Abstract
Motor fluctuations complicate the treatment of patients with Parkinson’s disease receiving levodopa. Extended-release carbidopa–levodopa has a pharmacokinetic profile that provides a more continuous levodopa serum concentration. Patients taking this formulation can expect longer duration of action and fewer doses per day, similar clinical improvement when compared to other levodopa formulations, and with a theoretically lower risk of developing motor fluctuations. Several studies, including three randomized control trials provide evidence for the efficacy, safety and tolerability of extended release carbidopa–levodopa in patients with both early and advanced Parkinson’s disease are reviewed here. Also provided is guidance for dosing of and conversion to extended release carbidopa–levodopa as well as a discussion of its place in the clinical practice.
Keywords: extended release carbidopa–levodopa, Rytary, Parkinson’s disease
Introduction
Carbidopa–levodopa (CD/LD) is the workhorse in the medical treatment of Parkinson’s disease (PD). However, as PD progresses, levodopa associated motor and non-motor fluctuations may develop.1 Motor fluctuations include early wearing off with exacerbation of motor symptoms and “on time” or peak dose dyskinesia; and non-motor fluctuations include neuropsychiatric, autonomic and sensory manifestations.1 After 5 years of levodopa therapy 75% of patients have drug related complications including troublesome fluctuations and dyskinesia in more than 50%.2 To address wearing off the frequency and strength of levodopa doses are increased with subsequent increase in plasma and striatal dopamine concentrations, which perpetuates dyskinesia. Such medication regimens can also negatively impact patient compliance. Pulsatile levodopa administration and higher doses of levodopa are key factors in the development of motor fluctuations.3–5 Continuous dopaminergic delivery has been shown to minimize motor complications in PD.6 Oral pharmacotherapy has aimed, not always successfully, to approximate such delivery.
In January 2015, the US Food and Drug Administration (FDA) approved Rytary extended-release (ER) capsules (IPX066 in clinical trials, Impax Laboratories, Hayward, California), an ER formulation of CD/LD for patients with PD.7 ER CD/LD (to be marketed as Numient) was also approved by the European Medicines Agency (EMA), with recommendations from the agency’s Committee for Medicinal Products for Human Use in November 2015.8 Despite its approval, Numient is not currently commercially available in the European Union. The capsules contain both immediate release (IR) and ER beads with the intention to provide rapid onset of effects, albeit with a more sustained duration than the IR formulation alone. With a more favorable pharmacological profile than its IR counterpart, ER CD/LD allows for reduced dose frequency and reduction in levodopa-associated fluctuations while delivering stable levodopa plasma concentrations.9 The formulation has proven efficacious in early, moderate and advanced PD.10
ER CD/LD has been tried against IR CD/LD11 and CD/LD plus entacapone (E) [a catechol-O-methyltransferase (COMT) inhibitor].12 The pharmacokinetic properties of ER CD/LD have been compared with other CD/LD formulations [IR, controlled release (CR), and with the addition of entacapone].13–15 CR CD/LD was developed to provide more sustained levodopa plasma concentrations than IR CD/LD,12 but results in unpredictable (and often incomplete) absorption and delayed clinical benefit.2,14–17 The addition of entacapone to IR CD/LD prolongs the half life (t1/2) of levodopa13 and reduces ‘off’ time but with large fluctuations in levodopa plasma concentrations.18,19 Both CR CD/LD and the addition of entacapone to CD/LD (as a separate tablet or combined as Stalevo, Novartis, Basel, Switzerland) extend the patient’s clinical benefit only modestly beyond the response to IR CD/LD.13 Neither option decreases the incidence of motor fluctuations.13 In the STRIDE-PD study, patients were randomized to receive CD/LD or CD/LD plus entacapone (CD/LD/E) and followed for 134 weeks.20 Counter intuitively, patients in the CD/LD/E group had a higher incidence of dyskinesia at the end of the study and a shorter time to developing dyskinesia.
Additional options with a goal of providing continuous dopaminergic delivery include rotigotine patch, ER pramipexole and ER ropinerole.21 The dopamine agonists have longer plasma t1/2 than levodopa and cause less pulsatile stimulation of dopamine receptors, but their use is often limited by side effects. Surgical options to ameliorate fluctuations include percutaneous gastrojejunostomy placement with use of CD/LD enteral suspension (levodopa–carbidopa intestinal gel, LCIG) via pump infusion as well as deep brain stimulation (DBS).22 The dopamine agonists and surgical options have not been formally tested against ER CD/LD.
Here we will review the pharmacological properties, therapeutic efficacy as well as safety and tolerability of ER CD/LD. Guidance for the initial dosing of ER CD/LD and its conversion from other formulations of CD/LD is included.
Pharmacological properties
Levodopa (L-3,4-dihydroxyphenylalanine) is the metabolic precursor of dopamine and is combined with carbidopa [an aromatic amino acid decarboxylase (AADC) inhibitor] to form IR CD/LD. After enteral absorption, levodopa is converted into dopamine by AADC and into 3-O-methyldopa by COMT. The addition of carbidopa to levodopa partially suppresses the peripheral metabolism of levodopa, reducing side effects such as nausea (Sinemet = Sine-Emesis = Without Vomiting), and maximizing levodopa transport into the central nervous system where its therapeutic potential can be realized.23 Active amino acid transporters mediate the small bowel intestinal absorption and blood brain barrier (BBB) penetration of levodopa into the central nervous system. Iterations of CD/LD, including CR CD/LD and CD/LD/E, involved modifications with a goal towards increased duration of effect and minimizing the potentiation of motor fluctuations by providing more stable levodopa concentrations. ER CD/LD is another step towards this goal.
ER CD/LD is packed into a capsule and is available in four dose strengths. Each capsule contains four components: IR, ER component 1, and ER component 2 (all of which contain both LD and CD as active ingredients), plus a functional excipient component, which contains tartaric acid as an acidifying agent designed to facilitate the absorption of LD.23 The combination of components creates an initial increase in plasma concentration (with the IR component) and provides a more sustained concentration (with the two ER components).23 Theoretically, the acidifying agent facilitates the maximal utilization of LD through more rapid absorption of LD prior to the drug travelling beyond the proximal expanse of the small intestine (where the bulk of LD absorption takes place) and by improving the absorption of LD beyond the duodenum and jejunum. The plasma concentration of LD over 12 h after ingesting the IR and both ER components individually as well as after ingesting a complete ER CD/LD capsule is shown in Figure 1.23 The pharmacokinetic profiles of each individual component and of a complete ER CD/LD capsule seen in the figure correspond to a single dose of 390 mg (two 195 mg capsules).
Figure 1.
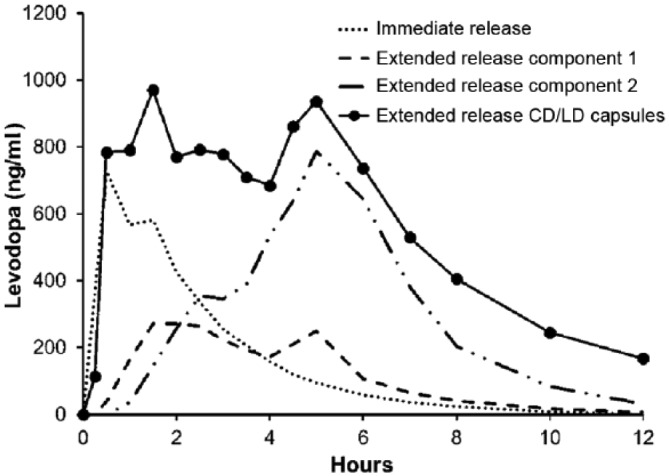
The graph depicts the pharmacokinetic profiles of each individual component and of a complete ER CD/LD capsule. CD/LD, carbidopa–levodopa. Reproduced from: Mittur et al.23; http://creativecommons.org/licenses/by-nc/4.0/
In healthy adults, the single-dose pharmacokinetics of ER CD/LD (two 48.75/195 mg capsules) was compared with IR CD/LD (25/100 mg), CR CD/LD (25/100 mg) and CD/LD/E (25/100/200 mg).15 Hsu and colleagues15 reported, after a single ER CD/LD dose, LD plasma concentrations reached an initial peak at 1 h, with a mean time to maximum concentration (Tmax) occurring around 4.5 h, and at 10 h the LD concentrations were less than 10% of the peak concentration. The initial increase in LD concentration in both the ER and IR CD/LD formulations was comparable. LD concentration was sustained in the ER formulation for 1.9–2.5 h longer than in the other products. Following ER CD/LD doses, less variability in LD plasma concentrations was seen compared with the IR, CR and CD/LD/E formulations and the decline in LD concentrations over time was less steep after ER CD/LD doses.15 The smoothness of the LD concentration–time curve mirrors the continuity of LD administration and theoretically imparts a reduced risk of motor complications in patients with PD.
In a 2011 open-label phase II study of ER CD/LD in patients with advanced PD, Hauser and colleagues24 reported comparable single-dose pharmacokinetic results to those reported by Hsu and colleagues15 in healthy participants. ER CD/LD allowed for rapid attainment and prolonged maintenance of therapeutic LD concentrations. ER CD/LD and IR CD/LD had a similar initial absorption rate with time to reach 50% Cmax of 0.78 and 0.76 h respectively.24 LD plasma concentrations were sustained above 50% of Cmax for 4 h with ER CD/LD and 1.4 h with IR CD/LD,24 providing a more durable effect. The LD bioavailability of ER relative to IR CD/LD was 74.5%. In a phase III crossover study, Stocchi and colleagues13 assessed the efficacy and safety of ER CD/LD against CD/LD/E in patients with advanced PD and reported the LD bioavailability of ER CD/LD relative to CD/LD/E to be 47%.
Hauser and colleagues24 also reported multidose pharmacokinetics of ER CD/LD compared with IR CD/LD in patients with advanced PD. This assessment was carried out over 12 h after 8 days of dose titration to maximize efficacy with minimal side effects. Over this assessment period 89% of patients took one or two ER CD/LD doses and 11% took three doses over 12 h. In the IR CD/LD cohort, 37% took one or two doses, 26% three doses, and 37% four or more doses. They calculated a fluctuation index for the ER and IR CD/LD, which correlated with the magnitude of the rise and fall of LD plasma concentrations relative to the average concentration. A lower fluctuation index implies an improved pharmacodynamics profile that may minimize Cmax (maximum concentration) related adverse effects. The fluctuation index for ER and IR CD/LD was 1.5 and 3.2 respectively.
Mittur and colleagues23 reported that on a dose-normalized basis, the Cmax values and area under the concentration–time cure (AUC) values for ER CD/LD are approximately 30% and 70% of the values for IR CD/LD. The mean duration of time (in hours) that the LD concentrations are sustained above 50% of Cmax after a single dose of ER CD/LD was 4.9 and 4 in healthy subjects and patients with PD respectively. Compared with other CD/LD formulations, ER CD/LD has lower intrasubject variability in Cmax and AUC values.
The effect of food on the pharmacokinetics of ER CD/LD has been evaluated.23,25 The sustained-release beads in the ER CD/LD capsule are designed to maintain integrity and minimize drug release when exposed to a pH below 7. These beads were mixed with Jell-O (pH 6.5) and Kozy-Shack Flan Crème Caramel Pudding (pH 6.1); the beads were washed after 30 min and then the amounts of remaining LD and CD were determined. Less than 4.5% of LD was lost in the process.23 Yao and colleagues25 compared the pharmacokinetics of a ER CD/LD dose (two capsules of 245 mg LD) under fasting conditions and when taken after a high-fat, high-calorie meal. The meal delayed the initial increase in LD concentration by approximately 2 h, reduced the Cmax by 21% and increased the AUC by 13% compared with the fasting state. High-fat meals may also lengthen the duration of benefit. Yao and colleagues25 also reported that sprinkling the contents of the ER CD/LD capsule onto apple sauce before ingestion did not affect the pharmacokinetics. Similar to all LD products, a proteinaceous meal may interfere with the absorption of LD by competing for binding sites on the intestinal, and probably more so, the BBB amino acid transporters.23
The effect of intrinsic factors, such as race, sex, age, body weight and renal function, on the pharmacokinetics of ER CD/LD can be extrapolated from prior studies.23 In the PD population with pharmacokinetic data about 95% are white, thus no assessment can be made on the effect of race in the PD population. In the healthy population, Cmax and AUC values were 10–17% higher in black compared to white subjects. The median time to peak LD concentration and the t1/2 (hours) of LD were similar in the two race groups. In healthy subjects and patients with PD, women had higher plasma concentration of LD compared with men. In healthy women the dose-normalized Cmax and AUC values were respectively 25% and 38% higher than in men. In women with PD the Cmax and AUC values were 35% and 37% higher than in men. In the healthy and PD populations, men and women had comparable median time to peak LD concentration and t1/2 of LD. Age affected the pharmacokinetic data in the healthy and PD populations, with more AUC variability in older patients. In patients with PD increased Cmax and AUC values (27% and 52% higher) were seen in those older than 65 years of age compared with those younger than 50 years of age. Increased weight correlated with reduced Cmax and AUC values and accounted for a substantial portion of the variability of these values in healthy and PD populations, with no difference in t1/2 noted. Decreased creatinine clearance increased AUC values in all subjects and accounted for some of the variability in Cmax and AUC values in all subjects.
Pharmacodynamics have the final word in the discussion of the pharmacology of ER CD/LD. Mao and colleagues14 characterized the pharmacokinetic–pharmacodynamic relationship of ER CD/LD with finger tapping, Unified Parkinson’s Disease Rating Scale (UPDRS) part III score, and the incidence of dyskinesia in patients with advanced PD. Their results were reported as Emax (describing the effect size) and as the half maximal effective concentration (EC50) of LD to improve finger tapping speed and UPDRS score or to induce dyskinesia. For finger tapping improvement the EC50 was 1590 ng/ml, for improved UPDRS score (Emax 63%) it was 812 ng/ml, and for dyskinesia it was 601 ng/ml. ER CD/LD and IR CD/LD have a similar concentration–effect relationship based on UPDRS III scores and finger tapping rate. Mao and colleagues26 report a similar effect on the natural history of disease progression in PD with exposure of LD-naïve patients with PD to ER CD/LD compared with previous studies of other formulations, with a progression of 11.6 UPDRS units per year. UPDRS combined part II and III scores were reduced by up to 76.7% (Emax) with an ED50 of 450 mg of LD per day.26
Therapeutic efficacy
The therapeutic efficacy of ER CD/LD has been measured in 3 phase-III, double-blind, randomized control trials (RTCs),12,13,27 which corroborated evidence from an earlier open-label randomized crossover study.24 A 9-month open-label extension study was completed to further assess the longer-term safety and efficacy of ER CD/LD.28 The results of these studies are further detailed in the following section and are summarized in Table 1.
Table 1.
Summarizes the efficacy results reported in trials of extended release carbidopa-levodopa.
| Study | Design | Study participants | Efficacy findings |
|---|---|---|---|
| Hauser et al.12 | Phase III, randomized, double-blind study; ER versus IR CD/LD | No. included in analysis: 368 Mean age: 63.2 Mean duration PD: 7.7 |
13.06% reduction in ‘off time’ 0.8 h more ‘on time’ without troublesome dyskinesia UPDRS I, II, III scores reduced by 4.05 Improved PGI-C, CGI-C, PDQ-39, mRS scores |
| Stocchi et al. 13 | Phase III, randomized, double-blind, double-dummy, crossover treatment trial; ER versus CD/LD+E | No. included in analysis: 84 Mean age: 64 Mean duration PD: 10 |
8.5% less ‘off time’ 1.4 h less ‘off time’ No difference in ‘on time’ with troublesome dyskinesia 2.4 less points UPDRS II+III |
| Pahwa et al.27 | Multicenter, multinational, randomized, double-blind, parallel-group, fixed-dose, placebo-controlled, 30-week study | No. included in analysis: 381 Mean age: 64–65 Mean duration PD: 2 |
Improved UPDRS II+III scores: 11.7, 12.9 and 14.9 points in the 145 mg, 245 mg and 390 mg three times daily groups Improved PDQ-39, PGI-I, CGI-I scores |
| Waters et al.28 | 9-month open-label extension trial with ER CD/LD | No. included in analysis: 567 (254 early and 313 advanced) Mean age: 64.1 Mean duration PD: 2.7 (early), 7.9 (advanced) |
UPDRS scores maintained from prior studies’ completion PGI scale: about 80% of patients were satisfied with their ER CD/LD |
| Tetrud et al.34 | Open-label study, conversion for CR plus IR CD/LD to ER CD/LD | No. included in analysis: 33 Mean age: 58.4 Mean duration PD: 8 |
PGI-C, 68.8% with at least minimal improvement CGI-C, 75% with at least minimal improvement |
CD/LD, carbidopa–levodopa; CGI-C, Clinician Global Impression of Change; E, entacapone; ER, extended release; IR, immediate release; mRS, modified Rankin Scale; PD, Parkinson’s disease; PDQ-39, 39-item Parkinson’s Disease Questionnaire; PGI-C, Patient Global Impression of Change.
In ADVANCE-PD,12 Hauser and colleagues compared ER CD/LD with IR CD/LD in a phase III randomized, double-blind trial. A total of 368 patients with PD and motor fluctuations completed the trial. The mean age of participants was 63.2 years and the mean duration of PD was 7.7 years. At the time of trial entry the mean daily ‘off time’ experienced by participants was 5.97 h. Patients underwent a 3-week open-label IR CD/LD dose-adjustment period, followed by a 6-week open-label ER CD/LD dose conversion period, and then were randomized into a 13-week treatment period with either ER CD/LD or IR CD/LD. They showed that ER CD/LD could be given less frequently; mean 3.6 doses per day compared with 5 doses in the IR group. In post hoc analysis, at the end of the 6-week dose conversion period from IR to ER CD/LD the ER dosage had increased from the recommended starting dosage by a mean of 21%.29 Despite the lower dose frequency, mean daily LD dose was higher in the ER CD/LD group (1630 mg) compared with the IR group (814.5 mg). At the end of the study, the ER group had a 13.06% reduction in ‘off time’ per day compared with 6.21% reduction in the IR group. This corresponded to 1.17 h greater reduction in ‘off time’ in the ER (2.18 h) compared with the IR group (1.01 h). Patients reported 0.8 h more ‘on time’ without troublesome dyskinesia (0.78 h without any dyskinesia) in the ER compared with the IR group. Mean ‘on time’ with troublesome dyskinesia did not differ between groups. Post hoc subgroup analysis of ADVANCE-PD showed that improvements in motor symptoms with ER CD/LD were not accompanied by increased ‘on time’ with troublesome dyskinesia.30–32 Mean scores on UPDRS parts I, II and III were improved by a greater amount in the ER CD/LD group (4.03 point difference compared with the IR group). The between-group difference in UPDRS part IV scores was not significant. Statistically significant improvement was noted in the Patient Global Impression of Change (PGI-C) and Clinician Global Impression of Change (CGI-C) scales, the modified Rankin Scale (mRS), and in the total score on the 39-item Parkinson’s Disease Questionnaire (PDQ-39) in the ER compared with the IR group.
In ASCEND PD, Stocchi and colleagues13 compared ER CD/LD with IR CD/LD+E or CD/LD/E in patients with advanced PD in a phase III, randomized, double-blind, double-dummy, crossover treatment trial. A total of 84 patients (83 were analyzed for diary-related endpoints), with mean age of 64 years and mean disease duration of 10 years, completed the study. Participants underwent a 6-week dose conversion from CD/LD/E or CD/LD+E to ER CD/LD, followed by two 2-week double-blind crossover periods (one to ER CD/LD and one to CD/LD+E). The primary efficacy endpoint was mean percent ‘off time’ based on patient diaries. The median daily LD dosage was 1495 mg in the ER CD/LD group and 600 mg in the CD/LD+E group. The median daily number of doses was three for ER CD/LD, five for IR CD/LD and four for entacapone (median daily dose of 800 mg). Patients in the ER group had a significantly lower mean percent ‘off time’ compared with the CD/LD+E group, 24% versus 32.5% respectively (p < 0.0001). This corresponded to a decrease from baseline of 34% versus 10%. ER CD/LD was favored over CD/LD+E in secondary endpoints, including mean ‘off time’ which decreased from 5.9 to 3.8 h per day and mean increase in ‘on time’ without troublesome dyskinesia from 9.8 to 11.4 h per day. This corresponded to 1.4 h less daily ‘off time’ in the ER CD/LD compared with the CD/LD+E group. No difference between groups was noted in ‘on time’ with troublesome dyskinesia. The sum of UPDRS part II and III scores during the ‘on’ state averaged 29.3 in the ER CD/LD group and 31.7 in the comparator group. Patient-reported preferences favored ER CD/LD over CD/LD+E, with 52.4% of patients preferring ER CD/LD, 27.4% preferring CD/LD+E and 20.2% expressing no preference.
In APEX-PD, Pahwa and colleagues27 evaluated the safety and efficacy of ER CD/LD in LD-naïve patients with early PD. This was a multicenter, multinational, randomized, double-blind, parallel-group, fixed-dose, placebo-controlled, 30-week study. A total of 381 patients with mean age of 64–65 years and mean disease duration of 2 years were randomized to four treatment groups. Patients received ER CD/LD 36.25/145 mg, 61.25/245 mg, 97.50/390 mg or identical placebo tablets three times a day. During the titration period, all treatment groups were initiated on 23.75/95 mg three times a day. On day 4, the dose of all treatment groups was increased to 36.25/145 mg three times a day. On day 8, the two groups destined for higher doses received 48.75/195 mg three times a day; and on day 15 this was increased to 61.25/245 mg three times a day. On day 22, the third treatment group’s dosage was increased to 97.50/390 mg three times a day. The primary outcome of APEX-PD was the change from baseline to week 30 in the sum score of UPDRS parts II and III. Secondary measures included UPDRS parts I–IV in various combinations, PGI-I, CGI-I and PDQ-39 scores. Improvement in UPDRS parts II and III scores compared with baseline were significant in all treatment groups compared with placebo (p < 0.0001). Mean improvements were 11.7, 12.9 and 14.9 points in the 145 mg, 245 mg and 390 mg three times a day groups respectively. Mean improvement in the placebo group was 0.6 points. Changes in scores of individual UPDRS parts (except in part IV) were significantly improved from baseline in all treatment groups at all time points compared with placebo (p < 0.05). Total PDQ-39 scores as well as PGI-I and CGI-I scales in all treatment groups were significantly improved compared with placebo. PDQ-39 scores were reduced by a mean of 4.4 (p = 0.02), 3.8 (p = 0.03) and 6 (p = 0.0008) in the 145 mg, 245 mg and 390 mg three times a day groups respectively. The percentage of patients reporting improvement ranged from 70.3% to 73.5% in the treatment groups compared with 33.7% in the placebo group; and the percentage of physicians reporting improvement in the patients ranged from 70.8% to 72.6% in the treatment groups compared with 27.2% for the placebo group (p < 0.0001).
Waters and colleagues28 completed a 9-month open-label extension trial with ER CD/LD composed of participants from ADVANCE-PD,12 APEX-PD27 and Hauser and colleagues’24 open-label crossover study. Patients had individualized dosing regimens of ER CD/LD. A total of 254 patients with early PD (from APEX-PD27) and 313 patients with advanced PD (from ADVANCE-PD12 and Hauser and colleagues24) completed the extension study. In patients with early PD, at the end of the 9 months the median total daily dose of LD from ER CD/LD was 720 mg, with correction for 70% relative bioavailability, corresponding to about 500 mg per day of IR LD. Seventy-eight percent of the patients in the early PD group maintained three times a day dosing. In patients with advanced PD, at the end of the extension period the median daily dose of ER LD was 1450 mg, corresponding to about 1015 mg per day of IR LD. The mean increase dose in the group over the 9-month period was 10%. At the end of the study, among patients with advanced disease, 45.2%, 42.5% and 10.6% were taking ER CD/LD three times daily, four times daily and five times daily respectively. Approximately 80% of patients with advanced disease completed the extension study without changing their dosing frequency. UPDRS and PGI results showed sustained improvement throughout the extension period. In patients with early PD previously receiving placebo, mean UPDRS scores were similar at the end of the extension study to those achieved by patients who received ER CD/LD in the antecedent APEX-PD study. At 9 months, mean sum of UPDRS parts II and III scores were 24 and 24.9 in patients who previously received ER CD/LD and placebo respectively. In patients with advanced PD, again similar mean sum scores were attained for patients who previously received IR or ER CD/LD in the ADVANCE-PD study, 28.1 and 28.2 respectively. On the PGI scale, about 80% of patients were satisfied with the ER CD/LD therapy at each extension time point.
Data from post hoc subgroup analysis from the ADVANCE-PD and ASCEND-PD studies are available. Espay and colleagues30 evaluated the influence of baseline disease severity on the efficacy of ER CD/LD. Subgroups were dichotomized to ‘more’ or ‘less’ severe based on median baseline ‘off time’ (5.67 h in ADVANCE-PD and 5.0 h in ASCEND-PD) and median combined UPDRS parts II and III score (32 in ADVANCE-PD and 30 in ASCEND-PD). Improvements in baseline UPDRS scores and ‘off time’ were seen in ER CD/LD compared with IR or CD/LD/E in both disease severity subgroups. In ADVANCE-PD, improvements in ‘off time’ were greater with ER compared with IR CD/LD in the more severe ‘off’ subgroup and in both UPDRS subgroups. Across disease severity subgroups, ER CD/LD improved UPDRS part II and III scores and ‘off time’ compared with IR CD/LD or CD/LD/E without significantly worsening troublesome dyskinesia.
LeWitt and colleagues31 evaluated the influence of concomitant PD medications on the efficacy of ER CD/LD using post hoc subgroup analysis of the ADVANCE-PD and ASCEND-PD cohorts. The concomitant use of amantadine, monoamine oxidase-B (MAO-B) inhibitors, or dopamine agonist did not diminish the efficacy or increase troublesome dyskinesias when ER CD/LD was compared with IR or CD/LD+E regimens. Gupta and colleagues32 also reported the consistent efficacy without worsening troublesome dyskinesia of ER CD/LD in patients taking amantadine, dopamine agonists or MAO-B inhibitors. They added that the use of adjunctive PD medications did not affect the final mean levodopa dose or dose frequency after conversion from IR CD/LD to ER CD/LD.
Dhall and colleagues33 evaluated the efficacy of ER CD/LD in the subgroup of patients from ADVANCE-PD with troublesome dyskinesia at baseline. In this cohort, ER CD/LD provided significant improvement in the sum of UPDRS part II and III scores and reduced ‘off time’ compared with IR CD/LD. These improvements were not accompanied by an increase in ‘on time’ with troublesome dyskinesia.
Tetrud and colleagues34 completed an open-label study in which 43 patients underwent conversion of a drug regimen from CR and IR CD/LD to ER CD/LD. Thirty-three patients completed the conversion and efficacy was reported as improved PGI-I and CGI-I findings in this cohort. At least minimal improvement was reported in 68.8% per PGI-I and 75% per CGI-I at the end of the conversion period. Twelve patients comprised a smaller cohort assessed more objectively. At 4 and 5 h after receiving a dose of ER CD/LD, UPDRS part III score improvements were significantly different in the ER compared with the CR CD/LD group. Mean ‘on time’ with troublesome dyskinesia was not significantly different between treatments.
Safety and tolerability
The above reviewed studies report on adverse events (AEs) associated with the administration of ER CD/LD and its comparators. The results suggest that ER CD/LD is similarly, if not better, tolerated than its IR CD/LD counterpart, but more AEs were reported with ER CD/LD compared with CD/LD+E. It should be noted that patients in these studies may be taking other PD-related medications including MAO-Is, dopamine agonists, amantadine and anticholinergic agents, which may confound reported AEs unless subgroup analysis is mentioned. The AEs reported in these studies are detailed in Table 2.
Table 2.
Summarizes the adverse events reported in trials of extended release carbidopa-levodopa.
| Study | Adverse events | Treatment-related serious adverse events | Dropout due to adverse event |
|---|---|---|---|
| Hauser et al.12
n = 368 |
ER dose conversion period 46% reported an AE Dyskinesia (6%) Nausea (5%) Headache; dizziness (4%) On/off phenomena; fall (3%) Dry mouth; anxiety; insomnia; constipation (2%) Double-blind treatment period 43% of those taking ER reported an AE Insomnia, nausea, fall (3%) Dizziness, dyskinesia, diarrhea, peripheral edema, URI, UTI, sleep disorder, weight loss (2%) Back pain, arthralgia (1%) Vomiting, depression (<1%) ICD (n = 3) |
ER dose conversion period Gait disturbance (n = 2) Dyskinesia (n = 2) Overdose (n = 1) Psychosis (n = 1) Double-blind treatment period Anxiety and psychosis (n = 1) |
ER dose conversion period 5% Double-blind treatment period n = 3 |
| Stocchi et al. (2014) n = 84 |
ER dose conversion period 30.9% reported an AE Nausea (7.3%), fall (2.7%), URI (2.7%), vomiting (2.7%), dyskinesia (0.9%), insomnia (0.9%) Randomized crossover period 20.2% reported an AE Dyskinesia (4.5%), insomnia (3.4%), confusional state (3.4%) Nausea, vomiting, fall (1.1%) |
No serious AEs were attributed to ER treatment | Dose conversion period n = 1 (dyspepsia, nausea, vomiting) Open-label period n = 1 (dyskinesia) |
| Pahwa et al.27
n = 381 |
68.5% reported AE 145 mg three times daily (56.3%) 245 mg three times daily (72.1%) 390 mg three times daily (71.4%) Nausea (15.7%) Headache (12.1%) Dizziness (11.8%) Insomnia (5.2%) Dyskinesia 145 mg three times daily (2.3%) 245 mg three times daily (3.8%) 390 mg three times daily (5.1%) |
None reported | 10.2% dropout due to AE Nausea, dizziness, vomiting, diarrhea, dyskinesia (n ⩾ 2) |
| Waters et al.28
n = 567 |
57.2% report at AE Total population Fall (5.2%) Dyskinesia (4.7%) Nausea (4.1%) Insomnia (3.9%) Early PD Fall (3.4%) Dyskinesia (1.9%) Nausea, insomnia (5.6%) Advanced PD Fall (6.6%) Dyskinesia (6.9%) Nausea (2.9%) Insomnia (2.6%) |
7% reported serious AEs Femoral neck fracture (n = 3) Fall, atrial fibrillation, gastritis, hyponatremia, spinal column stenosis, spinal osteoarthritis (n = 2) |
2.6% dropout due to AE Nausea, hallucinations, dizziness (n = 2) |
| Hsu et al.15
n = 22 |
Nausea (20.8%) Vomiting (8.3%) Headache (8.3%) |
None | N/A |
| Tetrud et al.34
n = 33 |
81.4% reported an AE >10% reported: UTI, nausea, anxiety and fall 9.3%: dyskinesia, URI |
Orthostatic hypotension (n = 1) Anxiety (n = 1) Exacerbation of parkinsonism (n = 1) |
16.3% dropout due to AE Nausea (n = 3) Abdominal pain, agitation, anxiety, confusional state, diarrhea, dyskinesia, dystonia, hallucination, headache, nausea, orthostatic hypotension, vision blurriness, vomiting (n ⩽ 2) |
AE, adverse event; ER, extended release; N/A, not applicable; PD, Parkinson’s disease; URI, upper respiratory infection; UTI, urinary tract infection.
To summarize, in early PD, the most common AE associated with ER CD/LD (with incidence greater than 5% of the study populations and greater than placebo) are nausea, dizziness, headache, insomnia, abnormal dreams, dry mouth, dyskinesia, anxiety, constipation, vomiting and orthostatic hypotension. In advanced PD the most common side effects (incidence greater than 5% and greater than IR CD/LD) are nausea and headache. Discontinuation of ER CD/LD should be considered in patients who develop excessive daytime sleepiness or sleep attacks. ER CD/LD is associated with an increased risk of dyskinesia, impulse control disorders, hallucinations or psychosis and cardiovascular ischemic events. Patients with psychosis or history of myocardial infarction or cardiac arrhythmia should be treated with caution.
Dosing and conversion
ER CD/LD is available in four doses of CD and LD at a ratio of 1:4. The doses are: 23.75/95, 36.25/145, 48.75/195 and 61.25/245 mg CD/LD.23 Based on the pharmacokinetic properties of ER CD/LD, when converting a patient from IR to ER CD/LD approximately twice the total daily dose of LD would be required.21 The peak LD concentration is not expected to be higher than following the patient’s prior IR CD/LD dose, despite the higher total dose of LD. When converting a patient from CD/LD plus entacapone, the initial ER CD/LD dose may need to be increased compared with conversion from IR CD/LD without entacapone.35 The initial dosing frequency of ER CD/LD is three times daily, but a maximum dosing frequency of five times daily, if tolerated, is suggested to maximize symptomatic control.34 The maximum recommended daily dose of ER CD/LD is 612.5/2450 mg.35 Rapid dose reduction or abrupt discontinuation is not recommended to avoid potential withdrawal effects, including hyperpyrexia, confusion and a neuroleptic malignant-like syndrome in severe cases.35,36
The official recommendation35 for starting ER CD/LD in a LD-naïve patient is to start with 23.75 mg/95 mg tablets, 1 tablet three times daily for 3 days, and may be increased to 35.25 mg/145 mg tablets three times daily, if clinically indicated, on day 4. In fact, the usual ER CD/LD maintenance dose in drug-naïve subjects in the pivotal APEX-PD study was 145 mg LD three times daily.27
For patients switching to ER CD/LD from other CD/LD formulations, the recommended starting dose can be determined using a dose-conversion table based on the total daily LD dose35 (Table 3). This table was used for initial dose conversion from IR CD/LD to ER CD/LD during the clinical trials, followed by titration according to clinical response, with patients tending to require twice the daily LD dosage from ER compared with the IR formulation.
Table 3.
Conversion from immediate-release carbidopa–levodopa to Rytary.
| Total daily dose of levodopa in immediate-release carbidopa–levodopa | Recommended starting dosage of Rytary |
|
|---|---|---|
| Total daily dose of levodopa in Rytary | Rytary dosing regimen | |
| 400–549 mg | 855 mg | 3 capsules Rytary 23.75 mg/95 mg taken three times daily |
| 550–749 mg | 1140 mg | 4 capsules Rytary 23.75 mg/95 mg taken three times daily |
| 750–949 mg | 1305 mg | 3 capsules Rytary 36.25 mg/145 mg taken three times daily |
| 950–1249 mg | 1755 mg | 3 capsules Rytary 48.75 mg/195 mg taken three times daily |
| ⩾1250 mg | 2340 mg or 2205 mg | 4 capsules Rytary 48.75 mg/195 mg taken three times daily or 3 capsules Rytary 61.25 mg/245 mg taken three times daily |
Reproduced from the Rytary Prescribing Information with permission from Impax Laboratories Incorporated.
Hauser37 describes a more mathematical approach that one could take in converting IR CD/LD or CD/LD/E to ER CD/LD. This approach utilizes knowledge of ER CD/LD pharmacokinetics. The goal is to match the Cmax of LD from one formulation to the other to provide the same motor benefit without increasing dyskinesia. The Cmax of ER CD/LD is about 30% of IR CD/LD, thus about three times the LD dosage should be administered per dose when converting from IR to ER formulations. The total LD dose for ER compared with IR CD/LD is expected (per clinical trials) to be twice as much. Therefore, if each dose administers three times as much LD, then doses can be administered two thirds as often. This is a less conservative approach to dose conversions.
Hauser37 also offers guidance for the conversion of CR CD/LD or CD/LD/E to ER CD/LD. CR CD/LD has 70% bioavailability compared with IR CD/LD, thus the initial ER CD/LD dose could be 30% less than the starting dose suggested by the IR to ER conversion table or by calculation based on pharmacokinetics. The conversion for CD/LD/E to ER CD/LD (without entacapone) will likely require higher doses of ER CD/LD to obtain patient satisfaction. Based on ASCEND-PD,13 a conversion ratio of 2.5 is suggested. This is a 25% increase over the suggested conversion from IR to ER CD/LD.
Tetrud and colleagues34 studied the conversion from CR CD/LD (in addition to IR CD/LD) to ER CD/LD. The mean LD conversion ratio was 1.8 in patients taking CR plus IR CD/LD (n = 30); and the mean LD conversion ratio was 1.5 in patients taking CR CD/LD alone (n = 3). The mean ER CD/LD dosing frequency was 3.5 times per day compared with 2.6 times per day of CR CD/LD plus 4.6 times per day of IR CD/LD (and 4.7 times per day for patients previously taking CR CD/LD alone). Based on the CGI ratings patients had improved clinical benefit on their new regimens with less frequency of doses.
To maximize continuation of ER CD/LD, it is suggested that patients be informed prior to conversion that further dose adjustments will likely be implemented (possibly in the near future).37 In clinical trials about 60% of patients converted from IR to ER CD/LD required higher doses than the conversion table suggested. Patients sensitive to changes in LD doses may report being under or over dosed, with worse parkinsonism or with dyskinesia respectively within days (or even one dose) of formulation conversion.37 If a single dose does not provide a satisfactory therapeutic response, then it should be increased; if the duration of each dose is not satisfactory, then the frequency of dosing should be increased.37 One could consider switching from IR to ER CD/LD one dose per day at a time.37 Each dose can be assessed for magnitude and duration of effect before a complete transition takes place.37 This should improve tolerability and maximize compliance with a full conversion.
ER CD/LD in the clinic
ER CD/LD is finding its place in clinical practice. If a patient with PD is having motor (or nonmotor) fluctuations while taking alternative formulations of CD/LD (IR, CR or with entacapone), then ER CD/LD can be offered in an attempt to smooth out the patient’s day. The ER formulation provides a more simplified drug regimen for the patient with less dosing frequency. The addition of medications such as dopamine agonists and MAO-B inhibitors can be avoided (as can their potential AEs). The use of ER CD/LD also negates the need for the addition of IR to CR CD/LD in patients with delayed onset. A patient may find more overnight relief from motor and nonmotor symptoms with ER CD/LD compared with IR and CR formulations. A given patient may find ER CD/LD more tolerable in terms of LD-related side effects compared with alternative formulations. The institution of ER CD/LD may delay the need for surgical options such as DBS and intestinal LCIG therapy. It is not known whether starting ER CD/LD early in patients with PD will cause less dyskinesia in the future compared with other formulations. Consensus positions among 11 movement disorder specialists on the optimization of ER CD/LD in PD have been reported.38
Conclusion
ER CD/LD is the newest iteration of CD/LD and has a favorable pharmacokinetic profile compared with other CD/LD formulations. This profile creates a smoother LD concentration–time curve and theoretically less pulsatile stimulation of striatal dopamine receptors. It is thought that motor fluctuations are caused by nonphysiologic fluctuations in LD plasma concentration.39 We know that high-fat, high-calorie meals can negatively impact the pharmacokinetic profile of ER CD/LD, and that if the need arises, the capsule can be opened and its contents swallowed with apple sauce with no decrement in effect. An individual’s intrinsic factors can influence the pharmacokinetics of ER CD/LD.
ER CD/LD was approved for use by the FDA and EMA based on these studies demonstrating its safety and efficacy in early and advanced PD. The reviewed studies involving pharmacokinetic and pharmacodynamics properties as well as randomized clinical trials assessing therapeutic efficacy and safety and a 9-month safety and efficacy study support ER CD/LD as an efficacious and well tolerated treatment for patients with PD. It may have benefits over other CD/LD formulations by minimizing fluctuations in LD plasma concentrations throughout the day, which may equate to less motor (and possibly nonmotor) symptomatic fluctuations. It has been proven to improve ‘off time’ without increase in time with bothersome dyskinesia. If successful in ameliorating fluctuations in a patient with PD, then ER CD/LD may delay the need for more invasive procedures, which provide even more continuous striatal stimulation, LCIG and DBS.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Jason Margolesky, University of Miami School of Medicine, Miami, FL, USA.
Carlos Singer, University of Miami School of Medicine, 1150 NW 14th St, Suite 609, Miami, FL 33136-1015, USA.
References
- 1. Aquino CC, Fox SH. Clinical spectrum of levodopa-induced complications. Mov Disord 2015; 30: 80–90. [DOI] [PubMed] [Google Scholar]
- 2. Fahn S, Jankovic J, Hallet M. Principles and practice of movement disorders. 2nd ed. Philadelphia: Elsevier Saunders, 2011. [Google Scholar]
- 3. Chase TN, Mouradian MM, Engber TM. Motor response complications and the function of striatal efferent systems. Neurology 1993; 43(Suppl. 6): S23–S27. [PubMed] [Google Scholar]
- 4. Chase TN. The significance of continuous dopaminergic stimulation in the treatment of Parkinson’s disease. Drugs 1998; 55(Suppl. 1): 1–9. [DOI] [PubMed] [Google Scholar]
- 5. Aviles-Olmos I, Martinez-Fernandez R, Foltynie T. L-Dopa-induced dyskinesias in Parkinson’s disease. Eur Neurol J 2010; 2: 91–100. [Google Scholar]
- 6. Wright BA, Waters CH. Continuous dopaminergic delivery to minimize motor complications in Parkinson’s disease. Expert Rev Neurother 2013; 13: 719–729. [DOI] [PubMed] [Google Scholar]
- 7. Impax. Impax pharmaceuticals announce FDA approval of RYTARY (carbidopa and levodopa) extended-release capsules for the treatment of Parkinson’s disease [press release]. Hayward, CA: Impax Pharmaceuticals; 2015. [Google Scholar]
- 8. European Medicines Agency. Numient: EPAR – Summary for the public, 2015. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002611/human_med_001934.jsp&mid=WC0b01ac058001d124.
- 9. Pilleri M, Antonini A. Novel levodopa formulations in the treatment of Parkinson’s disease. Expert Rev Neurother 2014; 14: 143–149. [DOI] [PubMed] [Google Scholar]
- 10. Hauser RA. IPX066: A novel carbidopa–levodopa extended-release formulation. Expert Rev Neurother 2012; 12: 133–140. [DOI] [PubMed] [Google Scholar]
- 11. Greig SL, McKeage K. Carbidopa–levodopa ER capsules (Rytary, Numient): a review in Parkinson’s disease. CNS Drugs 2016; 30: 79–90. [DOI] [PubMed] [Google Scholar]
- 12. Hauser RA, Hsu A, Kell S, et al. Extended-release carbidopa–levodopa (IPX066) compared with immediate-release carbidopa–levodopa in patients with Parkinson’s disease and motor fluctuations: a phase 3 randomised, double-blind trial. Lancet Neurol 2013; 12: 346–356. [DOI] [PubMed] [Google Scholar]
- 13. Stocchi F, Hsu A, Khanna S, et al. Comparison of IPX066 with carbidopa–levodopa plus entacapone in advanced PD patients. Parkinsonism Relat Disord 2014; 20: 1335–1340. [DOI] [PubMed] [Google Scholar]
- 14. Mao Z, Hsu A, Gupta S, et al. Population pharmacodynamics of IPX066: an oral extended-release capsule formulation of carbidopa–levodopa, and immediate-release carbidopa–levodopa in patients with advanced Parkinson’s disease. J Clin Pharmacol 2013; 53: 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsu A, Yao HM, Gupta S, et al. Comparison of the pharmacokinetics of an oral extended-release capsule formulation of carbidopa–levodopa (IPX066) with immediate-release carbidopa–levodopa (Sinemet), sustained-release carbidopa–levodopa (Sinemet CR), and carbidopa–levodopa-entacapone (Stalevo). J Clin Pharmacol 2015; 55: 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stocchi F, Quinn NP, Barbato L, et al. Comparison between a fast and a slow release preparation of levodopa and a combination of the two: a clinical and pharmacokinetic study. Clin Neuropharmacol 1994; 17: 38e44. [DOI] [PubMed] [Google Scholar]
- 17. Pahwa R, Lyons K, McGuire D, et al. Early morning akinesia in Parkinson’s disease: effect of standard carbidopa–levodopa and sustained-release carbidopa–levodopa. Neurology 1996; 46: 1059e62. [DOI] [PubMed] [Google Scholar]
- 18. Kuoppamäki M, Korpela K, Marttila R, et al. Comparison of pharmacokinetic profile of levodopa throughout the day between levodopa/carbidopa/entacapone and levodopa–carbidopa when administered four or five times daily. Eur J Clin Pharmacol 2009; 65: 443–455. [DOI] [PubMed] [Google Scholar]
- 19. LeWitt PA, Jennings D, Lyons KE, et al. Pharmacokinetic-pharmacodynamic crossover comparison of two levodopa extension strategies. Mov Disord 2009; 24: 1319–1324. [DOI] [PubMed] [Google Scholar]
- 20. Stocchi F, Rascol O, Kieburtz K, et al. Initiating levodopa–carbidopa therapy with and without entacapone in early Parkinson disease: the STRIDE-PD study. Ann Neurol 2010; 68: 18–27. [DOI] [PubMed] [Google Scholar]
- 21. DeMaagd G, Philip A. Parkinson’s disease and its management part 2: introduction to the pharmacotherapy of Parkinson’s disease, with a focus on the use of dopamine agents. P T 2015; 40: 590–600. [PMC free article] [PubMed] [Google Scholar]
- 22. Chaudhuri KR, Rizos A, Sethi K. Motor and nonmotor complications in Parkinson’s disease: an argument for continuous drug delivery? J Neural Transm 2013; 120: 1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mittur A, Gupta S, Modi N. Pharmacokinetics of Rytary, an extended-release capsule formulation of carbidopa–levodopa. Clin Pharmacokinet. Epub ahead of print 24 February 2017. DOI: 10.1007/s40262-017-0511-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hauser RA, Ellenbogen AL, Metman LV, et al. Crossover comparison of IPX06 and a standard levodopa formulation in advance Parkinson’s disease. Mov Disord 2011; 26: 2246–2252. [DOI] [PubMed] [Google Scholar]
- 25. Yao HM, Hsu A, Gupta S, et al. Clinical pharmacokinetics of IPX066: evaluation of dose proportionality and effect of food in healthy volunteers. Clin Neuropharmacol 2016; 39: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mao ZL, Modi NB. Dose-response analysis of the effect of carbidopa–levodopa extended-release capsules (IPX066) in levodopa-naıve patients with Parkinson disease. J Clin Pharm 2016; 56: 974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pahwa R, Lyons KE, Hauser RA, et al. Randomized trial of IPX066, carbidopa–levodopa extended release, in early Parkinson’s disease. Parkinsonism Relat Disord 2014; 20: 142–148. [DOI] [PubMed] [Google Scholar]
- 28. Waters CH, Nausieda P, Dzyak L, et al. Long-term treatment with extended-release carbidopa–levodopa (IPX066) in early and advanced Parkinson’s disease: a 9-month open-label extension trial. CNS Drugs 2015; 29: 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nausieda PA, Hsu A, Elmer L, et al. Conversion to IPX066 from standard levodopa formulation in advance Parkinson’s disease: experience in clinical trials. J Parkinsons Dis 2015; 5: 837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Espay AJ, Liang G, Sharma K, et al. The influence of baseline disease severity on the efficacy of IPX066, an extended-release formulation of carbidopa–levodopa, in advanced Parkinson’s disease. Mov Disord 2015; 30(Suppl. 1): abstract 211. [Google Scholar]
- 31. LeWitt P, Verhagen L, Rubens R, et al. The influence of concomitant medication on the efficacy of IPX066, an extended-release formulation of carbidopa–levodopa, in advanced Parkinson’s disease. Mov Disord 2015; 30(Suppl. 1): abstract 261. [Google Scholar]
- 32. Gupta S, Khanna S, Kell S, et al. Extended-release carbidopa–levodopa, IPX066: dosing and efficacy with concomitant medication use in Parkinson’s disease. Mov Disord 2016; 31(Suppl. 2): abstract 2028. [Google Scholar]
- 33. Dhall R, Struck L, Rubens R, et al. Efficacy of IPX066, an extended-release formulation of carbidopa–levodopa, in advanced Parkinson’s disease patients with troublesome dyskinesia. Mov Disord 2015; 30(Suppl. 1): abstract 207. [Google Scholar]
- 34. Tetrud J, Nausieda P, Kreitzman D, et al. Conversion to carbidopa and levodopa extended-release (IPX066) followed by its extended use in patients previously taking controlled-release carbidopa–levodopa for advanced Parkinson’s disease. J Neurol Sci 2017; 373: 116–123. [DOI] [PubMed] [Google Scholar]
- 35. Rytary Prescriber Information.RYTARY [package insert]. Hayward CA: Impax Laboratories, Inc.; documents.impaxlabs.com/rytary/pi.pdf (2016, accessed October 2016).
- 36. Hashimoto T, Tokuda T, Hanyu N, et al. Withdrawal of levodopa and other risk factors for malignant syndrome in Parkinson’s disease. Parkinsonism Relat Disord 2003; 9: S25–S30. [DOI] [PubMed] [Google Scholar]
- 37. Hauser RA. How to dose carbidopa and levodopa extended-release capsules (Rytary). Clin Med 2015; 1: 34–37. [Google Scholar]
- 38. Espay AJ, Pagan FL, Walter BL, et al. Optimizing extended-release carbidopa–levodopa in Parkinson disease Consensus on conversion from standard therapy. Neurol Clin Pract 2017; 7: 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Olanow CW, Obeso JA, Stocchi F. Continuous dopamine-receptor treatment of Parkinson’s disease: scientific rationale and clinical implications. Lancet Neurol 2006; 5: 677e87. [DOI] [PubMed] [Google Scholar]


