Abstract
Background:
Patients with coronary artery disease (CAD) had significantly lower bile acid excretion (BAE) compared with non-CAD patients, leading to the hypothesis that the inability to efficiently excrete bile acids leads to coronary atherosclerosis development. We investigated the long-term role of BAE in CAD development and related mortality in 50 patients with proven CAD compared with that of 50 patients with chest pain and no CAD (controls) matched for clinical and laboratory characteristics.
Methods:
All subjects received a 4-day standard diet that included ~500 mg of cholesterol. Fecal bile acids from 24-h stool collections were measured by gas liquid chromatography.
Results:
CAD patients excreted lower amounts of total bile acids than controls (p < 0.001), less deoxycholic acid (p < 0.0001) and less lithocholic acid (p < 0.01). BAE was the best significant independent laboratory factor that predicted CAD (p < 0.05). Mortality and CAD development rates were significantly lower for the controls at the 20-year follow up.
Conclusions:
These results showed that CAD patients had markedly decreased BAE levels compared with non-CAD controls. BAE <415 mg/day was associated with increased CAD long-term mortality. Impaired ability to excrete cholesterol might be considered an additional independent risk factor for CAD development.
Keywords: atherosclerosis, bile acids, coronary artery disease, high-density lipoprotein, low-density lipoprotein
Introduction
It is well accepted that cholesterol-lowering treatment (e.g. statins) favorably affects development and progression of atherosclerosis to only a limited extent in a significant number of patients.1,2
Synthesis of bile acids is a major route of cholesterol catabolism.3–6 The body produces about 800 mg of cholesterol per day and about half of that is used for bile acid synthesis, producing 400–600 mg daily. The relationship between fecal bile acid excretion (BAE) and coronary artery disease (CAD) has been debated for several decades, with some recent cross-sectional and prospective studies providing conflicting evidence with regards to the association between BAE and CAD-associated morbidity and mortality.3,7–13 The reasons for the increased CAD risk related to BAE remains, however, largely unexplained. An association between alteration in BAE and atherosclerosis has thus far been shown only in cross-sectional studies.13–22 Providing longitudinal information may help to understand the contribution of BAE to the cumulative risk load for CAD.
Cholic acid and chenodeoxycholic acid are the most abundant primary bile acids. These are synthesized by the liver. Secondary bile acids are the result of colonic bacterial action on primary bile acids.5 Secondary bile acids, deoxycholic acid and lithocholic acid, are the conjugated salts of primary BA-7-alpha-dehydroxylated derivatives. These account for over 90% of human bile acids.5
In addition to statins, low-density lipoprotein cholesterol (LDL-c) can be reduced by increasing bile acid waste and the compensatory hepatic up-regulation of bile acid synthesis.2,4,14,16,19–24 It is reasonable to speculate that reduced ability to convert cholesterol to bile acids would lead to body cholesterol excess.1,2,6–13,23–25 This assumption is supported by reports on animals that do not develop hypercholesterolemia or atherosclerosis despite being fed cholesterol-rich diets.2,11–14,20,23,25 Those animals reacted to the high cholesterol intake by excreting large amounts of bile acids, while animals with less efficient excretion of bile acids had increased plasma levels of cholesterol while on a high-cholesterol diet. The degree of hypercholesterolemia was inversely correlated to the rate of bile acid elimination.1,2,11–14,21,23,25
Simonen and Miettinen18 showed that males with heterozygous familial hypercholesterolemia (FH) and CAD excreted lower amounts of bile acids than those with FH and normal coronary arteries. Rajaratnam and colleagues demonstrated that postmenopausal women with CAD had inefficient fecal elimination of cholesterol.7,8 We found that the CAD patients excreted markedly lower amounts (approximately 50%) of fecal bile acids.15 The differences in excretion of bile acids were mainly due to lower excretion of deoxycholic and lithocholic acids.15
The purpose of this study was to explore the long-term effect of decreased BAE on mortality in relation to CAD during a 20-year follow-up period. By carrying out a longitudinal study we intended to verify whether the difference between patients with and without CAD remained unchanged, increased or decreased over the period of time.
Materials and methods
Study design
Subjects with symptoms suggestive of CAD were enrolled into the study and tested for the presence of CAD using coronary angiography. CAD-positive and CAD-negative patients were assayed for fecal BAE as described below. The follow-up period averaged 17 years for both groups.
Patient population
All patients hospitalized in our institution with chest pain suspected as being CAD (angina pectoris, unstable angina or myocardial infarction) were eligible for participation in this study, which was conducted from January 1995 through December 2015. They all underwent coronary catheterization according to clinical criteria (some of them after noninvasive tests, treadmill ergometry or a thallium scan) and were divided into two groups: those with confirmed CAD (demonstration of stenosis greater than 70% on coronary angiogram) and those with normal coronary arteries. Excluded were patients younger than 40 years of age (because of a low risk for CAD), those with psychiatric disease, malignancy, acute and end-stage renal failure (dialysis), hepatic disease, severe chronic obstructive pulmonary disease or acute infectious disease, and those receiving lipid-lowering drugs. A complete medical history was taken, and the medical charts of participants with previous visits to our clinic were reviewed in order to retrieve data on a history of CAD and cardiac risk factors – diabetes mellitus (DM), hyperlipidemia, hypertension, smoking and a body mass index (BMI) >25. In addition to a physical examination, the study candidates also underwent an electrocardiogram, laboratory tests (a complete blood count, glucose, renal function, liver enzyme, creatinine kinase) and a lipid profile in order to ensure they were clinically stable. All patients after coronary angiography were divided into two groups: those with confirmed CAD (Group 1) and those without CAD (Group 2, controls). Regarding concerns about the possibility that participants in the non-CAD group had symptomatic coronary atherosclerosis (i.e. producing pains), it is important to emphasize that members of this group had bona fide normal coronary arteries.
Group 1 patients were enrolled in the study on condition that they had not undergone any acute coronary event for at least a previous 3-month period in order to exclude any effect of infarction-related metabolic changes. The study was approved by the local ethics committee and each entrant signed an informed consent prior to enrolling (No. 0663-15-TLV.) Retrospective forms of long-term follow up and survival were based on electronic health records.
Study protocol
The study was approved by the Ethical Committee of Tel Aviv Sourasky Medical Center.
All patients performed a 24-h stool collection into special containers. A continuous non-absorbable marker was used to evaluate the quantity of stool excretion. Each subject received 12 capsules of 75 mg copper isothiocyanate to be taken one per meal for 4 days previous to stool collection, meant to serve as an internal standard for the purposes of calculation. A few patients did not have a bowel movement after three days of the internal standard (copper tablets) administration. In those cases copper was given for a few more days until a stool was passed.
One week prior to the ingestion of this internal standard, the patients received a standard in-hospital diet containing 490 mg/day of cholesterol.15,26 A high-cholesterol diet was well tolerated by all study participants without any discomfort or diarrhea. They were asked to record the contents of each meal consumed. Participants who did not conform to the strict diet were dropped from the study. Fecal collection was made only during the hospital stay of the patients admitted because of chest pain suggestive of ischemic heart disease. All blood samples were taken on the same day as feces collection.
Quantitative determination of the bile acids in feces
Determination of bile acids was done as previously described.15 This method allows for complete recovery and separation of bile acids, together with a high level of accuracy, reliability and simplicity in performance. The stools were collected throughout 24 h, and the amount of bile acids was determined per 225 mg of copper isothiocyanate that served as an internal standard.6,18,26,27 The method of determination of free bile acids and their fractions is described in detail in our earlier publication.15
Fecal cholesterol excretion in the present work was exceedingly low in comparison to total BAE. We deemed these negligible amounts of cholesterol to have no significant weight in the total sterol excretion. With a linear detection range of 1–150 μmol bile acids, we considered our methodology fairly sensitive and accurate. Coefficient of variations for bile acids measurements were: intra-assay 3% and inter-assay 5%.
In an attempt to simplify the assessment of BAE, the possibility of measuring blood level of surrogate markers for the excretion may appear useful. However, while several assays have been used to determine both total and individual bile acids in biological fluids, it should be recognized that less than 1% of the total bile acid pool is present in the peripheral blood (2–10 mmol/L) due to the high efficiency of the hepatic transport mechanism for bile acids. Moreover, serum BA level is predisposed to considerable variation due to alteration of liver function that is often undetectable by other liver function tests. Thus, it is not considered a reliable measure of metabolic risk for CAD.
Measurement of lipids content in the serum
Total cholesterol and triglyceride levels were measured enzymatically on a Siemens Advia 1650 Chemistry Analyser (Siemens Medical Solutions. Diamond Diagnostics - USA, Holliston, MA) using the manufacturer’s reagents.15
Statistical analysis
Categorical variables are reported as frequency and percentages, and continuous variables are reported as medians and interquartile ranges (IQR). Continuous variables were tested for a normal distribution histogram. Categorical variables were compared between patients with and without ischemic heart disease (IHD) by use of the Chi-square test or the Fisher exact test, and continuous variables by the independent samples t test or the Mann–Whitney U test.
Bile acids were divided into categories according to their median value. Kaplan–Meier plots were used to describe mortality during follow up in patients with and without IHD and in different bile acid categories. The log-rank test was used to compare them. The length of follow up was measured using the reverse censoring method. The univariate Cox regression was used to evaluate the association between bile acids, CAD status, potential confounders and mortality. Each bile acid was assessed by a separate multivariate Cox regression. The multivariate regression models included age, sex, IHD and variables with p < 0.2 in the univariate analysis. A two-tailed p < 0.05 level was considered statistically significant. Analyses were performed with SPSS (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, USA).
Results
A total of 67 males and 33 females (median age 62 years, IQR 54–68) participated in this prospective follow-up study. Fifty patients had been diagnosed with CAD (Group 1: 37 males, median age 62.5 years, IQR 58–68) and 50 patients were CAD-free (Group 2, controls): 30 males, median age 61 years (IQR 49–67), with no significant differences in age and gender (p = 0.123 and p = 0.726, respectively). Thirty CAD patient and 27 controls participants were included in a previous report.15
Table 1 lists patients’ clinical features, demographics, laboratory findings and CAD risk factors. There were no significant differences between the two groups in their BMI or in the prevalence of DM, hypertension, smoking or renal failure. They did, however, differ in total cholesterol and LDL-c levels. The controls had a slightly higher left ventricular ejection fraction (LVEF) that was clinically non-significant, although it was statistically significant compared with the control group (median 60 versus 55, p = 0.007). The BAEs of cholic and chenodeoxycholic acids were similar for both groups (p = 0.428 and p = 0.921, respectively). Patients with CAD had significantly lower excretion levels of deoxycholic (p < 0.001) and lithocholic acids (p = 0.004) compared with the controls.
Table 1.
Patients demographic, clinical and laboratory data.
| Study population (n = 100) |
IHD (n = 50) |
No IHD (n = 50) |
p-value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 62.0 (54.0–68.0) | 62.5 (58.0–68.3) | 61.0 (49.0–67.3) | 0.123 |
| Male, n (%) | 67 (67.0%) | 37 (68.5) | 30 (65.2) | 0.726 |
| Smoking, n (%) | 31 (31.0%) | 16 (29.6) | 15 (32.6) | 0.748 |
| BMI (kg/m2), median (IQR) | 27.0 (24.0–29.8) | 26.0 (23.0–29.3) | 28.0 (24.0–31.3) | 0.164 |
| Renal failure, n (%) | 20 (20.0) | 13 (24.1) | 7 (15.2) | 0.270 |
| Hypertension, n (%) | 40 (40.0) | 24 (44.4) | 16 (34.8) | 0.326 |
| Diabetes mellitus, n (%) | 32 (32.0) | 19 (35.2) | 13 (28.3) | 0.459 |
| LVEF, median (IQR) | 59.0 (55.0–60.0) | 55.0 (47.3–60.0) | 60.0 (55.0–60.0) | 0.007 |
| Cholic acid, median (IQR) | 14.0 (5.0–40.5) | 9.0 (5.0–32.3) | 19.4 (4.9–46.5) | 0.428 |
| Cholic acid >14 mg/24 h, n (%) | 48 (48.0) | 24 (44.4) | 24 (52.2) | 0.441 |
| Chenodeoxycholic acid, median (IQR) | 8.0 (0.0–21.0) | 8.0 (0.0–22.0) | 6.0 (0.0–20.3) | 0.921 |
| Chenodeoxycholic >8 mg/24 h, n (%) | 44 (44.0) | 25 (46.3) | 19 (41.3) | 0.616 |
| Deoxycholic acid, median (IQR) | 214 (148–374) | 170.5 (116.8–259.0) | 290.5 (188.5–444.0) | <0.001 |
| Deoxycholic acid >214 mg/24 h, n (%) | 49 (49) | 18 (33.3) | 31 (67.4) | 0.001 |
| Lithocholic, median (IQR) | 141.5 (82.3–209.5) | 94.5 (78.8–180.0) | 157.0 (131.3–234.3) | 0.004 |
| Lithocholic >141 mg/24 h, n (%) | 50 (50) | 19 (35.2) | 31 (67.4) | 0.001 |
| Total bile acid, median (IQR) | 416.0 (271.0–628.5) | 344.9 (225.3–523.3) | 597.5 (401.3–725.5) | <0.001 |
| Total bile acids >415 mg/24 h, n (%) | 50 (50) | 18 (33.3) | 32 (69.6) | <0.001 |
| Triglycerides, median (IQR) | 212.5 (167.0–263.5) | 210.5 (142.3–263.8) | 214.5 (183.0–264.4) | 0.516 |
| Triglycerides > mg/dL h, n (%) | 29 (29.0) | 15 (27.8) | 14 (30.4) | 0.770 |
| LDL, median (IQR) | 141.0 (118.3–179.8) | 162.5 (129.5–204.5) | 132.0 (113.5–145.0) | 0.001 |
| LDL >130 mg/dL h, n (%) | 65 (65.0) | 41 (75.9) | 24 (52.2) | 0.013 |
| HDL, median (IQR) | 47.5 (38.0–55.0) | 44.0 (36.0–54.3) | 49.0 (38.0–59.5) | 0.167 |
| HDL <45 mg/dL, n (%) | 47 (47.0) | 29 (61.7) | 18 (39.1) | 0.146 |
| Total cholesterol, median (IQR) | 238.0 (215.3–281.5) | 254.5 (216.0–298.0) | 232.0 (209.0–245.3) | 0.024 |
| Total cholesterol >200 mg/dL h, n (%) | 84 (84.0) | 47 (87.0) | 37 (80.4) | 0.369 |
HDL, high-density lipoprotein; IHD, ischemic heart disease; IQR, interquartile range; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction.
The mean follow up was 17.45 years (95% CI: 16.63–18.27), with a similar number of years in both groups (Group 1: 17.54 years, 95% CI 16.61–18.48; controls: 17.13 years, 95% CI 15.75–18.50). Table 2 shows the patients’ clinical, laboratory and demographic data so as to compare between survivors and patients who died during follow up. The patients who survived had markedly higher total bile, deoxycholic and lithocholic acid excretion levels (p < 0.001 for all comparisons). Table 3 lists the crude hazard ratios (HRs) of each predictor in association with cardiovascular mortality: only those ratios for total deoxycholic and lithocholic acids reached a level of significance (p < 0.001).
Table 2.
Patient survival according to clinical and laboratory characteristics.
| Deceased |
Alive |
Crude HR (95%CI) | p-value | |
|---|---|---|---|---|
| (n = 38) | (n = 62) | |||
| IHD, n (%) | 28 (73.7) | 26 (41.9) | 2.93 (1.41–6.07) | 0.004 |
| Age (years), median (IQR) | 66.0 (59.8–72.0) | 60.0 (50.0–66.0) | 1.21 (1.14–1.29) | <0.001 |
| Male, n (%) | 22 (57.9) | 45 (72.6) | 0.49 (0.26–0.94) | 0.032 |
| Smoking, n (%) | 10 (26.3) | 21 (33.9) | 1.05 (0.51–2.17) | 0.893 |
| Body mass index (kg/m2), median (IQR) | 26.0 (23.8–28.3) | 27.5 (23.8–31.5) | 0.94 (0.87–1.02) | 0.127 |
| Renal failure, n (%) | 9 (23.7) | 11 (17.7) | 1.10 (0.52–2.32) | 0.807 |
| Hypertension, n (%) | 16 (42.1) | 24 (38.7) | 1.08 (0.57–2.07) | 0.808 |
| Diabetes mellitus, n (%) | 14 (36.8) | 18 (29.0) | 1.39 (0.72–2.69) | 0.329 |
| LVEF, median (IQR) | 56.0 (48.0–60.0) | 60.0 (55.0–60.0) | 0.94 (0.89–0.97) | 0.001 |
| Cholic acid, median (IQR) | 7.5 (4.8–22.3) | 21.0 (6.5–46.5) | 0.99 (0.98–1.01) | 0.305 |
| Cholic acid >14 mg/24 h, n (%) | 13 (34.2) | 35 (56.5) | 0.54 (0.28–1.06) | 0.075 |
| Chenodeoxycholic acid, median (IQR) | 7.5 (0.0–22.0) | 8 (8.0–15.5) | 0.99 (0.97–1.02) | 0.586 |
| Chenodeoxycholic >8 mg/24 h, n (%) | 16 (42.1) | 28 (45.2) | 1.09 (0.58–2.08) | 0.764 |
| Deoxychcholic acid, median (IQR) | 154.5 (109.8–212.5) | 290.5 (185.5–482.5) | 0.993 (0.99–0.997) | <0.001 |
| Deoxychcholic acid >214 mg/24 h, n (%) | 9 (23.7) | 40 (64.5) | 0.27 (0.13–0.57) | 0.001 |
| Lithocholic acid, median (IQR) | 83.0 (59.6–131.3) | 184.0 (132.8–243.0) | 0.987 (0.982–0.992) | <0.001 |
| Lithocholic >141 mg/24 h, n (%) | 6 (15.8) | 44 (71.0) | 0.16 (0.07–0.38) | <0.001 |
| Total bile acid, median (IQR) | 277.0 (193.2–349.5) | 601.5 (415.0–734.5) | 0.994 (0.992–0.997) | <0.001 |
| Total bile acid >415 mg/24 h, n (%) | 4 (10.5) | 46 (74.2) | 0.09 (0.03–0.27) | <0.001 |
| Triglycerides, median (IQR) | 207.0 (173–264.8) | 214.5 (1496.0–264.5) | 1.001 (0.997–1.004) | 0.750 |
| Triglycerides >250 mg/dL, n (%) | 11 (28.9) | 18 (29.0) | 1.13 (0.56–2.29) | 0.727 |
| LDL, median (IQR) | 151.5 (125.5–183.5) | 132.5 (116.3–175.0) | 1.006 (1.000–1.013) | 0.068 |
| LDL >130 mg/, mg/dL n (%) | 28 (73.7) | 37 (59.7) | 1.64 (0.79–3.38) | 0.178 |
| HDL, median (IQR) | 42.0 (36.8–51.3) | 48.5 (38.0–59.0) | 0.98 (0.95–1.01) | 0.089 |
| HDL <45 mg/dL, n (%) | 22 (57.9) | 25 (40.3) | 1.45 (0.76–2.76) | 0.262 |
| Total cholesterol, median (IQR) | 246.0 (216.25–288.0) | 236.5 (215.0–276) | 1.005 (0.999–1.011) | 0.103 |
| Total cholesterol >200 mg/dL, n (%) | 32 (84.2) | 52 (83.9) | 0.99 (0.41–2.38) | 0.987 |
HDL, high-density lipoprotein; IHD, ischemic heart disease; IQR, interquartile range; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction.
Table 3.
Adjusted hazard ratio associated with cardiovascular mortality according to excreted total bile acids and their fractions.
| Adj. HR (95% CI)* | p-value | |
|---|---|---|
| Cholic acid >14 mg/24 h | 0.58 (0.26–1.32) | 0.197 |
| Chenodeoxycholic >8 mg/24 h | 0.88 (0.41–1.91) | 0.745 |
| Deoxycholic acid >214 mg/24 h | 0.46 (0.18–1.13) | 0.090 |
| Lithocholic >141 mg/24 h | 0.42 (0.15–1.17) | 0.099 |
| Total bile acid >415 mg/24 h | 0.14 (0.04–0.45) | 0.001 |
Adjusted to age, gender, BMI, CAD, LVEF, hypertension, smoking, total cholesterol, LDL, HDL, hemoglobin, creatinine.
HDL, high-density lipoprotein; IHD, ischemic heart disease; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction.
Figure 1 displays the patients’ cumulative survival rate according to CAD and in the non-CAD control group during the 20-year follow up: 76% of the controls were event-free compared with 16% of the CAD group (p = 0.002). Figure 2 illustrates survival among the patients according to the amounts of total bile acid excreted during 20 years of follow up. The vast majority (91.1%) of patients who excreted ⩾415 mg of bile acids survived for 20 years compared with 16% of those who excreted <415 mg. Figure 3 describes survival in patients according to the amounts of deoxycholic acid excretion during a 20-year follow up: 79.4% of the patients who excreted ⩾214 mg survived to the end of the 20-year follow-up period compared with 19.3% of the patients who excreted <214 mg and survived to the end of the 20-year follow-up period (p < 0.001). Figure 4 shows similar findings for the comparison of the fraction of lithocholic acid: it was 86.3% of the patients who stayed alive for 20 years and excreted ⩾141 mg compared with 18.4% of the patients who stayed alive for 20 years and excreted <141 mg (p < 0.001). Figure 5 shows differences in survival according to cholic acid excretion. The statistical cut-off point was 14 mg/24 h, and the differences in survival were: 64.0% of the patients who stayed alive for 20 years excreted ⩾14 mg versus 33.1% of the patients who stayed alive for 20 years excreted <14 mg (p = 0.65). There were no differences in survival when the groups were compared for chenodeoxycholic acid excretion (p > 0.05; Figure 6). The latter result can be explained by the fact that very small amounts of this fraction existed in total BAEs
Figure 1.
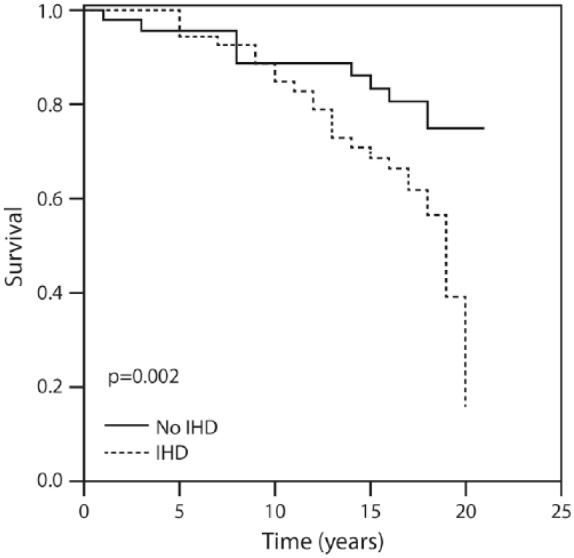
Twenty-year survival in patients with and without coronary artery disease.
Figure2.
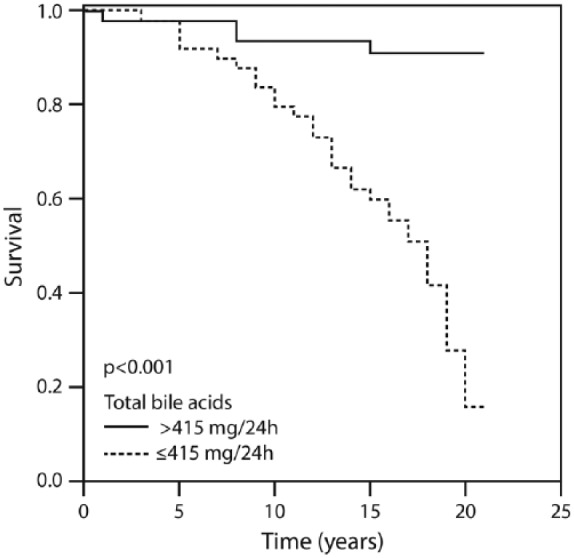
Twenty-year survival according to the amount of total bile acids above and below 415 mg/24 h.
Figure 3.
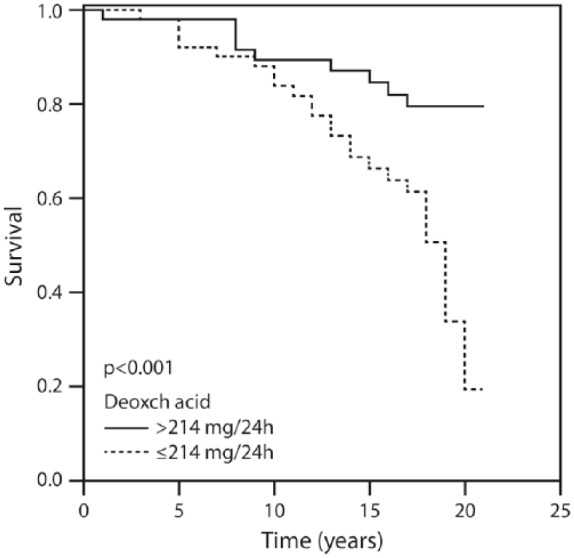
Twenty-year survival according to the amount of deoxycholic acid above and below 214 mg/24 h.
Figure 4.
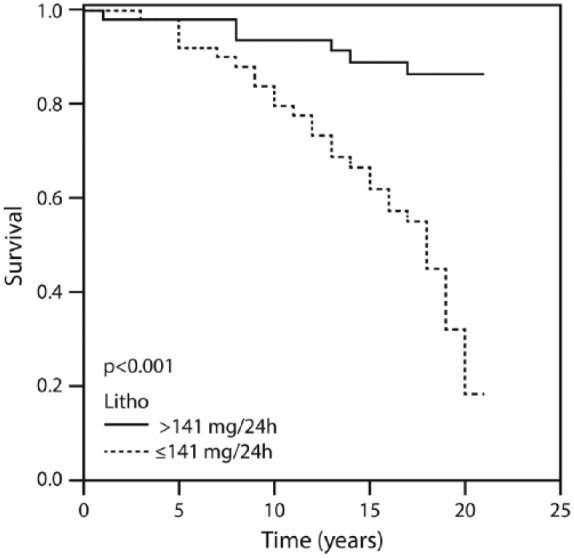
Twenty-year survival according to the amount of lithocholic acid above and below 141 mg/24 h.
Figure 5.
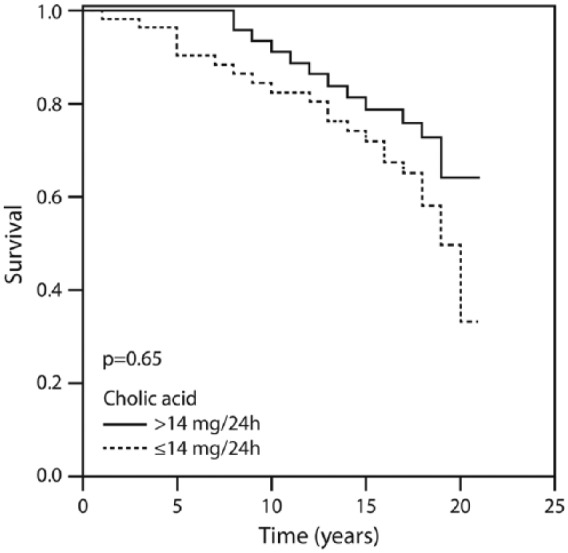
Twenty-year survival according to the amount of cholic acid above and below 14 mg/24 h.
Figure 6.
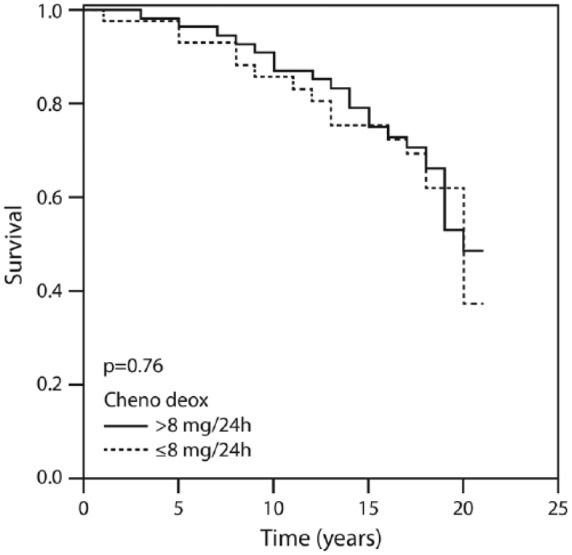
Twenty-year survival according to the amount of deoxycholic acid above and below 8 mg/24 h.
Multivariate regression analysis was used in order to define the clinical relevance of total bile acids in the CAD group. There was no correlation in any group between fecal bile acids and other lipid profile components. Table 3 displays adjusted HRs associated with cardiovascular mortality according to excreted total bile acids and their fractions adjusted to age, gender, BMI, CAD, LVEF, hypertension, smoking, total cholesterol, LDL, HDL, hemoglobin and creatinine. The clinical parameters of LVEF, BMI and presence of renal failure, and the laboratory parameters of lipid profile and BAE emerged as being significant (p < 0.05) independent factors that predicted CAD. Advanced age, male gender, LVEF, BMI, creatinine, hemoglobin and the major risk factors (DM, hypertension, smoking, hypercholesterolemia) for CAD did not correlate with BAEs. Regarding bile acids excretion-only, total bile acids and their two fractions as deoxycholic and lithocholic acids (but not cholic and chenodeoxycholic) correlated with CAD.
A total of 28 (56%) patients in Group 1 and 10 controls (20%) died during the 20-year follow up (p < 0.001). Four patients (8%) of Group 2 developed CAD during the follow up. Four study participants (8.0%) with a total bile acid level above the median (415 mg/dL) died compared with 34 patients (68.0%) with a total bile acid level <415 mg/dL (HR adjusted 0.14, 95% CI 0.04–0.45, p = 0.001). There was also a trend to associate between mortality and two main fractions of bile acid, deoxycholic acid and lithocholic acid that did not reach a level of significance in the multivariate analysis. Only 18% (9 patients) of the participants with a deoxycholic acid level >214 mg/dL died during the follow up, while 58% (29 patients) of those with a deoxycholic acid level <214 mg/dL died (HR 0.46, 95% CI 0.18–1.13, p = 0.09). The findings were similar for the participants with lithocholic acid levels >141 mg/dL, where 6 (12%) of them died compared with 32 (64%) with lithocholic acid levels <141 mg/dL (HR 0.42, 95% CI 0.15–1.17, p = 0.099).
Nine (18%) controls developed CAD events during the 20-year follow up, of whom 6 (12% of the controls) were on anti-hyperlipidemic treatment (started after determination of the BAE values).
Discussion
In the current investigation we found that mortality was significantly higher in individuals who excreted lower amounts of bile acids. Collected over a 20-year follow-up period, these findings support the hypothesis that CAD patients produce and eliminate lower levels of bile acids than non-CAD individuals, and that reduced production of bile acids, possibly leading to accumulation of cholesterol, could result in advanced atherosclerosis.3,6,15,17,18 The current investigation is in line with the few published human studies that reported increased fecal excretion of bile acids to have an atheroprotective effect.4,6,15,17,18 The majority of those investigations, however, were done on selected populations. Simonen and Miettinen18 showed that males with heterozygous FH and CAD excreted lower amounts of bile acids than controls with FH and normal coronary arteries. Rajaratnam and colleagues,17 however, showed that postmenopausal women with CAD had inefficient fecal elimination of bile acids. Our group studied earlier a mixed adult population with and without CAD and showed that CAD patients eliminated subnormal amounts of fecal bile acids.3 In the current investigation, we surveyed 50 CAD patients and 50 non-CAD patients with a follow-up period of up to 20 years. Our current findings support our earlier investigations and allow us to reach more firm conclusions on the crucial effect of the elimination of fecal bile acids in CAD development.
The remarkable differences in the amounts of excreted acids between the current study and control groups were found particularly in total bile acids, with deoxycholic acid as the main fraction. The difference was slightly less obvious, albeit significant, in the fraction of lithocholic acid, while there was no difference for cholic and chenodeoxycholic acids. Based on the current data, daily excretion of <415 mg total bile acids might be considered as a cut-off level for increased risk for CAD development. Amounts of deoxycholic acid <214 mg/24 h and lithocholic acid <141 mg/24 h can also be considered proatherogenic indicators.
Based on the significant differences seen only with secondary (deoxycholic and lithocholic) and not with primary bile acids (cholic and chenodeoxycholic), it would appear that gut bacteria-dependent metabolism of bile acids may be playing a role. Given that conjugated secondary bile acids are not absorbed and excreted in the feces, it is also likely that bacterial enzymes facilitating deconjugation would play a significant role. While these are speculations requiring further proof, it could open new lines of investigation related to the role of gut dysbiosis where increased deconjugation may limit fecal excretion.
We did not find any significant differences in the amounts of excreted bile acids between patients with and without hypercholesterolemia, DM, hypertension, smoking or family history of CAD (data not shown).
In contrast to total cholesterol, LDL-c and high-density lipoprotein, there was a correlation between the plasma triglycerides level and BAE, but only in the non-CAD group. This can be explained by rapid and more complete intestinal absorption of triglycerides due to an excess of intraluminal bile acids necessary for the emulsification of fats.
A significant percentage of patients were reported to develop atherosclerosis despite suppression of cholesterol levels by statin treatment.4,15,18 We reason that a greater decrease of cholesterol blood levels can be achieved by increasing cholesterol disposal. This is particularly true in the case of patients at high risk for CAD, and it can be accomplished by combining statins, ezetimibe, pcsk9 (proprotein convertase subtilisin/kexin type 9) inhibitor-evolocumab treatment with drugs affecting bile acid (and cholesterol) excretion, thus ensuring optimal management of dyslipidemia.
The objective of the current longitudinal study was to verify whether the differences in BAE between patients with CAD and non-CAD individuals persist, increase or decrease over an extended period of time. Importantly, some non-CAD patients can also be expected to develop coronary disease over a long period of follow up.
We consider that, in high-risk patients who cannot achieve optimal LDL cholesterol levels while on statin, ezetimibe or pcsk9 inhibitor, determination of BAE could help to evaluate whether adding a bile acid sequestrant may boost cholesterol lowering.
Study limitations
The sample was relatively small and this could be the reason why we did not find the impact of other bile acids, cholic and chenodeoxycholic acid, on patients’ survival. The vast majority of our data were collected before the widespread use of statins, but whatever statin use existed it would have led to underestimation of risk for the natural history of CAD. Limitations of this analysis include the absence of any information about statin use, and its intensity. Only mortality outcomes were recorded to the extent that nonfatal myocardial infarction (MI) occurred without subsequent CAD death during follow up. Thus, the risk for the combined outcomes of CAD death or nonfatal MI and total atherosclerotic cardiovascular disease would be underestimated in both groups. Our study included patients of predominantly European origin, thus limiting the generalizability of our findings. There are the usual limitations to this retrospective form of long-term follow up based on electronic health records as noted previously. Importantly, we do not know what lipid-regulating therapy was being used by the participants for the last 10 years of the study, and this limits the interpretation of data with regard to the magnitude of the 20-year benefit.
It is reasonable to assume that repeating stool analysis might yield more accurate results. However, stool sampling (described in the study protocol) is laborious and exceedingly challenging, meaning that repeating stool collection not only would intolerably burden participants but could result in a dropout rate risking the whole project. We thus opted for a single stool collection, using the same protocol published in two previous reports.15,26
It appears that BMI was not an independent determinant of BAE. [We found only weak correlation between BMI and cholic acid (r-0.2, p = 0.042).]
However, since BMI might affect BAE in an indirect fashion, the lack of homogeneity in BMI may limit the interpretation of our results.
Based on the significant differences seen only with secondary (deoxycholic and lithocholic) and not with primary bile acids (cholic and chenodeoxycholic), it would appear that gut bacteria-dependent metabolism of bile acids may be playing a role. Given that conjugated secondary bile acids are not absorbed and excreted in the feces, it is also likely that bacterial enzymes facilitating deconjugation would play a significant role. While these are speculations requiring further proof, additional lines of investigations related to the role of gut dysbiosis where increased deconjugation may limit fecal excretion are warranted.
Conclusion
The results of the present follow-up study showed significantly lower amounts of total bile acid, deoxycholic acid and lithocholic acid secretion in patients with CAD compared with non-CAD patients. Decreased BAE to a daily level <415 mg was associated with increased mortality.
In other words, decreased BAE was found to independently be associated with increased mortality when patients with CAD are compared with non-CAD individuals. This might support a role for BAE as an independent risk factor for CAD.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Gideon Charach, Department of Internal Medicine ‘C’, Tel Aviv Sourasky Medical Center, Affiliated to Tel Aviv University, Sackler Medical School, 6 Weizman Street, Tel Aviv 6423906, Israel.
Ori Argov, Department of Internal Medicine ‘C’, Tel Aviv Sourasky Medical Center, Affiliated to Tel Aviv University, Sackler Medical School, Israel.
Karyn Geiger, Department of Internal Medicine ‘C’, Tel Aviv Sourasky Medical Center, Affiliated to Tel Aviv University, Sackler Medical School, Israel.
Lior Charach, Department of Internal Medicine ‘C’, Tel Aviv Sourasky Medical Center, Affiliated to Tel Aviv University, Sackler Medical School, Israel.
Ori Rogowski, Department of Internal Medicine ‘C’, Tel Aviv Sourasky Medical Center, Affiliated to Tel Aviv University, Sackler Medical School, Israel.
Itamar Grosskopf, Department of Internal Medicine ‘C’, Tel Aviv Sourasky Medical Center, Affiliated to Tel Aviv University, Sackler Medical School, Israel.
References
- 1. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344: 1383–1389. [PubMed] [Google Scholar]
- 2. Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the asteroid trial. JAMA 2006; 295: 1556–1565. [DOI] [PubMed] [Google Scholar]
- 3. Bhat G, Rapp S, Beaudry J, et al. Inhibition of ileal bile acids transport and reduced atherosclerosis in apo E–/– mice by SC-435. J Lipid Res 2003; 44: 1614–1621. [DOI] [PubMed] [Google Scholar]
- 4. Izzat N, Deshazer M, Loose-Mitchell D. New molecular targets for cholesterol-lowering therapy. J Pharmacol Exp Ther 2000; 293: 315–320. [PubMed] [Google Scholar]
- 5. Chiang JY. Bile acids: regulation of synthesis. J Lipid Res 2009; 50: 1955–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rabinovich P, Guzachev A. Clinical significance of the cholesterol load test in atherosclerotic patients. Cardiologia 1987; 27: 44–48. [PubMed] [Google Scholar]
- 7. Rajaratnam R, Gylling H, Miettinen T. Serum squalene in postmenopausal women without and with coronary artery disease. Atherosclerosis 1999; 146: 61–64. [DOI] [PubMed] [Google Scholar]
- 8. Rajaratnam R, Gylling H, Miettinen T. Independent association of serum squalene and noncholesterol sterols with coronary artery disease in postmenopausal women. J Am Coll Cardiol 2000; 35: 1185–1191. [DOI] [PubMed] [Google Scholar]
- 9. Sudhop T, Gottwald B, von Bergmann K. Serum plant sterols as a potential risk factor for coronary disease. Metabolism 2002; 51: 1519–1521. [DOI] [PubMed] [Google Scholar]
- 10. Assmann G, Cullen P, Erbey J, et al. Plasma sitosterol elevation are associated with an increased incidence of coronary events in men; results of a nested case – control analysis of the Prospective Cardiovascular Münster (PROCAM) study. Nutr Metab Cardiovasc Dis 2006; 16: 13–21. [DOI] [PubMed] [Google Scholar]
- 11. Post SM, de Crom R, van Haperen R, et al. Increased fecal bile acid excretion in transgenic mice with elevated expression of human phospholipid transfer protein. Arterioscler Thromb Vasc Biol 2003; 23: 892–897. [DOI] [PubMed] [Google Scholar]
- 12. Lutton C. Cholesterol and bile acid dynamics: comparative aspects. Reprod Nutr Develop 1990; 30: 145–160. [PubMed] [Google Scholar]
- 13. Li H, Xu G, Shang Q, et al. Inhibition of ileal bile acid transport lowers plasma cholesterol levels by inactivating hepatic fanesoid X receptor and stimulating cholesterol 7alpha hydroxylase. Metabolism 2004; 53: 927–932. [DOI] [PubMed] [Google Scholar]
- 14. Lofland H, Clarkson T, Clair R, et al. Studies on the regulation of plasma cholesterol levels in squirrel monkeys of two genotypes. J Lipid Res 1972; 13: 39–47. [PubMed] [Google Scholar]
- 15. Charach G, Rabinovich P, Konikoff F, et al. Decreased fecal bile acid output in patients with coronary atherosclerosis. J Med 1998; 29: 125–136. [PubMed] [Google Scholar]
- 16. Gylling H, Hallikainen M, Rajaratnam RA, et al. The metabolism of plant sterols is disturbed in postmenopausal women with coronary artery disease. Metabolism 2009; 58: 401–407. [DOI] [PubMed] [Google Scholar]
- 17. Rajaratnam R, Gylling H, Mittinen T. Cholesterol absorption, synthesis and fecal output in postmenopausal women with and without coronary artery disease. Arterioscl Thromb Vasc Biol 2001; 21: 1650–1655. [DOI] [PubMed] [Google Scholar]
- 18. Simonen H, Miettinen T. Coronary artery disease and bile acid synthesis in familial hypercholesterolemia. Atherosclerosis 1987; 63: 159–166. [DOI] [PubMed] [Google Scholar]
- 19. Grundy S, Ahrens E, Miettinen T. Quantitative isolation and gas liquid chromatographic analysis of total bile acids. J Lipid Res 1965; 6: 397–410. [PubMed] [Google Scholar]
- 20. Angelin B, Carlson L. Bile acids and plasma high density lipoproteins: biliary lipid metabolism in fish eye disease. Eur J Clin Invest 1986; 16: 157–162. [DOI] [PubMed] [Google Scholar]
- 21. Yamuri Y, Murakami S, Ikeda K, et al. Fish and life style-related disease prevention: experimental and epidemiological evidence for anti atherogenic potential of taurine. Clin Exp Pharmacol Physiol 2004; 31(Suppl. 2): S20–S23. [DOI] [PubMed] [Google Scholar]
- 22. Morozova S, Suc-Royer I, Auwerx J. Cholesterol metabolism modulators in future drug therapy for atherosclerosis. Med Sci (Paris) 2005; 21: 53–58. [PubMed] [Google Scholar]
- 23. Poorman J, Buck R, Smith S, et al. Bile acid excretion and cholesterol 7 alpha hydroxylase expression in hypercholesterolemia-resistant rabbits. J Lipid Res 1993; 34: 1675–1685. [PubMed] [Google Scholar]
- 24. Princen H, Post S, Twisk J. Regulation of bile acid biosynthesis. Curr Pharmaceut Design 1997; 3: 59–84. [Google Scholar]
- 25. Machleder D, Ivandic B, Castellani L, et al. Complex genetic control of HDL levels in mice in response to an atherogenic diet: coordinate regulation of HDL levels and bile acid metabolism. J Clin Invest 1999; 99: 1406–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gilat T, Ronen O. Quantitative analysis of single stool samples using a continuous marker. Am J Dig Dis 1972; 17: 761–765. [DOI] [PubMed] [Google Scholar]
- 27. Batta A, Salen G, Rapole K, et al. Highly simplified method for gas-liquid chromatographic quantitation of bile acids and sterols in human stool. J Lipid Res 1999; 40: 1148–1154. [PubMed] [Google Scholar]


