Abstract
Background:
Curcumin, green tea polyphenols and selenium possess anti-inflammatory and anti-oxidant properties. Individually they have demonstrated some efficacy in animal models and human subjects with inflammatory bowel disease (IBD). To evaluate the efficacy and safety of Coltect [Curcumin (500 mg), green tea (250 mg) and selenium (100 µg)] in vivo and in patients with ulcerative colitis (UC).
Methods:
Each component was compared to placebo in a DSS mice colitis model. The efficacy was validated in a 2,4,6-trinitrobenzenesulfonic acid (TNBS) rat colitis model. Twenty patients with mild-to-moderate UC received two Coltect tablets twice daily for 8 weeks. Enrollees underwent sigmoidoscopy at study entrance and closure, and physical and laboratory evaluation at baseline, 4 and 8 weeks.
Results:
Coltect showed a synergistic therapeutic effect in the DSS and TNBS models. Disease activity was significantly higher in the placebo versus the treated group (p < 0.05). Selenium was the more active component. The contribution of green tea was minor. In the TNBS model, the Wallace scores for macroscopic lesions were 4.8 ± 1.5 (treatment) and 8.2 ± 0.5 (placebo) (p = 0.01). In humans, Coltect was well tolerated and effective. Fourteen subjects (70%) improved: nine (45%) went into complete remission, four (20%) experienced marked improvement and one (5%) experienced moderate improvement at the end of the trial. Clinical activity index decreased significantly at 4 and 8 weeks (p < 0.001). Two patients had no change in their symptoms, and one withdrew after 4 weeks. Flare-up in four subjects caused three to withdraw from the study after less than 4 weeks. Endoscopic improvement was observed in 11 (69%) patients, and four patients (25%) achieved complete remission.
Conclusions:
Coltect may serve as a first-line or add-on therapy in patients with mild-to-moderate UC.
Keywords: Coltect, curcumin, green tea, selenium, ulcerative colitis
Introduction
Curcumin (diferuloylmethane), an active ingredient in the food additive of Indian curry spice, is derived from the plant Curcuma Longa.1 It has been used for centuries as a remedy for a wide range of inflammatory diseases worldwide: its anti-inflammatory and anti-oxidative properties were demonstrated in several in vitro experiments on human lymphocytes and a gut epithelial cell line,2,3 as well as in an in vivo model of murine experimental colitis.4,5 In humans, curcumin reportedly maintained remission for up to 12 months in ulcerative colitis (UC) patients.6 In a recent study, Lang and colleagues showed that the addition of curcumin to mesalamine therapy was superior to the combination of placebo and mesalamine in inducing clinical and endoscopic remission in patients with mild-to-moderate active UC, without causing any apparent adverse effects.7 Curcumin is very safe and non-toxic, even when consumed in high doses (e.g. 8 g/day).8
Green tea extract is widely consumed worldwide as a herbal medicine for many illnesses. Its anti-oxidative and anti-inflammatory properties stem from its high concentration of various polyphenols. The most abundant catechin in green tea is epigallocatechin-3-gallate, which has been shown to have potent biological activities, including anti-oxidative,9 anti-inflammatory10 and anti-mutagenic11 effects in a variety of experimental models.
Selenium is an essential trace element for humans and animals. The therapeutic role of selenium has been largely attributed to its presence in selenoproteins, such as the 21st amino acid, selenocysteine, which functions to modulate pathways involved in inflammation. Epidemiological studies suggest an inverse relationship between selenium levels and the degree of inflammatory bowel disease (IBD).12 Although the actual mechanism is not fully understood, this observation is supported by studies showing that selenium deficiency exacerbated experimental colitis by affecting various signaling pathways involved in inflammation and oxidative stress, as well as by altering the gut microbiota.13
We have shown that the most effective ratios of the three ingredients for cancer prevention in a rat model are curcumin (500 mg), green tea (250 mg) and selenomethionine (100 µg) (Coltect, Israel).14 Herein, the efficacy of the combination is shown in the DSS model in mice, and validated in a 2,4,6-trinitrobenzenesulfonic acid (TNBS) model in rats. In the clinical setting our results suggest that Coltect may be an effective treatment in patients experiencing mild-to-moderate UC; however, in order to confirm its clinical efficacy larger trials are required.
Materials and methods
Animals
Male Wistar rats (6–8 weeks old) and C57BL/6J female mice (5 weeks old) were obtained from Harlan Laboratories (Rehovot, Israel). They were housed at the animal facility of the Tel-Aviv Sourasky Medical Center in plastic cages covered with metal grids (three animals per cage) in a temperature-controlled room (21–25°C) with a 12-h light/12-h dark cycle. The study was approved by the Institutional Committee for Animal Welfare of the Tel-Aviv Sourasky Medical Center (protocol number 1-12-09).14
Induction of colitis in murine models and experimental design
DSS-induced colitis in mice
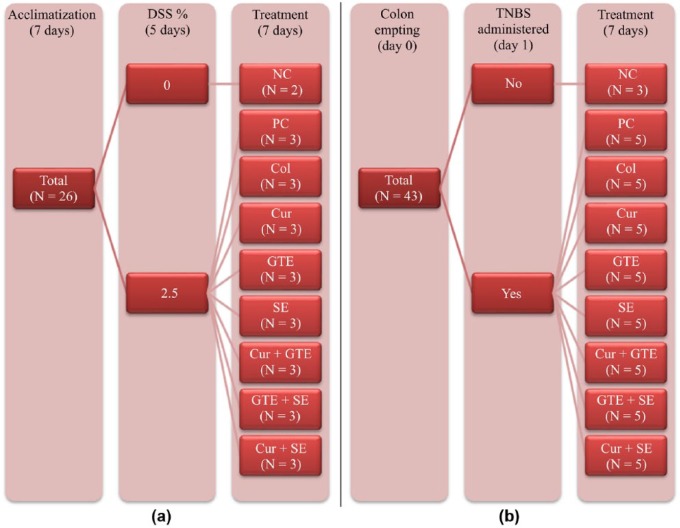
After 1 week of acclimation, the mice were exposed to 2.5% dextran sodium sulfate (DSS; MP Biomedicals, LLC, France) administered in the drinking water for 5 days ad libitum, followed by 2 days of tap water. Four mice received saline (2.5%) and served as the negative control group. The mice were randomly divided into eight subgroups (n = 6) that were treated as described in Figure 1(a). The tested formulas were administered in doses of 0.3 ml of tap water with 2% dimethyl sulfoxide by oral gavages. Severity of colitis was determined based on the following parameters:
Figure 1.
Flow charts of the (dextran sodium sulfate) DSS (a), and 2,4,6-trinitrobenzenesulfonic acid (TNBS) (b) colitis models.
Trinitrobenzenesulfonic acid-induced colitis in rats
The rats were weighed and then fasted for 24 h (for colon emptying) prior to the beginning of the experiment. On the day of the experiment, the rats were anesthetized by an intraperitoneal injection of halothane, and colitis was induced by intrarectal administration of 0.4 ml of a 25 mg/ml 2,4,6-trinitrobenzenesulfonic acid (TNBS) solution dissolved in 50% ethanol using an 8 cm long rubber catheter. Then, 0.4 ml of air was injected and the rats were kept in a Trendelenburg position for 30 seconds in order to ensure complete drainage of the TNBS solution from the catheter. Negative control rats (n = 3) received intrarectal administration of saline. The remaining rats were randomly divided into eight subgroups (n = 5) that were treated in all possible combinations of curcumin, green tea and selenomethionine, as described in Figure 1(b). Treatment was delivered by daily gavages of 0.5 ml solution starting from day 0.
All rats were sacrificed after 7 days. Their 10 cm distal colon was removed, opened longitudinally along the anti-mesenteric side and gently washed with ice-cold saline in order to remove all luminal content. The colon wet weight was measured, and colonic injury was immediately examined under a stereomicroscope and scored for visible damage. Macroscopic injury was based on a revised colonic damage scoring system as described by Wallace and colleagues.17
The induction and severity of colitis were evaluated by measuring:
daily body weight
colonic weight
macroscopic and histological score damage.
Phase 2A clinical trial
All participating patients signed a written consent form. The study was approved by the ethics committee of the Tel-Aviv Sourasky Medical Center and conducted in accordance with the Declaration of Helsinki [ClinicalTrials.gov identifier: NCT00793130].
Patients were eligible for enrollment in the study provided they were aged 18 years or older and had active mild-to-moderate UC [clinical activity index (CAI) 4–8] based on typical clinical endoscopic and histological criteria. Exclusion criteria (Table 1) included severe active UC, active chronic inflammatory or autoimmune disease other than UC (including Crohn’s disease), active infection (including viral infection), acute or chronic cardiac and/or renal failure, abnormal liver function, use of rectal corticosteroids within 4 weeks before study entry, known or suspected bleeding tendency, known thrombo-embolic events or current use of anticoagulants, infectious or ischemic colitis and a history of colorectal cancer. The subjects received a combined therapy of two tablets twice daily for 8 weeks. Each tablet contained curcumin (500 mg), green tea (250 mg) and selenium (100 µg).
Table 1.
Eligibility criteria.
| Inclusion criteria |
| Age 18–75 years |
| Active mild-to-moderate ulcerative colitis (4 < CAI ⩽ 8) |
| Hemoglobin >10 g/dL |
| Platelets count >100 103/µL |
| Normal liver functions |
| Normal renal functions |
| Willing to undergo sigmoidoscopy twice |
| Exclusion criteria |
| Severe active ulcerative colitis (CAI > 8) |
| Crohn’s disease |
| Active chronic inflammatory or autoimmune disease other than ulcerative colitis |
| Infectious/ischemic colitis |
| Active infection, including viral infection |
| Acute or chronic cardiac failure |
| Acute or chronic renal failure |
| Liver function abnormalities |
| Use of any corticosteroids within 4 weeks prior to study entry |
| Known thromboembolic disposition or current use of anticoagulants |
| Known or suspected bleeding tendency |
| Present or past colorectal cancer |
CAI, clinical activity index.
Patients included were those with new onset recently diagnosed disease as well as patients with chronic disease of a few years’ duration. None of the patients had received any medications (e.g. steroids, azathioprine and biological therapy) but to concomitant therapy with mesalamine.
Sigmoidoscopy was performed at study entrance and closure. Physical examination, determination of CAI, stool culture, complete chemical analysis of blood, C-reactive protein (CRP) and blood count were analyzed at baseline and at 4 and 8 weeks. The treating physician was asked to evaluate the global change in patient symptoms during each follow-up visit. The primary endpoints were improvement in CAI, as well as macroscopic (sigmoidoscopy) and microscopic (biopsy) scoring. Patients treated for other medical conditions (e.g. hypertension, diabetes mellitus, hypercholesterolemia, etc.) were asked not to make any changes in their regular treatment.
Statistics
The results are presented as a mean ± standard deviation. The Kruskal–Wallis test and χ2 tests were used to detect differences in continuous and categorical parameters between study groups, respectively. Two group comparisons were done using the Mann–Whitney U test for continuous variables and the Fisher exact test for categorical variables. Paired and unpaired tests were applied as appropriate. All statistics were performed using IBM SPSS software version 22.0 and Microsoft Excel 2010. A two-sided p-value of 0.05 or less was considered as being significant.
Results
The effect of the different components on DSS-induced colitis in mice
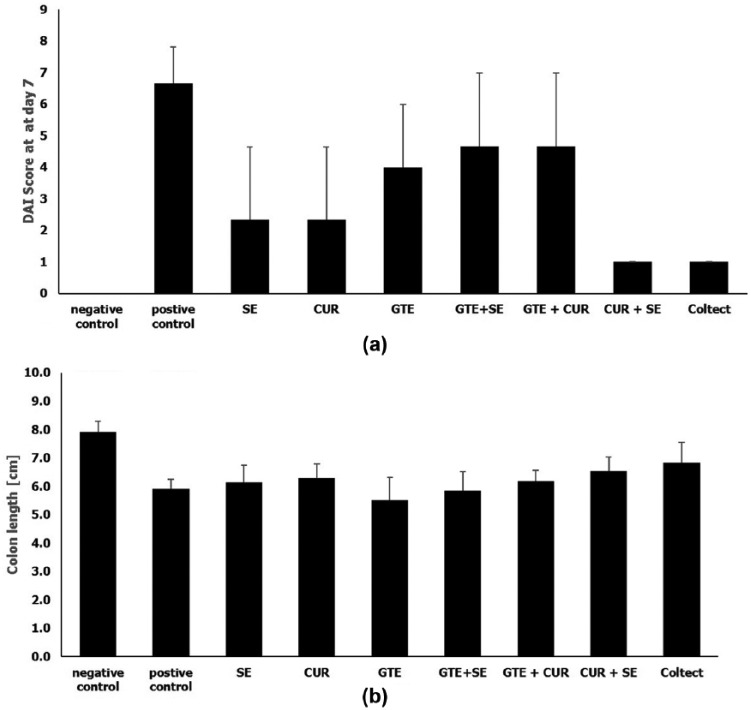
Improved histological score and a significantly lower DAI score (p < 0.05) were observed among mice treated with selenium (2.3 ± 2.3), curcumin (2.3 ± 2.3), a combination of selenium and curcumin (1.0 ± 0.0), and Coltect (1.0 ± 0.0) compared to the positive control group (6.67 ± 1.16) (Figure 2(a)). Green tea and the combination of green tea with either selenium or curcumin had a non-significant effect. Figure 2(b) depicts the mean colon lengths measured at day 7. Mice treated with green tea or green tea and selenium had the shortest colon length (5.5 ± 0.56 and 5.8 ± 0.29 cm, respectively), while those treated with the combination of selenium and curcumin or Coltect had the longest colon (6.53 ± 0.32 and 6.8 ± 0.29 cm, respectively, p < 0.05).
Figure 2.
Mean disease activity index (DAI) (a) and colonic length (b) on day 7 in the DSS colitis model.
CUR, curcumin; GTE, green tea; SE, selenium.
No significant changes in spleen weight were observed.
Effect of TNBS-induced colitis in rats
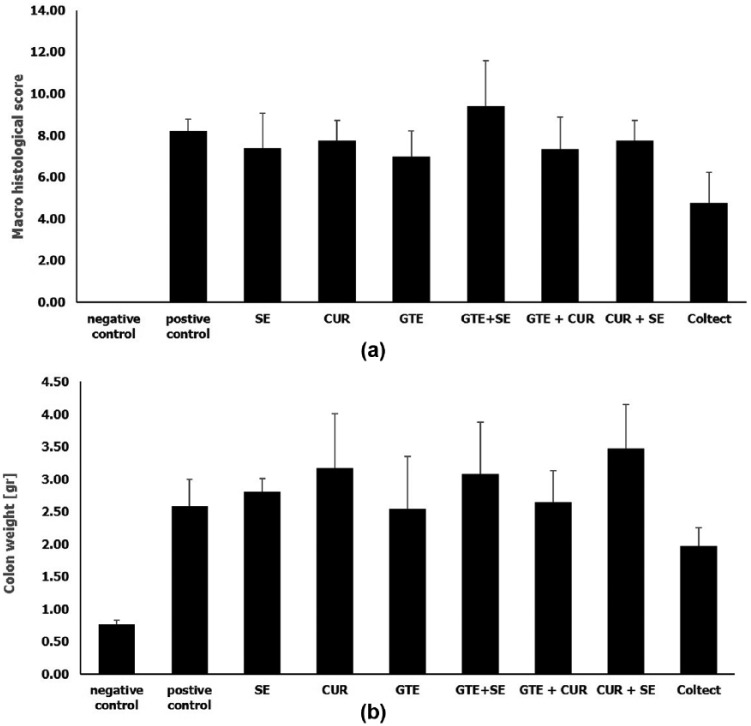
Figure 3 summarizes the macroscopic and histological damage score (Figure 3(a)) and the colonic weight (Figure 3(b)) for each group at the end of the experiment. The mean damage score in all study groups was lower than the positive control group (8.2 ± 0.5 p < 0.05). However, the histological improvement was statistically significant only among rats treated with Coltect (4.8 ± 1.5, p = 0.010). In addition, the combined drug was more effective in reducing colonic weight than the individual components.
Figure 3.
Mean macroscopic histological score (a) and colonic weight (b) in the TNBS colitis model.
CUR, curcumin; GTE, green tea; SE, selenium.
Clinical trial
Fifty-two UC patients were screened for their eligibility; 26 met the inclusion criteria for the trial. Twenty subjects signed the informed consent and entered the study (Figure 4). Nineteen patients were already receiving mesalamine upon study entry, and one patient had new disease onset. The participants’ baseline characteristics are summarized in Table 2. Overall, the clinical picture of 14 subjects (70%) improved: nine (45%) went into complete remission, four (20%) experienced marked improvement and one (5%) experienced moderate improvement at the end of the trial. Two patients had no change in their symptoms, and one of them withdrew after 4 weeks of treatment. Four subjects experienced exacerbation of their disease, which caused three of them to withdraw from the study after less than 4 weeks of treatment. These patients were included in the intention to treat but not in the per-protocol analysis.
Figure 4.
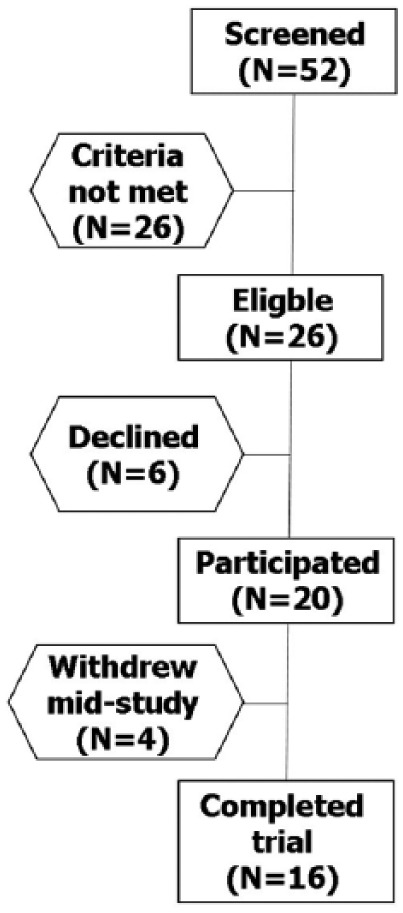
Flow chart of the phase 2A clinical trial.
Table 2.
Baseline characteristics of the Phase 2A clinical trial study population.
| Characteristic | n (mean) | ± SD/% | |
|---|---|---|---|
| Gender | Male | 12 | 60% |
| Female | 8 | 40% | |
| Age (years) | 43.10 | 14.0 | |
| Clinical activity index | 6.55 | 0.95 | |
| Number of weekly stools | <18 | 0 | 0% |
| 18–35 | 7 | 35% | |
| 36–60 | 11 | 55% | |
| >60 | 2 | 10% | |
| Blood in stool | Trace | 14 | 70% |
| Substantial | 6 | 30% | |
| Abdominal pain/cramps | None | 7 | 35% |
| Mild | 9 | 45% | |
| Moderate | 4 | 20% | |
| Global assessment of health | Good | 1 | 5% |
| Average | 17 | 85% | |
| Poor | 2 | 10% | |
| Hemoglobin | 13.45 | 1.50 | |
| C-reactive protein | 6.80 | 7.9 | |
| Erythrocyte sedimentation rate | 30.30 | 19.1 | |
| Extent of inflammation | Sigmoid | 8 | 40% |
| Splenic flexure | 10 | 50% | |
| Pan-colonic | 2 | 10% |
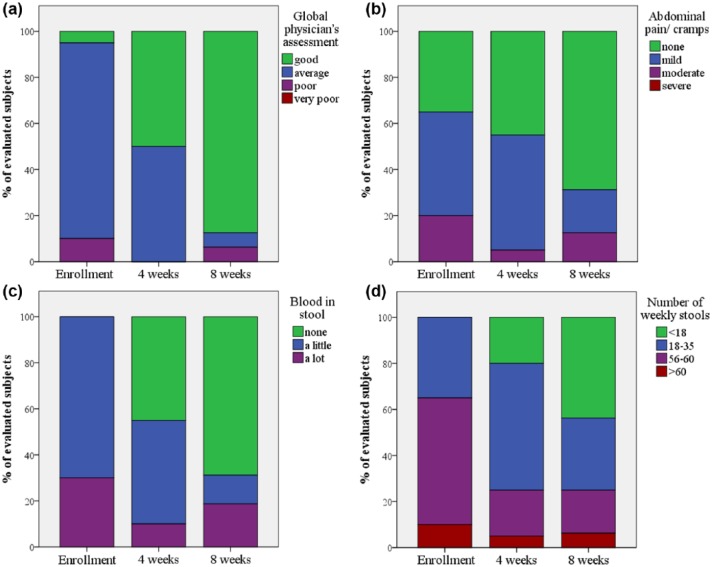
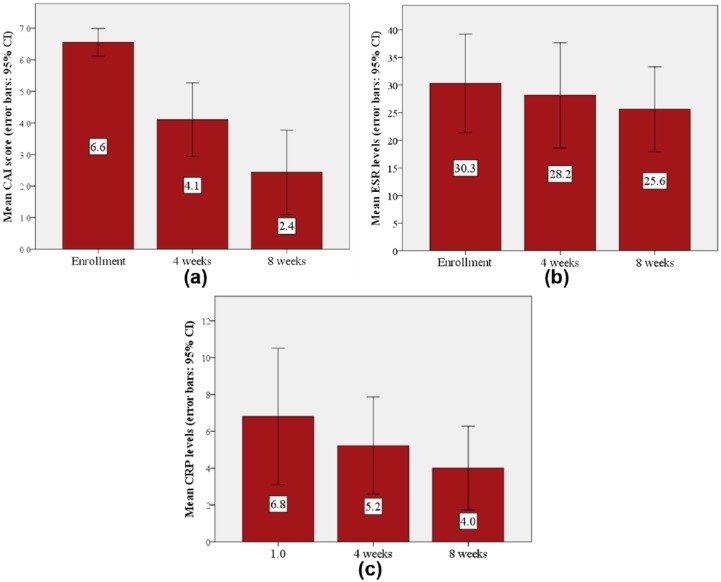
The investigator’s overall assessment was that the patients’ conditions had substantially improved, and were considered as being excellent in 14 out of the 16 patients who completed the study (Figure 5(a)). The patients’ symptoms had improved, with a decrease in abdominal pain, amount of blood in stool and the number of weekly stools (Figure 5(b)–(d), respectively). In the per-protocol analysis, the CAI scores (Figure 6(a)) were significantly reduced, as early as 4 weeks (p = 0.003). They further improved by a total of 4.19 ± 2.61 points on the last follow-up visit after 8 weeks of treatment (p < 0.001). The mean levels of both the CRP and erythrocyte sedimentation rate (ESR) normalized during the trial period (CRP: 6.8 versus 4.0; ESR: 30.3 versus 25.6) (Figure 6(b) and (c), respectively). There was no significant change in the hemoglobin levels. Endoscopic evaluation at the beginning and end of the study was available for 16 patients. Endoscopic improvement (defined as any drop ⩾1 in endoscopic component of the Mayo score18) was observed in 11 (69%) patients, and four patients (25%) achieved complete remission. The mean endoscopic score was significantly reduced from 1.69 ± 0.79 to 1.00 ± 0.73 (p = 0.012).
Figure 5.
Changes in distribution of clinical activity index score components of 16 patients: investigator’s global assessment (a), abdominal pain (b), blood in stool (c) and number of weekly stools (d).
Figure 6.
Comparison by visit of 16 patients of mean CAI score (a), CRP levels (b) and ESR levels (c).
CAI, clinical activity index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
Safety evaluation
No serious adverse events were reported. Five patients experienced mild adverse events, which included mild nausea and headache, transient increase in stool frequency and transient abdominal bloating. None of these events were considered to be drug related by the treating physician. No patient discontinued the trial because of drug-related side effects.
Discussion
The combination of curcumin, green tea and selenium is efficacious in improving the symptoms of colitis in murine models of experimental colitis and in patients with UC. The findings in murine models suggest a synergistic effect of Coltect’s components in controlling inflammation, making it superior to the use of each substance alone. The results of this phase 2A clinical trial suggest substantial improvement in symptoms, disease activity and mucosal healing in mild-to-moderate UC. Coltect can be used either as first-line therapy or as adjuvant therapy to a regimen of mesalamine.
Curcumin is known to be beneficial in patients with IBD.19 One of the advantages of curcumin, as a component in combination therapy, is the potential improvement in efficacy without hampering safety, especially if the drugs differ in their mode of action. Indeed, Lang and colleagues7 used a combination of curcumin and 5-amino salicylic acid in a randomized, multicenter, double-blind, placebo-controlled trial that showed efficacy of the combination in patients with mild-to-moderate UC.
The combination of these ingredients may be used in cancer prevention. Amantana and colleagues20 demonstrated that supplementing green tea with selenium increased the tea’s anti-mutagenic effects. We have previously shown that the combination of curcumin and celecoxib significantly and synergistically inhibited colorectal cancer carcinogenesis in vivo in experimental cancer models.21,22 The exact mechanism by which these ingredients play a role in conferring the observed clinical effect in active UC remains to be elucidated.
After confirming its efficacy in the DSS model in mice, the efficacy of the compound was further validated in a rat model of TNBS-induced colitis. The promising in vivo results led to a phase 2A clinical study. The extent and severity of the disease was meticulously evaluated clinically and endoscopically.
The small sample size, the short duration and the lack of post-study safety follow-up may not be sufficient to preclude rare side effects, and therefore limit our ability to fully evaluate Coltect’s safety profile. It is worthwhile mentioning that off-protocol follow-up suggests an excellent safety profile. Overall, Coltect is well tolerated and is not associated with an increased rate of adverse events. Moreover, the use of curcumin, selenium and green tea in other clinical trials has not demonstrated significant safety issues.7,23–25
The small number of human participants and the lack of a control group precludes the ability to estimate the role of a placebo effect, yet the high response rate observed in this small phase 2A study is well beyond the response rate of 12% among patients receiving placebo in similar previous studies.7 In addition, the objective improvement in both inflammatory markers and endoscopic evaluations observed in both murine models further supports the beneficial effect of Coltect in active UC.
A possible selection bias might have occurred due to mid-study withdrawal of four patients, although the reason for withdrawal was unrelated to the trial itself, including side effects.
One of the drawbacks of the current work is that although we were able to show the efficacy and safety of the Coltect compound, and that it is superior to each component alone in controlling inflammation, the underlying mechanism was not addressed nor investigated. Both curcumin and selenium were found to reduce colitis and produced an increased effect when used together, suggesting synergistic activity. Immunologic properties of curcumin include the inhibition of the nuclear factor kappa B pathway,4,26 tumor necrosis factor-alpha secretion27,28 and CD4 T-cell proliferation.3 The mechanism of selenium is not yet known. However, selenium (and selenoprotein) deficiency exacerbates experimental colitis by affecting various signaling pathways involved in inflammation and oxidative stress as well as by altering the gut microbiota.13
Coltect can be used as first-line therapy as well as an add-on to mesalamine.
It can enrich the limited options of first-line therapy in patients with mild-to-moderate disease severity. A natural effective therapy in the arsenal of weapons against UC is plausible, particularly in the setting of UC that mostly affects youngsters whose reluctance to use drugs is well documented.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: Drs. Arber and Katz have a patent issued for Coltect. Drs. Arber and Katz were part of the development team of Coltect, but did not receive any payment or other benefits for the present study. The other authors have no conflicts of interest to disclose.
Contributor Information
Shiran Shapira, The Integrated Cancer Prevention Center, Tel-Aviv Sourasky Medical Center, affiliated to Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Ari Leshno, The Integrated Cancer Prevention Center, Tel-Aviv Sourasky Medical Center, affiliated to Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Daniel Katz, MediGlobe Ltd, Israel.
Nitsan Maharshak, IBD Center, Department of Gastroenterology and Liver Diseases, Tel-Aviv Sourasky Medical Center, affiliated to Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Gil Hevroni, The Integrated Cancer Prevention Center, Tel-Aviv Sourasky Medical Center, affiliated to Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Maayan Jean-David, The Integrated Cancer Prevention Center, Tel-Aviv Sourasky Medical Center, affiliated to Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Sarah Kraus, The Integrated Cancer Prevention Center, Tel-Aviv Sourasky Medical Center, affiliated to Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Lior Galazan, The Integrated Cancer Prevention Center, Tel-Aviv Sourasky Medical Center, affiliated to Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Ilan Aroch, The Integrated Cancer Prevention Center, Tel-Aviv Sourasky Medical Center, affiliated to Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Dina Kazanov, The Integrated Cancer Prevention Center, Tel-Aviv Sourasky Medical Center, affiliated to Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Aharon Hallack, IBD Center, Department of Gastroenterology and Liver Diseases, Tel-Aviv Sourasky Medical Center, affiliated to Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Stewart Becker, IBD Center, Department of Gastroenterology and Liver Diseases, Tel-Aviv Sourasky Medical Center, affiliated to Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Mark Umanski, IBD Center, Department of Gastroenterology and Liver Diseases, Tel-Aviv Sourasky Medical Center, affiliated to Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Menachem Moshkowitz, The Integrated Cancer Prevention Center, Tel-Aviv Sourasky Medical Center, affiliated to Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel IBD Center, Department of Gastroenterology and Liver Diseases, Tel-Aviv Sourasky Medical Center, affiliated to Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Iris Dotan, IBD Center, Department of Gastroenterology and Liver Diseases, Tel-Aviv Sourasky Medical Center, affiliated to Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Nadir Arber, Head, the Integrated Cancer Prevention Center, Tel-Aviv Sourasky Medical Center, 6th Weizmann St., Tel-Aviv 6423906, Israel.
References
- 1. Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: from farm to pharmacy. BioFactors 2013; 39: 2–13. [DOI] [PubMed] [Google Scholar]
- 2. Midura-Kiela MT, Radhakrishnan VM, Larmonier CB, et al. Curcumin inhibits interferon-gamma signaling in colonic epithelial cells. Am J Physiol Liver Physiol 2012; 302: G85–G96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larmonier CB, Midura-Kiela MT, Ramalingam R, et al. Modulation of neutrophil motility by curcumin: implications for inflammatory bowel disease. Inflamm Bowel Dis 2011; 17: 503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Venkataranganna MV, Rafiq M, Gopumadhavan S, et al. NCB-02 (standardized Curcumin preparation) protects dinitrocholorobenzene-induced colitis through down-regulation of NFkappa-B and iNOS. World J Gastroenterol 2007; 13: 1103–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sugimoto K, Hanai H, Tozawa K, et al. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology 2002; 123: 1912–1922. [DOI] [PubMed] [Google Scholar]
- 6. Hanai H, Iida T, Takeuchi K, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol 2006; 4: 1502–1506. [DOI] [PubMed] [Google Scholar]
- 7. Lang A, Salomon N, Wu J, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol 2015; 13: 1444–1449. [DOI] [PubMed] [Google Scholar]
- 8. Lao CD, Ruffin MT, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med 2006; 6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst 1993; 85: 1038–1049. [DOI] [PubMed] [Google Scholar]
- 10. August DA, Landau J, Caputo D, et al. Ingestion of green tea rapidly decreases prostaglandin E2 levels in rectal mucosa in humans. Cancer Epidemiol Biomarkers Prev 1999; 8: 709–713. [PubMed] [Google Scholar]
- 11. Roy M, Chakrabarty S, Sinha D, et al. Anticlastogenic, antigenotoxic and apoptotic activity of epigallocatechin gallate: a green tea polyphenol. Mutat Res 2003; 523–524: 33–41. [DOI] [PubMed] [Google Scholar]
- 12. Barnett M, Bermingham E, McNabb W, et al. Investigating micronutrients and epigenetic mechanisms in relation to inflammatory bowel disease. Mutat Res 2010; 690: 71–80. [DOI] [PubMed] [Google Scholar]
- 13. Kudva AK, Shay AE, Prabhu SK. Selenium and inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 2015; 309: G71–G77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aroch I, Kraus S, Naumov I, et al. Chemopreventive effects of Coltect, a novel dietary supplement, alone and in combination with 5-aminosalicylic acid in 1,2-dimethylhydrazine-induced colon cancer in rats. Therap Adv Gastroenterol 2010; 3: 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murthy SNS, Cooper HS, Shim H, et al. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci 1993; 38: 1722–1734. [DOI] [PubMed] [Google Scholar]
- 16. Cooper HS, Murthy SN, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Investig 1993; 69: 238–249. [PubMed] [Google Scholar]
- 17. Wallace JL, Keenan CM, Gale D, et al. Exacerbation of experimental colitis by nonsteroidal anti-inflammatory drugs is not related to elevated leukotriene B4 synthesis. Gastroenterology 1992; 102: 18–27. [DOI] [PubMed] [Google Scholar]
- 18. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med 1987; 317: 1625–1629. [DOI] [PubMed] [Google Scholar]
- 19. Triantafyllidi A, Xanthos T, Papalois A, et al. Herbal and plant therapy in patients with inflammatory bowel disease. Ann Gastroenterol 2015; 28: 210–220. [PMC free article] [PubMed] [Google Scholar]
- 20. Amantana A, Santana-Rios G, Butler JA, et al. Antimutagenic activity of selenium-enriched green tea toward the heterocyclic amine 2-amino-3-methylimidazo[4,5-f]quinoline. Biol Trace Elem Res 2002; 86: 177–191. [DOI] [PubMed] [Google Scholar]
- 21. Shpitz B, Giladi N, Sagiv E, et al. Celecoxib and curcumin additively inhibit the growth of colorectal cancer in a rat model. Digestion 2007; 74: 140–144. [DOI] [PubMed] [Google Scholar]
- 22. Lev-Ari S, Strier L, Kazanov D, et al. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin Cancer Res 2005; 11: 6738–6744. [DOI] [PubMed] [Google Scholar]
- 23. Thomas R, Williams M, Sharma H, et al. A double-blind, placebo-controlled randomised trial evaluating the effect of a polyphenol-rich whole food supplement on PSA progression in men with prostate cancer: the U.K. NCRN Pomi-T study. Prostate Cancer Prostatic Dis 2014; 17: 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vinceti M, Dennert G, Crespi CM, et al. Selenium for preventing cancer. Cochrane Database Syst Rev 2014: CD005195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inoue M, Sasazuki S, Wakai K, et al. Green tea consumption and gastric cancer in Japanese: a pooled analysis of six cohort studies. Gut 2009; 58: 1323–1332. [DOI] [PubMed] [Google Scholar]
- 26. Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (digeruloylmethane) [corrected]. J Biol Chem 1995; 270: 24995–25000. [DOI] [PubMed] [Google Scholar]
- 27. Chen D, Nie M, Fan M, et al. Anti-inflammatory activity of curcumin in macrophages stimulated by lipopolysaccharides from porphyromonas gingivalis. Pharmacology 2008; 82: 264–269. [DOI] [PubMed] [Google Scholar]
- 28. Chan M. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochem Pharmacol 1995: 1551–1556. [DOI] [PubMed] [Google Scholar]