Abstract
The grass Ammophila breviligulata (American beachgrass) is known to host an endophyte of the genus Epichloë. Based on morphological characteristics it was originally identified as Acremonium typhinum var. ammophilae and is currently designated as Epichloë typhina var. ammophilae. However, the Epichloë species has not previously been identified based on DNA sequence data. Based on phylogenetic placement of beta-tubulin and translation elongation factor 1-alpha DNA sequences the endophyte is identified as a member of E. amarillans rather than E. typhina.
Keywords: Epichloë, Ammophila, Phylogeny
Introduction
Epichloë spp. (Clavicipitaceae, Ascomycota) are systemic fungal endophytes of many cool season grasses (Schardl et al., 2009; Tadych, Bergen & White Jr, 2014). Infection by these endophytes often provides numerous benefits to the host, such as insect, drought and disease resistance (Clarke et al., 2006; Kuldau & Bacon, 2008). The Epichloë endophyte found in some plants of American beachgrass, Ammophila breviligulata Fernald (Agrostidinae), was previously designated as Acremonium typhinum var. ammophilae White et Morgan-Jones, var. nov. (White Jr et al., 1992). The fungal species identification was based on morphological characteristics and was made before the current extensive molecular data on Epichloë spp. were available.
The nomenclature of the grass endophytes has since been revised. Based on 18S ribosomal DNA phylogeny, Glenn et al. (1996) proposed that the anamorphic grass fungal endophytes be reclassified from the genus Acremonium to the genus Neotyphodium. In 2011 the 18th International Botanical Congress ratified a proposal to consolidate anamorphic and teleomorphic fungal species based on the principle of “one fungus = one name” (Norvell, 2011). Leuchtmann et al. (2014) presented a comprehensive review of the known Epichloë spp., and proposed a realignment of the anamorphic Neotyphodium spp. with Epichloë. Based on the previous assignment (White Jr et al., 1992), the species of Epichloë infecting A. breviligulata was designated as E. typhina var. ammophilae (J.F. White and Morgan-Jones) J.F. White, comb. nov. (Leuchtmann et al., 2014). However, the A. breviligulata endophyte had not yet been subjected to any DNA sequence based analysis. Here we report the phylogenetic placement of the Epichloë endophyte of A. breviligulata based on beta-tubulin (tubB) and translation elongation factor 1-alpha (tefA) DNA sequences. In this analysis the endophyte from A. breviligulata is placed in a clade with E. amarillans, rather than E. typhina. We therefore propose that it be considered a member of E. amarillans.
Materials & Methods
Plant and fungal materials
Ammophila breviligulata (American beachgrass) cultivar ‘Cape’ plants were acquired through the USDA Plant Materials Center, Cape May, NJ. The cultivar Cape was developed from a single plant, which was vegetatively propagated, and was released by the Soil Conservation Service, USDA, in 1972 (Gaffney & Duell, 1974). This cultivar is the source of the endophyte previously described by White Jr et al. (1992). The endophyte from all plants of the Cape cultivar therefore originated from a single isolate. Plants were transplanted to pots in standard potting mix for growth in the greenhouse (Pro-mix BX Mycorrhizae, Quakertown, PA), watered to saturation as needed and fertilized weekly with a standard 20:20:20 fertilizer (Plantex 20–20–20 Classic; Master Plant-Prod Inc., Brampton, Ontario). Positive infection status was confirmed by plating surface-sterilized leaf sheath fragments and by microscopic observations, which followed previously published procedures (Bacon & White Jr, 1994; Florea, Schardl & Hollin, 2015).
The fungal endophyte was obtained by surface sterilizing innermost leaf sheath tissue and allowing the fungus to grow out from the host-grass tissue onto potato dextrose agar (PDA) plates. A plate was washed with sterile water and the water spread on a fresh PDA plate to isolate colonies arising from single spores. A single spore colony was isolated and then grown in potato dextrose broth for use in fungal genomic DNA extraction.
DNA isolation and amplification
Fungal DNA was isolated from 50 mg of tissue by using the Synergy 2.0 Plant DNA Extraction Kit (Ops Diagnostics, Lebanon, NJ, USA). Translation elongation factor 1 alpha (tefA) and beta-tubulin (tubB) sequences were amplified from the fungal DNA. The primers used for tubB gene amplification were tubB-exon1d-1 (5′-GAG AAA ATG CGT GAG ATT GT-3′) and tubB-exon4u-2 (5′GTT TCG TCC GAG TTC TCG AC-3′) and those for tefA gene amplification were tefA-exon1d-1 (5′GGG TAA GGA CGA AAA GAC-3′) and tefA-exon5u-1 (5′CGG CAG CGA TAA TCA GGA TAG-3′) (Moon et al., 2002). The 50 µL PCR reactions contained 0.2 µg of fungal genomic DNA, 40 picomoles of each forward and reverse primer (Integrated DNA Technologies, Inc., Coralville, IA, USA), and 25 µl of PrimeSTAR Max Premix (Clontech Laboratories, Mountain View, CA, USA). PCR was performed in a GeneAmp 9700 thermocycler (Applied Biosystems, Inc., Foster City, CA, USA) with 30 cycles of denaturation at 98 °C for 10 s, followed by 15 s annealing at 55 °C, and 2 min extension at 72 °C. The concentration of the PCR product was estimated by running a 5 µl aliquot on a 1% agarose gel and comparing the band intensity with that of the 1000 bp band in the HyperLadder 1kb marker (Bioline USA Inc., Taunton, MA, USA). The PCR products were sequenced directly (Genewiz, Inc., South Plainfield, NJ, USA). For each sequencing reaction, approximately 40 ng of PCR product was treated with 2 µl of ExoSAP-IT (USB Corp., Cleveland, OH, USA) to remove unincorporated primers and excess dNTPs. The ExoSAP-IT reaction was performed at 37 °C for 15 min followed by heating at 80 °C for 15 min to inactivate the enzymes. Sequencing was done in both directions.
Accession numbers
GenBank accession numbers for the tefA and tubB sequences are KX523126 and KX523127, respectively.
Phylogenetic analysis
The tubB and tefA sequences were aligned with those from non-hybrid Epichloë spp. available from the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/). The sequences included for comparison are those used previously in the analysis of E. typhina subsp. poae (see Tadych et al., 2012, Table 1). The Clustal-X program (Thompson et al., 1997) was used to align the sequences, and the alignment was modified manually to minimize gaps. The phylogenetic analysis was performed with the PAUP* program, version 4.0b10 for Macintosh. The phylogenetic analysis was done by using the maximum parsimony full heuristic search option set to random sequence addition, tree-bisection-reconnection (TBR) branch swapping, and Multrees on with 1000 bootstrap replications. Gaps were treated as missing data. The tubB tree was based on 469 total characters, of which 377 were constant, 17 variable characters were parsimony uninformative, and 75 variable characters were parsimony informative. The tefA tree was based on 804 total characters, of which 582 were constant, 45 variable characters were parsimony uninformative, and 177 variable characters were parsimony informative.
The sequences were also analyzed by the maximum likelihood method in the PAUP* program, which generated trees of similar topology to those of the maximum parsimony analyses (not shown). For the maximum likelihood analyses, the trees were generated with a fast heuristic search using the HKY85 model of sequence evolution, and 100 bootstrap replications.
Results & Discussion
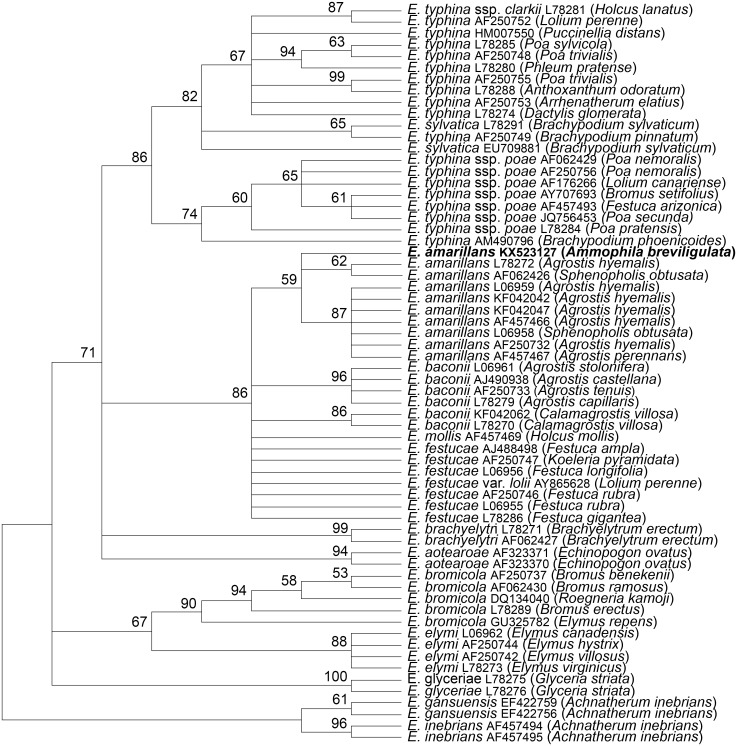
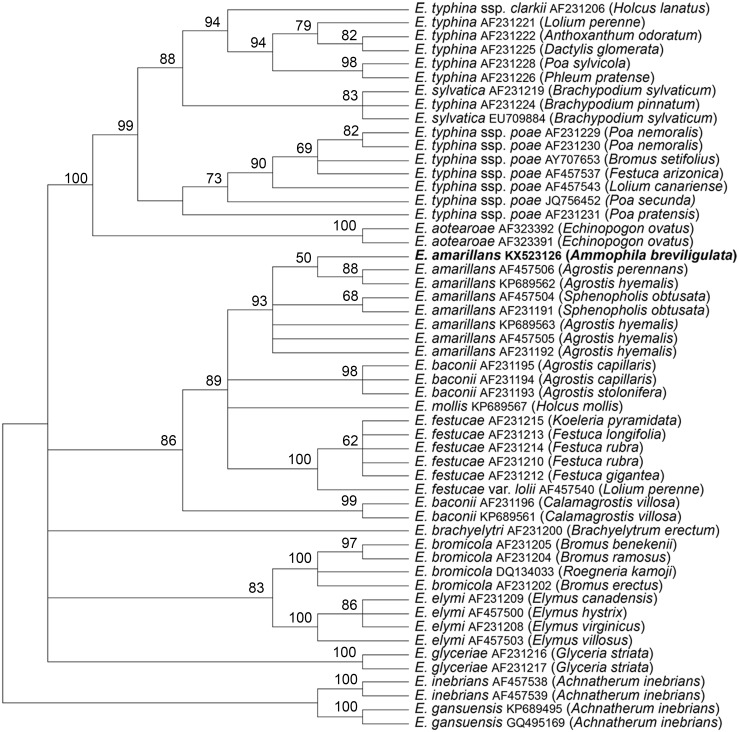
The tubB and tefA genes were chosen for analysis of the endophyte of A. breviligulata since sequences from many Epichloë spp. isolates are readily available at NCBI. There was no evidence of heterogeneity that would indicate that the endophyte had multiple gene copies typical for species of hybrid origin. Maximum parsimony phylogenetic analyses of endophyte tubB and tefA sequences are shown in Figs. 1 and 2, respectively. The species names are those presented in Leuchtmann et al. (2014). The E. gansuensis and E. inebrians sequences were designated as outgroups for rooting the trees since they are considered the basal Epichloë spp. (Ambrose, Koppenhöfer & Belanger, 2014; Chen et al., 2015). In both the tubB and tefA trees, the sequences from the endophyte of A. brevilligulata were placed in the E. amarillans clades.
Figure 1. Rooted 50% majority rule consensus maximum parsimony phylogenetic tree of tubB sequences.
The E. inebrians and E. gansuensis sequences were designated as outgroups for rooting the tree. The numbers at the nodes are the bootstrap percentages based on 1,000 replications. Sequence references are given by Epichloë species names, GenBank accession numbers, and the host grass species are given in parentheses. The E. amarillans isolate from A. breviligulata is identified by bolded text.
Figure 2. Rooted 50% majority rule consensus maximum parsimony phylogenetic tree of tefA sequences.
The E. inebrians and E. gansuensis sequences were designated as outgroups for rooting the tree. The numbers at the nodes are the bootstrap percentages based on 1,000 replications. Sequence references are given by Epichloë species names, GenBank accession numbers, and the host grass species are given in parentheses. The E. amarillans isolate from A. breviligulata is identified by bolded text.
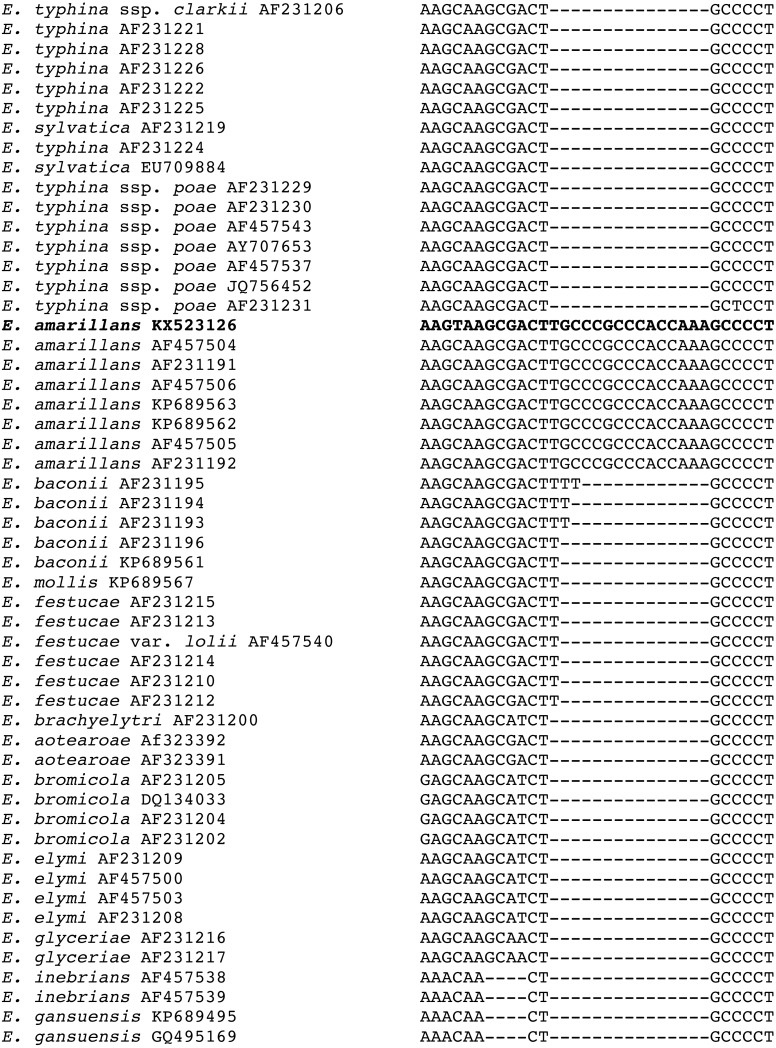
Additional support for the species assignment as E. amarillans comes from the presence of a shared 15 bp insert in the tefA sequence found only in other isolates of E. amarillans (Fig. 3). Shared indels are considered to be important phylogenetic characters (Pasko, Ericson & Elzanowski, 2011; Simmons & Ochoterena, 2000; Väli et al., 2008). A shared 19 bp deletion in tubB sequences was previously considered as supporting evidence that the E. typhina ssp. poae isolates infecting several different grass genera had a common progenitor (Tadych et al., 2012).
Figure 3. Sequence alignment of the region of the 15 bp insertion in the tefA sequences from the E. amarillans isolates.
The isolate from A. breviligulata is identified by bolded text.
A. breviligulata is ecologically important in shoreline dune building. Endophyte infected A. breviligulata was reported to exhibit greater vegetative growth and dune building relative to uninfected plants (Emery, Bell-Dereske & Rudgers, 2015) but was also correlated with reduced species richness at the field site (Rudgers et al., 2015). In these reports the endophyte was referred to as Epichloë sp.
In a survey of herbarium samples collected prior to 1971 (White Jr et al., 1992) and a survey of plants collected from natural dunes sites in Michigan and Indiana (Emery, Thompson & Rudgers, 2010), most A. breviligulata plants tested were not endophyte infected. However, the cultivar Cape, which is the source of the endophyte analyzed here, is highly infected (White Jr et al., 1992; Emery, Thompson & Rudgers, 2010). This cultivar is commonly used in dune revegetation. Because of the importance of A. breviligulata in dune restoration and the widespread dissemination of the endophyte infected cultivar Cape along the East Coast of the United States, it is important to know the taxonomic affiliation of the Epichloë endophyte that it is hosting. Based on the phylogenetic data presented here we propose that the endophyte of A. breviligulata pertains to the species E. amarillans.
Funding Statement
This work was supported by the Rutgers Center for Turfgrass Science and the USDA National Institute of Food and Agriculture Hatch project accession number 1007068 through the New Jersey Agricultural Experiment Station, Hatch project NJ12140. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Ian Drake conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
James F. White Jr conceived and designed the experiments, analyzed the data, wrote the paper, reviewed drafts of the paper.
Faith C. Belanger conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
DNA Deposition
References
- Ambrose, Koppenhöfer & Belanger (2014).Ambrose KV, Koppenhöfer AM, Belanger FC. Horizontal gene transfer of a bacterial insect toxin gene into the Epichloë fungal symbionts of grasses. Scientific Reports. 2014;4:5562. doi: 10.1038/srep05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon & White Jr (1994).Bacon CW, White Jr JF. Stains, media, and procedures for analyzing endophytes. In: Bacon CW, White Jr JF, editors. Biotechnology of endophytic fungi of grasses. Boca Raton: CRC Press; 1994. pp. 47–56. [Google Scholar]
- Chen et al. (2015).Chen L, Li X, Li C, Swoboda GA, Young CA, Sugawara K, Leuchtmann A, Schardl CL. Two distinct Epichloë species symbiotic with Achnatherum inebrians, drunken horse grass. Mycologia. 2015;107:863–873. doi: 10.3852/15-01. [DOI] [PubMed] [Google Scholar]
- Clarke et al. (2006).Clarke BB, White Jr JF, Hurley RH, Torres MS, Sun S, Huff DR. Endophyte-mediated suppression of dollar spot disease in fine fescues. Plant Disease. 2006;90:994–998. doi: 10.1094/PD-90-0994. [DOI] [PubMed] [Google Scholar]
- Emery, Bell-Dereske & Rudgers (2015).Emery SM, Bell-Dereske L, Rudgers JA. Fungal symbiosis and precipitation alter traits and dune building by the ecosystem engineer, Ammophila breviligulata. Ecology. 2015;96:927–935. doi: 10.1890/14-1121.1. [DOI] [PubMed] [Google Scholar]
- Emery, Thompson & Rudgers (2010).Emery SM, Thompson D, Rudgers JA. Variation in endophyte symbiosis, herbivory and drought tolerance of Ammophila breviligulata populations in the great lakes region. The American Midland Naturalist. 2010;163:186–196. doi: 10.1674/0003-0031-163.1.186. [DOI] [Google Scholar]
- Florea, Schardl & Hollin (2015).Florea S, Schardl CL, Hollin W. Detection and isolation of Epichloë species, fungal endophytes of grasses. Current Protocols in Microbiology. 2015;38 doi: 10.1002/9780471729259.mc19a01s38. 19A.1.1–19A.1.24. [DOI] [PubMed] [Google Scholar]
- Gaffney & Duell (1974).Gaffney FB, Duell RW. Registration of Cape American beachgrass. Crop Science. 1974;14:777. doi: 10.2135/cropsci1974.0011183X001400050052x. [DOI] [Google Scholar]
- Glenn et al. (1996).Glenn AE, Bacon CW, Price R, Hanlin RT. Molecular phylogeny of Acremonium and its taxonomic implications. Mycologia. 1996;88:369–383. doi: 10.2307/3760878. [DOI] [Google Scholar]
- Kuldau & Bacon (2008).Kuldau G, Bacon CW. Clavicipitaceous endophytes: their ability to enhance resistance of grasses to multiple stresses. Biological Control. 2008;46:57–71. doi: 10.1016/j.biocontrol.2008.01.023. [DOI] [Google Scholar]
- Leuchtmann et al. (2014).Leuchtmann A, Bacon CW, Schardl CL, White Jr JF, Tadych M. Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia. 2014;106:202–215. doi: 10.3852/13-251. [DOI] [PubMed] [Google Scholar]
- Moon et al. (2002).Moon CD, Scott B, Schardl CL, Christensen MJ. The evolutionary origins of three new Neotyphodium endophyte species from grasses indigenous to the Southern Hemisphere. Mycologia. 2002;94:694–711. doi: 10.2307/3761720. [DOI] [PubMed] [Google Scholar]
- Norvell (2011).Norvell LL. Fungal nomenclature. 1. Melbourne approves a new code. Mycotaxon. 2011;116:481–490. doi: 10.5248/116.481. [DOI] [Google Scholar]
- Pasko, Ericson & Elzanowski (2011).Pasko L, Ericson PGP, Elzanowski A. Phylogenetic utility and evolution of indels: a study in neognathous birds. Molecular Phylogenetics and Evolution. 2011;61:760–771. doi: 10.1016/j.ympev.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Rudgers et al. (2015).Rudgers JA, Bell-Dereske L, Crawford KM, Emery SM. Fungal symbiont effects on dune plant diversity depend on precipitation. Journal of Ecology. 2015;103:219–230. doi: 10.1111/1365-2745.12338. [DOI] [Google Scholar]
- Schardl et al. (2009).Schardl CL, Scott B, Florea S, Zhang D. Epichloë endophytes: clavicipitaceous symbionts of grasses. In: Deising H, editor. Plant relationships, The Mycota V. 2nd Edition Springer-Verlag; Berlin: 2009. pp. 275–306. [Google Scholar]
- Simmons & Ochoterena (2000).Simmons MP, Ochoterena H. Gaps as characters in sequence-based phylogenetic analyses. Systematic Biology. 2000;9:369–381. doi: 10.1093/sysbio/49.2.369. [DOI] [PubMed] [Google Scholar]
- Tadych et al. (2012).Tadych M, Ambrose KV, Bergen MS, Belanger FC, White Jr JF. Taxonomic placement of Epichloë poae sp. nov. and horizontal dissemination to seedlings via conidia. Fungal Diversity. 2012;54:117–131. doi: 10.1007/s13225-012-0170-0. [DOI] [Google Scholar]
- Tadych, Bergen & White Jr (2014).Tadych M, Bergen MS, White Jr JF. Epichloë spp. associated with grasses: new insights on life cycles, dissemination and evolution. Mycologia. 2014;106:181–201. doi: 10.3852/106.2.181. [DOI] [PubMed] [Google Scholar]
- Thompson et al. (1997).Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL-X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väli et al. (2008).Väli Ü, Brandström M, Johansson M, Ellegren H. Insertion-deletion polymorphisms (indels) as genetic markers in natural populations. BMC Genetics. 2008;9:8. doi: 10.1186/1471-2156-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White Jr et al. (1992).White Jr JF, Halisky PM, Sun S, Morgan-Jones G, Funk Jr CR. Endophyte-host associations in grasses. XVI. Patterns of endophyte distribution in species of the tribe Agrostideae. American Journal of Botany. 1992;79:472–477. doi: 10.2307/2445162. [DOI] [Google Scholar]