Abstract
The use of regenerative medicine to treat nervous system disorders like ataxia has been proposed to either replace or support degenerating neurons. In this study, we assessed the ability of human neural progenitor cells (hNPCs) to repair and restore the function of dying neurons within the spastic Han-Wistar rat (sHW), a model of ataxia. The sHW rat suffers from neurodegeneration of specific neurons, including cerebellar Purkinje cells and hippocampal CA3 pyramidal cells leading to the observed symptoms of forelimb tremor, hind-leg rigidity, gait abnormality, motor incoordination, and a shortened life span. To alleviate the symptoms of neurodegeneration and to replace or augment dying neurons, neuronal human progenitor cells were implanted into the sHW rats. At 30 d of age, male sHW mutant rats underwent subcutaneous implantation of an Alzet osmotic pump that infused cyclosporine (15 mg/kg/d) used to suppress the rat’s immune system. At 40 d, sHW rats received bilateral injections (500,000 cells in 5 µL media) of live hNPCs, dead hNPCs, live human embryonic kidney cells, or growth media either into the cerebellar cortex or into the hippocampus. To monitor results, motor activity scores (open-field testing) and weights of the animals were recorded weekly. The sHW rats that received hNPC transplantation into the cerebellum, at 60 d of age, displayed significantly higher motor activity scores and sustained greater weights and longevities than control-treated sHW rats or any hippocampal treatment group. In addition, cerebellar histology revealed that the transplanted hNPCs displayed signs of migration and signs of neuronal development in the degenerated Purkinje cell layer. This study revealed that implanted human progenitor cells reduced the ataxic symptoms in the sHW rat, identifying a future clinical use of these progenitor cells against ataxia and associated neurodegenerative diseases.
Keywords: stem cell, progenitor cell, ataxia, cerebellum, hippocampus, stereotactic transplantation, Purkinje cell
Introduction
Hereditary ataxias are neurological disorders that manifest a continuous decline in motor coordination due to neuronal death.1 In hereditary ataxias, cell death is due to gene mutations that develop into slowly progressing deterioration of motor coordination of mobility, speech, and eye movement.1 One common characteristic of hereditary ataxia is the progressive degeneration of neurons; in particular, Purkinje cells within the cerebellum.2 Present treatments only aim at slowing the advancement of the disease and offer only limited relief; however, regenerative medicine in the form of transplantation of stem cells has the potential to ameliorate the symptoms of ataxia.3,4
Cell replacement therapy represents an emerging field for treating neurodegenerative diseases, like ataxia.5 The goal of stem cell therapy is to target disorders that share the unifying feature of damaged or dying neurons and replace them with healthy new cells. Once these replacement cells are implanted within the desired brain region, it is hypothesized that these cells will migrate directly to the damaged tissue, develop into select neurons, and restore normal function. If not through direct replacement, then stem cell transplantation may also aid in reducing symptoms of these neurodegenerative diseases through other neuroprotective properties.3 For example, stem cells have been shown to enrich their surroundings with specific neurotrophic growth factors (i.e., glial cell line-derived neurotrophic factor [GDNF] brain-derived neurotrophic factor [BDNF], vascular endothelial growth factor [VEGF], and insulin-like growth factor [IGF] I), cleanse the environment of toxins, and reform neural circuitry to supplement the loss of neurons.6
Stem cell therapeutic treatments for ataxia have been demonstrated in both animal models and a few human clinical trials. Using a mouse model of spinocerebellar ataxia (SCA) type 2, intravenous injection of human mesenchymal stem cells yielded improvements in motor function on the rotarod and delayed the onset of ataxic symptoms through preservation of Purkinje cells.7 In another study, mouse neural stem cells (NSCs) were obtained from the brain of a normal adult mouse and transplanted into SCA type 1 ataxic mice displaying significant Purkinje cell loss.8 The animals treated with NSCs showed an increase in motor activity, recovery of molecular layer thickness, and increased survival of Purkinje cells. While no direct replacement of Purkinje cells by NSCs was detected, it was believed that these stem cells offered neuroprotection through direct contact with the surviving endogenous neurons.8 Similarly, a study by Mendoca et al.9 transplanted NSCs taken from neonatal mice into the cerebellums of adult Machado–Joseph disease mice. Their results showed an increased level in neurotropic factors, decreased neuronal loss, reduced neuroinflammation, and concomitant improvements in motor coordination.9
These animal studies suggested the hypothesis that NSCs have the potential to serve as regenerative therapy for human neurodegenerative diseases including ataxia. In 1 clinical trial, 16 patients diagnosed with different forms of SCA were intravenously and intrathecally infused to test the efficacy of umbilical cord mesenchymal stem cells (UCMSCs).10 The researchers monitored motor effects over 12 mo, and the resultant data showed that motor improvements in these patients were greatest at 3- and 6-mo postinfusion. At the end of the experiment, none of the patients suffered any serious side effects including no tumorigenesis.10 A different study used 24 ataxic patients diagnosed with either SCA or multiple system atrophy to examine the potential therapeutic effects of intrathecally injected UCMSCs.11 Follow-up analysis, conducted 6-mo postprocedure, revealed that patients initially showed improvements in motor coordination, fine motor movements, and dysarthria; however, these observed improvements were not persistent as 14 patients experienced regression to the same ataxic level observed prior to injection of UCMSCs.11 Finally, another clinical trial at Shandong Medical School involved 6 patients with hereditary cerebellar ataxia that had undergone fetal cerebellar tissue surgical grafts.12 This clinical study showed that neuron-replacing cell therapy appeared to restore at least some motor function to their ataxic patients.
Our laboratory studies the effectiveness of neuronal progenitor cell transplantations by examining the ability of these cells to survive in the adult brains of an animal model of ataxia. The spastic Han-Wistar (sHW) rat served as our animal model for ataxia as it suffers from an autosomal, recessive disorder that results in the neurodegeneration of cerebellar Purkinje cells and hippocampal CA3 pyramidal cells.13 Symptoms manifested in this animal model of ataxia are analogous to those seen in human patients, including forelimb tremors, hind-leg rigidity, gait abnormality, motor incoordination, muscle wasting, and a shortened life span (about 65 d).14 For our first study,15 we utilized a line of human neural progenitor cells (hNPCs), developed by Celavie Biosciences LLC (Oxnard, CA, USA) and were transplanted into the cerebellum of 40-d-old sHW rats. This recent study demonstrated that animals receiving hNPCs injections showed significant improvements in weight gain and motor activity compared to injection of dead progenitor cell controls, demonstrating the potential of these hNPCs to alleviate some symptoms caused by the sHW ataxia.15
Given the results of our previous study, we used bilateral stereotactic transplantation, into either the cerebellum or hippocampus, to demonstrate the ability of Celavie’s hNPCs to significantly improve weight, motor activity, and life expectancy. We also compared the effectiveness of bilateral implantations of hNPCs in the sHW rats with various controls, including dead neural progenitor cells (dNPC), a line of human embryonic kidney (HEK) cells, and human cell growth media (MED). In contrast to our previous methods study,15 which compared intra-arterial injections with direct unilateral injections into both brain regions (cerebellum and hippocampus) simultaneously, our present study examined bilateral injections into the cerebellum or hippocampus separately. This allowed us to test the effectiveness of implanted NPCs separately in the sHW rat cerebellum and hippocampus.
Materials and Methods
Animals
Male sHW rats (N = 104) were obtained from California State University, Northridge’s breeding colony. The experimental protocol (1516-019a) for this study was approved by the Institutional Animal Care and Use Committee at California State University, Northridge. For longevity studies, male sHW rat mutant siblings were randomly separated into either cerebellar (n = 40) or hippocampal (n = 40) groups for bilateral stereotactic injections. Both, the cerebellum and hippocampus treatment groups, received the same treatments, which were further divided into live hNPCs (cerebellum, n = 12, and hippocampus, n = 12), dead hNPCs (cerebellum, n = 12, and hippocampus, n = 12), live HEK cells (cerebellum, n = 8, and hippocampus, n = 8), or growth media injection (MED; cerebellum, n = 8, and hippocampus, n = 8). The sHW rats were housed in standard rat cages with access to Lab Diet 5001 rodent chow and water ad libitum. The colony room was maintained at a temperature of 22 °C ± 1 °C, with a 12/12-h light/dark cycle. To monitor effectiveness of treatment, motor activity scores (open-field testing) and weights of the sHW rats were recorded every fifth day, starting at 30 d. In addition, a separate group of normal male rats (n = 9) were tested (weight gain and motor activity assay) to compare against cell treatments applied to sHW mutants.
Cell Culture
hNPCs were obtained according to National Institutes of Health (NIH) Ethical Guidelines and have been characterized by a previous study.15 hNPCs were grown in culture medium consisting of animal-derived component-free (ADCF) minimum essential medium/Earle's balanced salt solution (MEM/EBSS) basal medium, supplemented with epidermal growth factor (Peprotech, Rocky Hill, NJ, USA), basic fibroblast growth factor (bFGF), insulin-like growth factor (IGF), transforming growth factor α (TGF-α), leukemia inhibiting factor (LIF) (Millipore, Temecula, CA, USA), N2 (Invitrogen, Carlsbad, CA, USA), and Gem 21 (Gemini Bioscience, Sacramento, CA, USA). The dNPCs were used to control for any paracrine effects of progenitor cell inoculations. The dNPCs were obtained from an hNPC population by placing them into a −20 °C freezer for 30 min to freeze and kill the cells, which were then placed in a −80 °C freezer for storage until use. Prior to inoculation in sHW rats, the suspended dNPCs in culture medium were thawed for 1 h. HEK 293 T cells were grown in a Roswell Park Memorial Institute (RPMI) growth media made up of sodium bicarbonate, fetal bovine serum (FBS), penicillin streptomycin (HyClone), sodium pyruvate (HyClone), and MEM nonessential amino acids (HyClone). For the growth media treatment group, the same RPMI growth media that was used to grow the HEK cells was injected to test for restorative properties in the sHW rats.
Neural Progenitor Cell Treatment
At 30 d of age, mutant sHW rats were sedated using 2.5% isoflurane and underwent subcutaneous implantation of an Alzet osmotic pump (Model 2004; 28-d duration) that infused cyclosporine (15 mg/kg/d) to suppress the rat’s immune system. Ten days later, at 40 d, sHW rats were anesthetized using chloral hydrate (350 mg/kg; Sigma, St. Louis, MO, USA) and received bilateral stereotactic injections of 500,000 live hNPCs/in 5 µL, 500,000 dead hNPCs/5 µL, 500,000 live HEK cells (293 T)/5 µL, or 5 µL of growth media (MED) either into the cerebellum (anterior-posterior [AP] = −11.0 mm, medial-lateral [ML] = ±2.0 mm, and dorsal-ventral [DV] = 5.5 mm) or the Cornu Ammonis 3 (CA3) region of the hippocampus (AP = −3.0 mm, ML = ±2.5 mm, DV = 3.5 mm).
Motor Activity Testing
An open-field (2 min) test was used by our lab14–17 to monitor whether hNPC treatment had any positive effects on the progressive decline in motor activity normally seen in these sHW rats. Beginning at 30 d of age (prior to pump implantation), all animals were tested every 5 d for the duration of the experiment for instinctive motor activity using the Michael L. Kaufman Activity Test System. The activity test consisted of placing the sHW rat in the center of the motor activity box (100 × 100 cm2 ABS black plastic open-field box) and allowing the rat to move around freely. The movement was recorded using a web camera (Logitech Pro 9000) coupled with Virtual Dub software (1.9.9). Movement data were analyzed by a 2-point converter system, which processes the motor activity recordings, 1 frame at a time. An open-field analyzer converted these data into a single motor activity score that summarizes the distance traveled (in centimeters) by each rat during three 2-min trials. Motor activity scoring (and measurement of weights) was performed by one of the authors (SZ) who was blind to all treatments.
Tissue Processing
To prepare brain tissue for histological analysis, at 60 d of age, a separate designated group of rats (n = 4 for the 3 sHW mutant cell treatments in each brain region) that were not included in data recording of weight, motor activity, and longevity were deeply anesthetized with chloral hydrate (400 mg/kg) and transcardially perfused with 0.9% perfusion saline solution followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer solution (PBS). The brains were harvested and postfixed in PFA for at least 2 d before being transferred to a 20% sucrose/4% PFA solution for 24 h to cryoprotect the brains prior to sectioning. Using a cryostat, brain slices (20 μm thickness) were obtained from the cerebellum (sagittal sections) and the hippocampus (coronal sections) and were processed via immunohistochemical staining to identify surviving human cells.
For immunohistochemistry analyses, the tissue was rinsed in a 10X wash buffer (Diagnostic Biosystems, Pleasanton, CA, USA) once for 5 min. Endogenous peroxidase blocking was achieved by washing the sections in a solution consisting of 0.36% β-D-glucose (Fisher Scientific, Waltham, MA, USA), 0.01% glucose oxidase (MP Biomedicals, Solon, OH, USA), and 0.013% sodium azide (Fisher Scientific) in 1X PBS (Fisher Scientific) for 60 min, proceeded by a 5-min rinse in wash buffer. Permeabilization was achieved by rinsing the tissue in wash buffer consisting of 0.1% Triton X-100 (Fisher Scientific) for 30 min, followed by a separate rinse in washing buffer for 5 min. Next, the tissue was washed with serum blocking solution consisting of 5% normal horse serum (Vector Laboratories, Burlingame, CA, USA) for 20 min. Next, tissue samples underwent a 15-min rinse of avidin-blocking solution (Vector Laboratories). Tissue samples were then washed for 15 min in a biotin-blocking solution (Vector Laboratories), followed by another 1-min rinse in wash buffer. After, samples were incubated with Human Nuclei MAB4383 (1:200; Millipore) in Antibody Diluent (Diagnostic Biosystems) for 60 min. After primary antibody incubation, tissue underwent a 5-min wash buffer rinse that was followed by a 45-min incubation with antimouse biotinylated secondary antibody (1:100; Vector Laboratories). Tissue was then rinsed for 5 min in wash buffer, incubated for 60 min in ABC kit solution (Vector Laboratories), and rinsed for another 5 min with wash buffer. The tissue was incubated in ImmPACT DAB Substrate (Vector Laboratories) for 5 min and then rinsed in wash buffer for 5 min.
At this point, some cerebellar tissue sections underwent additional immunostaining to identify the presence of calbindin, a Purkinje cell marker. To achieve dual staining, the tissue underwent another serum blocking wash with 5% normal goat serum (Vector Laboratories) for 20 min. Afterward, samples are incubated for 60 min with Calbindin Ab108404 (1:1000; Abcam) in Antibody Diluent. Tissue was then rinsed with immuno wash buffer and then incubated with antirabbit biotinylated secondary antibody (Vector Laboratories) for 45 min. Tissue was again rinsed for 5 min in wash buffer and then rinsed in another ABC kit solution for 60 min. This was followed by a 5-min wash buffer rinse, then a 5-min ImmPACT VIP Substrate (Vector Laboratories) incubation, and finally another 5-min immuno wash buffer rinse.
All tissue underwent the same final steps for background staining and mounting. Another incubation period of 1 min at 60 °C was needed for the background stain Methyl Green Counterstain (Vector Laboratories). After stain incubation, the tissue was washed in deionized (DI) water (Thermo Scientific, Waltham, MA, USA) for 2 min, then dehydrated in a series of alcohols, a 5-min dehydration in 95% ethanol (Decon Laboratories, King of Prussia, PA, USA), then two 5-min rinses in 100% ethanol (Arcos Organics, Waltham, MA, USA). Xylene (Fisher Scientific, Waltham, MA, USA) clearing was achieved with two 5-min rinses, and finally, the sections were placed on glass slides and cover slipped with mounting medium (Poly Sciences, Warrington, PA, USA). Sections were examined for the presence and location of human cells and calbindin under an Olympus BX60 fluorescent microscope with ToupView version 7.3 software.
Statistical Analysis
All values shown are means ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) tests were performed for longevity. We used Tukey’s pairwise post hoc analysis (SYSTAT analysis program) to determine differences among longevity means. For weight gain and motor activity test, we utilized repeated measure ANOVAs and then used Tukey’s pairwise post hoc analysis (SYSTAT analysis program) in order to detect differences in treatment means over time. Significance levels were set at P < 0.05 for all statistical tests.
Results
Our experiment tested the effectiveness of bilateral injections of live, human-derived, multipotent, neural progenitor cells (hNPCs) and compared their successes to a series of controls: dead hNPCs, other human embryonic cells (HEK), and growth media (MED). The effectiveness and survival of transplanted cells were ultimately determined by analysis of weight gain, motor activity scores, longevity, and histology.
Effects of Different Cell Treatments on sHW Weight Gain
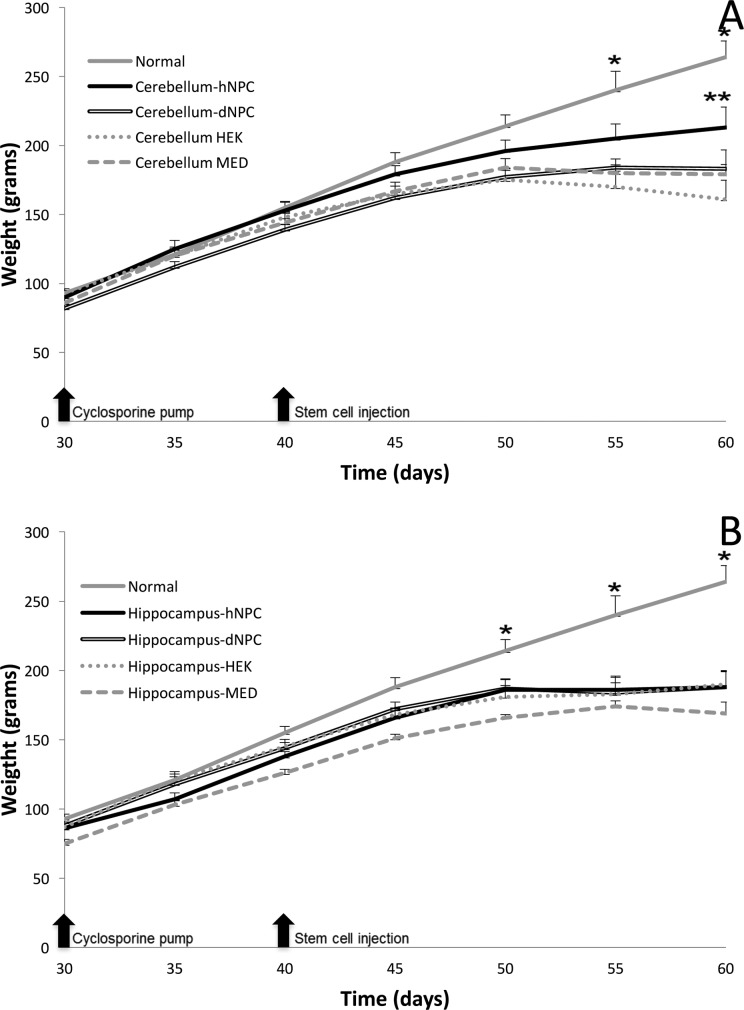
The experiment began with sHW rats at 30 d of age with the subcutaneous implantation of an osmotic pump releasing cyclosporine, and both weight gain and motor activity were recorded every 5 d until death. For weight gain, statistical significance (cerebellum treatments: F = 307.33, P < 0.001; hippocampus treatments: F = 227.77, P < 0.001) was observed over the 30-d experimental period. Normal, untreated rats showed significantly greater weight differences (Tukey’s, P < 0.05) compared to both cerebellar and hippocampal mutant treatment groups. In regard to mutant treatments, the effectiveness of the hNPCs can be seen as early as 50 d of age (10-d postimplantation) within the cerebellar hNPC group, with this treatment group deviating away (albeit nonsignificant) from all other mutant treatments (Fig. 1A). Among the cerebellum groups, post hoc analysis determined significant weight differences between the cerebellar hNPC and the other negative control treatment groups at 60 d of age (Fig. 1; Tukey’s, P < 0.05). In contrast, the other treatment groups (HEK and MED) displayed statistically similar trends in weight progression as the dNPC group: a slow increase, followed by a plateau period, then onset of a decline in weight statistically worse than weights observed in the cerebellar hNPC treatment rats (Fig. 1A). In contrast, we detected no statistical differences among any of the hippocampal mutants groups at any ages measured (Fig. 1B).
Figure 1.
Mean weight gain for mutant spastic Han-Wistar (sHW) rats bilaterally injected into either the cerebellum (A) or hippocampus (B) with human neural progenitor cells (hNPCs; cerebellum, n = 12; and hippocampus, n = 12), dead NPCs (dNPCs; n = 12), human embryonic kidney (HEK) cells (n = 8), or culture media (MED; n = 8). Additionally, untreated, normal HW rats (n = 9) were utilized as a positive weight gain control. (A) Untreated normal HW rats compared to the cerebellar hNPC group displayed statistically significant (F = 163.27, *P < 0.05) weight gain starting at 55 d. The bilateral injections of live hNPCs into the cerebellum elicited a statistically significant (Tukey’s, **P < 0.05) increase in weight gain in the sHW rats when compared to sHW rats injected with nonviable dNPCs. (B) Similar to the untreated normal versus cerebellar hNPC groups, the untreated normal group displayed increased weight gain when measured against hippocampal hNPC injections starting at 50 d (F = 89.83, *P < 0.05). The bilateral injections of live hNPCs into the hippocampus did not elicit a statistically significant increase (Tukey’s, P > 0.05) in weight gain in the treated sHW rats at any age measured. All values are means ± SEM.
Effects of Different Cell Transplants on sHW Motor Activity Scores
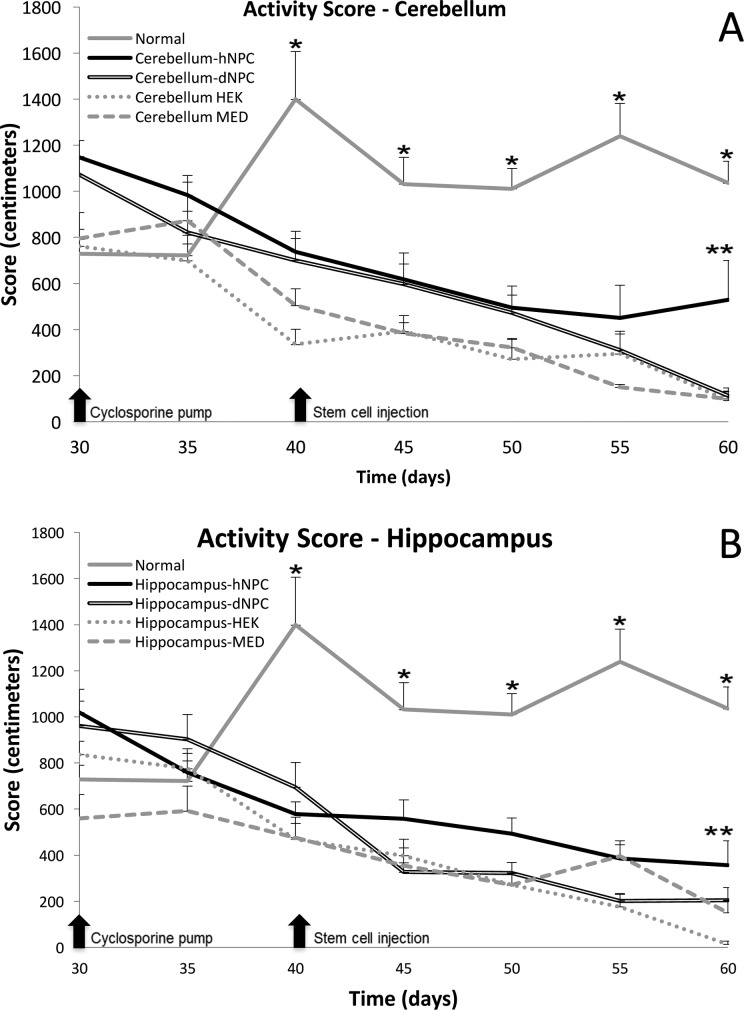
Due to the hereditary ataxia in the sHW rat, motor function is severely impaired within these mutants and steadily gets worse over their shortened life span.14 Open-field testing was used to assess and then compare the locomotor impairments and subsequent behavioral declines observed in the mutant sHW rat with different cell treatments. Similar to the weight gain data, statistical significance (cerebellum treatments: F = 28.46, P < 0.001; hippocampus treatments: F = 24.92, P < 0.001) was observed in the motor activity assay over the 30 d experimental period. As observed in previous studies, normal HW rats developed an upward motor activity trend while the mutant rats in contrast exhibited a progressive decline in motor ability regardless of treatment (Fig. 2). Similar to the weight gain data (Fig. 1), normal, untreated rats elicited significant motor activity differences (Tukey’s, P < 0.01) compared to both cerebellar and hippocampal mutant treatment groups, starting at 40 d. In the cerebellum group, the mutant data indicated that starting at 50 d of age, a distinctly different rate of decline began to emerge in the cerebellar hNPC-treated rats compared to the negative control mutants (Fig. 2A). A statistically significant increase in motor activity, compared to the negative controls, was observed at 55 d of age in only the cerebellar hNPC treatment group (Fig. 2A; Tukey’s, P < 0.05). Analyzing the results of the hippocampal mutant treatments (Fig. 2B), there was a small but still significant increase in the overall motor activity of the hNPC group (Tukey’s, P = 0.05), perhaps indicating a possible subtle progenitor cell aid to the degenerating hippocampal cells. The other treatments of HEK and MED, in both cerebellum and hippocampus, all displayed similar level of decreased motor activity that was typical of untreated sHW rats.15
Figure 2.
Open-field test was used to assess motor activity for untreated normal Han-Wistar (HW) rats (n = 9) or mutant spastic Han-Wistar (sHW) rats bilaterally injected into either the cerebellum (A) or hippocampus (B) with human neural progenitor cells (hNPCs; cerebellum, n = 12; and hippocampus, n = 12), dead NPCs (dNPCs; n = 12), human embryonic kidney (HEK) cells (n = 8), or culture media (MED; n = 8). (A) Analysis of activity scores between untreated normal HW rats and the cerebellar hNPC-treated group yielded significant differences starting at 40 d of age (F = 34.61; Tukey’s, *P < 0.05). The cerebellar hNPC-treatment group displayed statistically significant (Tukey’s, **P < 0.05) higher motor activity scores compared to the dNPC-treatment group at 60 d of age. (B) The same pattern of greater and sustained mobility is observed when contrasting the untreated normal group and all the hippocampal treated groups (F = 28.79; Tukey’s, *P < 0.05) starting at 40 d. Comparing the hippocampal hNPC- and dNPC-treatment groups, we did detect statistically significant differences (Tukey’s, **P < 0.05) in higher motor activity scores for the hNPC-treatment group at 60 d. All values are means ± SEM.
Effects of Different Cell Treatments on sHW Life Span
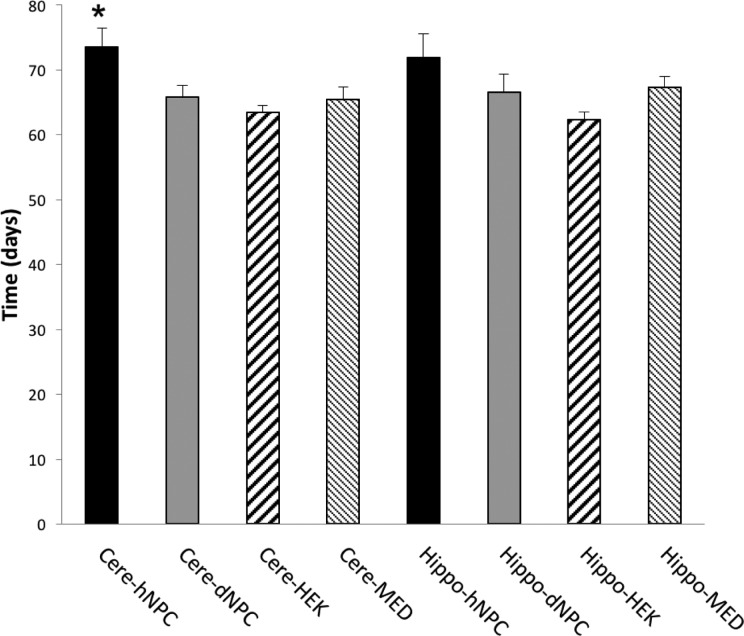
Following the same pattern as weight and motor activity analyses, only the cerebellar hNPC treatment group showed a statistically significant increase in survival time when compared to the other treatment groups (Fig. 3; F = 5.04, P < 0.05). The average life span of the sHW rat that received cerebellar bilateral injections of live hNPCs was 73.5 d compared to the average of the 65.7 d for the rats treated with dNPCs (Fig. 3). Comparable to the cerebellar hNPC group, the hippocampal hNPC injections also showed an increase (although not statistically significant when compared to the dNPC group), with an average life span of 71.9 d (Fig. 3; F = 5.04, P < 0.05). Results from the dNPC, HEK, and MED groups indicate that these treatments had no effect on decelerating the effects of neurodegeneration caused by the progressive onset of ataxia, with only hNPC therapy effectively increased life span, especially in the cerebellum.
Figure 3.
The mean longevity data were used to assess the effectiveness of bilateral injections into either the cerebellum or hippocampus with human neural progenitor cells (hNPCs; cerebellum, n = 12; and hippocampus, n = 12), dead NPCs (dNPCs; n = 12), human embryonic kidney (HEK) cells (n = 8), or culture media (MED; n = 8). The spastic Han-Wistar (sHW) rats that received injections of hNPCs into the cerebellum showed a statistically significant (F = 5.04; Tukey’s, *P < 0.05) increase in longevity when compared to sHW rats that received control injections into the cerebellum. The mean improvement in longevity of sHW rats that received hippocampal hNPC injection was slightly higher than the hippocampal dNPC group, but it was determined to be not statistically significant (Tukey’s, P > 0.05). All values are means ± SEM.
Immunohistochemical Analysis of Progenitor Cells
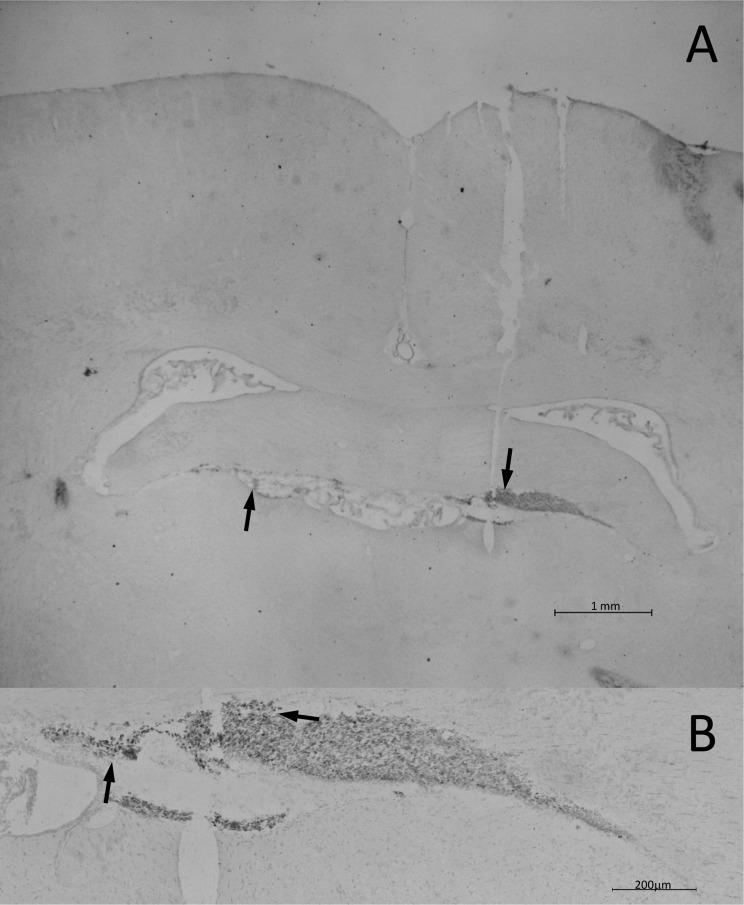
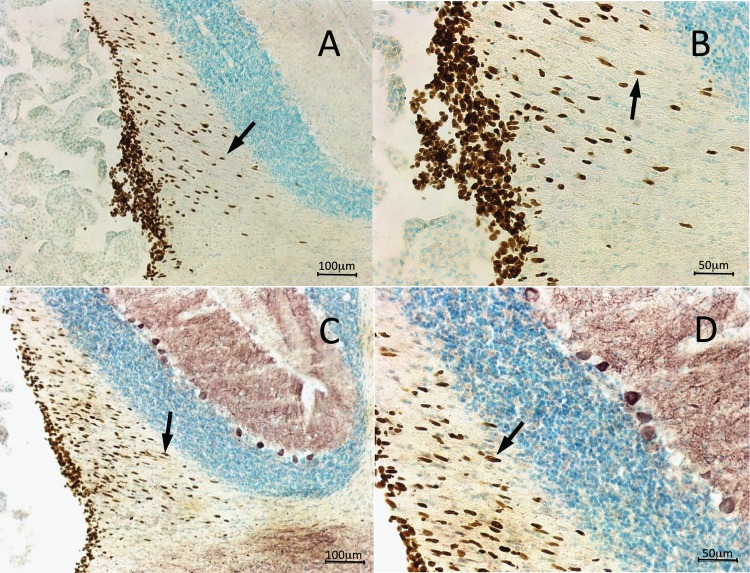
To examine the link between location and effectiveness of transplanted hNPCs, immunohistochemical staining was performed. A separate group of treated sHW rats was sacrificed and perfused at 60 d of age; brain tissue was sliced and then stained to search for surviving human cells was detected using an antihuman nuclear antibody. First, we confirmed the presence of surviving human cells in the HEK treatment group approximately 20-d posttreatment (Fig. 4A). Further examination indicated that the surviving HEK cells did not migrate away from the original injection site and instead formed a bolus of cells rostral to the hippocampus (Fig. 4B). Immunohistochemical analysis showed HEK cells survived but did not travel outside their injection site, correlating with the poor weight gain, motor activity, and longevity data exhibited by hippocampal HEK treatment group. Similar small boluses (clumps) of surviving HEK cells were also observed in the cerebellar HEK transplanted sHW rats (data not shown).
Figure 4.
Human nuclei immunohistochemical staining of the hippocampus of a 60-d-old spastic Han-Wistar rat that received stereotactic injections of live human embryonic kidney (HEK) cells. Arrows indicate human nuclei-labeled HEK cells clustered together in the hilus of the hippocampus. In contrast to the human neural progenitor cell transplant, HEK cells remained at the base of the injection site and had not migrated from their initial implant location. Human nuclei antibody at 1:200 dilution. (A) 10× Magnification; 1 mm scale bar. (B) 200× Magnification; 200 µm scale bar.
Figure 5 illustrates surviving labeled human cells in the hippocampus of two 60-d-old sHW rats that received bilateral injections of hNPCs. Figure 5A and C shows low magnification views of surviving hNPCs with dispersal of these human cells away from the inoculation site from the hilus toward the CA3 region, the locale of pyramidal cell neurodegeneration in the sHW rat. Along with reaching the degenerating CA3 region, staining revealed possible hNPC migration toward the CA2 region of the hippocampus. Higher magnification images show many labeled hNPCs had survived inside the sHW hippocampus (Fig. 5B and D).
Figure 5.
Human nuclei immunohistochemical staining of the hippocampus of a 60-d-old spastic Han-Wistar (sHW) rat that received bilateral injections of human neural progenitor cells (hNPCs). Arrows show surviving human nuclei-labeled hNPCs at the site of injection inside the hilus. The nuclei-labeled progenitor cells were observed to migrate away from the original inoculation site and dispersed throughout the CA3 region, the primary area of degeneration in the sHW rat. Human nuclei antibody at 1:200 dilution. (A and C) 100× Magnification; 100 µm scale bar. (B and D) 200× Magnification; 50 µm scale bar.
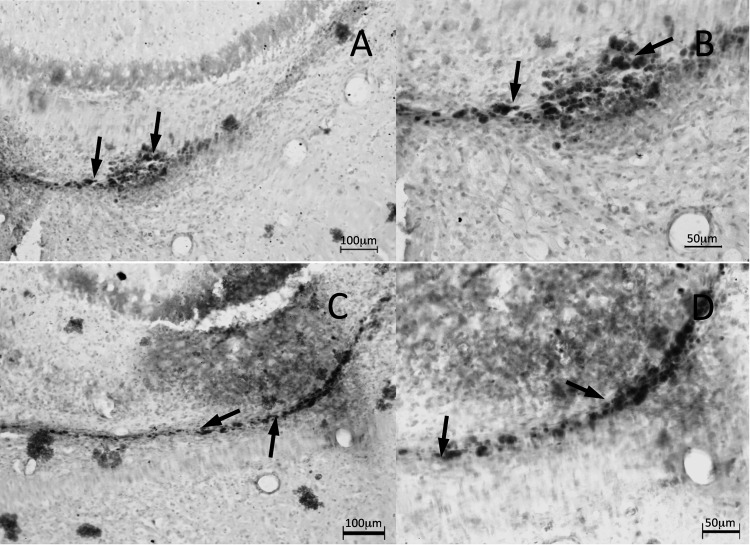
Similar to the immunohistochemical antihuman stain in the hippocampus, cerebellar hNPCs images also show significant survival and incorporation of hNPCs in the anterior lobe of folia III (site of one of the bilateral implants). However, in contrast with the survival of hNPCs in the hippocampus, cerebellar hNPCs display obvious migration patterns (Fig. 6A, low magnification; Fig. 6B, higher magnification). Staining indicated that hNPCs were migrating away from their site of transplantation in the white matter toward the granule cell layer and, most interesting, the Purkinje cell layer, the other area of neurodegeneration in the sHW rat (Fig. 6A and B). In addition, to begin characterizing possible development and maturation of the transplanted hNPCs in the 60-d-old sHW rat cerebellum, calbindin immunohistochemical staining was performed (Fig. 6C and D). Photomicrographs showed labeling of many hNPCs with anticalbindin (Fig. 6C). Also, labeled were the surviving Purkinje cells known to be calbindin positive in the adult rat cerebellum. Figure 6D shows a higher magnification view of calbindin-labeled human cells migrating away from the site of inoculation in the white matter and possibly en route to the neurodegenerating Purkinje cell region of the sHW rat.
Figure 6.
Immunohistochemical staining of the cerebellum of a 60-d-old spastic Han-Wistar rat that received stereotactic injections of live human neural progenitor cells (hNPCs) into the anterior lobe of folia III. Arrows in figures A and B show human nuclei-labeled NPCs migrating through the molecular layer toward the Purkinje cell layer. In figures C and D, both rat Purkinje cells and implanted hNPCs were labeled with calbindin antibodies. Human nuclei antibody at 1:200 dilution, calbindin antibody at 1:1000 dilution. (A and C) 100× Magnification; 100 µm scale bar. (B and D) 200× Magnification; 50 µm scale bar.
Discussion
Diseases like multiple sclerosis, amyotrophic lateral sclerosis, Parkinson’s disease, and ataxia have neurodegenerative effects as their defining characteristics, and more studies are demonstrating the therapeutic nature of stem cells in combating these disorders.7,18,19 Our study further demonstrates that hNPCs directly transplanted into the degenerating area were capable of slowing the progression of ataxic symptoms in the animal model, ultimately leading to increased longevity. Location of implantation was quite important as the cerebellar hNPC group showed significant motor improvements while the hippocampal group did not show as much effectiveness.
Progressive degeneration of Purkinje cells of the sHW rat directly leads to the decline in the motor coordination.14,16,17 Our data showed how stereotactically transplanting live hNPCs into the cerebellum significantly ameliorated the motor symptoms caused by progressive ataxia. Our results showed that the implantation of hNPCs was able to not only restore significant mobility (Fig. 2) but also increase weight gain (Fig. 1) in the sHW rats. With significantly higher weights and activity scores, the hNPCs triggered a longer life span in this treatment group (Fig. 3). Typical of untreated sHW rats are the symptoms of forelimb tremors, hind-leg rigidity, gait abnormality, motor incoordination, and muscle wasting.14 With the administration of hNPCs into the cerebellum, sHW rats displayed a noticeable deceleration in many of these features. The regression of hind-leg rigidity, coupled with better gait, the cerebellar hNPC-treated sHW rats were significantly more mobile at the end of the initial 20 d transplant period, correlating with statistically higher motor activity scores (Fig. 2). In addition to the increased motor activity, due to a dampening of these ataxic symptoms, higher weights can be attributed to a slower decline in muscle wasting, as mutants were able to retain more mass (Fig. 1). The administration of hNPCs to the cerebellar region also appeared to reduce the phenotypic tremors (R. Nuryyev, pers. comm.).
Histological analysis confirmed the survival, incorporation, and migration of hNPCs into the cerebellum (Fig. 6). Additionally, histology illustrated these migrating hNPCs tested positive for calbindin, similar to neighboring surviving Purkinje cells. Are the hNPCs becoming Purkinje cells or are they merely reflecting their progenitor nature? Future experiments in our lab will attempt to identify what these human cells become in the sHW rat cerebellum. Incorporation, survival, and subsequent migration of transplanted hNPCs toward the Purkinje cell layer can be considered as evidence that the injected cells follow local biochemical cues related to the pathogenesis of cerebellar ataxia and seemed to correlate with slowing or possibly reversing the progressive neurodegeneration.
The presence of calbindin expression in our hNPCs is an exciting discovery since calbindin has been shown to play a functional role in promoting neuronal differentiation of progenitor cells and stimulated neurite outgrowth.20 Typical of sHW rats is the degeneration of their GABAergic, calbindin-containing Purkinje cells through glutamate excitotoxicity, the outcome of which is the progressive loss of motor coordination and onset of ataxia.21 As shown in our experiment, bilateral injections of hNPCs into the cerebellum were able to alleviate these symptoms and offer some restoration of the mutant motor skills. A possible mechanism to explain the improvements witnessed in the cerebellar hNPC treatment group is the presence of calbindin from our hNPCs. This result suggests that a combination of neurotrophic factors promoted differentiation into calbindin-labeled cells just 3-wk posttransplantation in the 40-d-old sHW rat. This finding is quite interesting since flow cytometry has been used to verify that calbindin was not present in these specific NPCs prior to implantation (A. Kopyov, pers. comm.). The presence of calbindin some 20-d postimplantation may help explain why only the cerebellar treatment group saw significant improvements compared to the hippocampal treated rats.
Although histological evidence showed a movement of progenitor cells toward the Purkinje cell layer, no additional analysis was performed regarding the exact biochemical mechanisms responsible for hNPCs being able to activate pathways leading to the observed improvements in motor ability, weight gain, and ultimately longevity. Yet, other studies performed in vitro have shown that NSCs were able to elicit neuroprotective effects on neurons that were exposed to glutamate excitotoxicity and decreases the rate at which cell death occurred.22 NSCs implanted onto spinal cord sections resulted in axonal growth from motor neurons toward stem cells via neurotrophic secretions like glial cell-derived neurotrophic factor (GDNF) and nerve growth factor (NGF).23 In addition, adipose-derived mesenchymal stem cells were able to rescue neurons from early death due to glutamate excitotoxicity by secreting various neurotrophins (NTs), including VEGF, hepatocyte growth factor, BDNF, and NGF.24 Studies has shown that stem cells were able to express neurotrophic factors (bFGF, NT-3/4/5, BDNF, and NGF), which aid the local environment, ameliorating symptoms caused by neurodysfunction.23–25 Along with various stem cells, progenitor cells (like NPCs) also have the potential to induce proliferation and migration as well as offer protective properties via neutralization of apoptotic and inflammatory properties.26 While these studies have illustrated how neurotrophic factors help their surroundings, they do not address how these chemicals affect the stem cells themselves. A study examining cortical progenitor cells transplanted within the hippocampus unveiled that bFGF and NT-3 were key instigators of inducing differentiation in these progenitor cells into GABAergic calbindin-containing neurons.25
Additionally, implanted stem cells can offer protective properties specifically to Purkinje cells within a deteriorating cerebellum. By grafting mesenchymal stem cells into the cerebellum, dying Purkinje cells were replaced and rescued in a mouse model of ataxia.27 At 2-mo postsurgery, stem cells were shown to have integrated into the nervous system by migrating toward and into the Purkinje cell layer. Further histological assays showed that some of these mesenchymal stem cells had fused with Purkinje cells and were secreting Purkinje cell survival factors: BDNF, NT-3, or GDNF.27 In a study using a different ataxic mouse, one exhibiting Purkinje cell loss due to a mitochondrial mutation showed that the normal phenotype was rescued using NSCs.28 These stem cells did not replace the endogenous defective Purkinje cells, but rather supported the existing host cells by augmenting excessive levels of tissue plasminogen activator, originally caused by the mitochondrial dysfunction. These NSCs also helped promote dendritic growth and synaptogenesis that coalesced in the restoration of motor coordination circuitry in the ataxic mouse cerebellum.28 In addition, intravenous injections of human mesenchymal stem cells were able to increase the survival rate Purkinje cells in a mutated mouse model typically experiencing progressive Purkinje cell loss due to polyglutamate aggregates.7 Histological analysis revealed that mesenchymal stem cells injected through the tail vein had successfully migrated throughout the cerebellar white matter, molecular layer, and cortex.7 Finally, Neimann-Pick type C mouse mutants experience disruption to calcium homeostasis leading to Purkinje cell death.29 Mutant mice transplanted with bone marrow-derived mesenchymal stem cells exhibited a restoration to the calcium imbalance that correlated with the increased survival rate of the Purkinje cells.29
While bilateral stereotactic injections of hNPCs into the degenerating cerebellum showed alleviation of many of the ataxic symptoms in the sHW rats, similar injections of these cells into the degenerating hippocampus elicited only minimal therapeutic effects. Weight gain and longevity of these sHW rats remained similar to the dNPC, HEK, and MED groups (Figs. 1 and 2). Interestingly, the hippocampal hNPC treatment group at 60-d-old did display higher motor activity than dNPC, HEK, and MED control groups (Fig. 2). Thus, progenitor cell incorporation into the hippocampus was mostly ineffective (especially compared to cerebellar hNPC transplants). Previous observations of the sHW rat hippocampus have shown that as neurodegeneration progresses concurrent mossy fiber sprouting occurs.13 This reactive synaptogenesis of mossy fibers caused significant reconstruction of the sHW rat hippocampal circuitry. These previous results show significant synaptic reorganization, however, our data confirmed that the transplantation of hNPCs into the actively reconstructing hippocampus in sHW rats had no additional therapeutic effect.
Our rationale for using a dead progenitor cell (dNPC) group as a negative control was to account for any paracrine effects that might occur due to introducing complete but inactivated human progenitor cells into the rat brain. Weight gain (Fig. 1), motor activity scores (Fig. 2), and longevity (Fig. 3) showed that these dead progenitor cells were unable to stimulate any neuroprotective response. Similarly, the use of a living, yet nonneuronal cell line (HEK cells) also showed no positive effects on the various assays (weight gain, motor activity, and longevity) in the sHW rats. These 2 control experiments were used to eliminate the possibility that any improvements to the health of the sHW rats were due strictly to the introduction of any living or dead cells directly to the damaged area. Finally, another control inoculation used in our experiment was the injection of growth media; the goal was to determine that any positive effects documented were not caused by cellular growth factors present in the media. Results confirmed that the presence of growth factors from the media was not enough to stimulate any improvements in the ataxic rat.
The use of immunosuppressant agents, like cyclosporine, is an important factor when using human stem cells in a nonhuman, animal model, to avoid rejection of donor cells by the host immune system.30 Cyclosporine A is quite effective at disrupting the T cell costimulatory pathway and impairs proliferation, differentiation, and cytokine production rendering T cells to be inactive.31 To avoid unnecessary daily injections (which could result in unintended behavioral changes), our study chose to infuse cyclosporine chronically using an osmotic pump. To avoid any possible consequences (either positive or negative) of the hNPC treatment groups receiving cyclosporine, all groups, regardless of treatment, received a subcutaneous Alzet pump (filled with cyclosporine) implantation. No detectable changes in any subsequent measures were found due to the use of cyclosporine. In addition, a previous study demonstrated that residual levels of cyclosporine persist in the animal model for up to 15-d postdiscontinuation of use.32 This extended effect could be attributed to the improved weights and activity scores seen past the 28-d life span of the Alzet pump (at 58 d of age), along with the drop-off of these measurements past 70 d. Future studies will examine the potential benefits of replacing the cyclosporine pumps after 28-d depletion. The continued immune system suppression could give insight into how the hNPCs affect the ataxic symptoms on a longer time scale as well as allow the determination of cell types into which these progenitor cells have differentiated.
The sHW rats that had cerebellar injections of hNPCs were offered amelioration of the ataxic symptoms, however, the rats were not cured of this progressive genetic disorder. These progenitor cells were unable to correct the genetic defect that caused the disorder and, thus, NPC injection cannot be considered a true cure at this time. NPC injections either replaced degenerating neurons or, as suggested by our study, altered the cellular environment with various chemicals (like NTs) to compensate for the neurodysfunction. Progenitor cells, like Celavie’s hNPCs, have the ability to differentiate into neurons and thus can be applied to diseases characterized by progressive neurodegeneration. Our goal for this experiment was to test the efficacy of these cells within the deteriorating areas of the sHW rat, an animal model of ataxia. Our study illustrated that hNPCs bilaterally injected into the cerebellum were able to survive, migrate, and provide unknown neuroprotective effects to degenerating Purkinje cells. More specifically, injections of the live human NPCs into the cerebellum displayed an amelioration of ataxic symptoms including maintained weight, improved motor activity, and expanded longevity. We further supported the expanding role of progenitor cells as a form of regenerative medicine; live human-derived NPCs were a positive and effective treatment for the sHW rat’s neurodegenerative disorder.
Acknowledgments
The authors wish to thank the vivarium staff for animal care.
Authors’ Note: Oleg Kopyov, Alex Kopyov, Jessica Ochoa, and William Van Trigt are employees with Celavie Biosciences LLC, Oxnard, CA.
Ethical Approval: The experimental protocol (1516-019a) for this study was approved by the Institutional Animal Care and Use Committee (IACUC) at California State University, Northridge.
Statement of Human and Animal Rights: Rats were housed in standard rat cages with access to Lab Diet 5001 rodent chow and water ad libitum. The colony room was maintained at a temperature of 22 _C+1 _C, with a 12/12-h light/dark cycle.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Jayadev S, Bird TD. Hereditary ataxias: overview. Genet Med. 2013;15(9):673–683. [DOI] [PubMed] [Google Scholar]
- 2. Agler C, Nielsen DM, Urkasemsin G, Singleton A, Tonomura N, Sigurdsson S, Tang R, Linder K, Arepalli S, Hernandez D, et al. Canine hereditary ataxia in old English Sheepdogs and Gordon Setters is associated with a defect in the autophagy gene encoding RAB24. PLoS Genet. 2014;10(2):e1003991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piemonte F, Rossi F, Carletti B. Neuroprotection: the emerging concept of restorative neural stem cell biology for the treatment of neurodegenerative diseases. Curr Neuropharmacol. 2011;9(2):313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Erceg S, Moreno-Manzano V, Garita-Hernandez M, Stojkovic M, Bhattacharya SS. Concise review: stem cells for the treatment of cerebellar-related disorders. Stem Cells. 2011;29(4):564–569. [DOI] [PubMed] [Google Scholar]
- 5. Dantuma E, Merchant S, Sugaya K. Stem cells for the treatment of neurodegenerative diseases. Stem Cell Res Ther. 2010;1(5):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lunn JS, Sakowski SA, Hur J, Feldman EL. Stem cell technology for neurodegenerative diseases. Ann Neurol. 2011;70(3):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang YK, Chen MH, Chiang YH, Chen YF, Ma WH, Tseng CY, Soong BW, Ho JH, Lee OK. Mesenchymal stem cell transplantation ameliorates motor function deterioration of spinocerebellar ataxia by rescuing cerebellar Purkinje cells. J Biomed Sci. 2011;18(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chintawar S, Hourez R, Ravella A, Gall D, Orduz D, Rai M, Bishop DP, Geuna S, Schiffmann SN, Pandolfo M. Grafting neural precursor cells promotes functional recovery in an SCA1 mouse model. J Neurosci. 2009;29(42):13126–13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mendonca LS, Nobrega C, Hirai H, Kaspar BK, Pereira de Almeida L. Transplantation of cerebellar neural stem cells improves motor coordination and neuropathology in Machado-Joseph disease mice. Brain. 2014;138(2):320–335. [DOI] [PubMed] [Google Scholar]
- 10. Jin JL, Liu Z, Lu ZJ, Guan DN, Wang C, Chen ZB, Zhang J, Zhang WY, Wu JY, Xu Y. Safety and efficacy of umbilical cord mesenchymal stem cell therapy in hereditary spinocerebellar ataxia. Curr Neurovas Res. 2012;10(1):11–20. [DOI] [PubMed] [Google Scholar]
- 11. Dongmei H, Jing L, Mei X, Ling Z, Hongmin Y, Zhidong W, Li D, Zikuan G, Hengxiang W. Clinical analysis of the treatment of spinocerebellar ataxia and multiple system atrophy-cerebellar type with umbilical cord mesenchymal stromal cells. Cytotherapy. 2011;13(8):913–917. [DOI] [PubMed] [Google Scholar]
- 12. Wu CY, Bao XF, Zhang C, Zhang QL. Fetal tissue grafts for cerebellar atrophy in humans: a preliminary report In: Holtzman RNN, Stein BM, editors. Surgery of the spinal cord: potential for regeneration and recovery. New York (NY: ): Springer; 1992. p. 219–234. [Google Scholar]
- 13. Cohen RW, Cepeda C, Miyashiro-Turman JE, Levine MS. Development of morphological and physiological alterations in the hippocampus of the spastic Han-Wistar rat. Dev Brain Dysfunct. 1997;10(1):1–14. [Google Scholar]
- 14. Brunson KL, Khanna A, Cromwell HC, Cohen RW. Effect of the noncompetitive NMDA antagonists MK-801 and ketamine on the spastic Han-Wistar mutant: a rat model of excitotoxicity. Dev Neurosci. 2001;23(1):31–40. [DOI] [PubMed] [Google Scholar]
- 15. Uhlendorf TL, Nuryyev RL, Kopyov AO, Ochoa J, Younesi S, Cohen RW, Kopyov OV. Efficacy of two delivery routes for transplanting human neural progenitor cells (NPC) into spastic Han-Wistar rat, a model of ataxia. Cell Transplant. 2017;26(2):259–269. doi:10.3727/096368916X693527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Kummer BH, Cohen RW. Exercise-induced neuroprotection in the spastic Han Wistar rat: the possible role of brain-derived neurotrophic factor. BioMed Res Int. 2015;2015:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nisim AA, Hernandez CM, Cohen RW. The neuroprotective effects of non-NMDA antagonists in the cerebellum of the spastic Han Wistar mutant. Dev Neurosci. 1999;21(1):76–86. [DOI] [PubMed] [Google Scholar]
- 18. Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JWM, Petrou P, Ben-Hur T, Abramsky O, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67(10):1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Canesi M, Giordano R, Lazzari L, Isalberti M, Isaias IU, Benti R, Rampini P, Marotta G, Colombo A, Cereda E, et al. Finding a new therapeutic approach for no-option Parkinsonisms: Mesenchymal stromal cells for progressive supranuclear palsy. J Transl Med. 2016;14(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim JH, Lee JA, Song YM, Park CH, Hwang SJ, Kim YS, Kang BK, Son H. Overexpression of calbindin-d28 K in hippocampal progenitor cells increases neuronal differentiation and neurite outgrowth. FASEB J. 2007;329(3):409–420. [DOI] [PubMed] [Google Scholar]
- 21. von Bohlen Und Halbach O. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007;329(3):409–420. [DOI] [PubMed] [Google Scholar]
- 22. Geranmayeh MH, Baghbanzadeh A, Barin A, Salar-Amoli J, Dehghan MM, Rahbarghazi R, Azari H. Paracrine neuroprotective effects of neural stem cells on glutamate-induced cortical neuronal cell excitotoxicity. Adv Pharm Bull. 2015;5(4):515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lladó J, Haenggeli C, Maragakis NJ, Snyder EY, Rothstein JD. Neural stem cells protect against glutamate-induced excitotoxicity and promote survival of injured motor neurons through the secretion of neurotrophic factors. Mol Cell Neurosci. 2004;27(3):322–331. [DOI] [PubMed] [Google Scholar]
- 24. Lu S, Lu C, Han Q, Li J, Du Z, Liao L, Zhao RC. Adipose-derived mesenchymal stem cells protect PC12 cells from glutamate excitotoxicity-induced apoptosis by upregulation of XIAP through PI3-K/Akt activation. Toxicol. 2011;279(1-3):189–195. [DOI] [PubMed] [Google Scholar]
- 25. Pappas IS, Parnavelas JG. Neurotrophins and basic fibroblast growth factor induce the differentiation of calbindin-containing neurons in the cerebral cortex. Exp Neurol. 1997;144(2):302–314. [DOI] [PubMed] [Google Scholar]
- 26. Baraniak PR, McDevitt TC. Stem cells paracrine actions and tissue regeneration. Regen Med. 2010;5(1):121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jones J, Jaramillo-Merchán J, Bueno C, Pastor D, Viso-León M, Martínez S. Mesenchymal stem cells rescue Purkinje cells and improve motor functions in a mouse model of cerebellar ataxia. Neurobiol Dis. 2010;40(2):415–423. [DOI] [PubMed] [Google Scholar]
- 28. Li J, Imitola J, Snyder EY, Sidman RL. Neural stem cells rescue nervous Purkinje neurons by restoring molecular homeostasis of tissue plasminogen activator and downstream targets. J Neurosci. 2006;26(30):7839–7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee H, Lee JK, Min WK, Bae JH, He X, Schuchman EH, Jin HK. Bone marrow-derived mesenchymal stem cells prevent the loss of Niemann-Pick type C mouse Purkinje neurons by correcting sphingolipid metabolism and increasing sphingosine-1-phosphate. Stem Cells. 2010;28(4):821–831. [DOI] [PubMed] [Google Scholar]
- 30. Hovakimyan M, Müller J, Wree A, Ortinau S, Rolfs A, Schmitt O. Survival of transplanted human neural stem cell line (ReNcell VM) into the rat brain with and without immunosuppression. Ann Anat. 2012;194(5):429–435. [DOI] [PubMed] [Google Scholar]
- 31. Leitner J, Drobits K, Pickl WF, Majdic O, Zlabinger G, Steinberger P. The effects of cyclosporine A and azathioprine on human T cells activated by different costimulatory signals. Immunol Lett. 2011;140(1-2):74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brunner LJ, Bennett WM, Koop DR. Cyclosporine suppresses rat hepatic cytochrome P450 in a time-dependent manner. Kidney Int. 1998;54(1):216–223. [DOI] [PubMed] [Google Scholar]