Abstract
Crohn’s disease and ulcerative colitis are chronic inflammatory disorders of the gastrointestinal tract. Treatment options include biologic therapies; however, a proportion of patients lose response to biologics, partly due to the formation of anti-drug antibodies (ADAbs). Concomitant immunosuppressive agents reduce the development of ADAbs. This review article aims to assess the immunogenicity of biologic therapies and their clinical implications. A comprehensive literature search was conducted for articles published January 2009 to August 2015 reporting immunogenicity to adalimumab (ADM), certolizumab pegol (CZP), golimumab, infliximab (IFX), ustekinumab, and vedolizumab in inflammatory bowel disease (IBD). Eligible articles were reviewed and quality assessed by independent reviewers. Overall, 122 publications reporting 114 studies were assessed. ADAbs were reported for all agents, but the percentage of patients developing ADAbs was extremely variable, with the highest (65.3%) being for IFX administration to patients with IBD. ADAb presence was frequently associated with a reduction in primary efficacy and a loss of response, and, for IFX, an increase in adverse events (AEs). Lower serum levels of ADM, CZP and IFX were seen in ADAbs-positive rather than ADAbs-negative patients; pharmacokinetic data were unavailable for other therapies. Little information was available regarding the timing of ADAb development; studies reported their detection from as early as 10–14 days up to months after treatment initiation. Biologic therapies carry an intrinsic risk of immunogenicity, although reported rates of ADAbs vary considerably. The clinical implications of immunogenicity are a concern for effective treatment; further research, particularly into the more recently approved biologics, is required.
Keywords: anti-drug antibodies, biologic therapy, Crohn’s disease, immunogenicity, ulcerative colitis
Introduction
The two most common forms of inflammatory bowel disease (IBD) are Crohn’s disease (CD) and ulcerative colitis (UC). Both are chronic, immune-mediated inflammatory disorders of the gastrointestinal tract, characterized by abdominal pain, rectal bleeding, diarrhoea and fatigue.1–3 In 2012, CD was reported to affect between 319 (North America) and 322 (Europe) people per 100,000, and UC between 249 (North America) and 505 (Europe) people per 100,000, with the prevalence of both diseases reported to be increasing in both regions.4
Agents currently approved for the treatment of IBD include 5-aminosalicylic acid, corticosteroids, methotrexate (MTX), the thiopurines azathioprine and mercaptopurine and biologic therapies targeting tumour necrosis factor (TNF)-alpha and integrins.5–7 Both the American College of Gastroenterology and European Crohn’s and Colitis Organisation guidelines advise that treatment should be tailored to the individual, considering disease severity and location, with biologic therapies considered for those with moderate to severe disease who do not respond to, or are intolerant of, conventional therapy.5–9
Although biologic therapies represent a major advance in the treatment of IBD, and a high proportion of patients with moderate or severe disease respond well to biologics, at least 30% of patients fail to meet primary endpoints in clinical trials,10,11 and a proportion of patients lose response over time.12 Immunogenicity is recognized as a leading contributor to the loss of response to biologic therapies; as biologic agents are large, complex proteins, they trigger the formation of anti-drug antibodies (ADAbs) specific to the agent administered.13 Therefore, it is recommended that patients who develop ADAbs to a biologic therapy, with a consequent loss of response, should switch to a different agent with either the same or a different mechanism of action.14–16
Studies of the immunogenicity of biologics have reported variability in the rate of formation of ADAbs, and in the time post-exposure that ADAbs develop, possibly due to differing immunogenicity assay methodologies.13 A number of different techniques with varying specificity, sensitivity and cost are currently available to measure ADAbs. Assay techniques include enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA) and electrochemi-luminescence (ECL), with some methods optionally incorporating an acid-dissociation step to improve sensitivity and increase the chances of detecting ADAbs even in the presence of high levels of antigen.17–20
Aim
The objective of the current analysis was to evaluate and contextualize the immunogenicity of approved biologic therapies used to treat IBD in order to understand the clinical implications of the rate, timeline and impact of the development of an immunogenic response to biologics.
Methods
The methodological approach was described in a protocol and followed the recommendations and standards required by the UK National Institute for Health and Care Excellence (NICE) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Literature search
A comprehensive search strategy was developed, and online searches were conducted for relevant articles published in the period January 2009 to August 2015.
A number of electronic sources were searched: Medline® and Embase® via the Ovid interface; Cochrane Central Register of Trials (CENTRAL); Cochrane Database of Systematic Review (CDSR); NHS Economic Evaluation Database (NHS EED); Health Technology Assessment (HTA) Database; and Database of Abstracts of Reviews of Effects (DARE) via the Cochrane Library. In addition to the online search, key congresses, published systematic literature reviews (capturing suitable studies published prior to 2009) and the references in articles relevant to the topic of interest were hand-searched. Details of the search strategies are included as Supplementary Tables 1 and 2.
Study eligibility and screening
Randomized controlled trials (RCTs), non-RCTs and observational studies of patients with CD or UC were eligible. Case reports, case series, literature reviews, letters, commentaries and editorials were excluded. Studies were included if they reported data for the biologics adalimumab (ADM), certolizumab pegol (CZP), golimumab (GLM), infliximab (IFX), ustekinumab (UST) and vedolizumab (VDM), administered to patients with CD or UC either as monotherapy or in combination with conventional therapy.
Abstracts identified from the online search were screened for eligibility by a reviewer. A second, independent reviewer checked 10% of screened abstracts. Articles considered eligible were obtained and subjected to full-text review; any ineligible studies were excluded, with the rationale for exclusion documented. A second independent reviewer screened 20% of excluded publications and all eligible publications; any discrepancies between reviewers were resolved by consensus.
Data extraction and quality assessment
Relevant information was defined at the start of the study, and a data extraction table ( Supplementary Table 3) was developed to collect information obtained on full review of the selected articles. Selected articles were assessed for quality in accordance with the recommended methodology for systematic literature reviews.21 One reviewer screened all citations and full-text papers, which were then double-checked by an independent reviewer. Likewise, data extraction was conducted by a single reviewer, and subsequently validated by an independent reviewer. For RCTs, the quality of selected articles was assessed according to the NICE single technology appraisal manufacturer’s template22 and the Jadad scoring system.23 The quality of selected non-RCTs and observational studies was assessed using the Downs and Black instrument.24 Abstracts from conference proceedings were assessed using a modified version of the Downs and Black instrument. Studies scoring poorly on any of the quality assessments were excluded from the main analyses.
Results
Literature search
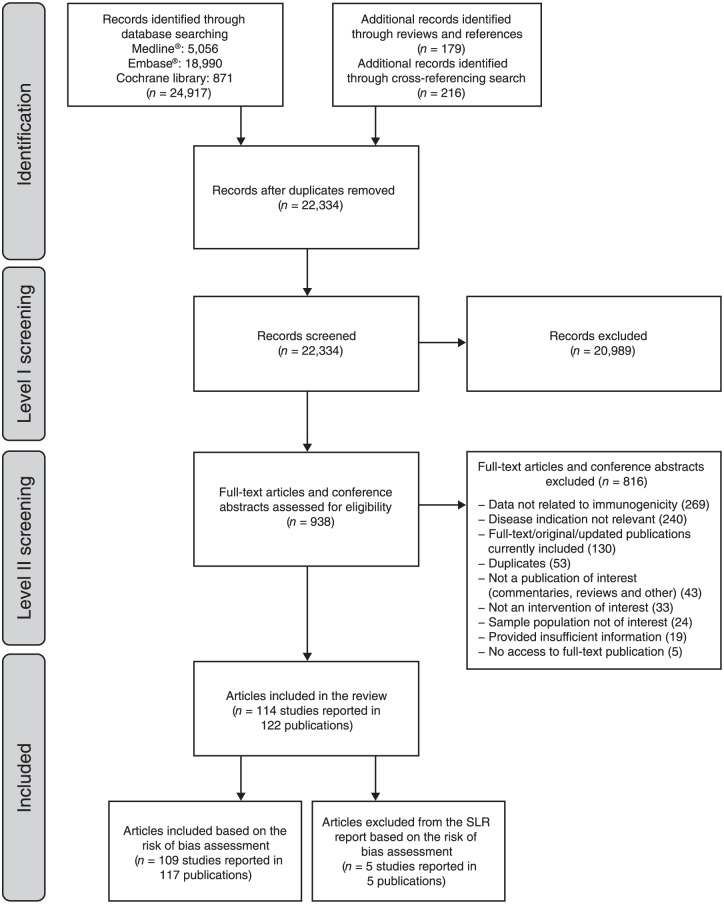
A total of 22,334 abstracts were identified and screened for eligibility; 938 articles were retrieved for full-text review, and 114 studies reported in 122 publications were finally assessed (Figure 1 and Supplementary Table 4).
Figure 1.
PRISMA flow diagram of screening and selection process.
n, number; SLR, systematic literature review.
Based on the full-text review of selected articles, five studies were excluded due to low-quality assessment scores. Details of these studies, and the reasons for their exclusion, are provided in Supplementary Table 5.
Over 90% of studies included in the review involved administration of ADM or IFX. Only a few studies were available for other agents; four, two, one and four studies were identified concerning CZP, GLM, UST and VDM treatment in IBD, respectively.
Rates of immunogenicity in patients receiving treatment with biologics
Rates of ADAb formation were extremely variable (Table 1). A brief summary of factors affecting the formation and detection of ADAbs is provided in Supplementary Table 6. A total of 12 RCTs and 62 non-RCTs or observational studies of IFX were reviewed, and rates of over 60% were observed in six of these studies. The highest rates of ADAb formation were associated with IFX in patients with CD or UC (Table 1). The highest rate for any other agent was 38% for ADM in one retrospective cohort study (Table 1).
Table 1.
| Biologic agent | All studies (n) | CD (n) | UC (n) | CD or UC (n) |
|---|---|---|---|---|
| Infliximabc | 0.0–65.3 (73) | 2.9–60.8 (22) | 6.1–41.0 (8) | 0.0–65.3 (43) |
| Adalimumab | 0.3–38.0 (22) | 0.3–35.0 (11) | 2.9–5.3 (3) | 14.0–38.0 (8) |
| Certolizumab pegol | 3.3–25.3 (4) | 3.3–25.3 (4) | – | – |
| Vedolizumab | 1.0–4.1 (4) | 1.0–4.1 (2) | 3.7 (1) | 4.0 (1) |
| Golimumab | 0.4–2.9 (2) | – | 0.4–2.9 (2) | – |
| Ustekinumab | 0.7 (1) | 0.7 (1) | – | – |
Only studies reporting rates of ADAbs were included (eight studies did not report specific proportions of patients developing ADAbs).
Immunogenicity analyses are product- and assay-specific.
One selected study was excluded from analysis as this had a small sample size (n = 28) and a high rate of immunogenicity (79%).
–, no publications available; ADAbs, anti-drug antibodies; CD, Crohn’s disease; n, number of studies; UC, ulcerative colitis.
Rates of immunogenicity in patients receiving treatment with infliximab
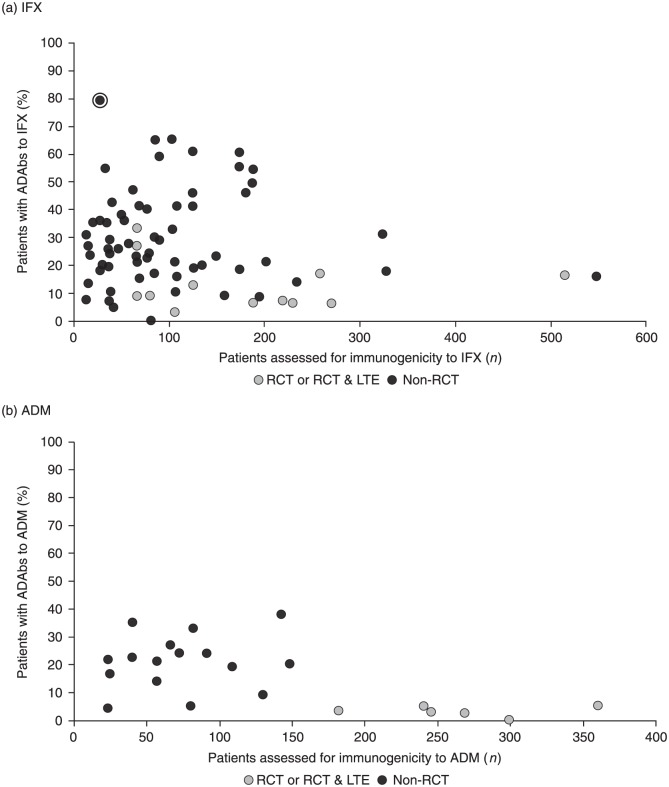
In total, 82 publications were identified, representing 78 studies on the immunogenicity of IFX; the majority of these (87.2%) were observational studies. Of the 78 studies reviewed, 74 reported the percentage of patients developing ADAbs to IFX. One of these studies25 was excluded from the analysis, as it reported a very high rate of immunogenicity (79%) based on a small sample size (n = 28). The percentage of patients with ADAbs to IFX varied widely, from 0% to 65.3% (Table 1 and Figure 2(a)).
Figure 2.
Rate of ADAbs formation to (a) IFX and (b) ADM.
Note: circled data point indicates study excluded from analysis as it had a small sample size (n = 28) and a high rate of immunogenicity (79%).
ADAbs, anti-drug antibodies; ADM, adalimumab; IFX, infliximab; LTE, long-term extension; n, number; RCT, randomized controlled trial.
Rates of immunogenicity in patients receiving treatment with adalimumab
A total of 25 publications were identified, representing 23 studies. As was the case with IFX, the majority of these (43.5%) were prospective observational studies. All but one of the studies reported the percentage of patients with CD or UC who developed ADAbs to ADM, which varied from 0.3% to 38% (Table 1 and Figure 2(b)).
Rates of immunogenicity in patients receiving treatment with certolizumab pegol, golimumab, ustekinumab and vedolizumab
CZP is approved for use in CD and not UC; therefore, in line with the approved indication, only studies reporting immunogenicity to CZP in CD were identified. These studies reported the percentage of patients developing ADAbs to CZP as ranging from 3.3% to 25.3% (Table 1). VDM is approved in both CD and UC, and identified studies reported immunogenicity developing in between 1% and 4.1% of patients (Table 1). GLM is not approved for use in CD. Two RCTs of GLM use in UC were identified, reporting that ADAbs were detected in 0.4% and 2.9% of patients (Table 1). In the single identified study of UST immunogenicity, 0.7% of patients in an RCT of UST in CD developed ADAbs (Table 1).
Impact of ADAb formation on treatment efficacy
In most of the included studies that evaluated efficacy, the presence of ADAbs was associated with a reduction in efficacy. Efficacy was assessed in a variety of ways, including Crohn’s Disease Activity Index (CDAI) response/remission, Mayo response, endoscopic improvement and treatment discontinuation. In studies of IFX, the proportion of patients achieving and maintaining a response was generally lower for patients with detected ADAbs than those without detected ADAbs ( Supplementary Table 7). ADAbs to ADM were also associated with reduced efficacy and a loss of response, together with a high rate of secondary treatment failure; these associ-ations were shown to be statistically significant in some studies ( Supplementary Table 8). In one study,26 discontinuation of ADM treatment was reported to be very high (83.3%) in patients with ADAbs ( Supplementary Table 8).
Impact of ADAb formation on treatment safety
In studies of IFX, AEs were more common in patients with ADAbs than in those without ADAbs. AEs observed included a higher proportion reporting infusion-related reactions. The studies for ADM, CZP, GLM, VDM or UST reported either no safety data segmented by ADAbs status or no increased safety concerns associated with the development of ADAbs.
Impact of ADAb formation on treatment pharmacokinetics
For studies included in this review in which serum levels of biologics were reported – ADM, CZP and IFX – ADAbs-positive patients had lower serum levels of the biologic than ADAbs-negative patients (Table 2).27–48
Table 2.
Serum levels of biologics in patients with and without ADAbs.
| Authors | Disease | Study design (number of patients assessed) | Biologic agent, sampling time and other specifications | Serum concentration of biologic agent (µg/mL)a | p value | |
|---|---|---|---|---|---|---|
| Patients with ADAbs | Patients without ADAbs | |||||
| Imaeda and colleagues35 | CD | Prospective cohort study (58) | IFX: trough | 0.2 | 3.4 | p < 0.01 |
| Levesque and colleagues36 | CD | Prospective cohort study (327) | IFX: 0 weeks IFX: 8 weeks |
1.5 1.6 |
6.4 7.4 |
– – |
| Vermeire and colleagues37 | CD | Prospective cohort study (174) | IFX: 4 weeks ADAbs conc. <8 µg/mL ADAbs conc. ⩾8 and <20 µg/mL ADAbs conc. ⩾20 µg/mL |
12.3 7.2 6.1 |
10.1 10.1 10.1 |
– – – |
| Stein and colleagues28 (CA) | CD | Prospective cohort study (69) | IFX: 26 weeks IFX: 52 weeks |
0.9 2.5 |
7.6 9.5 |
– – |
| Ainsworth and colleagues38 | CD | Retrospective cohort study (33) | IFX: 8 weeks Maintained responders Secondary non-responders Primary non-responders |
2.9 0.0 30.0 |
NR NR NR |
– – – |
| Hämäläinen and colleagues46 | CD or UC cohort | Prospective cohort study (28) | IFX: trough Non-responders Responders |
<0.1 >2.2 |
NR NR |
– – |
| Pallagi-Kunstár and colleagues39 | CD or UC cohort | Prospective cohort study (67) | IFX: trough | 2.7 | 3.9 | p = 0.015 |
| Paul and colleagues40 | CD or UC cohort | Prospective cohort study (103) | IFX: trough IFX monotherapy IFX plus immunosuppressants |
2.4 2.1 |
NR NR |
– – |
| Rivera and colleagues29 (CA) | CD or UC cohort | Prospective cohort study (69) | IFX: trough | 1.8 | 9.0 | p < 0.001 |
| Ungar and colleagues41 | CD or UC cohort | Prospective cohort study (125) | IFX: trough Responders Non-responders |
4.6 0.7 |
– – |
– – |
| Zitomersky and colleagues42 | CD or UC cohort | Prospective cohort study (134) | IFX: trough | 1.0 | 12.2 | p < 0.0001 |
| Vande Casteele and colleagues47 | CD or UC cohort | Retrospective cohort study (90) | IFX: 6 weeks | 5 | 27 | p = 0.003 |
| Frederiksen and colleagues30 (CA) | CD or UC cohort | Retrospective cohort study (189) | IFX: trough | 0.0 | 1.8 | p = 0.002 |
| Bodini and colleagues31 (CA) | CD | Prospective cohort study (23) | ADM: trough | 7.5 | 9.5 | p = 0.002 |
| Imaeda and colleagues43 | CD | Prospective cohort study (40) | ADM: trough | 5.5 | 16.0 | – |
| Yarur and colleagues32 (CA) | CD or UC cohort | Prospective cohort study (66) | ADM: timing unknown | 5.7 | 12.5 | – |
| Frederiksen and colleagues (CA)30
Frederiksen and colleagues48 |
CD or UC cohort | Retrospective cohort study (142) | ADM: trough | 0 | 8.3 | p < 0.0001 |
| Schreiber and colleagues44
Sandborn and colleagues27 Lichtenstein and colleagues45 Sandborn and colleagues33 |
CD | RCT (668) | CZP: 26 weeks 40 weeks 56 weeks 72 weeks 80 weeks |
6.1 1.0 0.9 1.5 1.6 |
23.8 9.0 8.7 8.7 9.4 |
– – – – – |
| Stefan and colleagues34 (CA) | CD | RCT and LTE (594) | CZP: trough | 2–4 | 8–12 | – |
All values presented to a maximum of one decimal place.
ADAbs, anti-drug antibodies; ADM, adalimumab; CA, conference abstract; CD, Crohn’s disease; conc., concentration; CZP, certolizumab pegol; IFX, infliximab; LTE, long-term extension; NR, not reported; RCT, randomized controlled trial; UC, ulcerative colitis.
Time course of immunogenicity
Minimal information was available regarding the earliest time at which immunogenicity was detected. Furthermore, the timing of dosing and subsequent testing for ADAbs varied between studies. The majority of studies reported ADAbs testing in serum taken prior to the next infusion (trough). ADAbs can occur as early as 10–14 days post-dosing, and this finding has been reported in some studies. However, ADAbs can also take months to reach detectable levels (Table 3).26,37,38,41,47,49–61
Table 3.
Time to detection of ADAbs.
| Author | Disease | Number of patients | ADAbs first detected | Biologic therapy |
|---|---|---|---|---|
| Baert and colleagues58
Magdelaine-Beuzelin and colleagues59 |
CD | 125 | After first infusion | IFX |
| Vermeire and colleagues37 | CD | 174 | After first infusion | IFX |
| Schatz and colleagues52 (CA) | CD and UC | 50 | Before second infusion | IFX |
| Ungar and colleagues41 | CD and UC | 125 | 2 weeks | IFX |
| Steenholdt and colleagues60
Steenholdt and colleagues51 |
CD and UC | 180 | Third infusion | IFX |
| Vande Casteele and colleagues47 | CD and UC | 90 | 4 weeks/fourth infusion | IFX |
| Vande Casteele and colleagues53 (CA) | CD and UC | 57 | 7 weeks | IFX |
| Ainsworth and colleagues38 | CD | 33 | 8 weeks | IFX |
| Vande Casteele and colleagues54 (CA) | CD and UC | 52 | 8 weeks/third infusion | IFX |
| Rosenthal and colleagues55 (CA) | CD and UC | 38 | <14 weeks | IFX |
| Colombel and colleagues50 | CD | 219 | 30 weeks | IFX |
| Hanauer and colleagues56 | CD | 299 | 2 weeks | ADM |
| West and colleagues61 | CD | 25 | 10 weeks | ADM |
| Karmiris and colleagues26
Baert and colleagues57 |
CD | 148 | 12 weeks | ADM |
| Sandborn and colleagues49 | UC | 721 | 6 weeks | GLM |
ADAbs, anti-drug antibodies; ADM, adalimumab; CA, conference abstract; CD, Crohn’s disease; GLM, golimumab; IFX, infliximab; UC, ulcerative colitis.
Pharmacoeconomics
We identified only one study that addressed pharmacoeconomic issues related to the development of immunogenicity to biologic therapy in IBD; this Danish study involved 69 patients with CD with secondary IFX failure receiving either IFX dose intensification (5 mg/kg every 4 weeks) or interventions based on serum IFX and IFX antibody levels.62 The authors concluded that monitoring of ADAbs to IFX in patients with CD was more cost-effective than dose escalation without drug monitoring and immunogenicity assessment.
Discussion
Development of immunogenicity occurs with all six of the biologic agents investigated in patients with IBD. Huge variability in the rate of immunogenicity was observed, depending on the timing of sampling, the assay technique (drug sensitive versus drug tolerant), the study population (agent-naïve or experienced and concomitant medications taken), the setting (RCTs versus observational study), and the criteria for a positive finding (a single sample or multiple samples above the defined threshold). Care must therefore be taken in the interpretation and comparison of these findings.
The timing of sampling (prior to or just after the next administration) greatly influences the detection rate. Most assays do not detect ADAbs in the presence of drug; since drug concentration is the lowest just before the next infusion, this is the optimal time to sample. This might be one explanation for the formation of ADAbs being reported to be lower in RCTs than in observational studies. Often, a limited number of time points were studied, and insufficient time was allowed for drug levels to decrease prior to sampling. However, it is also likely that improved assay techniques used in observational studies, together with the selection of patients with loss of response, led to higher levels of detection of ADAbs than in RCTs.
The immunogenicity reported for biologic agents used in IBD may adversely impact pharmacokinetics, efficacy and safety. Development of ADAbs to biologic therapy may therefore have a substantial impact on patients with IBD, as the consequent loss of efficacy and the increased potential for AEs is likely to result in a worsening of their condition, and a switch in their treatment may be required.6 Despite the range of treatments available for both CD and UC, the development of ADAbs to biologics, and the resultant impact, means that there is still an unmet treatment need in these diseases. Both European and US treatment guidelines require investigation of the immunogenic potential of biologic therapies when seeking marketing authorization for a new product for all indications, including CD and UC, and post-marketing monitoring of the development of immunogenicity.63,64
ADAbs produced in response to TNF inhibitors (TNFi) may be neutralizing or non-neutralizing, depending on the binding site. Neutralizing ADAbs produced in response to TNFi bind to the epitope binding (Fab′)2 region of the TNFi, thereby reducing the agents’ therapeutic activity.65 By contrast, non-neutralizing antibodies do not prevent binding of the agents to target molecules, and hence do not reduce the efficacy of biologic agents; they do, however, impact the pharmacokinetics, by accelerating clearance of the agent.66 It is also known that some ADAbs are transient in nature. These may appear at any time during treatment and seem to be of little clinical significance compared to persistent ADAbs.47,53,54
Giving biologic therapies in combination with concomitant immunosuppressive agents has been shown in several studies to reduce the development of ADAbs.67,68 One meta-analysis demonstrated that the use of concomitant immunosuppressants, primarily MTX, reduced the proportion of patients on IFX and ADM with detectable ADAbs by about 41% in those with rheumatoid arthritis, spondyloarthritis, psoriasis and IBD.67 However, the mechanism for this attenuation is not fully understood.68 Therefore, reducing the likelihood of a patient developing an immunogenic response to a biologic therapy may influence decisions made by the clinician in regard to the treatment prescribed for patients with IBD. There is evidence that scheduled treatment with IFX not only results in improved efficacy69 compared with episodic treatment, but that this is associated with a reduced level of immunogenicity.70 Clinicians should also consider administering a loading dose of a biologic at the commencement of therapy, because, in addition to achieving therapeutic levels rapidly, this has been reported to reduce immunogenicity,71 as has intravenous hydrocortisone treatment immediately prior to infusion with IFX.72
Potential limitations of the present study include the diversity of study design, patient populations, disease severity, concomitant therapies, assay methods and timing of the studies reviewed. This lack of standardization limited comparison across studies and between therapies. In addition, limited information was available for a number of the biologic agents included in the systematic literature search, with over 90% of studies reporting on IFX and ADM, reflecting the time these agents have been on the market compared to the other agents investigated. Not all studies reported data for some of the immunogenicity-related outcomes related to efficacy and safety, limiting conclusions on the impact of the development of ADAbs. It is also possible that rates of immunogenicity, as well as safety and efficacy outcomes, may have been underestimated in a number of the studies included in the review, due to insufficient sensitivity of the assay techniques used, small sample sizes, and limited follow-up of sampling (after wash-out of the biologic agent).
The potential clinical implication of lower biologic serum concentrations, reduced efficacy, and increased safety issues in the presence of immunogenicity is a concern for effective treatment and requires further study. In particular, more studies are required to assess the immunogenic potential of the more recently approved biologics. However, this analysis suggests that agents that elicit the lowest rate of immunogenicity may be preferential for treating CD and UC. Immunogenicity is one of the major causes for loss of response to biologic therapy,13 but immunogenicity must be assessed and interpreted in an appropriate manner. It is essential that research to improve understanding of immunogenicity and identify agents with limited immunogenic potential continues, in order to provide safe and effective treatment options.
Supplementary Material
Acknowledgments
The systematic literature review was conducted by Sadiq Lula and Carole Jones of Engage Scientific, a Division of Envision Pharma Group Ltd, and funded by Pfizer Inc.
The author contributions were as follows. SL was involved in study design, data collection and analysis. All authors were involved in data interpretation, and in manuscript drafting, reviewing and development. All authors read and approved the final manuscript, including the authorship list.
Footnotes
Funding: This work was supported by Pfizer Inc. Medical writing support, under the guidance of the authors, was provided by Carole Evans, PhD, on behalf of Complete Medical Communications, Macclesfield, UK and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015; 163: 461–464).
Conflict of interest statement: SV has received consulting fees from AbbVie, MSD, Takeda, Ferring, Genentech/Roche, Shire, Pfizer Inc, Galapagos, Mundipharma, Hospira, Celgene, Second Genome and Janssen; research grants from AbbVie, MSD and Takeda; and speaker fees from AbbVie, MSD, Takeda, Ferring, Dr Falk Pharma, Hospira and Tillot. AG has received speaker fees from AbbVie, MSD, Takeda, Pfizer, Hospira and Janssen Biologicals; consulting fees from UCB; and research grants from Pfizer Inc. SL is an employee of Engage Scientific, a Division of Envision Pharma Group Ltd, which acted as a paid consultant to Pfizer in connection with the conduct of the systematic literature review and interpretation of data. PA and AM are employees and stockholders of Pfizer Inc.
Contributor Information
Séverine Vermeire, Department of Gastroenterology, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium.
Ann Gils, Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium.
Paola Accossato, Pfizer Innovative Health, Pfizer S.r.l, Rome, Italy.
Sadiq Lula, Market Access Solutions, Envision Pharma Group Ltd, London, UK.
Amy Marren, Pfizer Innovative Health, Pfizer Inc, Collegeville, PA, USA.
References
- 1. Crohn’s & Colitis Foundation of America. What are Crohn’s & Colitis?, www.ccfa.org/what-are-crohns-and-colitis (2016, accessed 12 June 2017).
- 2. Crohn’s & Colitis UK. Ulcerative Colitis, www.crohnsandcolitis.org.uk/about-inflammatory-bowel-disease/ulcerative-colitis (2016, accessed 12 June 2017).
- 3. Crohn’s & Colitis UK. Crohn’s Disease, www.crohnsandcolitis.org.uk/about-inflammatory-bowel-disease/crohns-disease (2013, accessed 12 June 2017).
- 4. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46–54. [DOI] [PubMed] [Google Scholar]
- 5. Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 2010; 105: 501–523. [DOI] [PubMed] [Google Scholar]
- 6. Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn’s disease in adults. Am J Gastroenterol 2009; 104: 465–483. [DOI] [PubMed] [Google Scholar]
- 7. Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011; 60: 571–607. [DOI] [PubMed] [Google Scholar]
- 8. Dignass A, van Assche G, Lindsay JO, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis 2010; 4: 28–62. [DOI] [PubMed] [Google Scholar]
- 9. Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis 2012; 6: 991–1030. [DOI] [PubMed] [Google Scholar]
- 10. Panaccione R, Ghosh S. Optimal use of biologics in the management of Crohn’s disease. Therap Adv Gastroenterol 2010; 3: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park SC, Jeen YT. Current and emerging biologics for ulcerative colitis. Gut Liver 2015; 9: 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Silva PS, Nguyen DD, Sauk J, et al. Long-term outcome of a third anti-TNF monoclonal antibody after the failure of two prior anti-TNFs in inflammatory bowel disease. Aliment Pharmacol Ther 2012; 36: 459–466. [DOI] [PubMed] [Google Scholar]
- 13. Vincent FB, Morand EF, Murphy K, et al. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis 2013; 72: 165–178. [DOI] [PubMed] [Google Scholar]
- 14. Bendtzen K, Ainsworth M, Steenholdt C, et al. Individual medicine in inflammatory bowel disease: monitoring bioavailability, pharmacokinetics and immunogenicity of anti-tumour necrosis factor-alpha antibodies. Scand J Gastroenterol 2009; 44: 774–781. [DOI] [PubMed] [Google Scholar]
- 15. Vande Casteele N, Feagan BG, Gils A, et al. Therapeutic drug monitoring in inflammatory bowel disease: current state and future perspectives. Curr Gastroenterol Rep 2014; 16: 378. [DOI] [PubMed] [Google Scholar]
- 16. Guerra I, Chaparro M, Bermejo F, et al. Utility of measuring serum concentrations of anti-TNF agents and anti-drug antibodies in inflammatory bowel disease. Curr Drug Metab 2011; 12: 594–598. [DOI] [PubMed] [Google Scholar]
- 17. Bloem K, van Leeuwen A, Verbeek G, et al. Systematic comparison of drug-tolerant assays for anti-drug antibodies in a cohort of adalimumab-treated rheumatoid arthritis patients. J Immunol Methods 2015; 418: 29–38. [DOI] [PubMed] [Google Scholar]
- 18. Rivera R, Herranz P, Vanaclocha F. Clinical significance of immunogenicity in biologic therapy. Actas Dermosifiliogr 2014; 105: 1–4. [DOI] [PubMed] [Google Scholar]
- 19. Steenholdt C, Bendtzen K, Brynskov J, et al. Clinical implications of measuring drug and anti-drug antibodies by different assays when optimizing infliximab treatment failure in Crohn’s disease: post hoc analysis of a randomized controlled trial. Am J Gastroenterol 2014; 109: 1055–1064. [DOI] [PubMed] [Google Scholar]
- 20. Wolbink GJ, Vis M, Lems W, et al. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum 2006; 54: 711–715. [DOI] [PubMed] [Google Scholar]
- 21. Egger M, Juni P, Bartlett C, et al. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess 2003; 7: 1–76. [PubMed] [Google Scholar]
- 22. National Institute for Health and Clinical Excellence. Process and methods guide. Single technology appraisal: User guide for company evidence submission template, www.nice.org.uk/article/pmg24 (2015, accessed 12 June 2017).
- 23. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 24. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998; 52: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marits P, Landucci L, Sundin U, et al. Trough s-infliximab and antibodies towards infliximab in a cohort of 79 IBD patients with maintenance infliximab treatment. J Crohns Colitis 2014; 8: 881–889. [DOI] [PubMed] [Google Scholar]
- 26. Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn’s disease. Gastroenterology 2009; 137: 1628–1640. [DOI] [PubMed] [Google Scholar]
- 27. Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med 2007; 357: 228–238. [DOI] [PubMed] [Google Scholar]
- 28. Stein RE, Lee DY, Leonard MB, et al. The association between drug levels, anti-drug antibodies, and therapeutic response during infliximab therapy in pediatric Crohn’s disease. J Crohns Colitis 2014; 8: S434. [Google Scholar]
- 29. Rivera Rivera ED, Liao C, Van’t Hof K, et al. Correlation between infliximab levels (IFX) and antibody to infliximab (ATI) in pediatric patients with inflammatory bowel disease (IBD) with the commercially available assay using electrochemilumescense. Gastroenterology 2014; 146: S782–S783. [Google Scholar]
- 30. Frederiksen MT, Ainsworth MA, Brynskov J, et al. Antibodies against infliximab are associated with increased risk of anti-adalimumab antibody development in patients with inflammatory bowel disease. Gastroenterology 2014; 146: S238. [DOI] [PubMed] [Google Scholar]
- 31. Bodini G, Savarino V, Dulbecco P, et al. The influence of anti-adalimumab antibodies on adalimumab trough levels, TNF-alpha levels and clinical outcome. J Crohns Colitis 2014; 8: S42. [Google Scholar]
- 32. Yarur AJ, Deshpande AR, Sussman DA, et al. Serum adalimumab levels and antibodies correlate with endoscopic intestinal inflammation and inflammatory markers in patients with inflammatory bowel disease. Gastroenterology 2013; 144: S774–S775. [Google Scholar]
- 33. Sandborn WJ, Binion DG, Rubin D, et al. Antibodies against certolizumab pegol (CZP), plasma concentrations of CZP and efficacy in patients with Crohn’s disease receiving continuous CZP therapy with or without concomitant immunosuppressants. Am J Gastroenterol 2011; 106: S439–S440. [Google Scholar]
- 34. Stefan S, Sandborn WJ, Choi J, et al. Anti-drug antibodies separate responses of markers of inflammation (c-reactive protein, fecal calprotectin) in Crohn’s disease patients treated with certolizumab pegol. Inflamm Bowel Dis 2014; 20: S68. [Google Scholar]
- 35. Imaeda H, Andoh A, Fujiyama Y. Development of a new immunoassay for the accurate determination of anti-infliximab antibodies in inflammatory bowel disease. J Gastroenterol 2012; 47: 136–143. [DOI] [PubMed] [Google Scholar]
- 36. Levesque BG, Greenberg GR, Zou G, et al. A prospective cohort study to determine the relationship between serum infliximab concentration and efficacy in patients with luminal Crohn’s disease. Aliment Pharmacol Ther 2014; 39: 1126–1135. [DOI] [PubMed] [Google Scholar]
- 37. Vermeire S, Noman M, van Assche G, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut 2007; 56: 1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ainsworth MA, Bendtzen K, Brynskov J. Tumor necrosis factor-alpha binding capacity and anti-infliximab antibodies measured by fluid-phase radioimmunoassays as predictors of clinical efficacy of infliximab in Crohn’s disease. Am J Gastroenterol 2008; 103: 944–948. [DOI] [PubMed] [Google Scholar]
- 39. Pallagi-Kunstár É, Farkas K, Szepes Z, et al. Utility of serum TNF-alpha, infliximab trough level, and antibody titers in inflammatory bowel disease. World J Gastroenterol 2014; 20: 5031–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Paul S, Del Tedesco E, Marotte H, et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis 2013; 19: 2568–2576. [DOI] [PubMed] [Google Scholar]
- 41. Ungar B, Chowers Y, Yavzori M, et al. The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut 2014; 63: 1258–1264. [DOI] [PubMed] [Google Scholar]
- 42. Zitomersky NL, Atkinson BJ, Fournier K, et al. Antibodies to infliximab are associated with lower infliximab levels and increased likelihood of surgery in pediatric IBD. Inflamm Bowel Dis 2015; 21: 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Imaeda H, Takahashi K, Fujimoto T, et al. Clinical utility of newly developed immunoassays for serum concentrations of adalimumab and anti-adalimumab antibodies in patients with Crohn’s disease. J Gastroenterol 2014; 49: 100–109. [DOI] [PubMed] [Google Scholar]
- 44. Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med 2007; 357: 239–250. [DOI] [PubMed] [Google Scholar]
- 45. Lichtenstein GR, Thomsen OØ, Schreiber S, et al. Continuous therapy with certolizumab pegol maintains remission of patients with Crohn’s disease for up to 18 months. Clin Gastroenterol Hepatol 2010; 8: 600–609. [DOI] [PubMed] [Google Scholar]
- 46. Hämäläinen A, Sipponen T, Kolho KL. Serum infliximab concentrations in pediatric inflammatory bowel disease. Scand J Gastroenterol 2013; 48: 35–41. [DOI] [PubMed] [Google Scholar]
- 47. Vande Casteele N, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol 2013; 108: 962–971. [DOI] [PubMed] [Google Scholar]
- 48. Frederiksen MT, Ainsworth MA, Brynskov J, et al. Antibodies against infliximab are associated with de novo development of antibodies to adalimumab and therapeutic failure in infliximab-to-adalimumab switchers with IBD. Inflamm Bowel Dis 2014; 20: 1714–1721. [DOI] [PubMed] [Google Scholar]
- 49. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014; 146: 85–95. [DOI] [PubMed] [Google Scholar]
- 50. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010; 362: 1383–1395. [DOI] [PubMed] [Google Scholar]
- 51. Steenholdt C, Al-khalaf M, Brynskov J, et al. Clinical implications of variations in anti-infliximab antibody levels in patients with inflammatory bowel disease. Inflamm Bowel Dis 2012; 18: 2209–2217. [DOI] [PubMed] [Google Scholar]
- 52. Schatz SB, Prell C, Freudenberg F, et al. Correlation of infliximab levels and antibodies with clinical outcome in children with IBD. J Pediatr Gastroenterol Nutr 2011; 52: E45. [Google Scholar]
- 53. Vande Casteele N, Cuypers L, Singh S, et al. Transient versus sustained antibodies to infliximab: possibility to overcome low titer antibody responses by dose optimisation. J Crohns Colitis 2012; 6: S110. [Google Scholar]
- 54. Vande Casteele N, Cuypers L, Singh S, et al. Antibodies to infliximab can either be persistent or transient: a retrospective case-control study in IBD patients treated with infliximab maintenance therapy. Gastroenterology 2012; 142: S114. [Google Scholar]
- 55. Rosenthal C, Melmed G, Tripuraneni B, et al. Early infliximab trough levels predict remission at one year in pediatric IBD patients. Inflamm Bowel Dis 2012; 18: S5. [DOI] [PubMed] [Google Scholar]
- 56. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 2006; 130: 323–333. [DOI] [PubMed] [Google Scholar]
- 57. Baert F, Lockton S, Haunstein S, et al. Antibodies to adalimumab predict inflammation in Crohn’s patients on maintenance adalimumab therapy. Gastroenterology 2014; 146: S242. [Google Scholar]
- 58. Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 2003; 348: 601–608. [DOI] [PubMed] [Google Scholar]
- 59. Magdelaine-Beuzelin C, Vermeire S, Goodall M, et al. IgG1 heavy chain-coding gene polymorphism (G1m allotypes) and development of antibodies-to-infliximab. Pharmacogenet Genomics 2009; 19: 383–387. [DOI] [PubMed] [Google Scholar]
- 60. Steenholdt C, Bendtzen K, Brynskov J, et al. Cut-off levels and diagnostic accuracy of infliximab trough levels and anti-infliximab antibodies in Crohn’s disease. Scand J Gastroenterol 2011; 46: 310–318. [DOI] [PubMed] [Google Scholar]
- 61. West RL, Zelinkova Z, Wolbink GJ, et al. Immunogenicity negatively influences the outcome of adalimumab treatment in Crohn’s disease. Aliment Pharmacol Ther 2008; 28: 1122–1126. [DOI] [PubMed] [Google Scholar]
- 62. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014; 63: 919–927. [DOI] [PubMed] [Google Scholar]
- 63. European Medicine Agency. Guideline on immunogenicity assessment of biotechnology-derived therapeutic proteins, www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003946.pdf (2007, accessed 12 June 2017).
- 64. U.S. Food and Drug Administration. Guidance for industry: immunogenicity assessment for therapeutic protein products, www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM338856.pdf (2014, accessed 12 June 2017).
- 65. van Schie KA, Hart MH, de Groot ER, et al. The antibody response against human and chimeric anti-TNF therapeutic antibodies primarily targets the TNF binding region. Ann Rheum Dis 2015; 74: 311–314. [DOI] [PubMed] [Google Scholar]
- 66. van der Laken CJ, Voskuyl AE, Roos JC, et al. Imaging and serum analysis of immune complex formation of radiolabelled infliximab and anti-infliximab in responders and non-responders to therapy for rheumatoid arthritis. Ann Rheum Dis 2007; 66: 253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Garcês S, Demengeot J, Benito-Garcia E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: a systematic review of the literature with a meta-analysis. Ann Rheum Dis 2013; 72: 1947–1955. [DOI] [PubMed] [Google Scholar]
- 68. Jani M, Barton A, Warren RB, et al. The role of DMARDs in reducing the immunogenicity of TNF inhibitors in chronic inflammatory diseases. Rheumatology (Oxford) 2014; 53: 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology 2004; 126: 402–413. [DOI] [PubMed] [Google Scholar]
- 70. Caviglia R, Boskoski I, Cicala M. Maintenance treatment with infliximab for the management of Crohn’s disease in adults. Biologics 2009; 3: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Takeuchi T, Yamamoto K, Yamanaka H, et al. Post-hoc analysis showing better clinical response with the loading dose of certolizumab pegol in Japanese patients with active rheumatoid arthritis. Mod Rheumatol 2016; 26: 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Farrell RJ, Alsahli M, Jeen YT, et al. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn’s disease: a randomized controlled trial. Gastroenterology 2003; 124: 917–924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.