at a higher density (10 × 106/cm2) compared to a lower density (4 × 106/cm2). In bladders grafted with SIS seeded with 4 × 106 cells/cm2, usually small and irregularly distributed islands of muscle fibers were observed (Fig. 6B, E, G, J), while in bladders grafted with SIS seeded with 10 × 106 cells/cm2, robust smooth muscle bundles were formed (Fig. 6A, C, D, F).
Fig. 6.
Histological and immunohistochemical analysis of smooth muscle regeneration in urinary bladders augmented with small intestinal submucosa (SIS) seeded with 10 × 106 adipose-derived stem cells (ADSCs; first group; A, D), 4 × 106 ADSCs (second group; B, E), 10 × 106 bone marrow-derived mesenchymal stem cells (BM-MSCs; third group; C, F), 4 × 106 BM-MSCs (fourth group; G, J), SIS without cells (fifth group; H, K), and control (sixth group; I, L). Immunohistochemical staining of smooth muscle α-myosin heavy chain and hematoxylin and eosin (H&E) staining, light microscope, Scale bar 200 µm. Quantification of smooth muscle content in anti-smooth muscle myosin staining of reconstructed and control urinary bladders (M). Urinary bladders augmented with cell seeded with SIS showed significantly more smooth muscle content compared to urinary bladders augmented with SIS alone. *P < 0.05. Smooth muscle content did not differ significantly between the ADSCs and BM-MSCs groups (first vs. third and second vs. fourth, P > 0.05) but differed significantly between high (10 × 106) and low (4 × 106) cell groups (first vs. second and third vs. fourth, P < 0.05).
The quantitative morphometric analysis confirmed that smooth muscle content in bladders augmented with SIS seeded with 10 × 106 of ADSCs or BM-MSCs was comparable to the native bladder wall control (54.6 ± 7.6, 55.6 ± 4.9, and 55.9 ± 1.3, respectively, P > 0.05). Whereas, there were statistically significant differences in smooth muscle content between bladders reconstructed with SIS seeded with 4 × 106 of ADSCs or BM-MSCs and control as well as between unseeded SIS and control (P < 0.01; Fig. 6M).
Discussion
BM-MSCs and ADSCs display a similar morphology and surface marker profile as well as high potential for differentiation into the mesodermal lineages, which was confirmed in this study. However, it was also reported that these 2 MSC populations can differ in their therapeutic potential.25–27 In this study, we analyzed the ability of MSCs isolated from bone marrow and adipose tissue to regenerate the smooth muscles in tissue-engineered urinary bladders. Previously, it was demonstrated that both BM-MSCs and ADSCs stimulate detrusor regeneration.6–8,15,18–21,28 However, up to now, there were no studies comparing these 2 types of MSCs.
Our study confirmed that only cell-seeded grafts are able to regenerate smooth muscles in reconstructed urinary bladders. Urinary bladders augmented with unseeded grafts exhibited highly fibrotic tissue that was devoid of muscles. These observations are consistent with our previous outcomes and results of other authors who have also demonstrated that the use of unseeded grafts do not allow for proper urinary bladder regeneration.4–8 Some preclinical studies indicated that SIS-reconstructed bladders are histologically and functionally indistinguishable from the native functional bladder tissues.29–31 We and others showed that smooth muscle regeneration in bladders reconstructed with unseeded grafts is observed only in the border of the graft and native bladder tissue.15,32,33 Also, a clinical study on urinary bladder augmentation with unseeded SIS in 5 patients with bladder exstrophy clearly showed that unseeded SIS did not significantly improve bladder compliance and capacity and failed to provide long-term effective results. Histologically, the SIS-reconstructed human bladders had poor smooth muscle regeneration.34 The basis of these different findings remains unknown. A possible explanation could be the differences in animal models, graft sizes, or observation times. Histological analysis showed that urothelium in tissue-engineered bladders regenerated completely independently on the number or source of MSCs. These results are consistent with the previous observations of numerous authors which showed that urothelium regenerates spontaneously independently on the biomaterial used, cell presence, or graft area.3
The question is: how do MSCs enhance smooth muscle regeneration in tissue-engineered urinary bladders. The exact molecular mechanism of smooth muscle regeneration in tissue-engineered urinary bladders remains unknown. There are at least 2 possible mechanisms. Native SMCs from the remaining bladder wall stimulated by trophic factors secreted by MSCs migrate and cellularize the graft. SMCs arise from implanted MSCs, which differentiate into SMCs in the microenvironment of urinary bladders. In another study performed by our group on regeneration of porcine urinary bladders with ADSC-seeded BAM, we found that the number of ADSC-derived SMCs in urinary bladders in 3 mo following the reconstruction was very low. These results indicated that the predominant mechanism of smooth muscle regeneration is a trophic effect, not differentiation of implanted stem cells.35 Cells provide an antifibrotic effect by decreasing the biomaterial surface area for host fibroblasts and by supplying the cellular basis for the smooth muscle regeneration. Undifferentiated stem cells can trigger urinary bladder regeneration by secretion of trophic factors or anti-inflammatory cytokines. It was found that BM-MSCs modulate the immunologic milieu of the reconstructed bladder wall. BM-MSCs upregulate the expression of anti-inflammatory cytokines in reconstructed urinary bladders, which can prevent fibrosis and strengthen the regeneration process.7,36 The antifibrotic effect of both BM-MSCs and ADSCs in urinary bladder regeneration is clearly visible when we analyze the expression of the fibroblast marker, vimentin. In urinary bladders augmented with unseeded SIS, the expression of vimentin was ∼9 times higher than in the native bladder wall, in contrast to urinary bladders augmented with SIS seeded with 10 × 106 ADSCs where expression of vimentin was only ∼3 times higher than in the native one. Immunohistochemical anti-smooth muscle myosin staining indicated that both BM-MSCs and ADSCs had comparable potential for regeneration of urinary bladder detrusor and that smooth muscle content in tissue-engineered urinary bladder was highly dependent on the number of MSCs. In our previous studies, we found that 1 × 106 BM-MSCs per 1 cm2 of graft is not enough to fully regenerate the smooth muscle layer of reconstructed bladders.7 This finding encouraged us to use a higher number of cells for graft preparation. Ten million of cells per 1 cm2 of graft is a large number. However, when we prepare the graft for urinary bladder reconstruction, we have to take into account that a significant number of implanted cells die under the influence of toxic urine.37 In this study, we found that MSCs implanted in such a high number 3 mo postoperatively constituted only ∼30% of the total number of cells within the reconstructed bladder wall. This finding confirmed that a large number of cells died following the implantation. Bladders augmented with SIS seeded with 4 × 106 of ADSCs or BM-MSCs had significantly lower smooth muscle content compared to the control. However, when 10 × 106 of ADSCs or BM-MSCs were used, the smooth muscle abundance resembled the native level. There was also a significant difference in the organization of regenerated muscle fibers. In bladders augmented with SIS seeded with 4 × 106 of ADSCs or BM-MSCs, SMCs formed small bundles or single fibers irregularly distributed within the bladder wall. In bladders reconstructed with SIS seeded with 10 × 106 of ADSCs or BM-MSCs, SMCs formed massive smooth muscle bundles. However, there was no typical urinary bladder organization of smooth muscle layers: inner longitudinal, middle circular, and outer longitudinal. We hypothesize that this arrangement would change over a longer period of observation or could be directed by a scaffold.
Primary cultured MSCs were demonstrated to express messenger RNA (mRNA) for most SMC markers with the exception of smooth muscle myosin heavy chain (SM MHC).38–40 Therefore, it should also be considered that enhanced expression of SMC markers in MSC-seeded SIS groups could reflect the presence of MSCs, not smooth muscle regeneration. However, the expression of SM MHC, which is a selective marker for SMCs at both the mRNA and protein level, proved smooth muscle regeneration in tissue-engineered bladders. A large number of MSCs die immediately after the implantation under cytotoxic influence of urine37; therefore, the expression of analyzed markers arises from SMCs migrating from the native, remaining bladder wall. It is also unlikely that the phenotype of MSCs, which survived in the environment of the urinary bladder wall in 3 mo following the implantation, remained unchanged. Therefore, another option is that the expression of analyzed markers arises from ADSC-derived SMCs.
Interestingly, the RT-PCR gene expression analysis revealed that ADSCs have higher smooth muscle regenerative potential than BM-MSCs and that there is no significant difference in the expression of smooth muscle markers between bladders augmented with unseeded and 4 × 106 ADSC- or BM-MSC-seeded SIS. The expression of SM MHC, caldesmon, and vinculin was significantly higher in urinary bladders augmented with SIS seeded with 10 × 106 ADSCs compared to other groups but still lower compared to the native urinary bladder wall (∼60%, 30%, and 20%, respectively). Only expression of smoothelin in urinary bladders augmented with SIS seeded with 10 × 106 ADSCs, 4 × 106 ADSCs, and 10 × 106 BM-MSCs was comparable to the native urinary bladder wall. These results demonstrated that histologically proven regrowth of smooth muscle bundles is only halfway to regenerate the native bladder wall. Decreased expression of smooth muscle markers may be the result of the ongoing tissue remodeling and improper arrangement of smooth muscle fibers in reconstructed bladders.
Another question concerns functional recovery of reconstructed bladders. Previously, we found that histologically proven smooth muscle regeneration in tissue-engineered bladder guarantees contraction but not proper function.12 Chaotic smooth muscle arrangement observed in SIS-reconstructed bladders independently on the number or source of MSCs used will not assure the ability to contract and relax in repetitive cycles that provide proper micturition. Therefore, these issues should be addressed in future research.
Enhanced expression of several smooth muscle markers in bladders reconstructed with ADSCs is not enough to conclude that they have higher smooth muscle regenerative potential compared to BM-MSCs. However, compared to bone marrow, adipose tissue is more readily available, less invasive to harvest, and richer in MSCs. These characteristics together with comparable smooth muscle regenerative potential make ADSCs a better option for urinary bladder regeneration compared to BM-MSCs.
Conclusions
Taken together, these results indicated that smooth muscle regeneration in tissue-engineered urinary bladders is highly dependent on the number of MSCs used for the reconstruction. ADSCs and BM-MSCs have comparable potential to regenerate smooth muscle in tissue-engineered bladders.
Footnotes
Author Contributions: M.P. contributed to experimental design and performance of research, data analysis, and manuscript writing. A.J., K.W., M.R., L.B., J.A., M.B., A.H-B., M.G., M.F-B., A.M.G., T.K., and M.N. contributed to performance of research. A.M., J.M., A.G. contributed to data analysis. C.R. and T.D. critically revised the manuscript for important intellectual property.
Ethical Approval: This study was carried out in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Statement of Human and Animal Rights: Wistar rats were euthanized with an overdose of ketamine.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–1246. [DOI] [PubMed] [Google Scholar]
- 2. Pokrywczynska M, Jundziłł A, Adamowicz J, Drewa T. Tissue engineering-experimental method of urinary bladder regeneration. Postepy Hig Med Dosw. 2013;67:790–799. [DOI] [PubMed] [Google Scholar]
- 3. Pokrywczynska M, Adamowicz J, Sharma A, Drewa T. Human urinary bladder regeneration through tissue engineering-an analysis of 131 clinical cases. Exp Biol Med. 2014;239:264–271. [DOI] [PubMed] [Google Scholar]
- 4. Chung SY, Krivorov NP, Rausei V, Thomas L, Frantzen M, Landsittel D, Kang YM, Chon CH, Ng CS, Fuchs GJ. Bladder reconstitution with bone marrow derived stem cells seeded on small intestinal submucosa improves morphological and molecular composition. J Urol. 2005;174(1):353–359. [DOI] [PubMed] [Google Scholar]
- 5. Jack GS, Zhang R, Lee M, Xu Y, Wu BM, Rodríguez LV. Urinary bladder smooth muscle engineered from adipose stem cells and a three dimensional synthetic composite. Biomaterials. 2009;30(19):3259–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma AK, Hota PV, Matoka DJ, Fuller NJ, Jandali D, Thaker H, Ameer GA, Cheng EY. Urinary bladder smooth muscle regeneration utilizing bone marrow derived mesenchymal stem cell seeded elastomeric poly(1,8-octanediol-co-citrate) based thin films. Biomaterials. 2010;31(24):6207–6217. [DOI] [PubMed] [Google Scholar]
- 7. Pokrywczynska M, Jundzill A, Bodnar M, Adamowicz J, Tworkiewicz J, Szylberg L, Debski R, Marszalek A, Drewa T. Do mesenchymal stem cells modulate the milieu of reconstructed bladder wall? Arch Immunol Ther Exp. 2013;61(6):483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pokrywczynska M, Jundzill A, Adamowicz J, Kowalczyk T, Warda K, Rasmus M, Buchholz L, Krzyzanowska S, Nakielski P, Chmielewski T, et al. Is the poly (L-lactide-co-caprolactone) nanofibrous membrane suitable for urinary bladder regeneration? PLoS One. 2014;9(8):e105295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 10. Ribeiro A, Laranjeira P, Mendes S, Velada I, Leite C, Andrade P, Santos F, Henriques A, Grãos M, Cardoso CM, et al. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res Ther. 2013;4(5):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013;2013:130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adamowicz J, Juszczak K, Bajek A, Tworkiewicz J, Nowacki M, Marszalek A, Thor PJ, Chlosta P, Drewa T. Morphological and urodynamic evaluation of urinary bladder wall regeneration: muscles guarantee contraction but not proper function-a rat model research study. Transplant Proc. 2012;44(5):1429–1434. [DOI] [PubMed] [Google Scholar]
- 13. Adamowicz J, Kowalczyk T, Drewa T. Tissue engineering of urinary bladder– current state of art and future perspectives. Cent European J Urol. 2013;66(2):202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tian H, Bharadwaj S, Liu Y, Ma H, Ma PX, Atala A, Zhang Y. Myogenic differentiation of human bone marrow mesenchymal stem cells on a 3D nanofibrous scaffold for bladder tissue engineering. Biomaterials. 2010;31:870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma AK, Bury MI, Marks AJ, Fuller NJ, Meisner JW, Tapaskar N, Halliday LC, Matoka DJ, Cheng EY. A nonhuman primate model for urinary bladder regeneration using autologous sources of bone marrow-derived mesenchymal stem cells. Stem Cells. 2011;29(2):241–250. [DOI] [PubMed] [Google Scholar]
- 16. Su ZY, Li Y, Zhao XL, Zhang M. All-trans retinoic acid promotes smooth muscle cell differentiation of rabbit bone marrow-derived mesenchymal stem cells. J Zhejiang Univ Sci B. 2010;11(7):489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salem SA, Hwie AN, Saim A, Chee Kong CH, Sagap I, Singh R, Yusof MR, Md Zainuddin Z, Hj Idrus R. Human adipose tissue derived stem cells as a source of smooth muscle cells in the regeneration of muscular layer of urinary bladder wall. Malays J Med Sci. 2013;20(4):80–87. [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Lin HK, Frimberger D, Epstein RB, Kropp BP. Growth of bone marrow stromal cells on small intestinal submucosa: an alternative cell source for tissue engineered bladder. BJU Int. 2005;96(7):1120–1125. [DOI] [PubMed] [Google Scholar]
- 19. Kajbafzadeh AM, Tourchi A, Mousavian AA, Rouhi L, Tavangar SM, Sabetkish N. Bladder muscular wall regeneration with autologous adipose mesenchymal stem cells on three-dimensional collagen-based tissue-engineered prepuce and biocompatible nanofibrillar scaffold. J Pediatr Urol. 2014;10(6):1051–1058. [DOI] [PubMed] [Google Scholar]
- 20. Coutu DL, Mahfouz W, Loutochin O, Galipeau J, Corcos J. Tissue engineering of rat bladder using marrow-derived mesenchymal stem cells and bladder acellular matrix. PLoS One. 2014;9(12):e111966.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leite MT, Freitas-Filho LG, Oliveira AS, Semedo-Kuriki P, Laks M, Arias VE, Peixoto PS. The use of mesenchymal stem cells in bladder augmentation. Pediatr Surg Int. 2014;30(4):361–370. [DOI] [PubMed] [Google Scholar]
- 22. National Research Council. Guide for the care and use of laboratory animals. 8th ed Washington (DC: ): The National Academies Press; 2011. [Google Scholar]
- 23. Safford KM, Hicok KC, Safford SD. Neurogenic differentiation of murine and human adipose derived stromal cells. Biochem Biophys Res Commun. 2002;294(2):371–379. [DOI] [PubMed] [Google Scholar]
- 24. Lennon DP, Caplan AI. Isolation of rat marrow-derived mesenchymal stem cells. Exp Hematol. 2006;34(11):1606–1607. [DOI] [PubMed] [Google Scholar]
- 25. Kim Y, Kim H, Cho H, Bae Y, Suh K, Jung J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem. 2007;20(6):867–876. [DOI] [PubMed] [Google Scholar]
- 26. Elman JS, Li M, Wang F, Gimble JM, Parekkadan B. A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. J Inflamm (Lond). 2014;11(1):1 doi:10.1186/1476-9255-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rasmussen JG, Frøbert O, Holst-Hansen C, Kastrup J, Baandrup U, Zachar V, Fink T, Simonsen U. Comparison of human adipose-derived stem cells and bone marrow-derived stem cells in a myocardial infarction model. Cell Transplant. 2014;23(2):195–206. [DOI] [PubMed] [Google Scholar]
- 28. Zhu WD, Xu YM, Feng C, Fu Q, Song LJ, Cui L. Bladder reconstruction with adipose-derived stem cell-seeded bladder acellular matrix grafts improve morphology composition. World J Urol. 2010;28(4):493–498. [DOI] [PubMed] [Google Scholar]
- 29. Badylak SF, Kropp B, McPherson T, Liang H, Snyder PW. Small intestinal submucosa: a rapidly resorbed bioscaffold for augmentation cystoplasty in a dog model. Tissue Eng. 1998;4(4):379–387. [DOI] [PubMed] [Google Scholar]
- 30. Kropp BP, Eppley BL, Prevel CD, Rippy MK, Harruff RC, Badylak SF, Adams MC, Rink RC, Keating MA. Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology. 1995;46(3):396–400. [DOI] [PubMed] [Google Scholar]
- 31. Caione P, Capozza N, Zavaglia D, Palombaro G, Boldrini R. In vivo bladder regeneration using small intestinal submucosa: experimental study. Pediatr Surg Int. 2006;22(7):593–599. [DOI] [PubMed] [Google Scholar]
- 32. Landman J, Olweny E, Sundaram CP, Andreoni C, Collyer WC, Rehman J, Jerde TJ, Lin HK, Lee DI, Nunlist EH, et al. Laparoscopic mid sagittal hemicystectomy and bladder reconstruction with small intestinal submucosa and reimplantation of ureter into small intestinal submucosa: 1-year follow-up. J Urol. 2004;171(6 Pt 1):2450–2455. [DOI] [PubMed] [Google Scholar]
- 33. Zhang Y, Kropp BP, Lin HK, Cowan R, Cheng EY. Bladder regeneration with cell-seeded small intestinal submucosa. Tissue Eng. 2004;10(1–2):181–187. [DOI] [PubMed] [Google Scholar]
- 34. Caione P, Boldrini R, Salerno A, Nappo SG. Bladder augmentation using acellular collagen biomatrix: a pilot experience in exstrophic patients. Pediatr Surg Int. 2012;28(4):421–428. [DOI] [PubMed] [Google Scholar]
- 35. Pokrywczynska M, Jundziłł A, Buhl M, Balcerczyk D, Rasmus M, Warda K, Buchholz L, Kowalski F, Kwieciński P, Drewa T. Understanding the role of stem cells in urinary bladder regeneration—a preclinical study in a large animal model. Eur Urol Suppl. 2017:16:3. [Google Scholar]
- 36. Bury MI, Fuller NJ, Wethekam L, Sharma AK. Bone marrow derived cells facilitate urinary bladder regeneration by attenuating tissue inflammatory responses. Cent European J Urol. 2015;68(1):115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Adamowicz J, Kloskowski T, Tworkiewicz J, Pokrywczyńska M, Drewa T. Urine is a highly cytotoxic agent: does it influence stem cell therapies in urology? Transplant Proc. 2012;44(5):1439–1441. [DOI] [PubMed] [Google Scholar]
- 38. Tamama K, Sen CK, Wells A. Differentiation of bone marrow mesenchymal stem cells into the smooth muscle lineage by blocking ERK/MAPK signaling pathway. Stem Cells Dev. 2008;17(5):897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ball SG, Shuttleworth AC, Kielty CM. Direct cell contact influences bone marrow mesenchymal stem cell fate. Int J Biochem Cell Biol. 2004;36(4):714–727. [DOI] [PubMed] [Google Scholar]
- 40. Liu Y, Deng B, Zhao Y, Xie S, Nie R. Differentiated markers in undifferentiated cells: expression of smooth muscle contractile proteins in multipotent bone marrow mesenchymal stem cells. Dev Growth Differ. 2013;55(5):591–605. [DOI] [PubMed] [Google Scholar]