Abstract
Aims
To compare cardiac function when pacing from the right or left ventricular apex in patients with preserved left ventricular systolic function, at 1-year follow-up.
Methods
Prospective, multicentre centre randomizing conventional right ventricular apical (RVA) versus left ventricular apical (LVA) pacing using a coronary sinus lead in patients requiring ventricular pacing for bradycardia. Follow-up was performed using 3D-echocardiography at 6 and 12 months.
Results
A total of 36 patients (age 75.4 ± 8.7 years, 21 males) were enrolled (17 patients in the RVA group and 19 patients in the LVA group). A right ventricular lead was implanted in 8 patients in the LVA group, mainly because of high capture thresholds. There were no differences in the primary endpoint of LVEF at 1 year (60.4 ± 7.1% vs 62.1 ± 7.2% for the RVA and LVA groups respectively, P = 0.26) nor in any of the secondary endpoints (left ventricular dimensions, left ventricular diastolic function, right ventricular systolic function and tricuspid/mitral insufficiency). LVEF did not change significantly over follow-up in either group. Capture thresholds were significantly higher in the LVA group, and two patients had unexpected loss of capture of the coronary sinus lead during follow-up.
Conclusions
Left univentricular pacing seems to be comparable to conventional RVA pacing in terms of ventricular function at up to 1 year follow-up, and is an option to consider in selected patients (e.g. those with a tricuspid valve prosthesis).
Keywords: Ventricular pacing, Right ventricle, Left ventricle, Coronary sinus, Ventricular function
1. Introduction
It is well established that chronic right ventricular apical (RVA) pacing may have an adverse effect on left ventricular systolic function [1], [2] leading in the long term to adverse clinical outcome in such as heart failure [3], atrial fibrillation [4], [5], and even death [6]. As an alternative to RVA pacing, the interventricular septum or right ventricular outflow tract have been proposed to avoid these adverse effects, but results have been equivocal [7]. In the PACE trial [2], cardiac resynchronization therapy (CRT) has been shown to preserve left ventricular ejection fraction (LVEF) compared to an absolute decrease of 7% at 1 year with RVA pacing in patients with normal baseline systolic function. However, before CRT implantation can be routinely proposed in these patients, further evidence is required to support the findings and incremental cost related to CRT implantation compared to a dual chamber device needs to be addressed. Another alternative is pacing from the left ventricular apex (LVA), which has shown more favourable results in terms of left ventricular pump function than the RVA [8], [9]. The LVA can be paced either using a surgically-placed epicardial lead, or transvenously via transseptal puncture or the coronary sinus (CS). The first two alternatives have limitations, as they respectively require thoracotomy or are associated with a high risk of thrombo-embolic events [10]. Current implantation tools allow safe and successful implantation of left ventricular leads in >95% of patients [11]. Furthermore, positioning the left ventricular pacing lead in the distal anterior cardiac vein is usually technically easier than in a lateral or postero-lateral branch, without the risk of adverse effects such as phrenic nerve capture. The approach has the added advantage, compared to right ventricular pacing, of bypassing the tricuspid valve, which may prevent valve dysfunction associated with pacing leads [12].
Our aim was to compare chronic effects on LVEF resulting from transvenous pacing of the RVA versus the LVA in patients with preserved left ventricular systolic function.
2. Material and methods
The Right Versus Left Apical transvenous pacing for patients with preserved left ventricular systolic function (RIVELA) study was a physician-initiated multicentre, prospective, randomized, open label study conducted in Switzerland. Blinding was impossible due to group attribution being readily assessable by the chest X-ray, electrocardiogram or echocardiography. Randomization was performed 1:1 using a list of randomly permuted blocks into 2 groups stratified by baseline LVEF (≤/>55%). Patients randomized to the RVA group had a conventional pacing lead positioned at the RVA using a standard implantation technique. Those randomized to the LVA group had dedicated CS lead positioned as close as possible to the left ventricular apex (see Fig. 1). Inclusion criteria were: age >18 years; requirement for ventricular pacing according to current guidelines (including chronic atrial fibrillation); anticipated ≥50% daily ventricular pacing; LVEF ≥45% as evaluated by 2D-echocardiography, 3D-echocardiography, magnetic resonance imaging or radionuclide/contrast ventriculography. Patients were excluded it they had prior tricuspid valve replacement (annuloplasty was permitted), an intrinsic rhythm <30bpm, permanent atrial fibrillation scheduled to undergo ablation of the atrioventricular node, an echocardiographic window of insufficient quality for measuring LVEF, life expectancy of <1 year, pregnancy, or were unable/unwilling to sign a patient informed consent form.
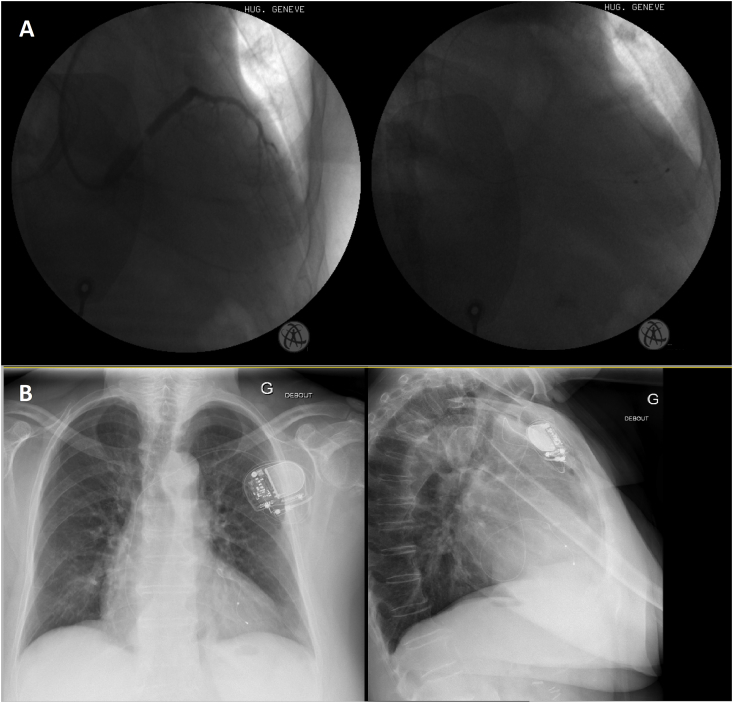
Fig. 1.
Examples of patients in the left ventricular apical group. A (left) Coronary sinus venogram showing possible target anterior cardiac and posterior veins with (right) final lead position in the posterior cardiac vein (postero-anterior views). B another patient with the coronary sinus lead in the anterior cardiac vein (postero-anterior and left lateral views).
Pacemakers were either dual-chamber (DDDR) or single-chamber (VVIR) St-Jude Medical (Sylmar, CA) models with the ventricular autocapture feature. The model of the bipolar right ventricular pacing lead was left to the discretion of the implanting physician, and was implanted at the apex according to standard implantation techniques. The bipolar left ventricular pacing lead was the St-Jude Medical Quickflex model, implanted distally as closely as possible to the apex e.g. via the anterior cardiac vein or postero-lateral vein. The CS was cannulated using dedicated guiding catheters as for standard CRT implantation. Programming of device parameters was left to the discretion of the study centre. There was no requirement to force ventricular pacing (e.g. by shortening AV intervals).
An echocardiogram (during ventricular pacing at 80bpm, to avoid changes in LVEF due to different heart rates) was performed within 72 h of implantation at 6 months and at 12 months' follow-up. A Philips (Gronigen, NL) iE33 echocardiograph was used for recording a standard and a real-time 3D echocardiogram (RT3DE). Two-dimensional, M-Mode, Doppler, tissue Doppler imaging (TDI) and 2D strain measurements were performed according to recommendations [13], [14]. RT3DE acquisition with a matrix-array transducer (X3-1 or X5-1) from the apical view was used for measuring left ventricular end-diastolic (LVEDV) and end-systolic (LVESV) volumes and LVEF. Raw data were analyzed by an echocardiography core lab at the University Hospital of Geneva by a single operator (H.M.) using Philips QLAB 9.1.
Device interrogation and clinical assessment were performed at each follow-up.
The primary endpoint was comparison of LVEF (measured by RT3DE) between groups at 1 year follow-up. Secondary endpoints were changes in echocardiographic parameters over time (including LVEF, LVEDV, LVESV, right ventricular function, severity of tricuspid and mitral regurgitation, tissue Doppler imaging) and evolution of pacemaker electrical parameters.
Data were entered on electronic case-report forms with monitoring by independent clinical trial units at each centre. The trial was registered on clinicaltrials.gov as NCT01535404 and conducted according to the declaration of Helsinki. Approval was obtained by the institutional ethics committees and each patient provided written informed consent.
2.1. Statistical analysis
In order to show a difference of 5% (in absolute terms) in LVEF between groups at 12 months with 90% power, a sample size of 172 patients. (86 in each group) was calculated. Evaluation was performed according to a modified intention-to-treat principle, with patients analyzed as initially randomized. No data imputation was performed for the main analyses, though the analysis of the primary endpoint was repeated, by conservatively imputing the worst observed LVEF in the LV group and the best observed LVEF in the RV group. Data are reported as mean ± standard deviation (SD) and the Mann Whitney U test and the Fisher exact test, for continuous and categorical variables respectively, were performed to verify that baseline characteristics were uniformly distributed. A generalized linear model was used to compare groups for the primary endpoint, with a Huber-White robust standard errors analysis to account for intra-centre correlation. The mean difference and 95% confidence interval (95%CI) were computed. Mixed models with random effect for centre and patient, and exchangeable correlation structure were used to compare changes over time of continuous echo and device data. A generalized linear model with identity link was used to compare rate of adverse events between groups. Two-sided P values of <0.05 were considered to be statistically significant. Analyses were performed by a statistician (C.K) using Stata 14.2 (StataCorp, College Station, TX, USA).
3. Results
A total of 36 patients (17 in the RVA group and 19 in the LVA group) were enrolled from the 5 centres during the period ranging from 15.5.2012 to 11.8.2014. Patient demographics are shown in Table 1. The study was terminated because of slow enrolment, due logistical challenges, and especially due to the advent of MRI-conditional devices (randomization to the LVA group precluded MRI-conditionality). A total of 8/19 (42%) patients randomized to the LVA group received a right ventricular lead instead. The reasons were high capture thresholds in 5 patients, CS dissection in 2 patients and pneumothorax in 1 patient.
Table 1.
Patient demographics at baseline.
| RVA (n = 17) | LVA (n = 19) | P | |
|---|---|---|---|
| Age (yrs) | 75.5 ± 5.3 | 75.3 ± 11.0 | 0.29 |
| Male | 12 | 9 | 0.48 |
| Syncope | 4 | 6 | 0.72 |
| Atrial arrhythmias | 1.00 | ||
| Chronic AF | 3 | 4 | |
| Paroxysmal AF/flutter | 4 | 2 | |
| Indication | 0.95 | ||
| Sinus dysfunction | 4 | 2 | |
| AVB I | 1 | 1 | |
| AVB II | 5 | 6 | |
| AVB III | 4 | 6 | |
| Slow AF | 3 | 4 | |
| Comorbidities | |||
| Hypertension | 4 | 3 | 0.68 |
| Ischemic heart disease | 2 | 2 | 1.00 |
| Diabetes | 5 | 7 | 0.73 |
| Renal dysfunction | 4 | 1 | 0.17 |
| Previous stroke | 1 | 2 | 1.00 |
| Previous neoplasia | 3 | 2 | 0.65 |
| Pacemaker type | |||
| single/dual chamber | 3/14 | 4/15 | 0.66 |
| Medication | |||
| ACEI | 4 | 5 | 1.00 |
| ARB | 3 | 4 | 1.00 |
| CCB | 4 | 7 | 0.48 |
| Digoxin | 1 | 1 | 1.00 |
| Diuretics | 6 | 8 | 0.74 |
| Nitrates | 0 | 1 | 1.00 |
| Baseline echography | |||
| LVEF (%) | 62.3 ± 8.3 | 62.6 ± 6.5 | 0.84 |
| LVEDV (ml) | 72.8 ± 23.5 | 65.4 ± 19.4 | 0.43 |
| LVESV (ml) | 28.2 ± 13.2 | 25.1 ± 11.0 | 0.51 |
AF = atrial fibrillation; ACEI = angiotensinogen converting enzyme inhibitor; ARB = angiotensin receptor blockers; AVB = atrioventricular block; CCB = calcium channel blockers; LVEF = left ventricular ejection fraction; LVEDV = left ventricular end-diastolic volume; LVESV = left ventricular systolic volume.
Total (skin-to-skin) procedure durations were 41 ± 11min in the RVA group and 91 ± 34min in the LVA group (P < 0.001); fluoroscopy durations were 4.6 ± 2.3min versus 18.1 ± 10.4min respectively (P < 0.001). All patients in the RVA group received a lead in the intended position. In patients in the LVA group who received a left ventricular lead, the lead was in the anterior cardiac vein in 8 patients (in a mid-ventricular position in 6 patients and in an apical position in 2 patients) and in a posterior or lateral vein in 3 patients (in a mid-ventricular position in 1 patient and in an apical position in 2 patients). None of the patients had phrenic nerve capture at the final lead position.
The paced QRS duration was 156 ± 23 ms for the RVA group and tended to be higher for the LVA group at 167 ± 22 ms (P = 0.22).
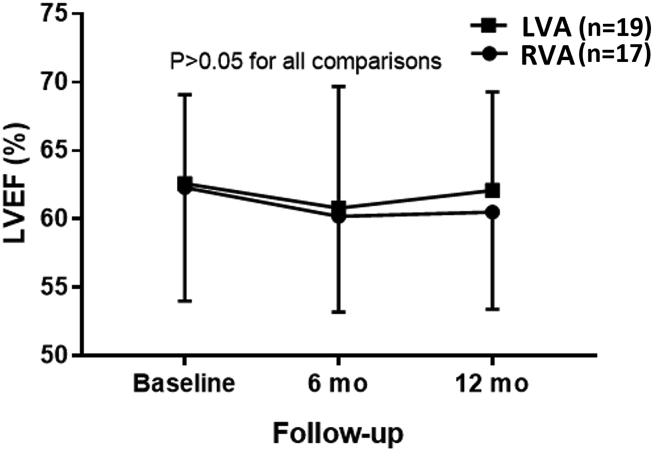
Full datasets were available for all patients at 6 months and for 33 patients (15 and 18 patients in the RVA and LVA groups respectively) at 12 months. There was no difference in the primary endpoint of LVEF between the groups at 12 months: 60.4 ± 7.1% vs 62.1 ± 7.2% for the RVA and LVA groups respectively (difference 1.7%, 95%CI -1.8 to 5.2, P = 0.26, see Fig. 2). After conservative imputation, the difference was 0.7% (95%CI -4.1 to 5.5). None of the echographic parameters (left ventricular remodelling, left ventricular diastolic function, right ventricular systolic function, tricuspid and mitral regurgitation etc.) showed any differences between or within the groups over time (see Table 2). Electrical parameters showed a significantly higher ventricular capture threshold in the LVA group (although values were comparable at 12 months) and lower sensing amplitudes (see Table 3).
Fig. 2.
Primary endpoint of left ventricular ejection fraction (LVEF) measured by 3D echocardiography at 1 year. Data are mean ± SD. There were no difference between groups at baseline, 6-month and 12 month follow-up, nor within each group over the different timepoints. LVA = left ventricular apex; RVA = right ventricular apex.
Table 2.
Comparison of echocardiographic parameters over time.
| Baseline |
6 months |
12 months |
P | ||||
|---|---|---|---|---|---|---|---|
| RVA | LVA | RVA | LVA | RVA | LVA | ||
| LVEF (%) | 62.3 ± 8.3 | 62.6 ± 6.5 | 60.2 ± 7.1 | 60.8 ± 8.9 | 60.5 ± 7.1 | 62.1 ± 7.2 | 0.59 |
| LVEDV (ml) | 72.8 ± 23.5 | 65.4 ± 19.4 | 85.9 ± 33.5 | 72.6 ± 27.9 | 81 ± 25 | 72 ± 25 | 0.77 |
| LVESV (ml) | 28.2 ± 13.2 | 25.1 ± 11.0 | 36.0 ± 20.9 | 30.2 ± 19.7 | 32.6 ± 14.1 | 28.1 ± 15.4 | 0.80 |
| LVEDD (mm) | 48.5 ± 6.5 | 46.4 ± 6.8 | 47.5 ± 6.3 | 47.5 ± 6.3 | 46 ± 6.6 | 45.9 ± 7.6 | 0.16 |
| LVESD (mm) | 30.8 ± 5.9 | 30.9 ± 6.2 | 32.6 ± 6.6 | 30.3 ± 7.6 | 30.5 ± 6.2 | 28.6 ± 6.0 | 0.67 |
| LVFS (%) | 36.6 ± 8.4 | 36.0 ± 7.5 | 32.4 ± 8.8 | 33.9 ± 11.7 | 33.7 ± 8.1 | 35.5 ± 9.1 | 0.77 |
| LVPS base (%) | −21.3 ± 7.1 | −19.9 ± 8.5 | −21.5 ± 9.1 | −25.5 ± 7.3 | −22 ± 5.1 | −24.8 ± 11.7 | 0.78 |
| LVPS mid (%) | −27.1 ± 8.4 | −29.6 ± 17.4 | −22.3 ± 7.8 | −26.2 ± 9.3 | −20.3 ± 12.2 | −18.1 ± 12.5 | 0.23 |
| LVPS apex (%) | −16.4 ± 8.2 | −22.5 ± 7.5 | −22.3 ± 9.8 | −21.0 ± 11.0 | −15.5 ± 17.2 | −22.3 ± 6.2 | 0.47 |
| RVFAC (%) | 48.1 ± 10.6 | 46.4 ± 6.8 | 41.5 ± 9.3 | 41.7 ± 7.0 | 42.2 ± 8.1 | 43.3 ± 8.9 | 0.70 |
| TAPSE (mm) | 19.1 ± 5.4 | 20.7 ± 4.9 | 19.0 ± 5.6 | 19.33 ± 4.0 | 17.9 ± 4.1 | 19.4 ± 2.9 | 0.30 |
| TA TDI (cm/s) | 12.2 ± 4.0 | 13.0 ± 3.2 | 11.1 ± 3.6 | 11.8 ± 2.4 | 11.0 ± 2.8 | 11.6 ± 2.2 | 0.13 |
| PASPG (mmHg) | 30.0 ± 13.1 | 23.2 ± 4.4 | 30.1 ± 12.4 | 25.8 ± 8.6 | 27.2 ± 7.1 | 26.8 ± 3.9 | 0.58 |
| E/e’ lateral | 10.0 ± 4.3 | 9.1 ± 4.1 | 11.4 ± 3.9 | 9.5 ± 4.1 | 11.0 ± 5.3 | 9.0 ± 3.6 | 0.57 |
| E/e’ septal | 15.3 ± 6.0 | 11.4 ± 4.6 | 14 ± 6.1 | 14.0 ± 5.3 | 12.6 ± 5.0 | 13.0 ± 4.6 | 0.54 |
| E/e’ average | 12.4 ± 4.8 | 10.3 ± 4.0 | 12.8 ± 4.5 | 11.5 ± 4.6 | 11.8 ± 4.9 | 11.1 ± 3.8 | 0.77 |
| LAS (cm2) | 21.3 ± 6.9 | 20.3 ± 5.0 | 22.1 ± 6.7 | 23.5 ± 14.2 | 20.8 ± 7.1 | 25.5 ± 18.9 | 0.76 |
| Grade 3/4 MR (%) | 29 | 26 | 35 | 16 | 29 | 21 | 0.71 |
| Grade 3/4 TR (%) | 29 | 11 | 35 | 26 | 29 | 42 | 0.18 |
LVEF = left ventricular ejection fraction; LVEDV = left ventricular end-diastolic volume; LVESV = left ventricular end-systolic volume; LVEDD = left ventricular end-diastolic dimension; LVFS = left ventricular fractional shortening; LVPS = left ventricular peak 2D strain; RVAC = right ventricular fractional area change; TAPSE = tricuspid annulus peak systolic excursion; TA TDI = tricuspid annulus tissue Doppler imaging peak velocity; PAPSG = pulmonary artery peak systolic pressure gradient; E/e’ average = average of septal and lateral mitral E/e’; LAS = left atrial surface in the apical 4 chamber view; MR = mitral regurgitation; TR = tricuspid regurgitation.
Table 3.
Comparison of electrical parameters over time.
| Baseline |
6 months |
12 months |
P | ||||
|---|---|---|---|---|---|---|---|
| RV | LV | RV | LV | RV | LV | ||
| V pacing (%) | 77.0 ± 37.7 | 83.4 ± 32.4 | 82.3 ± 26.0 | 66.7 ± 38.8 | 69.4 ± 37.8 | 72.6 ± 39.0 | 0.32 |
| V Capture threshold (V) | 0.53 ± 0.16 | 0.86 ± 0.70 | 0.63 ± 0.24 | 0.92 ± 0.66 | 0.72 ± 0.21 | 0.72 ± 0.34 | 0.035 |
| V impedance (Ohms) | 597 ± 116 | 633 ± 150 | 550 ± 130 | 501 ± 84 | 539 ± 125 | 541 ± 170 | 0.41 |
| V sensing (mV) | 10.0 ± 3.1 | 9.2 ± 3.6 | 10.4 ± 2.1 | 7.4 ± 3.3 | 10.3 ± 3.3 | 7.7 ± 4.1 | 0.007 |
Procedure- or device-related adverse events occurred in 7 patients who had been randomized to the LV group: 2 CS dissections (1 with pericardial effusion treated conservatively) and 1 pneumothorax (these 3 patients received a right ventricular lead), 2 acute pericarditis treated conservatively (both patients had atrial active-fixation leads which were held responsible for the complication), 2 patients with loss of left ventricular capture at 6 months' follow-up and 35 months after implantation. In the former case the patient no longer required ventricular pacing and declined revision, and in the latter patient a right ventricular lead had to be implanted due to complete loss of capture. Sensing thresholds and lead impedances were normal in both cases, without visible lead dislodgement. There were 2 admissions for heart failure in patients randomized to the RVA group, both were resolved with medical treatment. None of the patients died during the study.
4. Discussion
The RIVELA study is the first trial randomizing RVA to LVA for anti-bradycardia pacing in patients with preserved ventricular function. The main findings of our study were that 1) left uni-ventricular pacing via a CS tributary was comparable to traditional RVA pacing in terms of LVEF and secondary cardiac functional endpoints (left ventricular diastolic function and remodelling, right ventricular systolic function, mitral/tricuspid valve function) at 1-year follow-up 2) LVEF remained preserved in both groups over follow-up as compared to baseline 3) the rate of cross-over from LVA to RVA pacing at implantation was high, mainly due to elevated thresholds 4) there were significantly higher capture thresholds and lower sensing amplitudes with LVA pacing (although values were on average acceptable), with instances of unexpected loss of capture over follow-up.
The reports which showed superiority of LVA pacing compared to RV pacing were acute hemodynamic studies using surgically-placed epicardial leads in dogs [8], [9] or in a pediatric population [8], and may not reflect mid-term results in an adult population. However, only 4/19 (21%) patients who were in the LVA group actually received pacing from the apical region of the left ventricle, with the majority receiving left ventricular pacing from a mid-ventricular position due to CS tributary anatomy or were crossed over to RVA pacing at implantation for technical reasons. It is therefore impossible to draw definite conclusions on the effect of LVA pacing from our dataset. Nevertheless, our study shows that left univentricular pacing is feasible and that ventricular function is preserved over follow-up, making this an option to consider in selected patients, e.g. in case of a prosthetic tricuspid valve [15]. However, the implant procedure is more challenging than with RVA pacing, with longer procedure durations and greater fluoroscopic exposure. The 42% crossover rate from LVA to RVA was mainly due to high ventricular capture thresholds. CS leads are usually implanted with a success rate of >95% in the setting of cardiac resynchronization therapy, where higher thresholds may be accepted for treating heart failure. The indication for device implantation in our study was anti-bradycardia pacing, where asystole may result from loss of capture. This explains why a right ventricular lead was preferred to provide reliable pacing in case of high left ventricular capture thresholds. It may also be that CS lead implantation in the setting of preserved ventricular function without elevated right heart pressures may be more difficult, due to less dilation of tributary veins and smaller hearts (although this was not specifically evaluated). Electrical parameters were satisfactory overall in both groups, and there were no instances of evident ventricular lead dislodgment. Nevertheless, two patients in the LVA group had unexpected complete loss of ventricular capture (possibly due to micro-dislodgment), which casts a doubt on the reliability of this option in patients requiring ventricular pacing.
In the quest for alternative pacing sites to the RVA, pacing of the right interventricular septum has been explored, without conclusive results. More recently, left ventricular septal pacing using an investigational lead with a long electrically-active screw placed deep in the interventricular septum has shown promising results [16]. Also, there has lately been a revival in interest in His bundle pacing which is the most physiological means to electrically activate the ventricles [17].
4.1. Study limitations
The main limitation of our study is its small sample size. The crossover rate to right ventricular pacing in the LVA group was high, mitigating possible differences between the groups. Most patients who received a CS lead were paced from a mid-ventricular site (rather than from the apex), which may have impacted cardiac function. Follow-up was limited to 1 year, which may have been too short to show differences between treatment modalities. The patients were paced on average 67–83% of the time, and results may have differed had they been paced permanently.
5. Conclusions
Pacing of the left ventricle via a CS tributary is an alternative to RVA pacing and shows comparable outcome in terms of cardiac function over 1 year follow-up in patients with preserved baseline ventricular function. This is an option to consider in selected patients, e.g. those with prior tricuspid valve surgery, but does not otherwise seem to confer any advantage compared to conventional right ventricular pacing, with a more complex intervention and less reliable electrical parameters.
Funding
This is a physician-initiated study. Supplementary costs were neutralized by St-Jude Medical Switzerland.
Declarations of interest
H.B. has received institutional fellowship support from Abbott.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Leclercq C., Gras D., Le Helloco A., Nicol L., Mabo P., Daubert C. Hemodynamic importance of preserving the normal sequence of ventricular activation in permanent cardiac pacing. Am Heart J. 1995;129:1133–1141. doi: 10.1016/0002-8703(95)90394-1. [DOI] [PubMed] [Google Scholar]
- 2.Yu C.M., Chan J.Y., Zhang Q., Omar R., Yip G.W., Hussin A. Biventricular pacing in patients with bradycardia and normal ejection fraction. N Engl J Med. 2009;361:2123–2134. doi: 10.1056/NEJMoa0907555. [DOI] [PubMed] [Google Scholar]
- 3.Sweeney M.O., Hellkamp A.S. Heart failure during cardiac pacing. Circulation. 2006;113:2082–2088. doi: 10.1161/CIRCULATIONAHA.105.608356. [DOI] [PubMed] [Google Scholar]
- 4.Sweeney M.O., Bank A.J., Nsah E., Koullick M., Zeng Q.C., Hettrick D. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007;357:1000–1008. doi: 10.1056/NEJMoa071880. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney M.O., Hellkamp A.S., Ellenbogen K.A., Greenspon A.J., Freedman R.A., Lee K.L. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932–2937. doi: 10.1161/01.CIR.0000072769.17295.B1. [DOI] [PubMed] [Google Scholar]
- 6.Andersen H.R., Thuesen L., Bagger J.P., Vesterlund T., Thomsen P.E. Prospective randomised trial of atrial versus ventricular pacing in sick-sinus syndrome. Lancet (Lond. Engl.) 1994;344:1523–1528. doi: 10.1016/s0140-6736(94)90347-6. [DOI] [PubMed] [Google Scholar]
- 7.Barold S.S., Herweg B. Right ventricular outflow tract pacing: not ready for prime-time. J Interventional Cardiac Electrophysiol Int J Arrhythm Pacing. 2005;13:39–46. doi: 10.1007/s10840-005-0371-5. [DOI] [PubMed] [Google Scholar]
- 8.Vanagt W.Y., Verbeek X.A., Delhaas T., Mertens L., Daenen W.J., Prinzen F.W. The left ventricular apex is the optimal site for pediatric pacing: correlation with animal experience. Pacing Clin Electrophysiol. 2004;27:837–843. doi: 10.1111/j.1540-8159.2004.00544.x. [DOI] [PubMed] [Google Scholar]
- 9.Mills R.W., Cornelussen R.N., Mulligan L.J., Strik M., Rademakers L.M., Skadsberg N.D. Left ventricular septal and left ventricular apical pacing chronically maintain cardiac contractile coordination, pump function and efficiency. Circ Arrhythm Electrophysiol. 2009;2:571–579. doi: 10.1161/CIRCEP.109.882910. [DOI] [PubMed] [Google Scholar]
- 10.Morgan J.M., Biffi M., Geller L., Leclercq C., Ruffa F., Tung S. ALternate Site Cardiac ResYNChronization (ALSYNC): a prospective and multicentre study of left ventricular endocardial pacing for cardiac resynchronization therapy. Eur Heart J. 2016;37:2118–2127. doi: 10.1093/eurheartj/ehv723. [DOI] [PubMed] [Google Scholar]
- 11.Gras D., Bocker D., Lunati M., Wellens H.J.J., Calvert M., Freemantle N. Implantation of cardiac resynchronization therapy systems in the CARE-HF trial: procedural success rate and safety. Europace. 2007;9:516–522. doi: 10.1093/europace/eum080. [DOI] [PubMed] [Google Scholar]
- 12.Lin G., Nishimura R.A., Connolly H.M., Dearani J.A., Sundt T.M., III, Hayes D.L. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. J Am Coll Cardiol. 2005;45:1672–1675. doi: 10.1016/j.jacc.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins C., Moir S., Chan J., Rakhit D., Haluska B., Marwick T.H. Left ventricular volume measurement with echocardiography: a comparison of left ventricular opacification, three-dimensional echocardiography, or both with magnetic resonance imaging. Eur Heart J. 2009;30:98–106. doi: 10.1093/eurheartj/ehn484. [DOI] [PubMed] [Google Scholar]
- 14.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellikka P.A. Recommendations for chamber quantification: a report from the American society of Echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the european association of echocardiography, a branch of the european society of cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Sideris S., Drakopoulou M., Oikonomopoulos G., Gatzoulis K., Stavropoulos G., Limperiadis D. Left ventricular pacing through coronary sinus is feasible and safe for patients with prior tricuspid valve intervention. Pacing Clin Electrophysiol. 2016;39:378–381. doi: 10.1111/pace.12815. [DOI] [PubMed] [Google Scholar]
- 16.Mafi-Rad M., Luermans J.G., Blaauw Y., Janssen M., Crijns H.J., Prinzen F.W. Feasibility and acute hemodynamic effect of left ventricular septal pacing by transvenous approach through the interventricular septum. Circ Arrhythm Electrophysiol. 2016;9:e003344. doi: 10.1161/CIRCEP.115.003344. [DOI] [PubMed] [Google Scholar]
- 17.Sharma P.S., Ellenbogen K.A., Trohman R.G. Permanent His bundle pacing: the past, present, and future. J Cardiovasc Electrophysiol. 2017;28:458–465. doi: 10.1111/jce.13154. [DOI] [PubMed] [Google Scholar]