Abstract
Purpose:
The purpose of this study was to determine the clinical utility of an algorithm-based decision tool designed to assess risk associated with opioid use in the primary care setting.
Methods:
A prospective, longitudinal study was conducted to assess the utility of precision medicine testing in 1822 patients across 18 family medicine/primary care clinics in the United States. Using the profile, patients were categorized into low, moderate, and high risk for opioid use. Physicians who ordered testing were asked to complete patient evaluations and document their actions, decisions, and perceptions regarding the utility of the precision medicine tests.
Results:
Approximately 47% of primary care physicians surveyed used the profile to guide clinical decision-making. These physicians rated the benefit of the profile on patient care an average of 3.6 on a 5-point scale (1 indicating no benefit and 5 indicating significant benefit). Eighty-eight percent of all clinicians surveyed felt the test exhibited some benefit to their patient care. The most frequent utilization for the profile was to guide a change in opioid prescribed. Physicians reported greater benefit of profile utilization for minority patients. Patients whose treatment was guided by the profile had pain levels that were reduced, on average, 2.7 levels on the numeric rating scale.
Conclusions:
The profile provided primary care physicians with a useful tool to stratify the risk of opioid use disorder and was rated as beneficial for decision-making and patient improvement by the majority of physicians surveyed. Physicians reported the profile resulted in greater clinical improvement for minorities, highlighting the objective use of this profile to guide judicial use of opioids in high-risk patients. Significantly, when physicians used the profile to guide treatment decisions, patient-reported pain was greatly reduced.
Keywords: primary care, precision medicine, personalized medicine, opioid, pain management, opioid use disorder
Introduction
The prescription rate and sales of opioids has skyrocketed since 1999,1 resulting in a public health epidemic. The highest-using and most at-risk nonmedical opioid abusers more often obtain drugs from a doctor’s prescription than from any other source,2 with a staggering 26% prevalence of opioid use disorder (OUD) among primary care patients with noncancer-related chronic pain.3 Nearly half (44.5%) of all opioid prescriptions come from primary care groups (family practice physicians, internists, and general practitioners),4-6 and despite this, many primary care physicians lack confidence in prescribing opioids safely and feel unsure about detecting, predicting, and discussing prescription opioid abuse with their patients.7 These physicians have concerns about opioid misuse, medication side effects, and opioid addiction8-11 and feel they lack sufficient training.9,12 Not surprisingly, most opioid-prescribing physicians support medical school and clinician education in addiction and chronic pain9,13; however, such education is woefully inadequate. In addition to providing the best possible care to their patients, physicians are subject to legal retribution if their prescribing practices come under legal scrutiny.14
Physicians struggle with caring for patients who need pain relief, while considering the overall health and welfare of the patient. In a survey of more than 1000 primary care physicians, more than half believe that opioids are an effective treatment for managing noncancer chronic pain, and 83% of respondents attribute responsibility for prescription opioid abuse to physician-initiated prescriptions.13 Chronic pain conditions can result in prolonged use of opioids, leading to downstream addiction, which necessitates careful consideration regarding overprescribing opioids to manage long-term chronic pain.15 The lack of confidence in prescribing opioids, combined with physician beliefs about opioids as an effective, yet tricky, pain management option, reveals the importance of better guidance for prescribing clinicians.
The Centers for Disease Control and Prevention (CDC) released recent guidelines for primary care physicians prescribing opioids for chronic pain.3 The 3 main foci of the guidelines are (1) determining “when to initiate or continue pain management with opioids,” (2) managing “opioid selection, dosage, duration, and discontinuation,” and (3) “assessing risk and addressing harms of opioid use.”3 The third area recommends using Prescription Drug Monitoring Databases and urine drug testing (UDT) as means of assessing the risks of opioid use. Other screening tools, such as Screener and Opioid Assessment for Patients With Pain–Revised (SOAPP-R), Opioid Risk Tool (ORT), and brief risk interview, are available to physicians to gauge potential misuse of opioids by patients.2 However, these screening tools are based on subjective information.
Mounting evidence has described the significant role of genetics in predisposition to risk of opioid abuse, misuse, or addiction, yet regular clinical evaluation of genetic factors for this purpose remains to be adopted. The profile accurately stratifies patients into low-, moderate, and high-risk categories using a combination of objective genetic information, along with phenotypic risk factors in a propriety algorithm.16-18 While the clinical validity of the profile is described elsewhere,16-18 the profile is 42% genetic and 58% phenotypic (Supplementary Table 1) and performs with high accuracy, sensitivity, and specificity. In this study, we observed the clinical utility of the profile in primary care settings and show that physicians use profile results to accomplish the 3 main foci of the CDC guidelines.
Methods
Study Population
A prospective, longitudinal study was conducted to assess the clinical utility of profile testing in 1822 patients across 18 primary care clinics, or study sites, in the United States. Research at each study site was conducted by participating physicians (n = 35). All physicians were trained to understand the results of the profile prior to the start of the study. Additionally, patients were enrolled in the study by physicians based on medical necessity for the assessment of risk to OUD. This study (Protocols 1JUL14-62CR, 1JAN15-14CR, 1JAN15-20CR) was reviewed, approved, and overseen by Solutions IRB, an institutional review board licensed by the US Department of Health and Human Services, Office for Human Research Protections. All participants signed informed consent forms prior to data collection. Per protocol, exclusion criteria were significant diminished mental capacity, recent febrile illness that precludes or delays participation by more than 1 month, pregnancy or lactation, incomplete gene report, participation in a clinical study that may interfere with participation in this study, and anything that would place the individual at increased risk or preclude full compliance.
Data Collection
Genomic DNA was isolated from buccal swabs obtained from each patient using a proprietary DNA isolation technique and DNA isolation kit (Macherey Nagel GmbH & Co, KG, Duren, Germany), according to the manufacturer’s instructions. Genotyping was performed using predesigned TaqMan assays (Applied Biosystems, Foster City, California). Allele-specific fluorescence signals were distinguished by measuring end point 6-FAM or VIC fluorescence intensities at 508 and 560 nm, respectively, and genotypes were generated using Genotyper Software V 1.3 (Applied Biosystems). The DNA elution buffer was used as a negative control, and K562 cell line DNA (Promega Corporation, Madison, Wisconsin), was included in each batch of samples tested as positive control.
Phenotypic information was also collected, including whether patients had a personal history of alcoholism, personal history of illegal drug abuse, personal history of prescription drug abuse, family history of alcoholism, family history of illegal drug abuse, family history of prescription drug abuse, mental health disorders and/or depression, and age. Physicians who ordered precision medicine testing were asked to complete patient evaluations 2 times in the study: (1) during a baseline study visit, after profile results were available for review and (2) approximately 1 month after baseline, when physicians were conducting a follow-up to evaluate patient improvement. The evaluation form consisted of a 12-item checklist of actions or decisions in the patient’s treatment that the physician might have made using profile guidance (Supplementary Table 2) and was used to document the physician’s assessment of the validity and utility of the profile. The physician rated the benefit of the profile on clinical decision-making during the baseline visit and the benefit of the profile to patient outcomes during the follow-up. The rating was on a scale of 1 to 5 (1: no benefit, 5: significant benefit). There were 2167 ratings in total—1629 and 538 ratings for patients’ baseline and follow-up visits, respectively, which were assessed approximately a month apart. An aggregate benefit rating (referred to as benefit rating from here on) was calculated across both visits in order to have 1 rating per patient, as there was no difference in the rating distribution or mean rating between visits, nor were there any significant differences in the ratings when physicians evaluated patients at both visits versus just one.
At the follow-up visit, approximately 1 month after physicians implemented treatment changes due to profile test results, patients were asked to rate their level of pain before and after taking medications using the pain numeric rating scale (NRS). An NRS of 0 was equivalent to “no pain,” while an NRS of 10 was equivalent to “agonizing” pain.
The Algorithm
A profile score and its associated risk stratification was calculated for each subject. The profile algorithm is a patent-protected, validated measure of OUD risk.16-18 In short, it combines phenotypic and genotype information to calculate a risk score that correlates with high-, moderate, or low-risk stratifications of OUD,16-18 such that a score of 1 to 11 is associated with low risk, 12 to 23 with moderate risk, and ≥24 with high risk. The genetic markers used in the algorithm include 11 different single-nucleotide polymorphisms that have been implicated in opioid abuse, misuse, dependence, or addiction (Supplementary Table 1). This approach, which focuses on validated genetic variants, as opposed to comprehensive next-generation sequencing, is the preferred approach of many in the field.19 The phenotypic factors tested include an age of 16 to 45 years,20,21 personal history of alcohol abuse,22,23 personal history of illegal drug abuse,24,25 personal history of prescription drug abuse,26 and personal history of other mental health diseases including attention deficit disorder,27 obsessive compulsive disorder,28 bipolar disorder,29 and schizophrenia.30 The algorithm is 42% genetic information and 58% phenotypic information.16
Statistical Analyses
During the baseline visit, physicians decided whether or not to use the profile to guide patient care (Supplementary Table 2). First, we assessed whether physicians thought patients significantly benefitted from the profile. Patients were divided into 2 groups: guided and not guided, where guided patients had physicians who checked “yes” on at least one of the decisions listed in Supplementary Table 2. To assess any bias in physician use of the profile, logistic regression was used to test differences in the odds of patients receiving profile-guided care with age, gender, and race. To maintain sufficient sample size, race was categorized as African American, Caucasian, Hispanic, other, and those who declined to answer. We also checked whether there was bias due to profile test results. For simplification, in this study, risk category (low, moderate, or high) rather than the raw profile score was used in downstream analyses. Ordinal regression was also used to model the relationship between benefit ratings and profile scores (by risk category), adjusting for whether or not the physician used the profile to guide decisions.
To assess how beneficial the test was to guided patients, we applied ordinal logistic regression to determine association of benefit ratings with each specific decisions physicians made, adjusting for age, gender, and race of patients when appropriate. Odds ratios (ORs) reported for ordinal logistic regression are proportional odds comparing all possible consecutive ratings (ie, rating of 5 vs 4, 4 vs 3, etc).
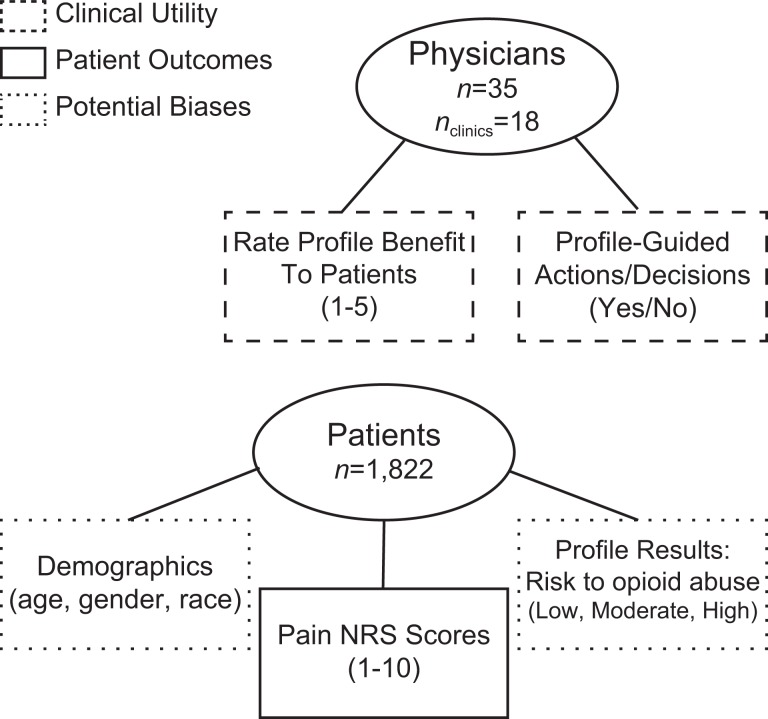
In addition to patient outcomes measured by the benefit rating by the physicians, we also examined patients’ self-reported NRS pain scores before and after receiving care from their physicians guided by profile results. The Wilcoxon signed rank test was used to determine whether there was significant change in patient NRS pain scores. All statistical analyses were performed with R version 3.2.5. P values ≤.05 were considered to be statistically significant. A summary of the data collected is shown in Figure 1.
Figure 1.
Summary of data collected in study from physicians and patients.
Results
Profile Guidance and Benefit to Patients
A total of 1822 patients were assessed in the study. During baseline and follow-up study visits, their physicians were asked to indicate how they used the profile results and rate the benefit of the profile on their patient care. Almost half (864, 47.4%) of patients’ physicians reported using the results of the profile to guide patient care decisions (Supplementary Table 2). The most frequent utilization of the profile included changing the opioid prescribed (n = 108) and using the information for referrals (n = 45; Table 1).
Table 1.
Benefit of the Profile by Patient Care Decisions Made by Physicians Who Used the Profile for Guidance.a
| Decision | n | % of Guided | P Value | OR | 95% CI |
|---|---|---|---|---|---|
| Decreased total opioid dose or frequency | 23 | 2.7 | 2.58 × 10− 5b | 15.2 | 4.9-671 |
| Changed opioid prescribed | 108 | 12.5 | 1.64 × 10− 5b | 5.2 | 2.5-11.3 |
| Increased total opioid dose or frequency | 25 | 2.9 | 2.66 × 10− 4b | 4.9 | 2.1-11.9 |
| Advised another provider to make changes in this patient’s prescriptions | 45 | 5.2 | 5.15 × 10− 4b | 2.9 | 1.6-5.4 |
| Started an opioid rotation | 13 | 1.5 | .55 | 1.4 | 0.5-4.4 |
| Switched from an opioid to a nonopioid pain medication | 19 | 2.2 | .62 | 1.3 | 0.5-3.1 |
| Spent more time with the patient | 846 | 97.9 | 4.99 × 10− 3b | 0.5 | 0.3-0.8 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aIn total, 864 (47.4%) patients received profile-guided decisions. Odds ratios are proportional odds after adjusting for age, gender, and race. Other decisions (Supplementary Table 2) not listed had n < 10.
bStatistical significance, P < .05.
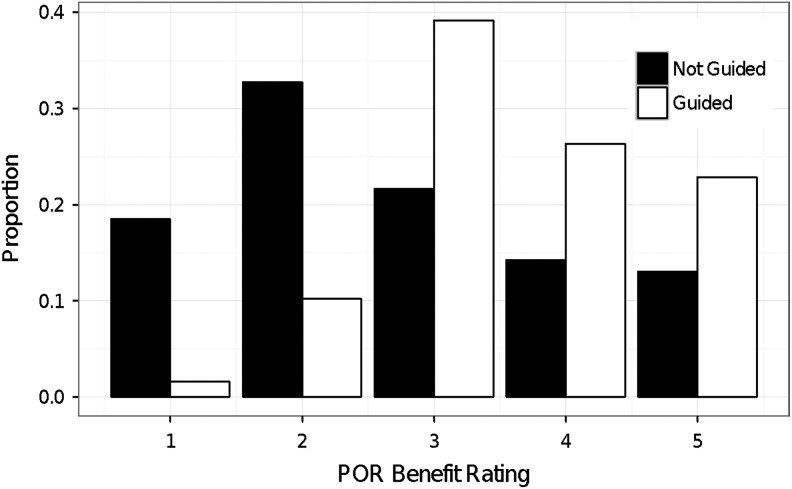
In analyzing the benefit to patients based on physician perception of how the test helped their patients (benefit ratings ranging from 1 to 5, where 1 was no benefit and 5 was significant benefit), physicians reported that overall 88% of patients benefited from the profile (benefit rating >1; Figure 2). Of physicians who used the profile to guide treatment decisions (listed in Supplementary Table 2), over 98% felt the profile provided at least some benefit to their patients (benefit rating >1), with an average benefit rating of 3.6 (standard deviation = 1.0; Figure 2). However, the benefit ratings varied depending on which actions were taken. From most to least significant (P ≤ .05), the following decisions were most associated with increased ratings: “Decided to titrate the patient off opioids,” “Changed opioid prescribed,” “Decreased total opioid dose or frequency,” “Increased total opioid dose or frequency,” and “Advised another provider to make changes in this patient’s prescriptions.” Only the decision, “Spent more time with the patient,” was significantly associated with decreased ratings, that is, physicians spent more time with patients whom they rated the profile was not as beneficial (Table 1).
Figure 2.
Physicians found more benefit to patient care when guided by the profile. Physicians were asked to rate the benefit of the profile to their patient on a scale of 1 to 5 (1: no benefit, 5: significant benefit). Physicians rated profile more 0.9 points higher for patient benefit if they used it to guide decisions (mean rating [SD]: not guided, 2.7 [1.3]; guided 3.6 [1.0]; total n = 1822). As ratings increased, physicians who used the profile to guide decisions were on average 4.40 times more likely to rate the benefit of the profile higher than physicians who did not make guided decisions. (P = 1.35 × 10−58, adjusted for age, gender, and race). Each bar represents the proportion of patients whose physician’s decisions were guided or not guided.
Physicians who made profile-guided decisions for their patients rated the benefit of the test 0.9 points higher than physicians who did not use the profile to guide decisions, though physicians who did not use the profile to guide patient care still reported some benefit from the profile results (3.6 vs 2.7; Figure 2). Physician ratings between patients who received guided and not guided decisions were associated with an adjusted proportional OR of 4.40 (P = 1.35 × 10−58). In other words, as ratings increased, physicians who used the profile to guide decisions were on average 4.40 times more likely to rate the benefit of the profile higher than physicians who did not make guided decisions.
Effect of Profile Score on Benefit to Patient Care
Overall, physicians rated the benefit of the profile higher if their patient’s profile score was also higher. If they used the profile to guide decisions, they were even more likely to rate it higher (Table 2). When modeling benefit ratings only with profile risk category for patients receiving guided decisions, ratings for patients who scored as moderate risk (profile score 12-23) were 1.3 times higher than those who scored as low risk (profile score <12, P = .002). Ratings for patients who scored as high risk (profile score ≥24) were 1.85 times higher than those who scored as low risk (P = .002). However, receiving guided decisions had an even greater impact, specifically 3.83 times more on benefit ratings than profile scores (P = 3.85 × 10−51).
Table 2.
Effect of Profile Score and Profile-Guided Decisions on Patient Care.a
| Total N (Guided/Not Guided) | Average Rating (Guided/Not Guided) | P Value | Adjusted OR | 95% CI | |
|---|---|---|---|---|---|
| Profile score category | |||||
| Lowb | 902 (402/500) | 3.0 (3.5/2.6) | |||
| Moderate | 831 (413/418) | 3.2 (3.6/2.8) | .002 | 1.30 | 1.10-1.54 |
| High | 89 (49/40) | 3.5 (3.7/3.2) | .002 | 1.85 | 1.26-2.72 |
| Guided | |||||
| Nob | 958 | 2.7 | |||
| Yes | 864 | 3.6 | 3.85 × 10− 51 | 3.83 | 3.22-4.57 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aOverall, there was a positive correlation between benefit ratings for patient care and profile scores. When modeling benefit ratings by profile risk category and if the patient received profile-guided care, benefit ratings for patients who scored as moderate risk were 1.3 times higher than those who scored as low risk (P = .002). Benefit for patients who scored as high risk were 1.85 times higher than those who scored as low risk (P = .002). Profile-guided decision-making had the greatest impact on patient benefit, increasing ratings by 3.83 times more than profile scores (P = 3.85 × 10−51).
bReference group for determining the adjusted proportional odds ratio.
Effect of Demographics on Profile Guidance and Benefit Ratings
On average, patients who received guided decisions were about 2 years older than those whose were not guided (P = .009). There was no gender bias between patients who received guided or not guided decisions (Table 3); however, compared to Caucasians, African Americans and Hispanics were less likely to receive guided decisions.
Table 3.
Odds of Receiving Profile-Guided Patient Care From Physicians Based on Demographics.a
| Demographic | Total n | P Value | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Age | 1822 | .009b | 1.008 | 1.002-1.01 |
| Gender: females vs males | ||||
| Females | 1034 | .097 | 1.17 | 0.97-1.41 |
| Males | 788 | |||
| Race: vs Caucasians | ||||
| Caucasians | 1178 | |||
| African American | 173 | .013b | 0.66 | 0.47-0.91 |
| Hispanic | 272 | .026b | 0.74 | 0.56-0.95 |
| Other | 126 | 4.72 × 10− 5b | 0.44 | 0.29-0.64 |
| Declined to answer | 73 | 2.61 × 10− 5b | 0.31 | 0.17-0.52 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aLogistic regression was used to model the odds of receiving guided decisions. Adjusted odds ratios and 95% CI reflect the OR after adjusting for covariates: guided or not guided, age, gender, and race. Compared to Caucasians, African Americans and Hispanics were less likely to receive guided decisions.
bStatistical significance, P < .05.
Among patients who received guided decisions, physicians were more likely to rate the profile as more beneficial if the patient was female as compared to male (adjusted OR = 1.40, P = .01) and Hispanic as compared to Caucasian (adjusted OR = 2.81, P = 1.31 × 10−15). Although not statistically significant, there was a trend that physicians of African American patients who received guided treatment also rated the profile more highly (adjusted OR = 1.49, P = .087). Interestingly, when considering all patients, regardless of whether or not they received guided decisions, ratings from physicians of African American patients were significantly higher than Caucasians (adjusted OR = 1.66, P = 7.48 × 10−4).
Overall Effect of the Profile on Patient Outcomes
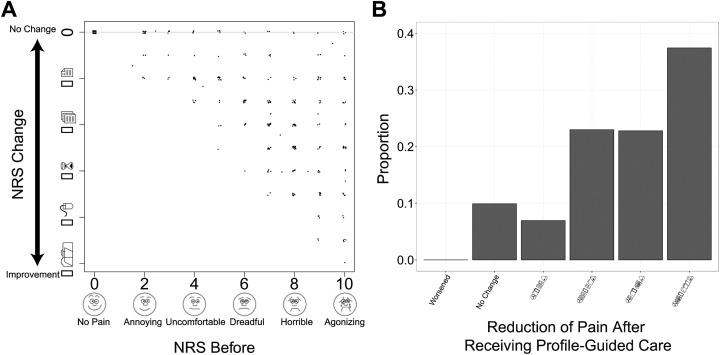
Approximately 1 month later, physicians implemented treatment changes due to profile test results; 505 (64%) patients who received profile-guided decisions completed the follow-up and also reported NRS pain scores rating their pain before and after taking medications. Numeric rating scale scores of 7 to 10 are considered severe pain, 4 to 6 moderate pain, and 1 to 3 mild pain.31 Specifically for this study, an NRS of 0 was equivalent to “no pain,” while an NRS of 10 was equivalent to “agonizing” pain. Any decrease in the NRS following guided treatment was considered to be an improvement. Overall, patients rated their pain levels to be on average 2.7 levels lower after receiving profile-guided care from their physicians (P = 2.61 × 10−55; Figure 3A). Based on the difference between pain NRS at the baseline and follow-up visits, 90% of patients reported some decrease in pain, while over 60% had at least a 50% reduction in pain. Patients whose physicians used the test results to guide treatment had reduced pain, in many cases going from severe or moderate pain down to low or no pain.
Figure 3.
The numeric rating scale (NRS) scores of patients at the follow-up visit whose physicians used the profile to guide patient care. A, Overall, patients rated their pain levels to be on average 2.7 levels lower after receiving profile-guided care from their physicians (P = 2.61 × 10−55). For plotting, noise was added to show individual data points. B, Based on the difference between pain NRS at the baseline and follow-up visits, 90% of patients reported some decrease in pain, while over 60% had at least a 50% reduction in pain.
Discussion
As many as 100 million Americans suffer from chronic pain,32 and in 2012, health-care providers wrote 259 million opioid prescriptions for pain treatment.33 The societal costs of the prescription opioid epidemic are staggering: 46 people a day die from an overdose of prescription painkillers33 and more than 1000 people a day are treated in emergency departments for mistreating prescription painkillers.34 Opioid use disorder costs the economy approximately US$52 to US$78.3 billion annually due to lost productivity, criminal justice costs, health-care costs, and drug abuse treatment costs.35,36
Primary care providers are at the frontline of the epidemic, treating patients suffering from pain, while ensuring their treatment does not lead to OUD, drug diversion, or overdose. Our study shows that the profile can guide primary care providers to make favorable clinical decisions about opioid treatment for their patients. Primary care physicians who used the profile test found it beneficial when making treatment decisions and rated the test higher than physicians who did not use the test. Those ratings were, on average, 0.9 points higher on a 5-point scale, which demonstrates that physicians benefit from having their decisions guided by the profile test results. Close to half of the patients in our study were given treatment that was guided by the results of the profile screening, and of those, nearly all patients’ physicians (98%) felt that the profile provided some benefit when adjustments were made to the treatment program. The largest benefit was reported to arise from clinical actions that involved reducing, changing, or eliminating the prescribed opioid. Physicians who did not use the test results to guide treatment decisions may have done so for several reasons—patients were low risk, and thus, test results simply confirmed physician behavior; physicians actually implemented changes based on the test results, such as increased frequency of urine drug screening, but did not consider it to be a “treatment action” and thus neglected to accurately record the guidance; or physicians decided to continue as treatment as usual and consider the results of the testing if necessary downstream.
Moreover, the use of the test to guide treatment was independent of the mean test score, which suggests utility for both low- and high-risk scores in clinical care. When physicians were not guided by the profile, it was more likely to be for patients who received “low-risk” results. This may indicate an underreporting of profile utilization, as the survey may not have captured the utility of negative results—for example, no change in treatment as a result of a “low risk” result may still be categorized as a guided action.
Additionally, this study found that there were differences in the benefit of the profile based on ethnicity and sex. Considerable evidence has shown variability in treatment and outcomes of pain-related conditions based on both these factors. Specifically, African Americans, compared to non-Hispanic whites suffer a greater burden of pain and pain-related suffering.37 Pain treatment approaches employed in multidisciplinary programs are less effective in improving symptoms in ethnic minorities,38 reinforcing the point that medicine must be patient-specific and consider multiple factors, including ethnicity. The higher profile benefit reported by primary care physicians for minorities in this study suggests that tailoring treatment with precision medicine may improve outcomes.
Most importantly, however, this study determined that using profile test results to guide treatment results in decreased pain for patients. Patient pain decreased, on average, 2.7 levels, as measured by the NRS, when physicians made treatment decisions based on the profile. This dramatic decrease in pain levels reflects the clinical utility of the profile in patient care. Furthermore, there are significant physical, psychological, and economic benefits to decreasing pain.
Physicians are advised to apply a systematic method when evaluating a patient for treatment with opioids.39 The consistent use of a screening method is recommended,9,40-42 followed by a review of the patient’s prescription history with PDMD,43 the use of a written treatment contract between physician and patient, and monitoring the patient’s drug use with random UDT.9,41,42,44,45 Currently available screening tools, such as SOAPP-R and ORT, depend solely on patient self-assessment to work effectively. The risk profile differs from these other screening tools because it provides objective OUD risk assessment based on genotypic and phenotypic data.16-18 Specifically in a primary care setting, a previous study16 demonstrated that the profile correctly identifies OUD with nearly 88% accuracy and high sensitivity and specificity. Furthermore, the profile was used to evaluate the selection, dosage, and discontinuation of opioids and found to be beneficial to do so for the majority of patients. This study demonstrates that primary care physicians can use the results of the profile to guide their decisions about how much they prescribe and to whom, thus accomplishing the 3 main foci of the CDC guidelines.
Study Limitations
One limitation of the study is possible selection bias, as there was no randomization. We attempted to account for as much bias as possible in the statistical analyses, but ultimately a randomized trial would eliminate selection bias. We are in the process of planning and executing a randomized trial. Additionally, we did not collect specific information about the study physicians and their practices, and thus, there may have been some treatment and practice differences that could affect the results of this study.
Conclusions
The prescription opioid epidemic makes it clear that there are a number of challenges for physicians who balance the pain management needs of their patients with avoiding risk of aberrant behavior to opioids. Recent CDC guidelines provide primary care physicians with a number of recommendations for prescribing opioids, including OUD risk assessment. This study demonstrates the profile provided primary care physicians with a useful tool to stratify risk of OUD. The profile was rated as beneficial for decision-making and patient improvement by the majority of physicians surveyed, with the most utilization for changing the prescribed opioid and the most significant benefits from changing the selection and dosage of opioids. Moreover, physicians reported the profile was most beneficial for patients at an increased risk of OUD, highlighting the objective use of this profile to guide judicial use of opioids in high-risk patients. Finally, and most significantly, patients whose physicians used the profile to guide treatment decisions had greatly reduced pain levels.
Supplementary Material
Author Biographies
Chee Lee is a skilled data scientist with a background in statistics and genomics. Her expertise lies in integrating population-level statistics, such as demographic, phenotypic, and electronic health records, with complex genomic data, including associations with genetic variants, gene expression, and epigenetics. Chee Lee received her PhD in Bioinformatics from the University of Michigan, and has almost a decade of experience conducting scientific research.
Maneesh Sharma, MD, is a Johns Hopkins University trained anesthesiologist and Interventional Pain Medicine specialist. He treats various orthopedic, neurologic and gastrointestinal injuries, diseases and degenerative conditions that cause chronic pain using surgical, medical, regenerative and integrative approaches.
Svetlana Kantorovich is a classically-trained neuroscientist who has conducted research in pain, epilepsy, cognitive dysfunction, and Parkinson’s disease. She earned her Bachelor’s degree from Washington University in St. Louis, her PhD in Neuroscience from the University of Florida, and completed her postdoctoral fellow training in neurobiology at the University of California, Irvine.
Ashley Brenton received her Bachelor’s Degree in Public Health Studies from the Johns Hopkins University, her PhD from University of California Davis in Entomology with a Designated Emphasis in Vector-Borne Diseases, and completed a viral pathogenesis fellowship at the Scripps Research Institute. Dr. Brenton has dedicated her career to the application of genomics to decrease major public health disease burden.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.L., S.K., and A.B. are employees of Proove Biosciences. M.S. is an investigator of a Proove-sponsored study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by Proove Biosciences, Inc.
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36(1):559–574. [DOI] [PubMed] [Google Scholar]
- 2. Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med. 2014;174(5):802–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. [DOI] [PubMed] [Google Scholar]
- 4. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen JH, Humphreys K, Shah NH, Lembke A. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176(2):259–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nwokeji ED, Rascati KL, Brown CM, Eisenberg A. Influences of attitudes on family physicians’ willingness to prescribe long-acting opioid analgesics for patients with chronic nonmalignant pain. Clin Ther. 2007;29(suppl):2589–2602. [DOI] [PubMed] [Google Scholar]
- 9. Jamison RN, Sheehan KA, Scanlan E, Matthews M, Ross EL. Beliefs and attitudes about opioid prescribing and chronic pain management: survey of primary care providers. J Opioid Manag. 2014;10(6):375–382. [DOI] [PubMed] [Google Scholar]
- 10. Potter M, Schafer S, Gonzalez-Mendez E, et al. Opioids for chronic nonmalignant pain. Attitudes and practices of primary care physicians in the UCSF/Stanford Collaborative Research Network. University of California, San Francisco. J Fam Pract. 2001;50(2):145–151. [PubMed] [Google Scholar]
- 11. Allen MJ, Asbridge MM, Macdougall PC, Furlan AD, Tugalev O. Self-reported practices in opioid management of chronic noncancer pain: a survey of Canadian family physicians. Pain Res Manag. 2013;18(4):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keller CE, Ashrafioun L, Neumann AM, Van Klein J, Fox CH, Blondell RD. Practices, perceptions, and concerns of primary care physicians about opioid dependence associated with the treatment of chronic pain. Subst Abus. 2012;33(2):103–13. [DOI] [PubMed] [Google Scholar]
- 13. Kennedy-Hendricks A, Busch SH, McGinty EE, et al. Primary care physicians’ perspectives on the prescription opioid epidemic. Drug Alcohol Depend. 2016;165:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Islam MM, McRae IS. An inevitable wave of prescription drug monitoring programs in the context of prescription opioids: pros, cons and tensions. BMC Pharmacol Toxicol. 2014;15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson HD, Dansie EJ, Kim MS, Moskovitz BL, Chow W, Turk DC. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14(6):613–627. [DOI] [PubMed] [Google Scholar]
- 16. Brenton A, Richeimer S, Sharma M, et al. Observational study to calculate addictive risk to opioids: a validation study of a predictive algorithm that detects opioid use disorder. Pharmacogenomics Pers Med. 2017;10:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farah R, Lee C, Kantorovich S, Smith GA, Meshkin B, Brenton A. Evaluation of a predictive algorithm that detects aberrant use of opioids in an addiction treatment centre. J Addict Res Ther. 2017;8(312):2. [Google Scholar]
- 18. Sharma M, Lee C, Kantorovich S, Tedtaotao M, Smith GA, Brenton A. Observational study to calculate addictive risk to opioids: a validation study of a predictive algorithm to evaluate opioid use disorder in a primary care setting. Health Serv Res Manag Epidemiol. 2017; 4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rybakowski JK, Dmitrzak-Weglarz M, Suwalska A, Leszczynska-Rodziewicz A, Hauser J. Dopamine D1 receptor gene polymorphism is associated with prophylactic lithium response in bipolar disorder. Pharmacopsychiatry. 2009;42(1):20–22. [DOI] [PubMed] [Google Scholar]
- 20. Chen TJ, Blum K, Payte JT, et al. Narcotic antagonists in drug dependence: pilot study showing enhancement of compliance with SYN-10, amino-acid precursors and enkephalinase inhibition therapy. Med Hypotheses. 2004;63(3):538–548. [DOI] [PubMed] [Google Scholar]
- 21. Nelson EC, Lynskey MT, Heath AC, et al. Association of OPRD1 polymorphisms with heroin dependence in a large case-control series. Addict Biol. 2014;19(1):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vaske J, Newsome J, Wright JP. Interaction of serotonin transporter linked polymorphic region and childhood neglect on criminal behavior and substance use for males and females. Dev Psychopathol. 2012;24(1):181–193. [DOI] [PubMed] [Google Scholar]
- 23. National Institute on Drug Abuse. Bringing the Full Power of Science to Bear on Drug Abuse and Addiction. National Institute on Drug Abuse; 2007. https://www.drugabuse.gov/bringing-power-science-to-bear-drug-abuse-addiction. Accessed January 1, 2017. [Google Scholar]
- 24. National Institute on Drug Abuse. What is Prescription Drug Abuse? National Institute on Drug Abuse; 2014. https://www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2016/prescription-opioid-heroin-abuse. Accessed January 1, 2017. [Google Scholar]
- 25. Cleland CM, Rosenblum A, Fong C, Maxwell C. Age differences in heroin and prescription opioid abuse among enrollees into opioid treatment programs. Subst Abuse Treat Prev Policy. 2011;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sproule B, Brands B, Li S, Catz-Biro L. Catz-Biro changing patterns in opioid addiction: characterizing users of oxycodone and other opioids. Can Fam Physician. 20009;55(1): 68–69.e5. [PMC free article] [PubMed] [Google Scholar]
- 27. Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355–362. [DOI] [PubMed] [Google Scholar]
- 28. Jang KL, Livesley WJ, Vernon PA. Alcohol and drug problems: a multivariate behavioural genetic analysis of co-morbidity. Addiction. 1995;90(9):1213–1221. [DOI] [PubMed] [Google Scholar]
- 29. Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20(1):1–17. [DOI] [PubMed] [Google Scholar]
- 30. Kern AM, Akerman SC, Nordstrom BR. Opiate dependence in schizophrenia: case presentation and literature review. J Dual Diagn. 2014;10(1):52–57. [DOI] [PubMed] [Google Scholar]
- 31. McCaffery M, Beebe A. Pain: Clinical Manual for Nursing Practice. Baltimore, MD: V.V. Mosby Company; 1993. [Google Scholar]
- 32. Institute of Medicine(US) Committee on Advancing Pain Research, Care, and Education Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 33. Centers for Disease Control and Prevention. CDC Vital Signs—Opioid Painkiller Prescribing. Centers for Disease Control and Prevention; 2016. https://www.cdc.gov/vitalsigns/opioid-prescribing/index.html. Accessed January 1, 2017. [Google Scholar]
- 34. Substance Abuse and Mental Health Services Administration. The DAWN Report: Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. 2013. https://www.samhsa.gov/data/sites/default/files/DAWN127/DAWN127/sr127-DAWN-highlights.html. Accessed January 1, 2017. [PubMed] [Google Scholar]
- 35. Hansen RN, Oster G, Edelsberg J, Woody GE, Sullivan SD. Economic costs of nonmedical use of prescription opioids. Clin J Pain. 2011;27(3):194–202. [DOI] [PubMed] [Google Scholar]
- 36. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Campbell CM, Edwards RR. Ethnic differences in pain and pain management. Pain Manag. 2012;2(3):219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Merry B, Campbell CM, Buenaver LF, et al. Ethnic group differences in the outcomes of multidisciplinary pain treatment. J Musculoskelet Pain. 2011;19(1):24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Agarin T, Trescot AM, Agarin A, Lesanics D, Decastro C. Reducing opioid analgesic deaths in America: what health providers can do. Pain Physician. 2015;18(3):F307–F322. [PubMed] [Google Scholar]
- 40. Chou R, Fanciullo GJ, Fine PG; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):112–130. [DOI] [PubMed] [Google Scholar]
- 41. Krashin D, Murinova N, Ballantyne J. Management of pain with comorbid substance abuse. Curr Psychiatry Rep. 2012;14(5):462–468. [DOI] [PubMed] [Google Scholar]
- 42. Miotto K, Kaufman A, Kong A, Jun G, Schwartz J. Managing co-occurring substance use and pain disorders. Psychiatr Clin North Am. 2012;35(2):393–409. [DOI] [PubMed] [Google Scholar]
- 43. Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 2011;12(5):747–754. [DOI] [PubMed] [Google Scholar]
- 44. Cheatle MD, O’Brien CP. Opioid Therapy in Patients with Chronic Noncancer Pain: Diagnostic and Clinical Challenges. Adv Psychosom Med. 2011;30:61–91. [DOI] [PubMed] [Google Scholar]
- 45. Atluri S, Akbik H, Sudarshan G. Prevention of opioid abuse in chronic non-cancer pain: an algorithmic, evidence based approach. Pain Physician. 2012;15(3 suppl):ES177–ES189. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.