Abstract
Brivaracetam (BRV), the n-propyl analogue of levetiracetam (LEV), is the latest antiepileptic drug (AED) to be licensed in Europe and the USA for the adjunctive treatment of focal-onset seizures with or without secondary generalization in patients aged 16 years or older. Like LEV, BRV binds to synaptic vesicle protein 2A (SV2A), but BRV has more selective binding and a 15- to 30-fold higher binding affinity than LEV. BRV is more effective than LEV in slowing synaptic vesicle mobilization and the two AEDs may act at different binding sites or interact with different conformational states of the SV2A protein. In animal models, BRV provides protection against focal and secondary generalized seizures and has significant anticonvulsant effects in genetic models of epilepsy. The drug undergoes first-order pharmacokinetics with an elimination half-life of 7–8 h. Although BRV is metabolized extensively, the main circulating compound is unchanged BRV. Around 95% of metabolites undergo renal elimination. No dose reduction is required in renal impairment, but it is recommended that the daily dose is reduced by one-third in hepatic dysfunction that may prolong half-life. BRV has a low potential for drug interactions. The efficacy and tolerability of adjunctive BRV in adults with focal-onset seizures have been explored in six randomized, placebo-controlled studies. These showed significant efficacy outcomes for doses of 50–200 mg/day. The most common adverse events reported were headache, somnolence, dizziness, fatigue and nausea. Patients who develop psychiatric symptoms with LEV appear to be at risk of similar side effects with BRV, although preliminary data suggest that these issues are likely to be less frequent and perhaps less severe. As with all AEDs, a low starting dose and slow titration schedule help to minimize side effects and optimize seizure control and thereby quality of life.
Keywords: antiepileptic drug, brivaracetam, epilepsy, focal-onset, seizures, synaptic vesicle protein 2A
Introduction
Epilepsy is the most common chronic neurological condition. Globally, 65 million individuals are affected1 and the diagnosis is made in an estimated 2.4 million people each year.2 Despite the availability of over 14 new antiepileptic drugs (AEDs) during the past three decades, repeated outcome analyses show that >30% fail to achieve prolonged seizure freedom with medical treatment.3–5 The introduction of novel agents is, therefore, welcome. Brivaracetam (BRV) is the latest AED to be licensed in Europe and the USA for the adjunctive treatment of focal-onset seizures with or without secondary generalization in patients aged 16 years or older. This article discusses the pharmacological properties of BRV, its performance in regulatory studies, details of its efficacy, tolerability and safety profiles and its place in everyday clinical practice.
Mechanisms of action and activity profile in animal models
BRV was discovered during a large-scale programme aimed at optimizing pharmacodynamic activity at a novel molecular AED target.6 It is the n-propyl analogue of levetiracetam (LEV; Figure 1), which acts as a high-affinity ligand for synaptic vesicle protein 2A (SV2A). SV2A is an integral transmembrane glycoprotein expressed in neurons and endocrine cells, which is involved in the modulation of synaptic vesicle exocytosis and neurotransmitter release.7 It also appears to have an important role in epileptogenesis, since SV2A deficiency in transgenic mice leads to increased seizure vulnerability.6
Figure 1.
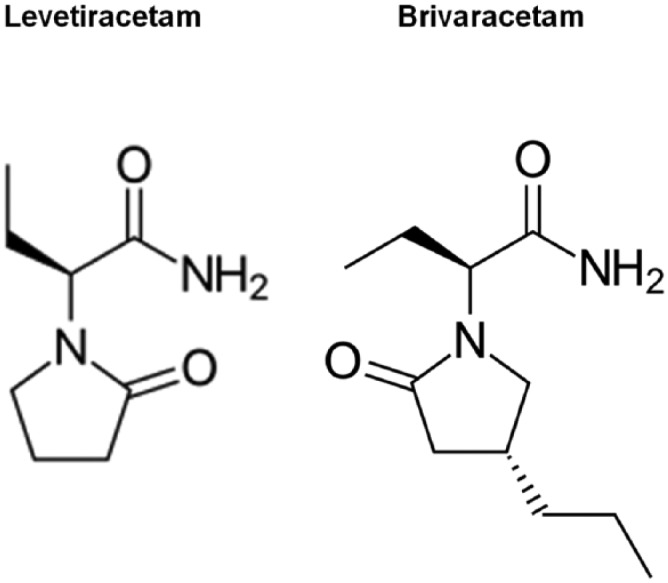
Chemical structures of levetiracetam and brivaracetam.
Like LEV, BRV binds to SV2A, but this molecule has more selective binding with the protein and a 15- to 30-fold higher binding affinity.8 The SV2A protein has been shown to be the primary molecular target of the anticonvulsant activity of both drugs.9,10 BRV enters into recycling synaptic vesicles, producing a frequency-dependent decrease of synaptic transmission at 100-fold lower concentrations than LEV.11 The drug has also been shown to be more effective than LEV in slowing synaptic vesicle mobilization.12 Radioligand binding studies suggest that LEV and BRV may act at different binding sites or interact with different conformational states of the SV2A protein.13 BRV also has inhibitory activity at voltage-gated sodium channels, but there is no evidence to suggest that this mechanism is relevant to its anticonvulsant properties.14
In animal models, including amygdala-kindled mice, BRV provides protection against focal and secondary generalized seizures, being more potent than LEV in protecting against secondary generalization.6 BRV has also had significant anticonvulsant effects in genetic models of epilepsy, including the Genetic Absence Rats from Strasbourg.15 There is evidence from a rat model of self-sustaining status epilepticus that BRV may also be an effective treatment in this situation.16,17 BRV crosses the blood–brain barrier much more rapidly than does LEV and reaches maximal brain concentration within minutes following intravenous administration.16 The use of BRV with diazepam in this model reduced frequency of spontaneous recurrent seizures, cumulative seizure time and spike frequency.17
Pharmacokinetics
When BRV was administered to healthy adult male volunteers, it was absorbed rapidly, with kinetics unaffected by the presence of food.18,19 The drug undergoes first-order pharmacokinetics with a volume of distribution close to total body water (VZ = 0.5 l/kg) and low plasma protein binding (17.5%).20 The elimination half-life is 7–8 h. The major route of metabolism is by hydrolysis of the acetamide group, which leads to the production of a carboxylic acid metabolite, constituting 34% of urinary excretion.19 Secondary routes of metabolism include hydroxylation mediated by cytochrome P450 2C19 (CYP2C19).20 Hydrolysis of the amide moiety results in the production of acid metabolites (34.2% of the radiolabelled dose). There is also CYP-mediated hydroxylation of the n-propyl side chain (15.9% of the dose). A hydroxyacid metabolite is formed by both of these pathways (15.2% of the dose). Glucuronic acid and taurine conjugates play a minor role in its elimination, with 8.6% of the dose being recovered unchanged in the urine.20 The three BRV metabolites (acid, hydroxyl and hydroxyacid) are all pharmacologically inactive.
Although BRV is extensively metabolized, the main circulating compound is the unchanged molecule, accounting for over 90% of the plasma radioactivity following an oral dose of 14C-labelled BRV in healthy humans.20 Renal clearance of unchanged BRV is low (0.04 ml/min/kg).19 Around 95% of metabolite elimination is via the kidneys, with an unchanged fraction of 8–11%.20,21
BRV clearance is increased in homozygous extensive metabolizers via CYP2C19, although this pathway appears to be insignificant compared with hydrolysis to the acid metabolite.22 There is no evidence of enzyme induction with this AED. Severe hepatic dysfunction may prolong its half-life to up to 17.4 h with BRV exposure being increased by 50–60%.23 It is recommended that the daily dose be reduced by one-third in these circumstances. No dosage reduction is required in patients with renal impairment, although there are no available data in patients on dialysis for end-stage renal disease. Pharmacokinetics are similar in children and adults.24 A paediatric dose adaptation of 2 mg/kg bodyweight twice daily with a maximum of 100 mg twice daily for bodyweight over 50 kg is predicted to ensure steady-state plasma concentrations in the same range as in adult patients receiving BRV 100 mg twice daily.
Fertility, pregnancy, teratogenicity and breast feeding
There are limited fertility, pregnancy and teratogenicity data regarding BRV in humans.25 In animal studies, BRV did not affect male or female fertility and no teratogenic potential was demonstrated in rat or rabbit models. Embryotoxicity was observed in rabbits at a maternal toxic dose of BRV with an exposure level eight-fold the clinical area under the curve exposure at the maximum recommended dose. In rats, BRV was shown to cross the placenta and to be excreted in breast milk with concentrations similar to maternal plasma concentrations. As with all women of childbearing potential with epilepsy, those taking BRV should receive counselling about these issues throughout their management and during pregnancy.26
Drug interactions
BRV has a low potential for drug interactions. There is the possibility for plasma concentrations to increase when the AED is given with inhibitors of CYP2C19, such as fluconazole and fluvoxamine, although adverse clinical consequences are thought to be unlikely.25 BRV approximately doubles the effect of alcohol on psychomotor function, attention and memory, although there is no pharmacokinetic interaction.27 In regulatory studies, when BRV was coadministered with LEV, there was no added efficacy, and no safety or tolerability issues were observed.28 Hepatic enzyme-inducing AEDs, such as phenobarbital, phenytoin and carbamazepine, reduce slightly BRV plasma concentrations but no dose adjustment is thought to be required.25,29 However, when BRV was given with carbamazepine, there was a 2.6-fold increase in carbamazepine epoxide concentrations.29 The clinical relevance of this potential interaction is thought to be minimal.30,31 In healthy subjects, rifampicin reduces the BRV area under the concentration curve and thus BRV dosing may need to be increased in patients taking these two drugs.32 The summary of product characteristics also advises caution when considering coadministration with other enzyme inducers, such at St. John’s wort.25 In vitro studies have shown that BRV may increase plasma concentrations of drugs metabolized by CYP2C19, such as lansoprazole, omeprazole and diazepam.25 A 20–30% reduction in the exposure of ethinylestradiol and levonorgestrel occurred with BRV 400 mg/day,33 but no interactions were observed with a dose of 100 mg/day.34 Concentrations of drugs transported by the transmembrane protein organic anion transporter OAT3 (acyclovir, bumetanide, ciprofloxacin, zidovudine, pravastatin, rosuvastatin, sitagliptin, famotidine, furosemide, penicillin G and methotrexate) may be reduced by 200 mg daily of BRV.25
Efficacy
The efficacy and tolerability of adjunctive BRV in adults with focal-onset seizures have been explored in six randomized, placebo-controlled studies (Table 1). During two phase IIb studies, adults with focal-onset seizures were randomized to receive BRV 5, 20 or 50 mg/day35 and 50 or 150 mg/day.36 In both studies the primary efficacy endpoint of percentage reduction in focal-onset seizures from baseline was statistically significant for patients receiving 50 and 150 mg/day, but not for smaller doses.
Table 1.
Randomized, placebo-controlled studies of brivaracetam in patients with focal-onset seizures (FOS).
| Study (Reference) |
ITT population | n (%) completed | Age range (years) | Duration of treatment (weeks) | Dose range (mg/day) | Percentage reduction in weekly FOS from baseline | Median percentage reduction in FOS frequency/week from baseline | Percentage ⩾50% responder rate | n (%) seizure-free |
|---|---|---|---|---|---|---|---|---|---|
| French and colleagues35
(NO1193) |
208 | 197 (94.7) | 16–65 | 7 | 5–50 | 50 mg/day – 22.1*
20 mg/day – 14.9 5 mg/day – 9.8 |
50 mg/day – 53.1*
20 mg/day – 42.6* 5 mg/day – 29.9 Placebo – 21.7 |
50 mg/day – 55.8*
20 mg/day – 44.2* 5 mg/day – 32* Placebo – 16.7 |
50 mg/day – 4 (7.7) 20 mg/day – 4 (7.7) 5 mg/day – 4 (7.7) Placebo – 1 (1.9) |
| Van Paesschen and colleagues36(NO1114) | 157 | 148 (94.3) | 16–65 | 10 | 50–150 | 150 mg/day – 16.3*
50 mg/day – 17.7* |
150 mg/day – 28.3 50 mg/day – 34.9* Placebo – 16.3 |
150 mg/day – 30.8 50 mg/day – 35.8* Placebo – 17.3 |
150 mg/day – 3 (5.8) 50 mg/day – 5 (9.4) Placebo – 1 (1.9) |
| Biton and colleagues37
(NO1253) |
396 | 392 (98.9) | 16–70 | 12 | 5–50 | 50 mg/day – 12.8*
20 mg/day – 4.1 5 mg/day – –0.9 |
50 mg/day – 30.5 20 mg/day – 22.5 5 mg/day – 20.0 Placebo – 17.8 |
50 mg/day – 32.7*
20 mg/day – 23.2 5 mg/day – 21.9 Placebo – 16.7 |
50 mg/day – 4 (4.0) 20 mg/day – 1 (1.0) 5 mg/day – 1 (1.1) Placebo – 0 (0) |
| Kwan and colleagues38
(NO1254) |
480 | 434 (90.4) | 16–70 | 16 | 50–150 | 50–150 mg/day – 7.3 | 50–150 mg/day – 26.9 Placebo – 18.9 |
50-150 mg/day – 30.3*
Placebo – 16.7 |
50–150 mg/day – 5 (1.5) Placebo – 0 (0) |
| Ryvlin and colleagues39
(NO1252) |
398 | 367 (92.2) | 16–70 | 12 | 20–100 | 100 mg/day – 11.7*
50 mg/day – 6.5 20 mg/day – 6.8 |
100 mg/day – 32.5*
50 mg/day – 26.8 20 mg/day – 30* Placebo – 17 |
100 mg/day – 36.0*
50 mg/day – 27.3 20 mg/day – 27.3 Placebo – 20.0 |
100 mg/day – 4 (4.0) 50 mg/day – 0 (0) 20 mg/day – 2 (2.0) Placebo – 0 (0) |
| Klein and colleagues40
(NO1358) |
760 | 696 (90.6) | 16–80 | 12 | 100–200 | 200 mg/day – 23.2*a
100 mg/day – 22.8*a |
200 mg/day – 35.6*b
100 mg/day – 37.2*b Placebo – 17.6 |
200 mg/day – 37.8*c
100 mg/day – 38.9*c Placebo – 21.6 |
200 mg/day – 10 (4.0)*
100 mg/day – 13 (5.2)* Placebo – 2 (0.8) |
Statistically significant difference in outcome between dose and placebo.
Percentage reduction over placebo in 28-day adjusted FOS frequency.
Median percentage reduction in FOS frequency from baseline during treatment period.
⩾50% responder rate for FOS frequency from baseline to the end of treatment period.
During four subsequent phase III studies patients received 5–200 mg/day BRV or placebo. Three studies employed fixed BRV doses37,39,40 and one was a flexible dosing trial.38 The percentage reduction in focal-onset seizures from baseline was statistically significant for patients receiving 100–200 mg/day in the three flexible dosing studies, and for patients taking 50 mg/day in study NO1253.35–37 This primary efficacy endpoint did not reach statistical significance in the flexible dose study in patients receiving BRV 50–150 mg/day,38 nor for lower doses of 5 mg and 20 mg/day.35,37,39 Statistically significant seizure freedom rates of 4.0–5.2% were achieved in patients treated with 100–200 mg BRV daily in the largest study.40 Health-related quality of life scores from patients participating in the fixed dose studies were stable or improved over the trials.41
Meta-analyses of adjunctive BRV for patients with refractory focal seizures have been undertaken.42,43 Results from 1639 adults who participated in five randomized, controlled studies found the pooled risk ratio of BRV versus placebo was 4.11 (95% CI 1.39–12.21) for seizure-free rates and 1.80 (95% CI 1.43–2.26) for 50% responder rates. These results were statistically significant, although the pooled risk ratio of 1.08 (95% CI 0.73–1.59) for withdrawal rates was not.42 The 50% responder rate for patients receiving BRV was significantly higher than placebo for daily doses of 20 mg, 50 mg and 100 mg, but not statistically significant for 5 mg or 150 mg. Patients randomized to receive BRV were statistically more likely to develop fatigue and somnolence than those receiving placebo.
The analysis of six randomized, controlled studies in which 2399 adults participated found the overall pooled risk ratios for seizure freedom and 50% responder rate were 4.74 (95% CI 2.00–11.25) and 1.79 (95% CI 1.51–2.12) respectively.43 Doses achieving a 50% or more reduction in seizures were 20–200 mg/day. Where BRV was administered to patients already receiving LEV, the AED was not effective in reducing seizure frequency by 50%. The authors postulated that this outcome may reflect the similar chemical structure and mechanism of action of the two AEDs. BRV was significantly more likely than placebo to be associated with irritability, fatigue, somnolence and dizziness.
Using an indirect comparison meta-analysis, the efficacy and tolerability of adjunctive BRV was compared to that of lacosamide, eslicarbazepine acetate and perampanel in patients with focal-onset seizures who participated in randomized, controlled trials.44 No significant differences were demonstrated using minimum (BRV 50 mg/day, eslicarbazepine acetate 800 mg/day, lacosamide 200 mg/day, perampanel 8 mg/day) and the highest effective tolerated doses (BRV 200 mg/day, eslicarbazepine acetate 1200 mg/day, lacosamide 400 mg/day, perampanel 12 mg/day).
An efficacy, tolerability and safety analysis concluded that studies provided Class 1 evidence that adjunctive BRV is effective in reducing focal-onset seizure frequency in adults with uncontrolled seizures.45 Efficacy in elderly people is comparable to that in younger subjects with no dosage adjustment required.46
Outcomes with BRV were evaluated in 47 (94% of ITT population) adults with Unverricht–Lundborg disease (genetically ascertained EPM1), who participated in two prospective, multicentre, double-blind phase III trials.47 Overall, 106 patients with moderate to severe myoclonus were randomized to receive BRV 50 mg/day, 150 mg/day or placebo in study NO1187 and 5 mg/day, 150 mg/day or placebo in study NO1236. Doses were titrated over 2 weeks with a treatment period of 12 weeks. NO1187 was completed by 47 of 50 patients; NO1236 by 54 of 56 randomized. There was no significant improvement in action myoclonus scores with the three BRV doses. However, the drug was well tolerated with high study completion rates. The authors postulated that perhaps doses were too small to produce improvements or that action myoclonus was not the optimal assessment tool for efficacy due to wide intra-patient variability.
Tolerability and safety
Dose-related adverse effects reported in the regulatory studies were classified by investigators as generally mild to moderate.48 The commonest comprised headache, somnolence, dizziness, fatigue and nausea.35–40,47 Overall, of 2218 patients randomized to receive BRV in these trials, 136 (6.1%) discontinued the drug due to adverse events.48 Pooled data from phase II and phase III studies suggested that long-term treatment with BRV was well tolerated.49
Psychiatric disorders were reported in less than 3% of study patients receiving BRV.35,37–40 The exception was the NO1114 study, analysis of which also showed that patients randomized to placebo were almost twice as likely to report psychiatric problems.36 Irritability, agitation, anxiety, insomnia, aggression and depression were the commonest symptoms. One patient receiving BRV in the NO1252 study had the drug withdrawn following an episode of psychosis.39 In the Unverricht–Lundborg studies, psychiatric symptoms were reported by 9 of 106 (8.5%) patients randomized in each of the BRV and placebo arms.47
Dosage and administration
BRV is available in 10 mg, 25 mg, 50 mg, 75 mg and 100 mg film-coated tablets and a bioequivalent 10mg/ml solution25 for oral administration.50,51 The tablets contain lactose and, therefore, are not suitable for patients with galactose intolerance, lactase deficiency or glucose–galactose malabsorption. The solution can be diluted in water or fruit juice and can be given by nasogastric or gastrostomy tube. It contains sorbitol (E420) and may not be suitable for patients with fructose intolerance. It also contains methylparahydroxybenzoate (E218), a paraben ester, which can produce allergic reactions. For patients requiring parenteral administration, there is a bolus injection solution or a 15-min intravenous infusion, both at a concentration of 10 mg/ml. The injection does not need to be diluted, although it may be mixed with 0.9% sodium chloride injection USP, lactated Ringer’s injection or 5% dextrose injection USP.
Use in clinical practice
BRV is currently licensed in Europe and the USA as an adjunctive treatment for patients with focal and /or secondary generalized seizures. Early in 2017, the manufacturer filed an application to the FDA for use of the AED as monotherapy for this indication, the outcome of which is awaited. The recommended starting dose is 50 mg/day or 100 mg/day in two divided doses. However, in clinical practice, this titration schedule has led to tolerability problems in some patients and thus a lower starting dose of 25 mg/day for 2 weeks, increasing to 25 mg twice daily for 2 more weeks, after which 50 mg twice daily is usually employed and events awaited. Thereafter, dosing is adjusted as clinically indicated with an initial upper target of brivaracetam 100 mg twice daily. Employing a low starting dose and slow titration schedule will help to minimize the risk of adverse effects, allowing dosing to be tailored for each individual patient and thereby improving the potential for optimal seizure control and better quality of life.
Post-marketing studies
Following the licensing of BRV, a number of projects have evaluated outcomes in everyday clinical settings. A German multicentre study included 262 children and adults with seizures refractory to AED treatment, who received 50–400 mg/day adjunctive BRV.52 Of the 192 patients for whom efficacy data were available at 6 months, 77 reported a 50% seizure reduction (including 29 seizure-free), 13 had a marginal seizure improvement, 50 had no change and 4 had an increase in seizures. Genetic generalized epilepsies were present in 19 patients. At 3 months three were seizure-free, eight had an improvement in seizure frequency, two reported no change and six had discontinued BRV. Absence seizures became completely controlled in all of six patients and myoclonia in four of eight patients. The most frequently reported adverse events were sedation, dizziness, mood changes, nausea and vomiting, irritability and aggression. During the study period, 68 (26%) of the 262 patients discontinued BRV, 41 (16%) due to side effects.
At the Kork Epilepsy Centre in Germany, data were prospectively collected from 101 adult patients with difficult-to-control (mainly focal-onset) seizures started on adjunctive BRV.53 On a median dose of 200 mg/day (range 50–400 mg/day), 28 (27.8%) patients demonstrated at least a 50% seizure reduction over 3 months, seven of whom were seizure-free. After 6 months the retention rate was 51.5%, with the main reason for discontinuation being lack of efficacy. The most frequent adverse events were dizziness and somnolence, but they only accounted for BRV discontinuation in three patients. Psychiatric problems, comprising irritability, aggression, depression and psychosis, each occurred in one patient.
Brivaracetam use in status epilepticus
A retrospective review of 205 patients with status epilepticus identified 11 patients who had been administered BRV for refractory and super-refractory status epilepticus, mainly due to vascular causes.54 Four patients had a history of epilepsy and were already taking AED treatment. BRV was started in initial doses of 50–400 mg/day with maintenance doses of 100–400 mg/day. Within 24 h of starting BRV resolution of status epilepticus occurred in three patients.
Switching to brivaracetam from levetiracetam
Given that LEV and BRV are both ligands of SV2A, there has been interest in how patients fare when switched from the older to the newer AED. At a single centre in Germany, 43 patients with focal-onset seizures were switched abruptly from LEV (3 monotherapy) to BRV in ratios of 15:1 and 20:1. There were no immediate adverse consequences.53 Of these, 26 (60%) discontinued BRV and restarted LEV, 20 due to lack of efficacy (5 with worsening seizures) and 6 because of tolerability issues. BRV was continued by 17 (40%) patients, 3 of whom reported reduced irritability with one experiencing an improvement in somnolence. In a German multicentre study, 133 patients were switched from LEV to BRV (median ratio 15:1; range 2:1–40:1), the majority in one day.52 At 3 months, 18 (13.5%) were seizure-free, 36 (27.1%) had improved seizure outcomes and 31 (23.3%) patients discontinued BRV. Of the 51 patients switched to BRV because of adverse events associated with LEV, an improvement was reported in 30 (58.8%). Of patients who had behavioural problems with LEV, 75% reported no such issues with BRV. However, these individuals were statistically more likely to also have behavioural problems with BRV. In the two studies, 41 patients were switched to BRV doses higher than the recommended 200 mg/day. Of these, nine patients had an improvement in seizure frequency, but two had a subsequent dose reduction due to dizziness53 and seven discontinued BRV thereafter.52
Non-psychotic behavioural adverse events were evaluated in a phase IIIb open-label, multicentre study.55 Adults who had all experienced behavioural problems within 16 weeks of receiving LEV were switched overnight to BRV, which was then prescribed over a 12-week period with doses titrated to 50–200 mg/day. Of 29 patients, 20 showed a marked or moderate improvement in behavioural adverse effects. BRV was discontinued by three patients – two due to side effects and one because of lack of efficacy.
Conclusion
BRV is a novel AED which acts as a high-affinity ligand for SV2A with evidence to suggest that it modulates vesicle activity. It has more potency for SV2A than LEV and may affect the protein differently. BRV has efficacy for focal and secondary generalized seizures. Animal models and some open-label human data provide encouraging results for genetic epilepsies. BRV carries a low potential for drug interactions. Most common side effects comprise headache, somnolence, dizziness, fatigue and nausea. Patients who develop psychiatric symptoms with LEV appear to be at risk of similar side effects with BRV, although preliminary data suggest that these issues are likely to be less frequent and perhaps less severe. As with all AEDs, a low starting dose and slow titration schedule help to minimize side effects and optimize seizure control and thereby quality of life.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: Martin Brodie serves on the scientific advisory boards of Eisai Ltd, UCB Pharma, GlaxoSmithKline, Lundbeck, Bial, GW Pharmaceuticals and Takeda. He is on the speakers’ bureau for Eisai Ltd, UCB Pharma, GlaxoSmithKline and Lundbeck and has accepted travel grants for scientific meetings from Eisai Ltd, UCB Pharma and Lundbeck.
Linda Stephen has received lecture fees and support for travel to congresses from UCB Pharma and Eisai Ltd.
Contributor Information
Linda J. Stephen, Epilepsy Unit, West Glasgow ACH, Dalnair St, Glasgow, G3 8SJ, Scotland.
Martin J. Brodie, Epilepsy Unit, West Glasgow Ambulatory Care Hospital, Glasgow, Scotland
References
- 1. Moshé SL, Perucca E, Ryvlin P, et al. Epilepsy: new advances. Lancet 2015;385: 884–898. [DOI] [PubMed] [Google Scholar]
- 2. WHO. Epilepsy factsheet, www.who.int/mediacentre/factsheets/fs999/en/ (accessed November 2017).
- 3. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000; 342; 314–319. [DOI] [PubMed] [Google Scholar]
- 4. Brodie MJ, Barry SJE, Bamagous GA, et al. Patterns of treatment response in newly diagnosed epilepsy. Neurology 2012; 78: 1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Z, Brodie MJ, Liew D, et al. Unchanged outcomes in newly diagnosed epilepsy. JAMA Neurol in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klitgaard H, Matagne A, Nicolas J-M, et al. Brivaracetam: rationale for discovery and preclinical profile of a selective SV2A ligand for epilepsy treatment. Epilepsia 2016; 57: 538–548. [DOI] [PubMed] [Google Scholar]
- 7. Kaminski RM, Matagne A, Leclercq K, et al. SV2A protein is a broad-spectrum anticonvulsant target: functional correlation between protein binding and seizure protection in models of both partial and generalized epilepsy. Neuropharmacology 2008; 54: 715–720. [DOI] [PubMed] [Google Scholar]
- 8. Gillard M, Fuks B, Leclercq K, et al. Binding characteristics of brivaracetam, a selective, high affinity SV2A ligand in rat, mouse and human brain: relationship to anticonvulsant properties. Eur J Pharmacol 2011; 664: 36–44. [DOI] [PubMed] [Google Scholar]
- 9. Baijalieh SM, Peterson K, Shinghal R, et al. SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science 1992; 257: 1271–1273. [DOI] [PubMed] [Google Scholar]
- 10. Feany MB, Lee S, Edwards RH, et al. The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell 1992; 70: 861–867. [DOI] [PubMed] [Google Scholar]
- 11. Matagne A, Margineau D-G, Kenda B, et al. Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A. Br J Pharmacol 2008; 154: 1662–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang X, Bognar J, Jr, He T, et al. Brivaracetam augments short-term depression and slows vesicle recycling. Epilepsia 2015; 56: 1899–1909. [DOI] [PubMed] [Google Scholar]
- 13. Wood MD, Gillard M. Evidence for a differential interaction of brivaracetam and levetiracetam with the synaptic vesicle 2A protein. Epilepsia 2017; 58: 255–262. [DOI] [PubMed] [Google Scholar]
- 14. Niespodziany I, André VM, Leclère N, et al. Brivaracetam differentially affects voltage-gated sodium currents without impairing sustained repetitive firing in neurons. CNS Neurosci Ther 2015; 21: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tai KK, Truong DD. Brivaracetam is superior to levetiracetam in a rat model of post-hypoxic myoclonus. J Neural Transm (Vienna) 2007; 114: 1547–1551. [DOI] [PubMed] [Google Scholar]
- 16. Nicolas J-M, Hannestad J, Holden D, et al. Brivaracetam, a selective high-affinity synaptic vesicle protein 2A (SV2A) ligand with preclinical evidence of high brain permeability and fast onset of action. Epilepsia 2016; 57: 201–209. [DOI] [PubMed] [Google Scholar]
- 17. Niquet J, Suchomelova L, Thompson K, et al. Acute and long-term effects of brivaracetam and brivaracetam–diazepam combinations in an experimental model of status epilepticus. Epilepsia 2017; 58: 1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sargentini-Maier ML, Rolan P, Connell J, et al. The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after single increasing oral doses in healthy males. Br J Clin Pharmacol 2007; 63: 680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rolan P, Sargentini-Maier LM, Pigeolet E, et al. The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after multiple increasing oral doses in healthy men. Br J Clin Pharmacol 2008; 66: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sargentini-Maier LM, Espié P, Coquette A, et al. Pharmacokinetics and metabolism of 14C-brivaracetam, a novel SV2A ligand, in healthy subjects. Drug Metab Dispos 2008; 36: 36–45. [DOI] [PubMed] [Google Scholar]
- 21. Mumoli L, Palleria C, Gasparini S, et al. Brivaracetam: review of its pharmacology and potential use as adjunctive therapy in patients with partial onset seizures. Drug Des Devel Ther 2015; 9: 5719–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stockis A, Watanabe S, Rouits E, et al. Brivaracetam single and multiple rising oral dose study in healthy Japanese participants: influence of CYP2C19 genotype. Drug Metab Pharmacokinet 2014; 29: 394–399. [DOI] [PubMed] [Google Scholar]
- 23. Stockis A, Sargentini-Maier ML, Horsmans Y. Brivaracetam disposition in mild to severe hepatic impairment. J Clin Pharmacol 2013; 53: 633–641. [DOI] [PubMed] [Google Scholar]
- 24. Schoemaker R, Wade JR, Stockis A. Brivaracetam population pharmacokinetics in children with epilepsy aged 1 month to 16 years. Eur J Clin Pharmacol 2017; 73: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. European Medicines Agency Brivaracetam SPC. www.medicines.org.uk/emc/medicine/31452 (accessed November 2017).
- 26. SIGN. Epilepsy and women’s health. In SIGN 143: diagnosis and management of epilepsy in adults. Edinburgh: SIGN, 2015, pp.31–48, www.sign.ac.uk/assets/sign143.pdf (accessed November 2017). [Google Scholar]
- 27. Kruithof AC, Watanabe S, Peeters P, et al. Pharmacological interactions between brivaracetam and ethanol in healthy males. J Psychopharmacol. Epub ahead of print 20 September 2016. DOI: 10.1177/0269881116665326. [DOI] [PubMed] [Google Scholar]
- 28. Hoy SM. Brivaracetam: a review in partial-onset (focal) seizures in patients with epilepsy. CNS Drugs 2016; 30: 761–772. [DOI] [PubMed] [Google Scholar]
- 29. Stockis A, Chanteux H, Rosa M, et al. Brivaracetam and carbamazepine interaction in healthy subjects and in vitro. Epilepsy Res 2015; 113: 19–27. [DOI] [PubMed] [Google Scholar]
- 30. Stockis A, Sargentini-Maier ML, Brodie MJ. Brivaracetam and carbamazepine interaction study in adult patients with epilepsy, with or without valproate coadministration. Epilepsy Res 2016; 128: 163–168. [DOI] [PubMed] [Google Scholar]
- 31. Brodie MJ, Fakhoury T, McDonough B, et al. Brivaracetam-induced elevation of carbamazepine-epoxide levels: a post hoc analysis from the clinical development programme. Epilepsy Res in press. [DOI] [PubMed] [Google Scholar]
- 32. Stockis A, Watanabe S, Scheen AJ, et al. Effect of rifampicin on the disposition of brivaracetam in human subjects: further insights into brivaracetam hydrolysis. Drug Metab Dispos 2016; 44: 792–799. [DOI] [PubMed] [Google Scholar]
- 33. Stockis A, Rolan P. Effect of brivaracetam (400 mg/day) on the pharmacokinetics and pharmacodynamics of a combination oral contraceptive in healthy women. J Clin Pharmacol 2013; 53: 1313–1321. [DOI] [PubMed] [Google Scholar]
- 34. Stockis A, Watanabe S, Fauchoux N. Interaction between brivaracetam (100 mg/day) and a combination oral contraceptive: a randomized, double-blind, placebo-controlled study. Epilepsia 2014; 55: e27–e31. [DOI] [PubMed] [Google Scholar]
- 35. French JA, Costantini C, Brodsky A, et al. Adjunctive brivaracetam for refractory partial-onset seizures: a randomized, controlled trial. Neurology 2010; 75: 519–525. [DOI] [PubMed] [Google Scholar]
- 36. Van Paesschen W, Hirsch E, Johnson M, et al. Efficacy and tolerability of adjunctive brivaracetam in adults with uncontrolled partial-onset seizures: a phase IIb, randomized, controlled trial. Epilepsia 2013; 54: 89–97. [DOI] [PubMed] [Google Scholar]
- 37. Biton V, Berkovic SF, Abou-Khalil B, et al. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double-blind, placebo-controlled trial. Epilepsia 2014; 55: 57–66. [DOI] [PubMed] [Google Scholar]
- 38. Kwan P, Trinka E, Van Paesschen W, et al. Adjunctive brivaracetam for uncontrolled focal and generalized epilepsies: results of a phase III, double-blind, randomized, placebo-controlled, flexible-dose trial. Epilepsia 2014; 55: 38–46. [DOI] [PubMed] [Google Scholar]
- 39. Ryvlin P, Werkahn KJ, Blaszczyk B, et al. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: results from a double-blind, randomised, placebo-controlled trial. Epilepsia 2014; 55: 47–56. [DOI] [PubMed] [Google Scholar]
- 40. Klein P, Schiemann J, Sperling MR, et al. Randomized, double-blind, placebo-controlled, multicenter, parallel-group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial-onset seizures. Epilepsia 2015; 56: 1890–1898. [DOI] [PubMed] [Google Scholar]
- 41. Brandt C, Borghs S, Elmoufti S, et al. Health-related quality of life in double-blind phase III studies of brivaracetam as adjunctive therapy of focal seizures: a pooled, post-hoc analysis. Epilepsy Behav 2017; 69: 80–85. [DOI] [PubMed] [Google Scholar]
- 42. Ma J, Huang S, You C. Adjunctive brivaracetam for patients with refractory partial seizures: a meta-analysis of randomized placebo-controlled studies. Expert Opin Pharmacother 2015; 16: 1755–1767.26165169 [Google Scholar]
- 43. Lattanzi S, Cagnetti C, Foschi N, et al. Brivaracetam add-on for refractory focal epilepsy: a systematic review and meta-analysis. Neurology 2016; 86: 1344–1352. [DOI] [PubMed] [Google Scholar]
- 44. Brigo F, Bragazzi NL, Nardone R, et al. Efficacy and tolerability of brivaracetam compared to lacosamide, eslicarbazepine acetate, and perampanel as adjunctive treatments in uncontrolled focal epilepsy: results of an indirect comparison meta-analysis of RCTs. Seizure 2016; 42: 29–37. [DOI] [PubMed] [Google Scholar]
- 45. Ben-Menachem E, Mameniskiene R, Quarato P, et al. Efficacy and safety of brivaracetam for partial-onset seizures in 3 pooled studies. Neurology 2016; 87: 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brodie MJ, Whitesides J, Schiemann J, et al. Tolerability, safety and efficacy of brivaracetam for focal seizures in older patients: a pooled analysis from three phase III studies. Epilepsy Res 2016; 127: 114–118. [DOI] [PubMed] [Google Scholar]
- 47. Kälviäinen R, Genton P, Andermann E, et al. Brivaracetam in Unverricht–Lundborg disease (EPM1): results from two randomized, double-blind, placebo-controlled studies. Epilepsia 2016; 57: 210–221. [DOI] [PubMed] [Google Scholar]
- 48. Zhu L, Chen D, Tao C, et al. The adverse event profile of brivaracetam: a meta-analysis of randomized controlled trials. Seizure 2017; 45: 7–16. [DOI] [PubMed] [Google Scholar]
- 49. Toledo M, Whitesides J, Schiemann J, et al. Safety, tolerability, and seizure control during long-term treatment with adjunctive brivaracetam for partial-onset seizures. Epilepsia 2016; 57: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 50. Stockis A, Hartstra J, Mollet M, et al. Bioavailability and bioequivalence comparison of brivaracetam 10, 50, 75, and 100 mg tablets and 100 mg intravenous bolus. Epilepsia 2016; 57: 1288–1293. [DOI] [PubMed] [Google Scholar]
- 51. Klein P, Biton V, Dilley D, et al. Safety and tolerability of adjunctive brivaracetam as intravenous infusion or bolus in patients with epilepsy. Epilepsia 2016; 57: 1130–1138. [DOI] [PubMed] [Google Scholar]
- 52. Steinig I, von Podewils F, Möddel G, et al. Postmarketing experience with brivaracetam in the treatment of epilepsies: a multicenter cohort study from Germany. Epilepsia 2017; 58: 1208–1216. [DOI] [PubMed] [Google Scholar]
- 53. Steinhoff BJ, Bacher M, Bucurenciu I, et al. Real-life experience with brivaracetam in 101 patients with difficult-to-treat epilepsy: a monocenter survey. Seizure 2017; 48: 11–14. [DOI] [PubMed] [Google Scholar]
- 54. Strzelczyk A, Steinig I, Willems LM, et al. Treatment of refractory and super-refractory status epilepticus with brivaracetam: a cohort study from two German university hospitals. Epilepsy Behav 2017; 70: 177–181. [DOI] [PubMed] [Google Scholar]
- 55. Yates SL, Fakhoury T, Liang W, et al. An open-label, prospective, exploratory study of patients with epilepsy switching from levetiracetam to brivaracetam. Epilepsy Behav 2015; 52: 165–168. [DOI] [PubMed] [Google Scholar]


