Abstract
Potassium-competitive acid blocker (P-CAB) is a class of drug that competitively blocks the potassium-binding site of H+, K+-adenosine triphosphate (ATP)ase. Although the history of this class of drugs started over 30 years ago, clinical use of two P-CABs, revaprazan and vonoprazan, were only recently approved in Korea and Japan, respectively. Among them, vonoprazan has several advantages over conventional proton-pump inhibitors (PPIs), including rapid onset of action, long duration of acid suppression, fewer interindividual variations in terms of acid suppression, and minimum dietary influence on its action. These advantages of vonoprazan have been proved in clinical trials conducted for license approvals for several acid-related diseases. In this review article, current evidence of vonoprazan in the management of gastroesophageal reflux disease (GERD) will be summarized. Since the clinical trial data, as well as postmarketed clinical data, have consistently demonstrated superiority of vonoprazan over conventional PPIs in terms of achieving healing of mucosal breaks and maintaining the healing, it may provide an excellent, if not complete, option for fulfilling some of the unmet needs for current GERD therapy. The safety problem of vonoprazan is also discussed, as more pronounced hypergastrinemia inevitably ensues with its use.
Keywords: gastroesophageal reflux disease, potassium-competitive acid blocker, proton pump inhibitor, safety, vonoprazan
Introduction
Gastroesophageal reflux disease (GERD) is currently the most prevalent acid-related disease in Western countries.1 While it was once thought of as a rare disease in Asian countries, GERD has now been recognized as an emerging disease, prevalence of which has reached over 10% in Japan, Taiwan, and India.2,3 Proton pump inhibitors (PPIs) remain the mainstay for the management of GERD. However, 10–20% of patients with Los Angeles C and D (LA-C/D) grade esophagitis do not heal despite 8 weeks of continuous double-dose PPI therapy.4 Moreover, it has been well documented that achieving complete symptomatic relief with PPI is more difficult than simply healing mucosal breaks, resulting in dissatisfaction of current therapy in about one third of patients with GERD.5,6 To improve therapeutic gain, a histamine H2 receptor antagonist (H2RA) before sleep, in addition to twice daily PPI was proposed for controlling nocturnal acid breakthrough (NAB).7 Despite successful control of NAB in the short term, the efficacy of the add-on H2RA eventually declined due to development of tolerance to H2RA.8 For the daytime heartburn control, tentative sealing of ‘acid pocket’ occurring after meals with sodium alginate–antacid formulation has been reported to reduce post-prandial acid reflux.9,10 Obviously, however, this approach cannot be employed for NAB. Baclofen, a gamma-aminobutyric acid (GABA-B) receptor agonist, showed some effects on reducing reflux by tightening the lower esophageal sphincter (LES),11 but adverse events such as drowsiness limit its use. Long-acting PPIs such as tenatoprazole12 and modified-release (MR) formulation of dexlansoprazole MR improved 24 h pH control over conventional lansoprazole.13 The former drug has not been marketed yet and the MR formulation of enantiomer of lansoprazole has not achieved full success for healing and symptom control in patients with GERD despite improved metabolic profile of enantiomer, dual-release formulation, and increased dosage, as compared with conventional lansoprazole.14 One of the important limitations of dexlansoprazole MR is inadequate control during night time of pH > 4, even with higher doses.13 Patients who continuously suffer from symptomatic or unhealed esophagitis under PPI therapies may require more invasive endoscopic or surgical therapies. Hence, there are definite unmet needs for more efficient therapies for managing GERD.
In Japan, vonoprazan, a novel potassium-competitive acid blocker (P-CAB), which may overcome some of the limitations of current PPI therapy, was approved for insurance coverage in 2014 and marketed in 2015 for treatment of gastric and duodenal ulcers, erosive esophagitis, eradication of Helicobacter pylori and prevention for recurrence in nonsteroidal anti-inflammatory-drug (NSAID) or low-dose-aspirin (LDA) ulcer. In this review, I will summarize current evidence on the efficacy and safety of vonoprazan focusing on the management of GERD.
What are potassium-competitive acid blockers?
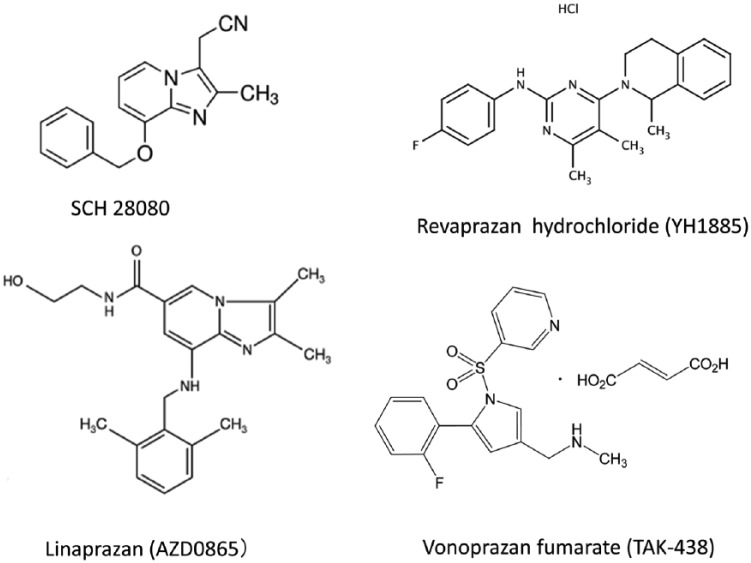
The first drug categorized as a P-CAB, SCH 28080 (Figure 1), was developed as an antisecretory drug more than 30 years ago.15,16 It was later found that SCH 28080 was a competitive ligand of the K+ site of H+, K+-adenosine triphosphate (ATP)ase,17,18 the mode of action distinct from PPIs that requires acid-catalyzed activation and covalent binding as sulfhydryl agents to H+, K+-ATPase. However, clinical development of SCH 28080 was stopped due to hepatotoxicity. Since then, another drug, AZD0865 (linaprazan) was developed. AZD0865 is a potent, but reversible, inhibitor of H+, K+-ATPase, with rapid onset of action.19 In phase II and III trials, it exhibited similar efficacy with esomeprazole 40 mg in terms of healing of esophagitis and for symptom control for nonerosive reflux disease (NERD).20,21 However, further clinical development was suspended as the efficacy was not superior to esomeprazole and there was a concern on hepatotoxicity during these trials.20,21
Figure 1.
Chemical structure of potassium-competitive acid blockers.
Currently, only two P-CABs, revaprazan and vonoprazan, are marketed.
P-CAB, potassium-competitive acid blocker.
Among drugs categorized in P-CABs, the first P-CAB used in clinical practice was revaprazan (YH1885, Revanex®) (Figure 1), which was first marketed in South Korea.22,23 In common with previous P-CABs, it showed a rapid onset of action. However, the acid suppression with revaprazan was not superior to conventional PPIs, as the mean intragastric pH was pH 3.3 and 3.9 on the first and the seventh day, respectively, in healthy male volunteers given 200 mg of revaprazan. Furthermore, the pH > 4 holding time of the dose of revaprazan (200 mg) chosen for clinical use was < 12 h,23 which was similar or even inferior to the value reported in conventional PPIs24 (Table 1). Indeed, the efficacy of healing of ulcers after endoscopic submucosal dissection with revaprazan was similar to 20 mg of rabeprazole.25 No publication was found for GERD treatment with revaprazan by PubMed search. Considering that the pH > 4 holding time is an important predictor of healing erosive esophagitis,26 it may be presumed that the advantage of using revaprazan over PPI would be small, if any. Indeed, revaprazan has not yet been approved for GERD therapy from the regulatory authority (see Acknowledgments).
Table 1.
pH > 4 holding time (%) with proton-pump inhibitors and potassium-competitive acid blockers.
| PPI/P-CAB | Dose (mg) | Single-dose studies | Multiple-dose | |
|---|---|---|---|---|
| Omeprazole* | 20 | 30.4 | 48.7 ± 20.5 | |
| Esomeprazole* | 20 | 32.5 ± 9.2 | 56.3 ± 7.4 | |
| 40 | 43.1 ± 17.8 | 64.6 ± 15.2 | ||
| Lansoprazole* | 15 | 28.1 ± 10 | 45.9 ± 14.3 | |
| 30 | 39.1 ± 12.8 | 55.1 ± 14.4 | ||
| Rabeprazole* | 10 | 29.8 ± 13.2 | 51.2 ± 13.1 | |
| 20 | 42.8 ± 15.9 | 57.7 ± 14.2 | ||
| Revaprazan# | 150 | 39.4 ± 22.7 | 39.9 ± 17.0 | |
| 300 | 61.7 ± 14.2 | 65.6 ± 12.0 | ||
| Vonoprazan§ | 10 | JP | 38.4 ± 22.3 | 63.3 ± 8.7 |
| UK | 43.1 ± 21.2 | 60.2 ± 19.1 | ||
| 20 | JP | 63.3 ± 17.9 | 83.4 ± 16.7 | |
| UK | 62.7 ± 16.8 | 85.2 ± 12.3 | ||
| 40 | JP | 85.3 ± 8.3 | 100 ± 00 | |
| UK | 85.6 ± 7.4 | 93.2 ± 10.5 | ||
The second P-CAB introduced in actual clinical use is vonoprazan fumarate (TAK-438), which was marketed in Japan in early 2015 and has gained popularity because of its superior properties to conventional PPIs in terms of rapid onset of action, longer duration of action, consistent acid suppression irrespective of CYP2C19, and, most importantly, more potent acid suppression.
Currently, a phase III clinical trial [ClinicalTrials.gov identifier: NCT03006874] for reflux esophagitis is conducted in Korea to compare the safety and efficacy of new P-CAB, tegoprazan (RQ-00000004/CJ-12420) 50 and 100 mg with 40 mg of esomeprazole, the details of which are yet to be published.
Vonoprazan fumarate, a potassium-competitive acid blocker with proven track records for gastroesophageal reflux disease
Vonoprazan fumarate has a different chemical structure defined as a pyrrole derivative which is different from previous P-CABs such as SCH 28080 and AZD0865 that have benzimidazole ring or revaprazan having pyrimidine ring27 (Figure 1). Another notable difference from previous P-CABs is that vonoprazan has alkaline pKa of 9.06, which enabled its high level of accumulation in acid space such as intracellular canaliculi of the parietal cells.28 In animal experiments, vonoprazan highly accumulated in the gastric gland both in the resting and in the active state; remarkably, the ratio of accumulation in the resting gastric gland was higher than that in the actively secreting gastric gland, as compared with lansoprazole.29 The accumulation of vonoprazan in the resting gland might account for its rapid onset of action although further experiments are necessary to examine whether vonoprazan can accumulate in the tubulovesicles in the resting parietal cells, and inactivate H+, K+-ATPase. Furthermore, vonoprazan selectively accumulated in the gastric corpus mucosa, particularly in parietal cells as shown in an autoradiography study,30 verifying specific targeting of this drug. It is rapidly absorbed and reaches to the maximum plasma level (Cmax) within 2 h but the plasma half-life (t1/2) was much longer (t1/2: about 7 h with 20 mg of vonoprazan taken on fasting) than conventional PPIs having much shorter half-lives (t1/2 = 1–2 h), as shown in the pharmacokinetic profile31–33 (Table 2, Figure 2), enabling once-daily dose for clinical use. As it does not require acid-catalyzed activation, similar efficacy of acid suppression with vonoprazan was shown irrespective of the administration before or after breakfast31,32 (Figure 2). Most importantly, vonoprazan exhibited potent, long-lasting acid suppression starting from the first day of administration which was dose-dependent and further increase in 7 days. At 40 mg, vonoprazan achieved almost total achlorhydria in both Japanese and UK patients34 (Table 1, Figure 3). Thus, the acid suppression with vonoprazan was much quicker and more profound than conventional PPIs and this effect started from the first day of administration. Even at 7 days, when PPIs achieve steady-state acid suppression, vonoprazan showed superiority to esomeprazole (20 mg) or rabeprazole (10 mg)35 (Figure 4) in terms of the 24 h pH > 4 holding time.
Table 2.
Pharmacokinetic property of proton-pump inhibitors and potassium-competitive acid blockers (single oral dose).
| Drug | Dose (mg) | tmax (h) | Cmax (µmol/l) | AUC (µmol·h/l) | t1/2 (h) |
|---|---|---|---|---|---|
| Omeprazole | 20 | 1–4 | 0.23–23.2 | 0.58–3.47 | 0.5–1.2 |
| Esomeprazole | 20 | 1–3.5 | 2.1–2.4 | 4.2 | 1.3–1.6 |
| 40 | 4.7–5.1 | 12.6 | |||
| Lansoprazole | 30 | 1.2–2.1 | 1.62–3.25 | 4.6–13.5 | 0.9–2.1 |
| Rabeprazole | 20 | 1.14 | 1.14 | 2.22 | 0.6–1.4 |
| Revaprazan | 200 | 2.1 ± 1.3 | 361.4 ± 124.1* | 1343.1 ± 365.9** | 2.4 ± 0.2 |
| Vonoprazan | 10 | 1.75 | 9.7 ± 2.1* | 60.1 ± 9.0** | 6.95 ± 1.03 |
| 20 | 1.50 | 25.0 ± 5.6* | 160.3 ± 38.6** | 6.85 ± 0.80 |
Figure 2.
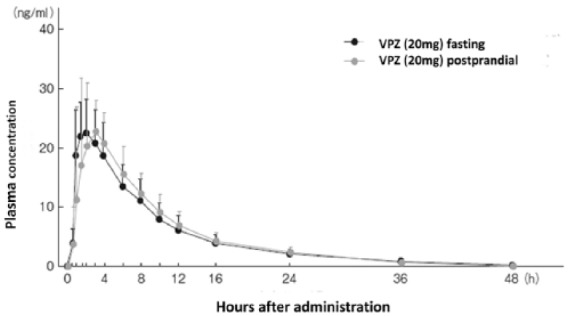
Plasma concentration of vonoprazan after a single oral dose of 20 mg.
Pharmacokinetics after an oral dose of 20 mg vonoprazan given either before or after breakfast were similar. Elimination half-life (t1/2) of vonoprazan was also similar irrespective of the timing of prescription. The t1/2 was about 7.7 h in this experiment, which is much longer than conventional proton-pump inhibitors (see Table 2). This data was adopted from Pharmaceuticals and Medical Devices Agency of Japan.32
Figure 3.
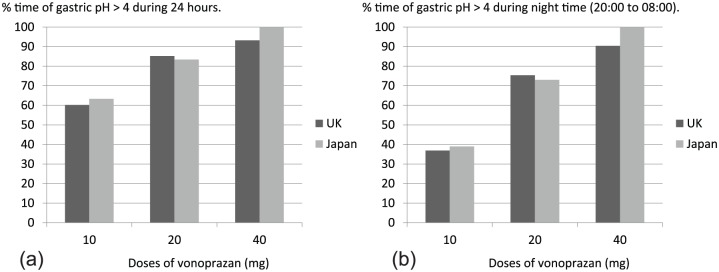
Response for pH > 4 holding time with various doses of vonoprazan.
Vonoprazan dose dependently prolonged 24 h pH > 4 holding time in healthy young male volunteers. The proportion of time (%) of gastric pH > 4 in 24 h (a) and night time (20:00 to 08:00) (b) after a single morning dose for 7 days was depicted using data from Jenkins and colleagues.34 As noted, differences between volunteers from the UK and Japan were small in both profiles. Notably, a single morning dose of vonoprazan effectively increased night time pH > 4 holding time, if the higher dose was used.
Figure 4.
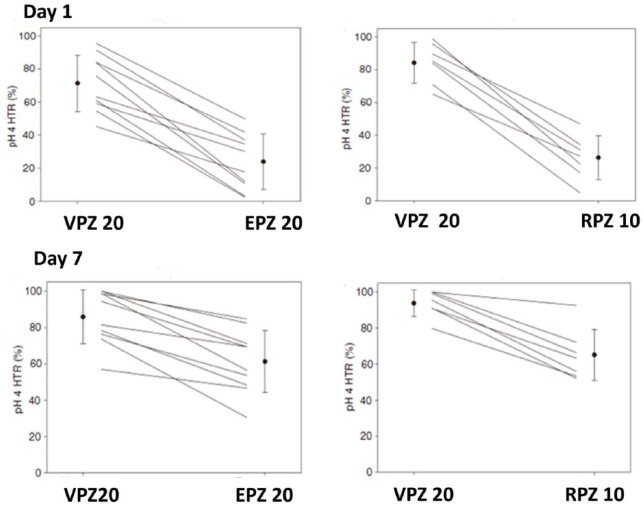
pH > 4 holding time on the 1st and 7th day.
24 h pH > 4 holding time of vonoprazan was compared with proton-pump inhibitors (esomeprazole and rabeprazole). VPZ at the dose of 20 mg showed rapid (from the 1st day) and more potent gastric acid suppression throughout the 7 days than 20 mg of esomeprazole or 10 mg of rabeprazole. In the study comparing VPZ 20 mg with EPZ 20 mg, % of pH > 4 holding time on the 1st and 7th day was 71.4 ± 17.0 and 85.8 ± 14.7% for VPZ, 23.9 ± 16.9 and 61.2 ± 17.1% for EPZ, respectively. In the study comparing VPZ 20 mg with RPZ 10 mg, % of pH > 4 holding time on the 1st and 7th day was 84.2 ± 12.4 and 93.8 ± 7.3% for VPZ, 26.3 ± 13.4 and 65.1 ± 14.2% for RPZ, respectively. Data are from Sakurai and colleagues.35
VPZ, vonoprazan; EPZ, esomeprazole; RPZ, rabeprazole; HTR, holding time rate.
Other investigators also demonstrated that gastric acid suppression with vonoprazan 20 mg given either as once or twice per day was superior to esomeprazole 20 mg given either as once or twice per day irrespective of CYP2C19 status.36 One of the unmet needs, NAB, therefore, can be controlled by vonoprazan because pH > 4 holding time in the night time was about 75%, and over 90% with a single morning dose of 20 mg and 40 mg, respectively,34 and almost 100% when 20 mg was given in two divided doses.36
Vonoprazan is mainly metabolized in the liver via CYP3A4 of cytochrome P45031,32 (Figure 5), which is distinct from PPIs whose metabolisms are mostly through CYP2C19 pathway. Interference of the metabolism of vonoprazan with clarithromycin, a potent inhibitor of CYP3A4, supports that the major metabolic disposition pathway of vonoprazan occurs through CYP3A4 in humans.37 Since CYP2C19 status has clinically relevant differences between individuals with extensive metabolizer phenotype versus poor metabolism in terms of the efficacy of PPIs and also influences drug activation process via CYP2C19 such as thienopyridines. Since thienopyridines are often used together with PPIs, this raised a serious concern, as the antiplatelet effect of thienopyridines, if compromised, can lead to grave outcomes, which prompted the US Food and Drug Administration to issue a warning. Metabolic disposition of vonoprazan, less dependent on CYP2C19, provides smaller interindividual variations in the efficacy and less drug interactions through this pathway. However, drugs metabolized through the CYP3A4 pathway can interfere with the metabolism, and conversely, vonoprazan can affect the drugs metabolized through this pathway requiring attention.32,37
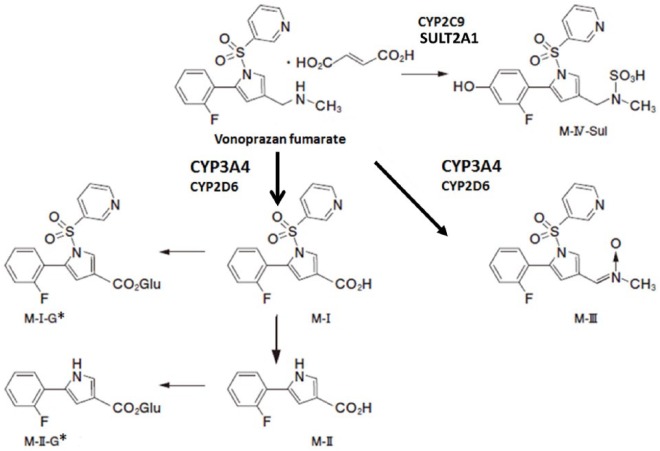
Figure 5.
Metabolic disposition pathways of vonoprazan.
The major route of metabolic disposition of vonoprazan was reported via CYP3A4, which converts vonoprazan to compounds M-I and M-III (thick arrows). Although CYP2D6, CYP2C9 and CYP2C19, and sulfotransferase2A1 (SULT2A1) also participate in metabolic disposition, their roles are relatively minor. M-I-G* and M-II-G* were inferred glucuronic-acid-conjugated (G) products from M-I, and M-II, respectively. Data are taken from the Pharmaceuticals and Medical Devices Agency of Japan32 with some modifications.
Effect of vonoprazan for gastroesophageal reflux disease (GERD)
A phase II clinical trial comparing various doses (5–40 mg once per day) of vonoprazan with lansoprazole (30 mg once per day) showed excellent therapeutic effect on erosive esophagitis, reflecting longer 24 h gastric pH > 4 holding time with vonoprazan.38 Remarkably, 20 and 40 mg of vonoprazan almost completely healed erosions in patients with high grades of erosive esophagitis (LA-C/D) in 4 weeks, whereas healing rate in LA-C/D esophagitis with lansoprazole at 4 weeks was 87%. No serious adverse events were observed in this trial, even with the highest tested dose of vonoprazan (40 mg). Based on the phase II results, the pivotal randomized controlled phase III trial comparing daily dose of 20 mg of vonoprazan with lansoprazole 30 mg for 8 weeks was conducted. This trial was designed to demonstrate the noninferiority of vonoprazan against lansoprazole 30 mg in healing erosive esophagitis as a required step for getting insurance approval from the regulatory authority. Another purpose of this trial was to study a long-term safety and efficacy of vonoprazan. Patients who were healed by 8 weeks of both arms (vonoprazan and lansoprazole) of therapy were assigned to a maintenance dose of either 10 or 20 mg of vonoprazan and followed up for up to 52 weeks.39 The first phase of this trial looking at healing rate of erosive esophagitis as the primary outcome recapitulated the results of the phase II trial. Overall healing rate at 8 weeks with 20 mg of vonoprazan and 30 mg of lansoprazole was 99.0% and 95.5%, respectively, thus noninferiority of vonoprazan against lansoprazole was verified. Actually, post hoc analysis showed that vonoprazan was significantly better in healing erosive esophagitis than lansoprazole. In a subgroup analysis, vonoprazan scored a significantly higher healing rate (98.7% at 8 weeks) as compared with lansoprazole 30 mg (87.5% at 8 weeks) in those with severe esophagitis (LA-C/D) (Figure 6). In the latter part of this trial studying a long-term efficacy and safety of maintenance dose of vonoprazan 10 or 20 mg per day, recurrence rates at 52 weeks with either dose of vonoprazan were similar and <10% (Table 3). Another trial comparing efficacy of lansoprazole 15 mg, the dose approved for maintenance therapy for GERD in Japan, versus vonoprazan 10 and 20 mg to prevent recurrence of erosive esophagitis for 24 weeks, was conducted. Although the results of this trial have not been published yet, either dose of vonoprazan was clearly superior to lansoprazole 15 mg in preventing recurrence of esophagitis according to the government report32 (Table 3). Based on these two trials, 10 mg of vonoprazan was approved for maintenance therapy to prevent recurrence of erosive reflux disease in Japan. In case of failure with this dose, however, 20 mg of vonoprazan can be used for maintenance. In previous reports, clinical data on maintaining healing of esophagitis with lansoprazole 15 mg, or esomeprazole 10 mg were published.40,41 Considering similarity with the clinical trial data comparing vonoprazan with lansoprazole32 and the similarity with independent PPI data, vonoprazan 10 mg seemed to show better preventive effect in maintaining healing of erosive esophagitis (Table 3). Moreover, recurrence rates reported in the maintenance therapy with 10 or 20 mg of vonoprazan was numerically lower than those reported for maintenance therapy using regular doses of PPIs.42,43 Because of many demographic factors such as ethnicity, obesity, and severity of erosive reflux disease in the patients enrolled, this difference should not be interpreted that vonoprazan maintenance with 10 or 20 mg is better than maintenance therapy with regular doses of PPIs. Thus, direct head-to-head comparisons between vonoprazan and regular-dose PPIs are necessary to ascertain whether vonoprazan actually is superior to regular-dose PPIs for maintaining healing. It is unfortunate that recurrence rates according to the baseline severity of reflux disease were not reported in the latter part of this trial. Such information would help physicians understand that vonoprazan should be used for long-term maintenance in patients with severe grade esophagitis, often refractory to PPI maintenance. Although more information is necessary, low rate of recurrence with vonoprazan corroborated well with the data that pH > 4 holding time was important in preventing relapse of healed reflux esophagitis,44 and provided us with a new armament to protect esophageal mucosal break.
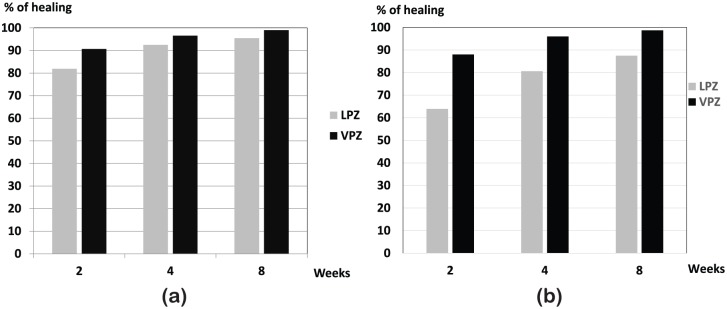
Figure 6.
Efficacy of vonoprazan 20 mg for healing erosive esophagitis.
Overall healing rate in patients with esophagitis of vonoprazan 20 mg once daily was compared with that of lansoprazole 30 mg once daily. Noninferiority of vonoprazan against lansoprazole was verified (a) in patients with severe grade of esophagitis (grade C/D of the Los Angeles classification), vonoprazan achieved significantly faster and higher healing (b) depicts healing rate of LA-grade C/D.
LPZ, lansoprazole; VPZ, vonoprazan.
Table 3.
Recurrence rate of erosive esophagitis during maintenance therapy (up to 52 weeks) in Japanese patients.
Vonoprazan was also shown to exhibit an excellent healing for PPI-resistant esophagitis,45–47 indicating its potential role for fulfilling an unmet need for treating difficult reflux esophagitis.
Unfortunately, in terms of symptom control, another important therapeutic goal for the management of GERD, detailed results were not provided in the phase III trial of vonoprazan. The only statement given on the control of reflux or regurgitation symptoms in this report was that there were no differences between vonoprazan and lansoprazole.39 No account for the symptomatic control during the maintenance phase of this trial was given, either. Considering the early onset of acid control with vonoprazan, it would be interesting to know whether it provides early symptomatic control over conventional PPIs. However, the performance of vonoprazan may not be so promising in terms of symptomatic control. Indeed, the first randomized placebo-controlled trial comparing vonoprazan 10 mg or 20 mg in patients with NERD failed to show superiority over placebo on the primary outcome measure of the proportion of heartburn-free days.48 In this trial, NERD patients were classified into two subgroups: patients with no mucosal change (Grade N) and those with minimal mucosal changes such as mucosal redness, or turbidity (Grade M). In the latter group, presence of regurgitation of gastric acid has been strongly indicated, as chromostaining or narrow-band imaging could disclose elements of mucosal damages not identifiable by conventional white light endoscopy.49 Vonoprazan 20 mg showed significant improvement in heartburn symptoms in the grade M subgroup, as expected. It also decreased the mean severity of heartburn. Since the results of the first trial was close to showing significant difference, a second RCT [ClinicalTrials.gov identifier: NCT02954848] with vonoprazan 10 mg in patients with NERD is currently underway and may be reported in the future. In a smaller-scale retrospective study to examine the effect of vonoprazan on GERD symptoms, successful control of symptoms achieved by 20 mg of vonoprazan was not so impressive: 75% in patients with erosive esophagitis and 60% in patients with NERD.50 Consistent with this report, vonoprazan was shown to provide symptom resolution in about half of reflux symptoms in PPI-refractory GERD patients.51 These rather suboptimal response rates indicate causes other than acid are responsible for symptom generation in this group of patients. This problem has been highlighted as therapeutic performance of PPI for NERD patients being inferior to that for erosive esophagitis.52 Several reasons have been given to explain this unsatisfactory performance of PPIs: increased sensitivity to acid or weakly acidic reflux, motor dysfunction, and perception disorder in the central nervous system are some of the explanations. For instance, patients with functional heartburn are defined as a group of patients whose symptoms are unrelated to acid reflux. Therefore, these patients should be managed by other modalities, although a portion of the patients may respond to PPIs.53
A recent report also showed that vonoprazan might be effective in patients with eosinophilic esophagitis who were refractory to PPI therapy.54 Further detailed study is warranted to confirm this preliminary finding.
Safety of vonoprazan
Throughout the course of clinical development, safety of vonoprazan has been surveyed, with particular attention to liver toxicity, as this has been a problem with previous P-CABs.30,33,34 Overall, no serious drug-related treatment-emerged adverse events (TEAEs) were noticed during the clinical trials, including those for GERD. In the abovementioned phase III trial,39 the safety profile of vonoprazan in the maintenance phase up to 52 weeks was similar to that of lansoprazole.34 It should be noted that the rate of abnormality in liver function tests in groups assigned to vonoprazan were similar to that of lansoprazole during the 8 weeks. No increase in liver toxicity was noticed during the long-term phase of the study in either group of vonoprazan, 10 or 20 mg.39
Since vonoprazan suppressed acid inhibition more profoundly than PPIs, increase in serum gastrin level was far greater in patients with vonoprazan than lansoprazole 30 mg. During the 8 weeks of trial, serum gastrin in the group taking vonoprazan 20 mg elevated about one-and-a-half times (about 300 pg/ml) of lansoprazole 30 mg. At the end of the chronic maintenance phase (52 weeks) of continuous administration of vonoprazan either 10 or 20 mg, further increase in serum gastrin was noted in both groups: 317.5 ± 336.42 to 777.6 ± 678.64 (pg/ml) in vonoprazan 20 mg group, and 291.0 ± 219.59 to 514.4 ± 435.53 (pg/ml) in vonoprazan 10 mg, respectively. In animal carcinogenicity experiments using a much higher dose of vonoprazan (5 mg/kg in rat to 6 mg/kg in mouse) for 2 years, increased incidence of gastric endocrine tumor was reported in rodents.31,32 As rodents have been well known for their susceptibility to developing gastric endocrine tumors with acid suppression by histamine H2RA or PPI, this should be interpreted cautiously, considering extremely high doses of vonoprazan. In the long-term prevention trial for GERD, slight increase in gastric endocrine cells was noted at 52 weeks in both vonoprazan 10 and 20 mg groups.39 For the moment, the longest safety data available for continuous administration for vonoprazan in this regard, are from the low-dose aspirin (LDA) prevention trial with 10 mg of vonoprazan spanning up to 104 weeks. As with maintenance therapy for reflux esophagitis, no gastric endocrine cell tumors were reported, despite higher gastrin level in the patients enrolled in the LDA-prevention trial.55 Despite the safety data so far obtained in vonoprazan trials, cautious monitoring of endocrine cell hyperplasia and potential development of endocrine cell tumors is mandatory when continuous use of vonoprazan is required, since the development of carcinoid tumor was reported with prolonged use (>10 years in most cases) of PPI.56 As has been a problem with PPIs, bacterial overgrowth in the gut will inevitably ensue because of the loss of protection conferred by gastric acid. Indeed, profound change in the microbiota was recently reported with 4-week administration of 20 mg of vonoprazan in young healthy volunteers.57 Long-term consequences of the altered gut microbiota for the host metabolism and health need further vigilance.
As drug absorption can be influenced by gastric acid, coprescription of vonoprazan with atazanavir or rilpivirine is contraindicated due to diminished absorption of these drugs.
PPIs have been widely used for nearly 3 decades and their overall safety has been established. However, a number of safety concerns associated with their long-term use have been raised (Table 4). Recent guidelines from the American Gastroenterological Association did not recommend increasing intake of calcium, vitamin B12, or magnesium beyond the recommended dietary allowance or monitoring of bone mineral density, serum creatinine, magnesium, or vitamin B12.58 So far, no report concerning these nutritional, metabolic, renal and infectious problems has been published on vonoprazan. Nevertheless, continuous attention should be required for picking up such adverse events associated with vonoprazan, considering its capability of more profound and prolonged acid suppression.
Table 4.
Adverse events associated with chronic proton-pump inhibitor use.
| Causes | Diseases |
|---|---|
| Hypochlorhydria | Small intestinal bacterial overgrowth |
| Clostridium difficile infection | |
| Pneumonia | |
| Spontaneous bacterial peritonitis | |
| Malabsorption | Iron deficiency anemia |
| Hypocalcemia, osteoporosis, fracture | |
| Hypomagnesemia | |
| Vitamin B12 deficiency | |
| Hypergastrinemia | Gastric endocrine hyperplasia |
| Carcinoid tumor (gastric neuroendocrine tumor) | |
| Others | Interstitial nephritis |
| Collagenous colitis | |
| Myocardial infarction | |
| Dementia |
Conclusion
Introduction of more potent, long-acting vonoprazan into clinical use would fulfill at least a part, if not all, of unmet needs of GERD management. For instance, it has a potential to overcome the problem of nocturnal acid breakthrough because acid suppression with a single high dose of vonoprazan or two divided regular doses could cover the night period in the majority of healthy patients. Moreover, efficacy of vonoprazan for patients having a more severe grade of esophagitis in the phase III clinical trial in Japan and for PPI-refractory cases was documented in a small case series, again indicating a promise for fulfilling one of the unmet needs for GERD therapy.39,45–47 Further large-scale, prospective, randomized controlled studies with PPI as a comparator, however, are imperative to verify this potential. For maintaining healing, vonoprazan, even in a low dose showed an excellent result. We need to explore which of the strategies, vonoprazan first and stepped down to PPI; PPI first and stepped up to vonoprazan; or continuous vonoprazan with dose reduction after healing, would be more advantageous in terms of patient satisfaction, required cost and long-term safety concerns associated with hypergastrinemia and hypochlorhydria.
Another unmet need with conventional PPIs has been a rather poor symptomatic control in patients with NERD. Unfortunately, very few data are available with vonoprazan, requiring further evidence in this aspect of managing GERD.
Of course, we need to carefully watch the long-term safety of vonoprazan, including the development of gastric neuroendocrine tumors, influence on nutrient deficiency, increase in infection, and alteration of gut microbiota among others.
For the moment, vonoprazan is available only in Japan, but an international trial of comparing vonoprazan with lansoprazole in patients with erosive reflux disease in Asia, including China, Korea, Taiwan and Malaysia is underway. It is expected that it would be launched for use for GERD patients in some of the Asian countries in 2018. Considering highly efficient eradication results with vonoprazan-based triple therapy for Helicobacter pylori,59 further expanded license for this indication is expected, as the disease burden associated with this infection, such as gastric cancer, is high in this part of the world. A phase II clinical trial of vonoprazan in Europe has been initiated and that of the USA is under consideration, where the demand for better therapy for GERD patients is high. Thus, vonoprazan might have a potential to play a pivotal role in the management of GERD internationally in the future.
Acknowledgments
I am grateful to Professor Nayoung Kim, Bundang Seoul National University Hospital, for providing me with the information on revaprazan.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: KS has received lecture fees from Takeda Pharma Inc., EA Pharma and Astra Zeneca.
References
- 1. El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014; 63: 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fock KM, Talley N, Goh KL, et al. Asia-Pacific consensus on the management of gastro-oesophageal reflux disease: an update focusing on refractory reflux disease and Barrett’s oesophagitis. Gut 2016; 65: 1402–1415. [DOI] [PubMed] [Google Scholar]
- 3. Kinoshita Y, Adachi K, Hongo M, et al. Systematic review of the epidemiology of gastroesophageal reflux disease in Japan. J Gastroenterol 2011; 46: 1092–1103. [DOI] [PubMed] [Google Scholar]
- 4. Richter JE, Kahrilas PJ, Johanson J, et al. Efficacy and safety of esomeprazole compared with omeprazole in GERD patients with erosive esophagitis: a randomized controlled trial. Am J Gastroenterol 2001; 96: 656–665. [DOI] [PubMed] [Google Scholar]
- 5. Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut 2012; 61: 1340–1354. [DOI] [PubMed] [Google Scholar]
- 6. Bytzer P. What makes individuals with gastroesophageal reflux dissatisfied with their treatment? Clin Gastroenterol Hepatol 2009; 7: 816–822. [DOI] [PubMed] [Google Scholar]
- 7. Xue S, Katz PO, Banerjee P, et al. Bedtime H2 blockers improve nocturnal gastric acid control in GERD patients on proton pump inhibitors. Aliment Pharamacol Ther 2001; 15: 1351–1356. [DOI] [PubMed] [Google Scholar]
- 8. Facker WK, Ours TM, Vaezi MF, et al. Long-term effect of H2RA therapy on nocturnal acid breakthrough. Gastroenterology 2002; 122: 625–632. [DOI] [PubMed] [Google Scholar]
- 9. Rohof WO, Bennink RJ, Smout AJPM, et al. An alginate-antacid formulation localizes to the acid pocket to reduce acid reflux in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2013; 11: 1585–1591. [DOI] [PubMed] [Google Scholar]
- 10. Kahrilas P, McColl K, Fox M, et al. The acid pocket: a target for treatment in reflux disease? Am J Gastroenterol 2013; 108: 1058–1064. [DOI] [PubMed] [Google Scholar]
- 11. Cossentino MJ, Mann K, Ambruster SP, et al. Randomised clinical trial: the effect of baclofen in patients with gastro-esophageal reflux-a randomised prospective study. Aliment Pharmacol Ther 2012; 35: 1036–1044. [DOI] [PubMed] [Google Scholar]
- 12. Hunt R, Armstrong D, Yaghoobi M, et al. The pharmacodynamics and pharmacokinetics of S-tenatoprazole-Na 30 mg, 60 mg and 90 mg vs. esomeprazole 40 mg in healthy male subjects. Aliment Pharmacol Ther 2010; 31: 648–657. [DOI] [PubMed] [Google Scholar]
- 13. Metz DC, Vakily M, Dixit T, et al. Review article: dual delayed release formulation of dexlansoparazole MR, a novel approach to overcome the limitations of conventional single release proton pump inhibitor therapy. Aliment Pharmacol Ther 2009; 29: 928–937. [DOI] [PubMed] [Google Scholar]
- 14. Sharma P, Shaheen NJ, Perez MC, et al. Clinical trials: healing of erosive oesophagitis with dexlansoprazole MR, a proton pump inhibitor with a novel dual delayed-release formulation-results from two randomized controlled studies. Aliment Pharmacol Ther 2009; 29: 731–741. [DOI] [PubMed] [Google Scholar]
- 15. Ene MD, Khan-Daneshmend T, Roberts CJC. A study of the inhibitory effects of SCH 28080 on gastric secretion in man. Br J Pharmacol 1982; 76: 389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Long JF, Chiu PJS, Derelanko MJ, et al. Gastric antisecretory and cytoprotective activities of SCH 28080. J Pharmacol Exp Ther 1983; 226; 114–120. [PubMed] [Google Scholar]
- 17. Keeling DJ, Laing SM, Senn-Bilfinger J. SCH 28080 is a luminally acting, K+-site inhibitor of the gastric (H+ + K+)-ATPase. Biochem Pharmacol 1988; 37: 2231–2236. [DOI] [PubMed] [Google Scholar]
- 18. Keeling DJ, Taylor AG, Schudt C. The binding of a K+ competitive ligand, 2-methyl, 8-(phenylmethoxy)imidazo(1,2-a)pyridine 3-acetonitrile, to the gastric (H++K+)-ATPase. J Biol Chem 1989; 264: 5545–5551. [PubMed] [Google Scholar]
- 19. Gedda K, Briving C, Svensson K, et al. Mechanism of action of AZD0865, a K+ competitive inhibitor of H+, K+-ATPase. Biochem Pharmacol 2007; 73: 198–205. [DOI] [PubMed] [Google Scholar]
- 20. Kahrilas PJ, Dent J, Lauristen K, et al. A randomized, comparative study of three doses of AZD0865 and esomeprazole for healing of reflux esophagitis. Clin Gastroenterol Hepatol 2007; 5: 1385–1391. [DOI] [PubMed] [Google Scholar]
- 21. Dent J, Kahrilas P, Hatlebakk J, et al. A randomized, comparative trial of a potassium-competitive acid blocker (AZD0865) and esomeprazole for the treatment of patients with nonerosive reflux disease. Am J Gastroenterol 2008; 103: 20–26. [DOI] [PubMed] [Google Scholar]
- 22. Yu KS, Bae KS, Shon JH, et al. Pharmacokinetic and pharmacodynamics evaluation of a novel proton pump inhibitor, YH1885, in healthy volunteers. J Clin Pharamacol 2004; 44: 73–82. [DOI] [PubMed] [Google Scholar]
- 23. Kim HK, Park SH, Cheung DY, et al. Clinical trial: inhibitory effect of revaprazan on gastric acid secretion in healthy male subjects. J Gastroenterol Hepatol 2010; 25: 1618–1625. [DOI] [PubMed] [Google Scholar]
- 24. Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol 2009; 65: 19–31. [DOI] [PubMed] [Google Scholar]
- 25. Kim YG, Jang BI, Kim TN. A matched case-control study of a novel acid-pump antagonist and proton-pump inhibitor for the treatment of iatrogenic ulcers caused by endoscopic submucosal resection. Gut Liver 2010; 4: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bell NJV, Burget D, Howden CW, et al. Appropriate acid suppression for the management of gastroesophageal reflux disease. Digestion 1992; 51(Suppl. 1): 59–67. [DOI] [PubMed] [Google Scholar]
- 27. Arikawa Y, Nishida H, Kurasawa O, et al. Discovery of a novel pyrrole derivative 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfony)-1H-pyrrol-3-yl]-N-methylmethanamine fumarate (TAK-438) as a potassium-competitive acid blocker (P-CAB). J Med Chem 2012; 55: 4446–4456. [DOI] [PubMed] [Google Scholar]
- 28. Shin JM, Inatomi N, Munson K, et al. Characterization of a novel potassium-competitive acid blocker of the gastric H, K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridine-3-ylsulfony)-1H-pyrrol-3-yl]-N-methyl methanamine fumarate (TAK-438). J Pharmacol Exp Therap 2011; 339: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsukawa J, Hori Y, Nishida H, et al. A comparative study on the modes of action of TAK-438, a novel potassium-competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. Biochem Pharmacol 2011; 81: 1145–1151. [DOI] [PubMed] [Google Scholar]
- 30. Matsukawa J, Kogame A, Tagawa Y, et al. Radiographic localization study of a novel potassium-competitive acid blocker, vonoprazan, in the rat gastric mucosa. Dig Dis Sci 2016; 61: 1888–1894. [DOI] [PubMed] [Google Scholar]
- 31. Echizen H. The first-in-class potassium-competitive acid blocker, vonoprazan fumarate: pharmacokinetic and pharmacodynamics consideration. Clin Pharmacokinet 2016; 55: 409–418. [DOI] [PubMed] [Google Scholar]
- 32. Pharmaceuticals and Medical Devices Agency of Japan. Drug approval review for vonoprazan fumarate (in Japanese), the Pharmaceuticals and Medical Devices Agency of Japan, http://www/pmda/go.jp/ (2014, accessed 25 August 2017).
- 33. Shin JM, Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil 2013; 19: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jenkins H, Sakurai Y, Nishimura A, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy subjects. Aliment Pharmacol Ther 2015; 41: 636–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20mg compared with esomeprazole 20mg or rabeprazole 10mg in healthy adult male subjects-a randomised open-label cross- over study. Aliment Pharmacol Ther 2015; 42: 719–730. [DOI] [PubMed] [Google Scholar]
- 36. Kagami T, Sahara S, Ichikawa H, et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole with reference to CYP2C19. Aliment Pharmacol Ther 2016; 43: 1048–1059. [DOI] [PubMed] [Google Scholar]
- 37. Jenkins H, Jenkins R, Patat A. Effect of multiple oral doses of the potent CYP3A4 inhibitor clarithromycin on the pharmacokinetics of a single oral dose of vonoprazan: a phase I, open-label, sequential design study. Clin Drug Invest 2017; 37: 311–316. [DOI] [PubMed] [Google Scholar]
- 38. Ashida K, Sakurai Y, Nishimura A, et al. Randomised clinical trial: a dose-ranging study of vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the treatment of erosive esophagitis. Aliment Pharmacol Ther 2015; 42: 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ashida K, Sakurai Y, Hori T, et al. Randomised clinical trial: vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther 2016; 43: 240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kawamura M, Ohara S, Koike T, et al. Cytochrome P450 2C19 polymorphism influences the preventive effect of lansoprazole on the recurrence of erosive esophagitis. J Gastroenterol Hepatol 2007; 22: 222–226. [DOI] [PubMed] [Google Scholar]
- 41. Kinoshita Y, Miwa H, Kasugai K. Efficacy and safety of esomeprazole, compared with omeprazole, in maintenance therapy for reflux esophagitis-a phase III, multicenter randomized, double-blind trial. Nihon Shokakibyo Gakkai Zasshi 2013; 110: 1428–1438 (in Japanese with English abstract). [PubMed] [Google Scholar]
- 42. Hatleback JG, Berstad A. Lansoprazole 15 and 30 mg daily in maintaining healing and symptom relief in patients with reflux esophagitis. Aliment Pharmacol Ther 1997; 11: 365–372. [DOI] [PubMed] [Google Scholar]
- 43. Lauritsen K, Devière J, Bigard MA, et al. Esomeprazole 20 mg and lansoprazole 15mg in maintaining healed reflux oesophagitis: metropole study results. Aliment Pharmacol Ther 2003; 17: 333–341. [DOI] [PubMed] [Google Scholar]
- 44. Johnson DA, Katz PO, Levine D, et al. Prevention of relapse of healed reflux esophagitis is related to the duration of intaragastric pH>4. J Clin Gastroenterol 2010; 44: 475–478. [DOI] [PubMed] [Google Scholar]
- 45. Iwakiri K, Sakurai Y, Shiino M, et al. A randomized, double-blind study to evaluate the acid-inhibitory effect of vonoprazan (20 mg and 40 mg) in patients with proton-pump inhibitor-resistant erosive esophagitis. Therap Adv Gastroenterol 2017; 10: 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hoshino S, Kawami N, Takenouchi N, et al. Efficacy of vonoprazan for proton pump inhibitor-resistant reflux esophagitis. Digestion 2017; 95: 156–161. [DOI] [PubMed] [Google Scholar]
- 47. Yamashita H, Kanamori A, Kano C, et al. The effects of switching to vonoprazan, a novel potassium-competitive acid blocker, on gastric acidity and reflux patterns in patients with erosive esophagitis refractory to proton pump inhibitors. Digestion 2017; 96: 52–59. [DOI] [PubMed] [Google Scholar]
- 48. Kinoshita Y, Sakurai Y, Shiino M, et al. Evaluation of the efficacy and safety of vonoprazan in patients with nonerosive gastroesophageal reflux disease: a phase III, randomized, double-blind, placebo-controlled, multicenter study. Curr Ther Res Clin Exp 2016; 81–82: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Falk GW. Is conventional endoscopic identification of non-erosive reflux disease adequate? Digestion 2008; 78(Suppl. 1): 17–23. [DOI] [PubMed] [Google Scholar]
- 50. Asaoka D, Nagahara A, Hojo M, et al. Efficacy of a potassium-competitive acid blocker for improving symptoms in patients with reflux esophagitis, non-erosive reflux disease, and functional dyspepsia. Biomed Rep 2017; 6: 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Okuyama M, Nakahara K, Iwakura N, et al. Factors associated with potassium-competitive acid blocker non-response in patients with proton pump inhibitor-refractory gastroesophageal reflux disease. Digestion 2017; 95: 281–287. [DOI] [PubMed] [Google Scholar]
- 52. Chey WD. Endoscopy-negative reflux disease: concepts and clinical practice. Am J Med 2004; 117: 36S–43S. [DOI] [PubMed] [Google Scholar]
- 53. Hachem C, Shaheen NJ. Diagnosis and management of functional heartburn. Am J Gastroenterol 2016; 111: 53–61. [DOI] [PubMed] [Google Scholar]
- 54. Ishimura N, Ishihara S, Kinoshita Y. Sustained acid suppression by potassium-competitive acid blocker (P-CAB) may be an attractive treatment candidate for patients with eosinophilic esophagitis. Am J Gastroenterol 2016; 111: 1203–1204. [DOI] [PubMed] [Google Scholar]
- 55. Kawai T, Oda K, Funao N, et al. Vonoprazan prevents low-dose aspirin-associated ulcer recurrence: randomised phase 3 study Gut Published Online First: 01 December 2017. doi: 10.1136/gutjnl-2017-314852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nandy N, Hanson JA, Strickland RG, et al. Solitary gastric carcinoid tumor associated with long-term use of omeprazole: a case report and review of the literature. Dig Dis Sci 2016; 61: 708–712. [DOI] [PubMed] [Google Scholar]
- 57. Otsuka T, Sugimoto M, Inoue R, et al. Influence of potassium-competitive acid blocker on the gut microbiome of Helicobacter pylori-negative healthy individuals. Gut 2017; 66: 1723–1725. [DOI] [PubMed] [Google Scholar]
- 58. Murakami K, Sakurai Y, Shiino M, et al. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 2016; 65: 1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Freedberg DE, Kim LS, Yang YX. The risk and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology 2017; 152: 706–715. [DOI] [PubMed] [Google Scholar]